Abstract
Inflammation mechanisms play a critical role in muscle homeostasis, and in Muscular Dystrophies (MDs), the myofiber damage triggers chronic inflammation which significantly controls the disease progression. Immunomodulatory strategies able to target inflammatory pathways and mitigate the immune-mediated damage in MDs may provide new therapeutic options. Owing to its capacity of influencing the immune response and enhancing tissue repair, stem cells’ secretome has been proposed as an adjunct or standalone treatment for MDs. In this review study, we discuss the challenging points related to the inflammation condition characterizing MD pathology and provide a concise summary of the literature supporting the potential of perinatal stem cells in targeting and modulating the MD inflammation.
1. Muscular Dystrophies and Inflammation
Muscular Dystrophies (MDs) encompass a group of rare, inheritable genetic myopathies, characterized by progressive muscle weakness, muscle degeneration, and fibro-adipose tissue replacement [1]. According to the World Health Organization (WHO), MDs collectively affect approximately 1 in 3500 to 5000 live male births globally, with Duchenne Muscular Dystrophy (DMD) being the most prevalent form. These conditions impose significant economic and social burdens on patients, families, and healthcare systems. The pathogenetic mechanism of MDs is rarely due to a single gene defect; rather, it typically results from complex changes in multiple molecular networks [2]. One of the main challenges in managing MDs is the interplay between muscle degeneration and immune responses [3]. Inflammation plays a dual role in muscle homeostasis; indeed, even though it is essential for the activation and differentiation of muscle progenitor cells, during tissue repair, persistent inflammation can exacerbate muscle degeneration [4]. In MDs, the recurrent release of intracellular muscle components following myofiber damage triggers chronic inflammation, contributing significantly to disease progression [5]. MDs exhibit distinct immune profiles, driven by varying immunomodulatory molecules that regulate the immune reaction to muscle damage. Immune responses are typically characterized by tissue infiltration of macrophages, neutrophils, and both helper and cytotoxic T-lymphocytes [6]. For example, M1 macrophages, which are associated with a pro-inflammatory profile, promote the degradation of damaged muscle through the release of cytokines like Tumor Necrosis Factor (TNF-α), Interleukin 1β (IL-1β), and Interleukin 6 (IL-6) [7], facilitating the clearance of necrotic cells [8]. On the contrary, M2 macrophages release anti-inflammatory cytokines (e.g., IL-4 and IL-10) to suppress the inflammatory response and promote tissue repair [9,10]). This delicate balance between immune-mediated muscle degeneration and regeneration underscores the complexity of MD pathogenesis [3]. In Duchenne Muscular Dystrophy (DMD), muscle biopsies exhibit calcium deposits, varied sized and hypercontracted fibers, internal nuclei, fibrosis, and fatty infiltration [1]. The mutations in the dystrophin gene lead to chronic activation of the immune system [11] and both innate and adaptive immune responses play pivotal roles in exacerbating muscle damage [12]. Macrophages and cytotoxic T-cells release pro-inflammatory cytokines, such as TNF-α and Interferon γ (IFNγ), perpetuating muscle degeneration and fibrosis [13]. Therapeutic interventions targeting macrophages, TGF-β signaling, and fibrosis have shown promise in preclinical models of DMD [12]. Moreover, the mitochondrial component in DMD pathogenesis is increasingly recognized as a critical factor in disease progression. Dysfunctional mitochondria contribute to Oxidative Stress, impaired calcium homeostasis, and energy deficits, exacerbating myofiber damage. This mitochondrial dysfunction is intricately linked to chronic inflammation and the activation of immune responses, further worsening muscle degeneration [14]. Additionally, Dubinin et al. [15] identified the mitochondrial permeability transition (MPT) pore as a key contributor to mitochondrial dysfunction in dystrophin-deficient mdx mice and demonstrated the therapeutic potential of alisporivir, a non-immunosuppressive MPT pore inhibitor, in preserving mitochondrial function and alleviating cardiomyopathy in DMD. Limb-girdle muscular dystrophies (LGMDs) also involve immune activation. In LGMD, R5 and R9 inflammation is a prominent feature, and patients often respond to corticosteroid treatment, suggesting that immune cells such as B-cells, CD4+, and CD8+ T-cells contribute to disease pathology [16,17]. Dysferlinopathies, that feature inflammatory infiltrates, myonecrosis, and regeneration, including Miyoshi myopathy and LGMD type 2R are marked by defective membrane repair mechanisms, leading to immune cell infiltration, particularly macrophages [18]. Inflammatory cytokines such as TNF-α and iNOS are elevated, and NF-κB signaling is implicated in disease progression [19]. Notably, the complement system plays a distinct role in dysferlinopathies, with the Membrane Attack Complex (MAC) localizing to muscle fibers, an absent feature in DMD, indicating disease-specific immune mechanisms [20]. Congenital Muscular Dystrophies (CMDs) exhibit inflammation like in DMD, with macrophages dominating the inflammatory infiltrates [21] and marked dystrophic changes with striking fatty infiltration. Lamininopathies, such as Merosin-Deficient Congenital Muscular Dystrophy (MDC1A), are associated with early immune cell invasion and activation of TLR and NF-κB signaling pathways [22]. Laminin α-2-deficient dystrophy shows fatty infiltration, with complete absence or patchy reduction in laminin α-2. In Facioscapulohumeral Muscular Dystrophy (FSHD), around 30–40% of patients exhibit muscle inflammation, with macrophages and T-cells accumulating in the muscle tissue [23]. Other MDs, such as Emery-Dreifuss Muscular Dystrophy (EDMD) and Oculopharyngeal Muscular Dystrophy (OPMD), also involve immune responses, though to varying degrees [24]. In EDMD, which displays fiber size variation, internal nuclei, fibrosis, and altered myofibril orientation [25], macrophages and T-cells have been observed in muscle tissues, and inflammatory markers like TNF-α and IL-6 in muscle biopsies are elevated [26]. In OPMD, the peculiar accumulation of nuclear aggregates in muscle fibers activates an immune response, with macrophages and CD8+ T-cells contributing to muscle inflammation and fibrosis [25,27]. Myotonic Dystrophy (MD) type 1 and 2, although primarily associated with muscle wasting and multi-systemic involvement, also show evidence of immune activation [28]. Muscle biopsies demonstrate irregularity in muscle fiber size, rows of internal nuclei, muscle fibrosis, and myofibril orientation perpendicular to the muscle fiber. However, elevated muscle-related levels of pro-inflammatory cytokines and macrophage infiltration suggest a role for the immune system in developing muscle degeneration and fibrosis, especially in cardiac and respiratory muscles [29]. A description of the main characteristics, molecular mechanisms, and inflammatory involvement of the mentioned MD is provided in Table 1.

Table 1.
Muscular Dystrophies key features, mechanism, and related-immune responses.
Across these diverse forms of MD, the chronic activation of immune responses and the resulting inflammation and fibrosis are common pathological features [30]. While treatments targeting inflammatory pathways are primarily symptomatic, they may become curative if capable of modulating the molecular alterations underlying the disease [31]. Cell-based therapies, anti-inflammatory drugs, and immunomodulatory agents are at the forefront of ongoing research aimed at mitigating the immune-mediated damage in these diseases [32]. Pharmacological interventions remain a cornerstone in the management of MDs, particularly Duchenne Muscular Dystrophy (DMD). Glucocorticoids, such as prednisone, prednisolone, deflazacort, and vamorolone, are commonly employed for their anti-inflammatory properties. These agents function by inhibiting the NF-κB pathway, leading to prolonged ambulation, improved pulmonary function, and delayed cardiomyopathy onset in DMD patients [33]. Despite their benefits, long-term glucocorticoid use is associated with adverse effects, including growth retardation, bone demineralization, and metabolic disturbances. Consequently, there is a pressing need for alternative therapies with improved safety profiles. Emerging pharmacological strategies focus on novel agents such as tamoxifen, which has demonstrated potential in modulating muscle degeneration pathways [34]. Additionally, gene therapy approaches, including exon skipping and stop codon readthrough, aim to restore dystrophin function, offering promising avenues for disease modification [35]. While these innovative therapies are under investigation, their long-term efficacy and safety remain to be fully established. In particular, by targeting and repairing specific genomic sequences, CRISPR-Cas9 holds the potential for long-term disease amelioration [36]. Despite its promise, it faces significant challenges, including off-target effects, delivery inefficiencies, and ethical concerns surrounding germline editing. Additionally, this approach does not directly address the inflammatory milieu that perpetuates muscle damage, highlighting the need for complementary therapies [37]. Moreover, the role of gut microbiota seems to be relevant as a potential means of therapeutic intervention. Gut microbiota are increasingly recognized for their influence on systemic inflammation and muscle homeostasis [38]. Dysbiosis, or an imbalance in the microbial community, has been implicated in exacerbating inflammation in MDs. Interventions aimed at restoring a healthy microbiota through probiotics, prebiotics, or dietary modifications have shown potential in reducing inflammatory markers. Moreover, the complex interplay between gut and muscles requires further elucidation to optimize therapeutic outcomes [39]. Finally, recent findings highlight the therapeutic potential of the stem cell secretome in modulating inflammation and promoting tissue repair in various congenital pathologies. Tung et al. [40] describe the diverse bioactive components of the secretome, including cytokines, growth factors, and extracellular vesicles, which collectively contribute to its regenerative and immunomodulatory properties. These mediators can target pathological mechanisms, offering a foundation for exploring similar strategies in MD. Given the growing potential of perinatal stem cells in inflammation modulation, this review aims to highlight the challenges associated with the inflammatory conditions characterizing MDs pathology and explore how perinatal stem cells and their secretome could contribute to their regulation.
2. Skeletal Muscle Recovery: Challenges and Therapeutic Strategies
Chronic inflammation remains a major driver of muscle damage [2,41]. Reactive oxygen species (ROS) are key contributors to the process, making them ideal targets for therapeutic intervention [42], acting as both central players and byproducts of the inflammatory process. ROS production is triggered by mutations in muscle-regulating proteins, leading to myofiber damage and the accumulation of inflammatory cells. In Muscular Dystrophies, non-selective channels like connexin hemichannels increase cytosolic Ca2+, activating proteases and mitochondrial dysfunction. ROS overproduction leads to Ca2+ leak, oxidation of lipids, proteins, and DNA, and disruption of NF-κB and Nrf2 signaling, contributing to muscle degeneration [24]. Anti-inflammatory and antioxidant agents, particularly those with fewer side effects compared to glucocorticoids, are essential for long-term management [43]. Recent advances in stem cell-based therapies, including induced pluripotent stem cells (iPSCs), have generated optimism for treating MDs [32,44,45].A list of stem cells involved in cell therapy approaches for MD is shown in Table 2. However, clinical translation of stem cell-based therapies remains limited by issues such as immune rejection and the reduced viability of transplanted cells derived from the hostile inflammatory environment [46]. A diverse range of cell types has been investigated for their myogenic potential, including myoblasts [47], muscle precursor cells, CD133+ cells [48], Bone Marrow Mononuclear Cells (BM MNCs) [49], mesoangioblasts [50], and Mesenchymal Stem Cells (MSCs) ([51] Cells such as Side Population (SP) cells, Muscle-Derived Stem Cells (MDSCs), myogenic-endothelial progenitors, and pericytes have shown the capacity to differentiate into skeletal muscle tissue in both in vitro and in vivo studies [52,53]. However, due to the progressive replacement of muscle with connective and adipose tissue occurring in advanced MD stages, most therapies would be ineffective if started late, making early intervention essential. As efforts to optimize cell-based therapies, emerging research has shifted focus toward the paracrine effects of stem cells, particularly their secretome [54]. The secretome, comprising growth factors, cytokines, and extracellular vesicles (EVs) released by stem cells [55], has demonstrated significant pro-therapeutic potential. Rather than relying solely on cell replacement, leveraging the bioactive molecules within the secretome may provide an alternative approach to modulating the muscle environment, reducing inflammation, and promoting tissue regeneration [56]. The secretome’s capacity to influence the immune response and enhance tissue repair, without the risks associated with cellular transplantation, has been proposed as an adjunct or even a standalone therapeutic strategy in MD treatment. This review will focus on recent advances in the use of secretome derived from perinatal tissues, particularly the amniotic membrane and umbilical cord, for treating MDs acting on their inflammatory features.

Table 2.
Stem cell types, sources, and roles in muscular dystrophy therapy.
3. Stem Cell Secretome: Immunomodulatory and Potential Therapeutic Features
A secretome consists of soluble paracrine agents secreted by cells in free form or in EVs, including cytokines, growth factors, and regulatory nucleic acids, which play a key role in tissue regeneration [57]. Research shows that the therapeutic benefits of stem cells are mediated by the factors they secrete, highlighting the secretome as a promising cell-free alternative to direct stem cell therapy [58]. It has shown potential in promoting tissue repair and modulating the immune system [59], with promising results in treating skeletal muscle injuries, spinal disc degeneration, skin wounds [60], liver disorders [61], cardiovascular diseases [47,62], neurodegenerative conditions [63], and osteoarthritis [64].
3.1. The Perinatal Cells Secretome
Perinatal tissues, derived from term placentas and fetal annexes, are exposed to high metabolic demands that increase mitochondrial activity and generate Reactive oxygen species (ROS), leading to Oxidative Stress (OS) and inflammation. Specifically, from the innermost layer on, these tissues include the amniotic fluid, amniotic membrane, chorionic membrane, chorionic villi, umbilical cord (including Wharton’s jelly), and placental basal plate (comprising both maternal and fetal cells [65]. Several in vitro studies have demonstrated that perinatal cells interact with both the innate and adaptive immune systems, targeting T and B lymphocytes, macrophages, dendritic cells, neutrophils, and natural killer cells [66]. Mesenchymal Stem Cells (MSCs) and Amniotic Epithelial Cells (AECs) derived from perinatal tissues exhibit a remarkable capacity to modulate the immune response, making them attractive candidates for therapeutic use [67]. AECs from the placenta, known for their immunoprivileged phenotype, play a critical role in maintaining maternal–fetal tolerance [68,69]. Their low expression of HLA class IA, absence of HLA class II, and expression of non-classical molecules, such as HLA-G, HLA-E, and HLA-F, enable them to suppress natural killer (NK) cell cytotoxicity and modulate dendritic and T cell activity [70]. These cells also secrete anti-inflammatory factors such as IL-10 and Prostaglandin E2 (PGE2), promoting an anti-inflammatory macrophage phenotype while counteracting fibrosis. Indeed, the immunosuppressive capability of the AEC-derived secretome seems to be a promising agent for reducing inflammation and tissue damage in MDs [71]. Similarly, MSCs are known to exhibit a therapeutic responsive polarization, allowing them to adopt either pro- or anti-inflammatory phenotypes depending on their microenvironment [72]. Preconditioning MSCs with pathogen-associated molecular patterns (PAMPs) like lipopolysaccharides (LPSs) enhances their anti-inflammatory effects, as demonstrated by the production of EVs with superior immunomodulatory properties [73]. Moreover, Wharton’s Jelly-MSCs (WJ-MSCs) share characteristics with bone marrow-derived MSCs (BM-MSCs) and Embryonic Stem Cells (ESCs) [72,74]. Compared to adult MSCs, WJ-MSCs exhibit superior “stemness” due to minimal exposure to environmental factors and genetic alterations. These qualities, along with their reduced teratoma risk, make them promising candidates for clinical use [75]. WJ-MSCs’ secretome plays critical roles in cellular homeostasis, anti-inflammation, and immunomodulation [76,77]. Recent studies [78] indicate that the secretome of WJ-MSCs enhances neutrophil function and longevity, with potential therapeutic applications in neutropenia or chronic granulomatous disease [78]. For instance, WJ-MSCs preconditioned with LPS produce EVs that accelerate wound healing and reduce Oxidative Stress in diabetic models [79]. This highlights the potential of secretome for decreasing inflammation and enhancing tissue regeneration without requiring direct cell transplantation. The culture of MSCs in 3D settings has emerged as a promising strategy to optimize their paracrine activity [80]). By replicating the in vivo niche and promoting intercellular interactions, 3D spheroid cultures significantly enhance the secretion of key immunomodulatory factors such as transforming growth factor-beta 1 (TGF-β1), IL-6, Tumor Necrosis Factor-Stimulated Gene-6 (TSG-6), and PGE2 [81]. Moreover, dynamic 3D cultures yield a higher number of EVs with enhanced anti-inflammatory effects, reducing CD8+ T cell proliferation and Oxidative Stress while promoting wound closure. The 3D spatial organization of MSCs not only maintains their low immunogenicity but also increases their therapeutic potential, making the 3D aggregate method an exciting avenue for future clinical applications [82]. In addition to their immunomodulatory properties, the antioxidant potential of the stem cell secretome is crucial in mitigating the effects of Oxidative Stress (OS), which is a significant contributor to muscle degeneration in dystrophic conditions. Perinatal cells, particularly AECs and MSCs, are susceptible to OS-induced damage which leads to cellular senescence, DNA damage, and impaired cell function [83]. Studies have demonstrated that the antioxidant components of secretome can counteract ROS, protect cell membranes, and prevent apoptosis [84,85]. Optimizing culture conditions and employing antioxidant strategies may further enhance the therapeutic efficacy of perinatal-derived secretomes [86]. Clinical trials exploring the use of the WJ-MSC secretome in various conditions, including type 1 diabetes mellitus (T1DM) [87], have shown promising results [88]. For instance, early-phase trials suggest that secretome-based therapies may help in restoring insulin production in newly diagnosed T1DM patients [89]. These findings underscore the potential of the stem cell-derived secretome as a versatile therapeutic tool, extending beyond traditional cell therapy to treat a range of degenerative diseases, including MDs. As the field of regenerative medicine evolves, the use of cell-free therapies such as the stem cell secretome offers a compelling alternative to conventional methods [90]. By harnessing their immunomodulatory and antioxidant properties, secretomes may provide a safer, ethically favorable, and effective treatment option for managing inflammation and Oxidative Stress, key drivers of muscle degeneration in dystrophic patients. This insight has spurred interest in engineering or preconditioning MSCs to enhance their therapeutic potential, particularly by refining their secretory profile, especially the extracellular vesicles (EVs) [91].
3.2. Extracellular Vesicles: Main Players of the Stem Cell Secretome
Extracellular vesicles (EVs) are bilayer membrane-bound structures rich in bioactive molecules like lipids, nucleic acids and proteins. EVs are generally classified into three types: exosomes (Exo), microvesicles (MVs), and apoptotic bodies, differentiated by their origin, size, cargo, function, and mechanisms of release [92]. Today, EVs are recognized as main mediators of intercellular communication, extending beyond traditional cell–cell interactions and secreted molecules. EVs influence recipient cells by either interacting via ligand–receptor mechanisms or by fusing and transferring their bioactive content directly into the target cell cytosol, modifying its physiological state. EVs can also release their contents into the extracellular environment, allowing further signaling modulation [93]. EVs can be isolated from multiple biological fluids like blood, urine, plasma, breast milk, and amniotic fluid and tissues such as brain and lung tumors, as well as perinatal sources like the placenta [94]. Under normal conditions, EVs contribute to the regulation of physiological processes like immune responses, coagulation, angiogenesis, apoptosis, and cellular homeostasis [95]. On the other hand, they also play a significant role in the onset and progression of various diseases, including cancer, neurodegeneration, infections, and cardiovascular disorders [96,97]. Owing to their immunomodulatory properties, EV-based therapies are being investigated for the treatment of inflammatory diseases, autoimmune conditions, and cancer [98]. A deeper understanding of the mechanisms through which EVs exert their immunomodulatory effects is currently being investigated to specifically identify their bioactive cargo and target cells [99].
The role of EVs as the sole mediators of the immunomodulatory effects of the secretome has been disputed by other studies pointing out that the immunomodulatory effect is exerted by the secretome in toto and not by secreted factors directly conveyed by EVs. Papait et al. [100] reported that the immunomodulatory effects of the hAMSCs secretome are primarily due to factors in the CM rather than the EVs themselves, which, although internalized by immune cells, did not show significant effects at their original concentrations. Others have shown that EVs have a reduced impact compared to their parental MSCs [101,102], or an immunosuppressive action on T cells [103], and some even found no effect of EVs at all [104]. Similarly, research investigating the impact of MSC-derived EVs on B cells has produced conflicting findings [105]. Indeed, Carreras-Planella et al. reported that MSC-EVs were unable to stimulate naïve B cell expansion or decrease memory B cells. While MSC-EVs induced CD24ʰⁱ CD38ʰⁱ B cells at levels comparable to MSCs, they did not generate true regulatory B cells (Bregs) as they failed to produce IL-10. These findings suggest that MSCs influence B cell modulation through soluble factors other than EVs. In Conforti’s study [101], MSC co-culture led to a statistically significant rise in IL-10 and TGF-β, alongside a reduction in GM-CSF and IFN-γ, compared to EVs incubation. Their findings suggest that EVs exhibit a diminished immunomodulatory impact on T-cell proliferation and antibody production in vitro, relative to their cellular counterpart, not provoking a significant clinical response in most GvHD patients. These discrepancies may arise from methodological differences that make comparisons across studies challenging.
4. Advances in Secretome from Perinatal Cells for MDs Treatment
Perinatal cells and their secretome have shown strong immunomodulatory potential [106,107], a property closely linked to their ability to support tissue regeneration [107], especially in conditions characterized by acute or chronic inflammation, such as MDs. A recent study by Sandonà [108] demonstrated that the human amniotic mesenchymal stem cell (hAMSC) secretome directly impacts the muscle stem cell (MuSC) niche by enhancing progenitor cell proliferation and differentiation, typically compromised in DMD muscles. By employing a neutral sphingomyelinase inhibitor to block EV release from hAMSCs, they revealed distinct roles for the hAMSC secretome and its EV fraction in muscle regeneration, indicating that freely secreted factors from the conditioned medium (CM) primarily support MuSC proliferation, as evidenced by a higher increase in nuclei count, while EVs are more effective in driving MuSC differentiation, as shown by a higher myotube fusion index. Significantly, local treatment with hAMSC-derived EVs greatly facilitated muscle regeneration in dystrophic mice, reducing muscle fibrosis and improving muscle function. This treatment enhanced myofiber size, and led to increased formation of new myofibers and to a greater number of proliferating MuSCs within the fibers themselves. Additionally, conditioned medium and exosomes from placental MSCs showed similar enhancement of myoblast differentiation and reduced fibrogenic gene expression in DMD models, though placental MSCs are less specifically characterized compared to amniotic MSCs [109]. This study also explores the factors mediating the hAMSC secretome’s regenerative effects. Interestingly, the CM of hAMSCs is rich in metalloproteinase (MMP) inhibitors (TIMP1 and TIMP2), which counteract MMPs involved in DMD progression. Moreover, CM hAMSC contains Growth Differentiation Factor 15 (GDF15), a myomitokine with a role in energy metabolism but with a still debated impact on muscle diseases. Moreover, hAMSC-derived EVs carry miRNAs that have a critical role in muscle differentiation and regeneration, such as miR-26a, which targets Smad1 and Smad4 in the TGF-β/BMP pathway, a well-known inhibitor of differentiation, as well as miR-214, which negatively regulates Ezh2 protein, accelerating skeletal muscle cells differentiation. These results align with other studies showing that EVs from fetal and placental stem cells can reduce fibrosis and support muscle repair in MuSCs [108]. Another study showed that WJ-MSC EVs improved angiogenesis and myogenesis, reduced fibrosis, and directed inflammation aimed to muscle repair, with a notable increase in M2-like pro-regenerative macrophage numbers compared to controls in a volumetric muscle loss murine model [110]. Bier et al. [109] showed that placenta-derived exosomes have potential for treating DMD. They studied the effects of placenta-derived mesenchymal stem cells (PL-MSCs) and their exosomes on muscle cells from both healthy controls and DMD models. Treatment with PL-MSCs or their exosomes improved muscle cell differentiation, reduced fibrosis-related genes, and increased utrophin levels, which may help to restore muscle function. The therapeutic effects were linked to exosomal miR-29c, which promotes muscle repair and reduces fibrosis. By silencing miR-29c, these benefits were diminished, highlighting the important role of this miRNA. These results confirmed the previous ones by Nakamura [111] demonstrating that MSC exosomes promote myogenesis and angiogenesis in vitro, and muscle regeneration in an in vivo model of muscle injury, an effect partly mediated by miRNAs, such as miR-494. In addition, another relevant example of exosomes-derived miRNA activity in the MD context has been shown by Sandonà et al. [54]. They demonstrated that EVs released by mesenchymal cells like fibro–adipogenic progenitors (FAPs) mediate miRNAs transfer to MuSCs. In addition, when dystrophic FAPs are treated with HDAC inhibitors (HDACis), they release EVs containing increased levels of specific miRs, such as miR-206, a muscle-specific miR, that cooperatively target key biological processes related to muscle regeneration, fibrosis reduction, and inflammation control. In DMD patients and “mdx mice”, exposure to HDACis raises miR-206 levels in FAP-released EVs, enhancing muscle regeneration and reducing fibrosis. When EVs are transplanted into dystrophic muscles, they activate and expand MuSCs, boosting regeneration while inhibiting both fibrosis and inflammation. Inhibiting individual miRs, such as miR-206, reveals that it is essential for EV-induced MuSC expansion and muscle regeneration. Furthermore, the combined activity of HDACi-induced miRs contributes to the overall beneficial effects of these EVs. In the context of inflammation, Noonin and Thongboonkerd [112] demonstrated that miR-181c in exosomes from human Umbilical Cord MSCs (hUCMSCs) reduces TLR4 expression and NFκB activation, lowering pro-inflammatory cytokine levels [113]. Fibrosis, a mechanism for which it is necessary to focus on muscular disorders, could find its remedy, as demonstrated by Hodge et al. [61]. They found out that amniotic epithelial cells (hAECs) and their CM reduced liver fibrosis and injury in a mouse model. Eventually, besides secretomes or ECVs alone, co-cultures of perinatal stem cells and myoblasts also showed a positive effect in treating myopathies-related pathological effects. Kwon et al. [114] highlighted the protective role of hWJ-MSCs in preventing muscle cell death. WJ-MSCs significantly reduced apoptosis in mouse skeletal muscle myoblasts (C2C12 cell line) under serum deprivation conditions, knowing that antibody analysis revealed high levels of chemokine XCL1 secretion. XCL1 treatment effectively inhibited apoptosis in both serum-starved and lovastatin-treated C2C12 cells, but not in other cell lines. Knockdown of XCL1 in WJ-MSCs confirmed its pivotal role in this anti-apoptotic effect. Additionally, XCL1 treatment improved muscle defects in a zebrafish myopathy model, suggesting its potential as a therapeutic agent for muscle diseases [114]. Furthermore, Kono et al. confirmed the paracrine effect of mouse MSCs on the inflammatory response of LPS-activated C2C12 cells. IL-6 production from LPS-activated C2C12 cells was significantly increased after co-culturing with MSCs. Moreover, IL-6 and Inducible Nitric Oxide Synthase (iNOS) mRNA expression was notably upregulated in C2C12 cells cocultured with MSCs, while TNF-α and IL-1β mRNA expression was reduced.
5. Discussion and Future Outlooks
Recent research revealed how the immune system plays a significant role in MDs, showing insights into the disease mechanisms behind MDs and the interplay between muscle and the immune system, opening new therapeutic avenues for possible treatments of MDs. Understanding immune system interactions with dystrophic muscle has led to the development of new treatment strategies beyond traditional immunosuppressants regimens. To date, MuSC-derived myoblasts, mesoangioblasts (MABs), CD133+ cells, MSCs, and CDCs have been evaluated in clinical trials for MDs. Despite some positive indications in terms of safety or functional/histological recovery, these therapeutic options appear still preliminary. As efforts to enhance cell-based therapies continue, emerging research has increasingly focused on the paracrine effects of stem cells, especially how their secretome can influence the immune system. This literary review aimed to analyze the immunomodulatory and antioxidant potential of perinatal stem cell-derived secretomes and their EVs as potential therapeutic approaches for MDs treatment. The stem cell secretome derived from perinatal tissues is already known to support tissue regeneration, relying on its adaptable immunomodulatory activity. A graphic summary of this review study is represented in Figure 1. The amniotic membrane and umbilical cord are valuable sources of MSCs and AECs, excellent candidates for obtaining immunomodulatory secretomes. For example, the secretome from hAMSCs enhances MuSC function, with free factors supporting MuSC proliferation and EVs promoting differentiation, muscle regeneration, reduced fibrosis, and increased myofiber formation in dystrophic mice [108]. In addition, EVs from hWJ-MSCs improved angiogenesis and myogenesis and reduced fibrosis in muscle injury models [110]. They also increased M2-like macrophages, which are associated with tissue repair. Exosomal miRNAs (e.g., miR-29, miR-26, miR-214) play a significant role in enabling these processes [108,109,111]. However, there are conflicting findings regarding the impact of purified EVs compared to their parental cell effects, with some research suggesting that EVs are less effective or even non-immunosuppressive on certain immune cells [101,102,105]. To advance clinical applications and next-generation stem cell-EV therapy, the ideal stem cell source should be chosen according to its paracrine potential and its ease of isolation. Perinatal stem cells like AECs and WJ-MSCs, which are isolated from delivery waste clinical material, along with the lack of ethical concern, and consistent expansion and cryopreservation, proved to be optimal candidates over adult MSCs and Embryonic Stem Cells as sources for future EVs-based paracrine therapy. Nowadays, the clinical applications of EVs remain challenging due to the lack of standardized protocols to produce vesicles for use in human therapy. There are open debates about the diversity and preparation of stem cell EVs and consequently about the selection of methods for EV isolation and purification. Future challenges include the availability of standardized quality tests, and the precision improvement of in vitro and in vivo functional assays will be essential. With ongoing advancements, these factors could greatly impact the consistency and reliability of cell-free therapies, soon offering renewed hope to patients affected by skeletal muscle disorders.
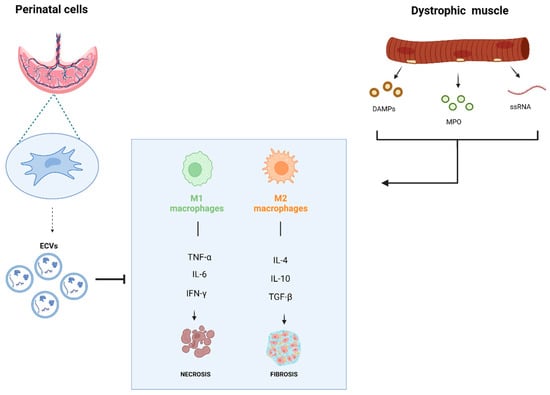
Figure 1.
Interplay between perinatal cells and the dystrophic muscles. The effect of dystrophic muscle on macrophage M1/M2 activation and associated cytokines production and the influence of perinatal cell secretome.
Author Contributions
Conceptualization, P.M. and R.C.; methodology, R.C.; investigation, S.P. and S.L.; data curation, S.P. and F.P.; writing—original draft preparation, S.P., S.L. and R.C.; writing—review and editing, S.P., R.C., G.P., S.L., P.M., G.C. and F.A.; visualization, S.L.; supervision, F.A.; project administration, G.C.; funding acquisition, G.C. and F.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Consorzio Interuniversitario per le Biotecnologie, by PRIN Project “Progetto ‘Study of myopathy 3D models for anti-inflammatory treatment strategy’, codice proposta: 2022NTZWFR_001—CUP: J53D23003240006 Finanziato dall’Unione Europea—NextGenerationEU a valere sul Piano Nazionale di Ripresa e Resilienza (PNRR)—Missione 4 Istruzione e ricerca—Componente 2 Dalla ricerca all’impresa—Investimento 1.1, Avviso Prin2022 indetto con DD N. 104 del 2/2/2022” granted to G.C. and F.A. and by Fondazione Cassa di Risparmio di Bologna (CARISBO) Project “Bioprinted cell models of muscular dystrophies for screening of inflammatory markers—BioMoDy” granted to F.A.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| AECs | Amniotic Epithelial Cells |
| BM MNCs | Bone Marrow Mononuclear Cells |
| BM-MSCs | Bone Marrow-Mesenchymal Stem Cells |
| CD133+ Cells | Hematopoietic Stem/Progenitor Cells Expressing CD133 |
| CMDs | Congenital Muscular Dystrophies |
| DM | Myotonic Dystrophy |
| DMD | Duchenne Muscular Dystrophy |
| EDMD | Emery-Dreifuss Muscular Dystrophy |
| ESCs | Embryonic Stem Cells |
| FSHD | Facioscapulohumeral Muscular Dystrophy |
| hAMSC | human amniotic mesenchymal stem cell |
| HDAC inhibitors: | Histone deacetylase inhibitors |
| HLA | Human Leukocyte Antigen |
| IFNγ | Interferon Gamma |
| IL-10 | Interleukin 10 |
| IL-1β | Interleukin 1 Beta |
| IL-4 | Interleukin 4 |
| IL-6 | Interleukin 6 |
| iNOS | Inducible Nitric Oxide Synthase |
| LGMD R5 | Limb-Girdle Muscular Dystrophy Type R5 |
| LGMD R9 | Limb-Girdle Muscular Dystrophy Type R9 |
| LGMD | Limb-Girdle Muscular Dystrophy |
| LPS | lipopolysaccharide |
| MABs | mesongioblasts |
| MAC | Membrane Attack Complex |
| MDC1A | Merosin-Deficient Congenital Muscular Dystrophy |
| MDs | Muscular Dystrophies |
| MDSCs | Muscle-Derived Stem Cells |
| MSCs | Mesenchymal Stem Cells |
| MuSC | muscle stem cell |
| NF-κB | Nuclear Factor Kappa B |
| OPMD | Oculopharyngeal Muscular Dystrophy |
| OS | Oxidative Stress |
| PGE2 | Prostaglandin E2 |
| PL-MSCs | placenta-derived mesenchymal stem cells |
| ROS | Reactive oxygen species |
| SP Cells | Side Population Cells |
| TGF-β | Transforming Growth Factor β |
| TLR | Toll-like Receptor |
| TNF-α | Tumor Necrosis Factor Alpha |
| TSG-6 | Tumor Necrosis Factor-Stimulated Gene-6 |
| WJ-MSCs | Wharton’s Jelly-Mesenchymal Stem Cells |
References
- Mercuri, E.; Bönnemann, C.G.; Muntoni, F. Muscular Dystrophies. Lancet 2019, 394, 2025–2038. Available online: www.thelancet.com (accessed on 3 February 2025).
- Bouché, M.; Muñoz-Cánoves, P.; Rossi, F.; Coletti, D. Inflammation in Muscle Repair, Aging, and Myopathies. BioMed Res. Int. 2014, 2014, 821950. [Google Scholar] [CrossRef] [PubMed]
- Tidball, J.G.; Welc, S.S.; Wehling-Henricks, M. Immunobiology of Inherited Muscular Dystrophies. Compr. Physiol. 2018, 8, 1313–1356. [Google Scholar] [CrossRef]
- Yang, W.; Hu, P. Skeletal Muscle Regeneration is Modulated by Inflammation; Elsevier Pte Ltd.: Singapore, 2018; Volume 13, pp. 25–32. [Google Scholar] [CrossRef]
- Tidball, J.G. Regulation of muscle growth and regeneration by the immune system. Nat. Rev. Immunol. 2017, 17, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Tidball, J.G.; Wehling-Henricks, M. Damage and inflammation in muscular dystrophy: Potential implications and relationships with autoimmune myositis. Curr. Opin. Rheumatol. 2005, 6, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, L.; Berardinelli, M.G.; De Pasquale, L.; Nicoletti, C.; D’Amico, A.; Carvello, F.; Moneta, G.M.; Catizone, A.; Bertini, E.; De Benedetti, F.; et al. Functional and Morphological Improvement of Dystrophic Muscle by Interleukin 6 Receptor Blockade. EBioMedicine 2015, 2, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Villalta, S.A.; Nguyen, H.X.; Deng, B.; Gotoh, T.; Tidball, J.G. Shifts in macrophage phenotypes and macrophage competition for arginine metabolism affect the severity of muscle pathology in muscular dystrophy. Hum. Mol. Genet. 2008, 18, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Kharraz, Y.; Guerra, J.; Mann, C.J.; Serrano, A.L.; Muñoz-Cánoves, P. Macrophage Plasticity and the Role of Inflammation in Skeletal Muscle Repair. Mediat. Inflamm. 2013, 2013, 491497. [Google Scholar] [CrossRef] [PubMed]
- Sulahian, T.H.; Högger, P.; E Wahner, A.; Wardwell, K.; Goulding, N.J.; Sorg, C.; Droste, A.; Stehling, M.; Wallace, P.K.; Morganelli, P.M.; et al. Human monocytes express CD163, which is upregulated by IL-10 and identical to p155. Cytokine 2000, 12, 1312–1321. [Google Scholar] [CrossRef] [PubMed]
- De Paepe, B.; De Bleecker, J.L. Cytokines and Chemokines as Regulators of Skeletal Muscle Inflammation: Presenting the Case of Duchenne Muscular Dystrophy. Mediat. Inflamm. 2013, 2013, 540370. [Google Scholar] [CrossRef] [PubMed]
- Evans, N.P.; Misyak, S.A.; Robertson, J.L.; Bassaganya-Riera, J.; Grange, R.W. Immune-Mediated Mechanisms Potentially Regulate the Disease Time-Course of Duchenne Muscular Dystrophy and Provide Targets for Therapeutic Intervention. PM R 2009, 1, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Kranig, S.A.; Tschada, R.; Braun, M.; Pöschl, J.; Frommhold, D.; Hudalla, H.; Patry, C. Insights Image for “Dystrophin deficiency promotes leukocyte recruitment in mdx mice”. Pediatr. Res. 2020, 87, 798. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, L.; Villa, C.; Molinaro, D.; Torrente, Y.; Farini, A. The Immune System in Duchenne Muscular Dystrophy Pathogenesis. Biomedicines 2021, 9, 1447. [Google Scholar] [CrossRef]
- Dubinin, M.V.; Starinets, V.S.; Talanov, E.Y.; Mikheeva, I.B.; Belosludtseva, N.V.; Serov, D.A.; Tenkov, K.S.; Belosludtseva, E.V.; Belosludtsev, K.N. Effect of the Non-Immunosuppressive MPT Pore Inhibitor Alisporivir on the Functioning of Heart Mitochondria in Dystrophin-Deficient mdx Mice. Biomedicines 2021, 9, 1232. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Rodia, M.T.; Pacilio, S.; Angelini, C.; Cenacchi, G. LGMD D2 TNPO3-Related: From Clinical Spectrum to Pathogenetic Mechanism. Front. Neurol. 2022, 13, 840683. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, E.; Rojas-García, R.; De Luna, N.; Pou, A.; Brown, R.H.; Illa, I. Inflammation in dysferlin myopathy: Immunohistochemical characterization of 13 patients. Neurology 2001, 57, 2136–2138. [Google Scholar] [CrossRef] [PubMed]
- Cohen, T.V.; Cohen, J.E.; Partridge, T.A. Myogenesis in dysferlin-deficient myoblasts is inhibited by an intrinsic inflammatory response. Neuromuscul. Disord. 2012, 22, 648–658. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baek, J.-H.; Many, G.M.; Evesson, F.J.; Kelley, V.R. Dysferlinopathy Promotes an Intramuscle Expansion of Macrophages with a Cyto-Destructive Phenotype. Am. J. Pathol. 2017, 187, 1245–1257. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, K.; Zabojszcza, J.; Carl, M.; Taubert, S.; Lass, A.; Harris, C.L.; Ho, M.; Schulz, H.; Hummel, O.; Hubner, N.; et al. Increased Susceptibility to Complement Attack due to Down-Regulation of Decay-Accelerating Factor/CD55 in Dysferlin-Deficient Muscular Dystrophy. J. Immunol. 2005, 75, 6219–6225. [Google Scholar] [CrossRef] [PubMed]
- Jeudy, S.; Wardrop, K.E.; Alessi, A.; Dominov, J.A. Bcl-2 Inhibits the Innate Immune Response during Early Pathogenesis of Murine Congenital Muscular Dystrophy. PLoS ONE 2011, 6, e22369. [Google Scholar] [CrossRef]
- Gerbino, A.; Forleo, C.; Milano, S.; Piccapane, F.; Procino, G.; Pepe, M.; Piccolo, M.; Guida, P.; Resta, N.; Favale, S.; et al. Pro-inflammatory cytokines as emerging molecular determinants in cardiolaminopathies. J. Cell. Mol. Med. 2021, 25, 10902–10915. [Google Scholar] [CrossRef] [PubMed]
- Dahlqvist, J.R.; Andersen, G.; Khawajazada, T.; Vissing, C.; Thomsen, C.; Vissing, J. Relationship between muscle inflammation and fat replacement assessed by MRI in facioscapulohumeral muscular dystrophy. J. Neurol. 2019, 266, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- González-Jamett, A.; Vásquez, W.; Cifuentes-Riveros, G.; Martínez-Pando, R.; Sáez, J.C.; Cárdenas, A.M. Oxidative Stress, Inflammation and Connexin Hemichannels in Muscular Dystrophies. Biomedicines 2022, 10, 507. [Google Scholar] [CrossRef] [PubMed]
- Becker, N.; Moore, S.A.; Jones, K.A. The inflammatory pathology of dysferlinopathy is distinct from calpainopathy, Becker muscular dystrophy, and inflammatory myopathies. Acta Neuropathol. Commun. 2022, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mahon, N.; Glennon, J.C. The bi-directional relationship between sleep and inflammation in muscular dystrophies: A narrative review. Neurosci. Biobehav. Rev. 2023, 150, 105116. [Google Scholar] [CrossRef]
- Yamashita, S.; Hara, K.; Tawara, N. AUTOIMMUNE & INFLAMMATORY NMD. Neuromuscul. Disord. 2021, 31, S54–S55. [Google Scholar] [CrossRef]
- Johansson, Å.; Carlström, K.; Carlström, C.; Ahré, N.B.O.; Cederquist, K.; Krylborg, E.; Forsberg, H.; Olsson, T. Abnormal Cytokine and Adrenocortical Hormone Regulation in Myotonic Dystrophy. J. Clin. Endocrinol. Metab. 2000, 85, 3169–3176. [Google Scholar] [CrossRef]
- Nakamori, M.; Hamanaka, K.; Thomas, J.D.; Wang, E.T.; Hayashi, Y.K.; Takahashi, M.P.; Swanson, M.S.; Nishino, I.; Mochizuki, H. Aberrant Myokine Signaling in Congenital Myotonic Dystrophy. Cell Rep. 2017, 21, 1240–1252. [Google Scholar] [CrossRef]
- Villa, C.; Farini, A.; Torrente, Y. Editorial: Inflammation in muscular dystrophies: Mediators, mechanisms, and therapeutics. Front. Immunol. 2024, 15, 1470266. [Google Scholar] [CrossRef]
- Petrof, B.J.; Podolsky, T.; Bhattarai, S.; Tan, J.; Ding, J. Trained immunity as a potential target for therapeutic immunomodulation in Duchenne muscular dystrophy. Front. Immunol. 2023, 14, 1183066. [Google Scholar] [CrossRef]
- Singh, S.; Singh, T.; Kunja, C.; Dhoat, N.S.; Dhania, N.K. Gene-editing, immunological and iPSCs based therapeutics for muscular dystrophy. Eur. J. Pharmacol. 2021, 912, 174568. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Chen, Z.; Yu, Y.; Zhang, N.; Jiang, H.; Zhang, G.; Zhang, Z.; Zhang, B. Current Pharmacological Strategies for Duchenne Muscular Dystrophy. Front. Cell Dev. Biol. 2021, 9, 689533. [Google Scholar] [CrossRef]
- Botti, V.; Menzel, O.; Staedler, D. A state-of-the-art review of tamoxifen as a potential therapeutic for duchenne muscular dystrophy. Front. Pharmacol. 2022, 13, 1030785. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.C.; Wood, M.J.A.; Davies, K.E. Therapeutic approaches for Duchenne muscular dystrophy. Nat. Rev. Drug Discov. 2023, 22, 917–934. [Google Scholar] [CrossRef] [PubMed]
- Chemello, F.; Olson, E.N.; Bassel-Duby, R. CRISPR-Editing Therapy for Duchenne Muscular Dystrophy. Hum. Gene Ther. 2023, 34, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Uddin, F.; Rudin, C.M.; Sen, T. CRISPR Gene Therapy: Applications, Limitations, and Implications for the Future. Front. Oncol. 2020, 10, 1387. [Google Scholar] [CrossRef]
- Picca, A.; Fanelli, F.; Calvani, R.; Mulè, G.; Pesce, V.; Sisto, A.; Pantanelli, C.; Bernabei, R.; Landi, F.; Marzetti, E. Gut Dysbiosis and Muscle Aging: Searching for Novel Targets against Sarcopenia. Mediat. Inflamm. 2018, 2018, 7026198. [Google Scholar] [CrossRef] [PubMed]
- Ticinesi, A.; Milani, C.; Lauretani, F.; Nouvenne, A.; Mancabelli, L.; Lugli, G.A.; Turroni, F.; Duranti, S.; Mangifesta, M.; Viappiani, A.; et al. Gut microbiota composition is associated with polypharmacy in elderly hospitalized patients. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Tung, S.; Delavogia, E.; Fernandez-Gonzalez, A.; Mitsialis, S.A.; Kourembanas, S. Harnessing the therapeutic potential of the stem cell secretome in neonatal diseases. Semin. Perinatol. 2023, 47, 151730. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.; Li, Y.-L. Inflammation balance in skeletal muscle damage and repair. Front. Immunol. 2023, 14, 1133355. [Google Scholar] [CrossRef]
- Pole, A.; Dimri, M.; Dimri, G.P. Oxidative stress, cellular senescence and ageing. AIMS Mol. Sci. 2016, 3, 300–324. [Google Scholar] [CrossRef]
- Henríquez-Olguín, C.; Altamirano, F.; Valladares, D.; López, J.R.; Allen, P.D.; Jaimovich, E. Altered ROS production, NF-κB activation and interleukin-6 gene expression induced by electrical stimulation in dystrophic mdx skeletal muscle cells. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2015, 1852, 1410–1419. [Google Scholar] [CrossRef] [PubMed]
- Biressi, S.; Filareto, A.; Rando, T.A. Stem cell therapy for muscular dystrophies. J. Clin. Investig. 2020, 130, 5652–5664. [Google Scholar] [CrossRef] [PubMed]
- Ghori, F.F.; Wahid, M. Induced pluripotent stem cells from urine of Duchenne muscular dystrophy patients. Pediatr. Int. 2021, 63, 1038–1047. [Google Scholar] [CrossRef]
- Baldari, S.; Di Rocco, G.; Piccoli, M.; Pozzobon, M.; Muraca, M.; Toietta, G. Challenges and Strategies for Improving the Regenerative Effects of Mesenchymal Stromal Cell-Based Therapies. Int. J. Mol. Sci. 2017, 18, 2087. [Google Scholar] [CrossRef]
- Song, K.; Wang, Y.; Sassoon, D. Expression of Hox-7.1 in Myoblasts Inhibits Terminal Differentiation and Induces Cell Transformation. Nature 1992, 360, 477–481. Available online: https://www.nature.com/articles/360477a0 (accessed on 3 February 2025). [CrossRef] [PubMed]
- Torrente, Y.; Belicchi, M.; Marchesi, C.; D’antona, G.; Cogiamanian, F.; Pisati, F.; Gavina, M.; Giordano, R.; Tonlorenzi, R.; Fagiolari, G.; et al. Autologous Transplantation of Muscle-Derived CD133 + Stem Cells in Duchenne Muscle Patients. Cell Transplant. 2007, 16, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Badhe, P.; Sharma, A.; Sane, H.; Paranjape, A.; Bhagwanani, K.; Gokulchandran, N. Autologous bone marrow mononuclear cell transplantation in Duchenne muscular dystrophy. Am. J. Case Rep. 2014, 15, 128–134. [Google Scholar] [CrossRef]
- Cossu, G.; Previtali, S.C.; Napolitano, S.; Cicalese, M.P.; Tedesco, F.S.; Nicastro, F.; Noviello, M.; Roostalu, U.; Sora, M.G.N.; Scarlato, M.; et al. Intra-arterial transplantation of HLA-matched donor mesoangioblasts in Duchenne muscular dystrophy. EMBO Mol. Med. 2015, 7, 1513–1528. [Google Scholar] [CrossRef]
- Świątkowska-Flis, B.; Zdolińska-Malinowska, I.; Sługocka, D.; Boruczkowski, D. The Use of Umbilical Cord-Derived Mesenchymal Stem Cells in Patients with Muscular Dystrophies: Results from Compassionate Use in Real-Life Settings. Stem Cells Transl. Med. 2021, 10, 1372–1383. [Google Scholar] [CrossRef]
- Alviano, F.; Fossati, V.; Marchionni, C.; Arpinati, M.; Bonsi, L.; Franchina, M.; Lanzoni, G.; Cantoni, S.; Cavallini, C.; Bianchi, F.; et al. Term amniotic membrane is a high throughput source for multipotent mesenchymal stem cells with the ability to differentiate into endothelial cells in vitro. BMC Dev. Biol. 2007, 7, 11. [Google Scholar] [CrossRef]
- Péault, B.; Rudnicki, M.; Torrente, Y.; Cossu, G.; Tremblay, J.P.; Partridge, T.; Gussoni, E.; Kunkel, L.M.; Huard, J. Stem and Progenitor Cells in Skeletal Muscle Development, Maintenance, and Therapy. Mol. Ther. 2007, 15, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Sandonà, M.; Consalvi, S.; Tucciarone, L.; De Bardi, M.; Scimeca, M.; Angelini, D.F.; Buffa, V.; D’Amico, A.; Bertini, E.S.; Cazzaniga, S.; et al. HDAC inhibitors tune miRNAs in extracellular vesicles of dystrophic muscle-resident mesenchymal cells. EMBO Rep. 2020, 21, e50863. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kandoi, S.; Misra, R.; Vijayalakshmi, S.; Rajagopal, K.; Verma, R.S. The Mesenchymal Stem Cell Secretome: A new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev. 2019, 46, 1–9. [Google Scholar] [CrossRef]
- Eleuteri, S.; Fierabracci, A. Insights into the Secretome of Mesenchymal Stem Cells and Its Potential Applications. Int. J. Mol. Sci. 2019, 20, 4597. [Google Scholar] [CrossRef] [PubMed]
- Uhlén, M.; Karlsson, M.J.; Hober, A.; Svensson, A.S.; Scheffel, J.; Kotol, D.; Zhong, W.; Tebani, A.; Strandberg, L.; Edfors, F.; et al. The human secretome. Sci. Signal. 2019, 12, eaaz0274. [Google Scholar] [CrossRef] [PubMed]
- Gunawardena, T.N.A.; Rahman, M.T.; Abdullah, B.J.J.; Abu Kasim, N.H. Conditioned media derived from mesenchymal stem cell cultures: The next generation for regenerative medicine. J. Tissue Eng. Regen Med. 2019, 13, 569–586. [Google Scholar] [CrossRef]
- Sandonà, M.; Di Pietro, L.; Esposito, F.; Ventura, A.; Silini, A.R.; Parolini, O.; Saccone, V. Mesenchymal Stromal Cells and Their Secretome: New Therapeutic Perspectives for Skeletal Muscle Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 652970. [Google Scholar] [CrossRef]
- Montero-Vilchez, T.; Sierra-Sánchez, Á.; Sanchez-Diaz, M.; Quiñones-Vico, M.I.; Sanabria-de-la-Torre, R.; Martinez-Lopez, A.; Arias-Santiago, S. Mesenchymal Stromal Cell-Conditioned Medium for Skin Diseases: A Systematic Review. Front. Cell Dev. Biol. 2021, 9, 654210. [Google Scholar] [CrossRef] [PubMed]
- Hodge, A.; Andrewartha, N.; Lourensz, D.; Strauss, R.; Correia, J.; Goonetilleke, M.; Yeoh, G.; Lim, R.; Sievert, W. Human Amnion Epithelial Cells Produce Soluble Factors that Enhance Liver Repair by Reducing Fibrosis While Maintaining Regeneration in a Model of Chronic Liver Injury. Cell Transplant. 2020, 29, 963689720950221. [Google Scholar] [CrossRef] [PubMed]
- Ranganath, S.H.; Levy, O.; Inamdar, M.S.; Karp, J.M. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012, 10, 244–258. [Google Scholar] [CrossRef]
- da Silva, A.V.; Serrenho, I.; Araújo, B.; Carvalho, A.M.; Baltazar, G. Secretome as a Tool to Treat Neurological Conditions: Are We Ready? Int. J. Mol. Sci. 2023, 24, 16544. [Google Scholar] [CrossRef] [PubMed]
- van Buul, G.M.; Villafuertes, E.; Bos, P.K.; Waarsing, J.H.; Kops, N.; Narcisi, R.; Weinans, H.; Verhaar, J.A.; Bernsen, M.R.; van Osch, G.J. Mesenchymal stem cells secrete factors that inhibit inflammatory processes in short-term osteoarthritic synovium and cartilage explant culture. Osteoarthr. Cartil. 2012, 20, 1186–1196. [Google Scholar] [CrossRef] [PubMed]
- Silini, A.R.; Di Pietro, R.; Lang-Olip, I.; Alviano, F.; Banerjee, A.; Basile, M.; Borutinskaite, V.; Eissner, G.; Gellhaus, A.; Giebel, B.; et al. Perinatal Derivatives: Where Do We Stand? A Roadmap of the Human Placenta and Consensus for Tissue and Cell Nomenclature. Front. Bioeng. Biotechnol. 2020, 8, 610544. [Google Scholar] [CrossRef] [PubMed]
- Balbi, C.; Piccoli, M.; Barile, L.; Papait, A.; Armirotti, A.; Principi, E.; Reverberi, D.; Pascucci, L.; Becherini, P.; Varesio, L.; et al. First Characterization of Human Amniotic Fluid Stem Cell Extracellular Vesicles as a Powerful Paracrine Tool Endowed with Regenerative Potential. Stem Cells Transl. Med. 2017, 6, 1340–1355. [Google Scholar] [CrossRef] [PubMed]
- Paris, F.; Pizzuti, V.; Marrazzo, P.; Pession, A.; Alviano, F.; Bonsi, L. Perinatal Stem Cell Therapy to Treat Type 1 Diabetes Mellitus: A Never-Say-Die Story of Differentiation and Immunomodulation. Int. J. Mol. Sci. 2022, 23, 14597. [Google Scholar] [CrossRef] [PubMed]
- Parolini, O.; Caruso, M. Review: Preclinical studies on placenta-derived cells and amniotic membrane: An update. Placenta 2011, 32, S186–S195. [Google Scholar] [CrossRef] [PubMed]
- Wassmer, C.; Lebreton, F.; Bellofatto, K.; Bosco, D.; Berney, T.; Berishvili, E. Generation of insulin-secreting organoids: A step toward engineering and transplanting the bioartificial pancreas. Transpl. Int. 2020, 33, 1577–1588. [Google Scholar] [CrossRef]
- Morandi, F.; Marimpietri, D.; Görgens, A.; Gallo, A.; Srinivasan, R.C.; El-Andaloussi, S.; Gramignoli, R. Human Amnion Epithelial Cells Impair T Cell Proliferation: The Role of HLA-G and HLA-E Molecules. Cells 2020, 9, 2123. [Google Scholar] [CrossRef]
- Fathi, I.; Miki, T. Human Amniotic Epithelial Cells Secretome: Components, Bioactivity, and Challenges. Front. Med. 2022, 8, 763141. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.L.; Chan, S.T.; Lo, C.Y.; A Deane, J.; A McDonald, C.; Bernard, C.C.; Wallace, E.M.; Lim, R. Amnion cell-mediated immune modulation following bleomycin challenge: Controlling the regulatory T cell response. Stem Cell Res. Ther. 2015, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Ti, D.; Hao, H.; Tong, C.; Liu, J.; Dong, L.; Zheng, J.; Zhao, Y.; Liu, H.; Fu, X.; Han, W. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J. Transl. Med. 2015, 13, 308. [Google Scholar] [CrossRef] [PubMed]
- Bongso, A.; Fong, C.; Gauthaman, K. Taking stem cells to the clinic: Major challenges. J. Cell. Biochem. 2008, 105, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- El Omar, R.; Beroud, J.; Stoltz, J.F.; Menu, P.; Velot, E.; Decot, V. Umbilical cord mesenchymal stem cells: The new gold standard for mesenchymal stem cell-based therapies? Tissue Eng. Part B Rev. 2014, 20, 523–544. [Google Scholar] [CrossRef] [PubMed]
- Anzalone, R.; Iacono, M.L.; Loria, T.; Di Stefano, A.; Giannuzzi, P.; Farina, F.; La Rocca, G. Wharton’s Jelly Mesenchymal Stem Cells as Candidates for Beta Cells Regeneration: Extending the Differentiative and Immunomodulatory Benefits of Adult Mesenchymal Stem Cells for the Treatment of Type 1 Diabetes. Stem Cell Rev. Rep. 2011, 7, 342–363. [Google Scholar] [CrossRef]
- Lanzoni, G.; Linetsky, E.; Correa, D.; Cayetano, S.M.; Alvarez, R.A.; Kouroupis, D.; Gil, A.A.; Poggioli, R.; Ruiz, P.; Marttos, A.C.; et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: A double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl. Med. 2021, 10, 660–673. [Google Scholar] [CrossRef] [PubMed]
- Drobiova, H.; Sindhu, S.; Ahmad, R.; Haddad, D.; Al-Mulla, F.; Al Madhoun, A. Wharton’s jelly mesenchymal stem cells: A concise review of their secretome and prospective clinical applications. Front. Cell Dev. Biol. 2023, 11, 1211217. [Google Scholar] [CrossRef] [PubMed]
- Marrazzo, P.; Crupi, A.N.; Alviano, F.; Teodori, L.; Bonsi, L. Exploring the roles of MSCs in infections: Focus on bacterial diseases. J. Mol. Med. 2019, 97, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Kouroupis, D.; Correa, D. Increased Mesenchymal Stem Cell Functionalization in Three-Dimensional Manufacturing Settings for Enhanced Therapeutic Applications. Front. Bioeng. Biotechnol. 2021, 9, 621748. [Google Scholar] [CrossRef] [PubMed]
- Bartosh, T.J.; Ylöstalo, J.H.; Mohammadipoor, A.; Bazhanov, N.; Coble, K.; Claypool, K.; Lee, R.H.; Choi, H.; Prockop, D.J. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc. Natl. Acad. Sci. USA 2010, 107, 13724–13729. [Google Scholar] [CrossRef]
- Yuan, X.; Sun, L.; Jeske, R.; Nkosi, D.; York, S.B.; Liu, Y.; Grant, S.C.; Meckes, D.G.; Li, Y. Engineering extracellular vesicles by three-dimensional dynamic culture of human mesenchymal stem cells. J. Extracell. Vesicles 2022, 11, e12235. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J. Oxygen, the Janus gas; its effects on human placental development and function. Am. J. Anat. 2009, 215, 27–35. [Google Scholar] [CrossRef]
- Ezquer, M.; Urzua, C.A.; Montecino, S.; Leal, K.; Conget, P.; Ezquer, F. Intravitreal administration of multipotent mesenchymal stromal cells triggers a cytoprotective microenvironment in the retina of diabetic mice. Stem Cell Res. Ther. 2016, 7, 42. [Google Scholar] [CrossRef]
- Stavely, R.; Nurgali, K. The emerging antioxidant paradigm of mesenchymal stem cell therapy. Stem Cells Transl. Med. 2020, 9, 985–1006. [Google Scholar] [CrossRef]
- Marrazzo, P.; Angeloni, C.; Freschi, M.; Lorenzini, A.; Prata, C.; Maraldi, T.; Hrelia, S. Combination of Epigallocatechin Gallate and Sulforaphane Counteracts In Vitro Oxidative Stress and Delays Sternness Loss of Amniotic Fluid Stem Cells. Oxidative Med. Cell. Longev. 2018, 2018, 5263985. [Google Scholar] [CrossRef]
- Hu, J.; Yu, X.; Wang, Z.; Wang, F.; Wang, L.; Gao, H.; Chen, Y.; Zhao, W.; Jia, Z.; Yan, S.; et al. Long term effects of the implantation of Wharton’s jelly-derived mesenchymal stem cells from the umbilical cord for newly-onset type 1 diabetes mellitus. Endocr. J. 2013, 60, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Wu, Z.; Xu, X.; Liao, L.; Chen, J.; Huang, L.; Wu, W.; Luo, F.; Wu, C.; Pugliese, A.; et al. Umbilical Cord Mesenchymal Stromal Cell with Autologous Bone Marrow Cell Transplantation in Established Type 1 Diabetes: A Pilot Randomized Controlled Open-Label Clinical Study to Assess Safety and Impact on Insulin Secretion. Diabetes Care 2016, 39, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Pizzuti, V.; Paris, F.; Marrazzo, P.; Bonsi, L.; Alviano, F. Mitigating Oxidative Stress in Perinatal Cells: A Critical Step toward an Optimal Therapeutic Use in Regenerative Medicine. Biomolecules 2023, 13, 971. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.Z.; Kucia, M.; Jadczyk, T.; Greco, N.J.; Wojakowski, W.; Tendera, M.; Ratajczak, J. Pivotal role of paracrine effects in stem cell therapies in regenerative medicine: Can we translate stem cell-secreted paracrine factors and microvesicles into better therapeutic strategies? Leukemia 2012, 26, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.A.; Chan, L.K.W.; Hung, L.C.; Phoebe, L.K.W.; Park, Y.; Yi, K.-H. Clinical Applications of Exosomes: A Critical Review. Int. J. Mol. Sci. 2024, 25, 7794. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Turturici, G.; Tinnirello, R.; Sconzo, G.; Geraci, F. Extracellular membrane vesicles as a mechanism of cell-to-cell communication: Advantages and disadvantages. Am. J. Physiol. Physiol. 2014, 306, C621–C633. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, W.; Gomez-Lopez, N.; Erez, O.; Romero, R.; Margolis, L. Extracellular vesicles generated by placental tissues ex vivo: A transport system for immune mediators and growth factors. Am. J. Reprod. Immunol. 2018, 80, e12860. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kang, M.-H.; Jeyaraj, M.; Qasim, M.; Kim, J.-H. Review of the Isolation, Characterization, Biological Function, and Multifarious Therapeutic Approaches of Exosomes. Cells 2019, 8, 307, Erratum in Cells 2021, 10, 462. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.-Y.; Papoutsakis, E.T. Extracellular vesicles: Exosomes, microparticles, their parts, and their targets to enable their biomanufacturing and clinical applications. Curr. Opin. Biotechnol. 2019, 60, 89–98. [Google Scholar] [CrossRef]
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience 2015, 65, 783–797. [Google Scholar] [CrossRef] [PubMed]
- Robbins, P.D.; Morelli, A.E. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 2014, 14, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Marrazzo, P.; Pizzuti, V.; Zia, S.; Sargenti, A.; Gazzola, D.; Roda, B.; Bonsi, L.; Alviano, F. Microfluidic Tools for Enhanced Characterization of Therapeutic Stem Cells and Prediction of Their Potential Antimicrobial Secretome. Antibiotics 2021, 10, 750. [Google Scholar] [CrossRef]
- Papait, A.; Ragni, E.; Cargnoni, A.; Vertua, E.; Romele, P.; Masserdotti, A.; Orfei, C.P.; Signoroni, P.B.; Magatti, M.; Silini, A.R.; et al. Comparison of EV-free fraction, EVs, and total secretome of amniotic mesenchymal stromal cells for their immunomodulatory potential: A translational perspective. Front. Immunol. 2022, 13, 960909. [Google Scholar] [CrossRef] [PubMed]
- Conforti, A.; Scarsella, M.; Starc, N.; Giorda, E.; Biagini, S.; Proia, A.; Carsetti, R.; Locatelli, F.; Bernardo, M.E. Microvescicles Derived from Mesenchymal Stromal Cells Are Not as Effective as Their Cellular Counterpart in the Ability to Modulate Immune Responses In Vitro. Stem Cells Dev. 2014, 23, 2591–2599. [Google Scholar] [CrossRef]
- Del Fattore, A.; Luciano, R.; Pascucci, L.; Goffredo, B.M.; Giorda, E.; Scapaticci, M.; Fierabracci, A.; Muraca, M. Immunoregulatory Effects of Mesenchymal Stem Cell-Derived Extracellular Vesicles on T Lymphocytes. Cell Transplant. 2015, 24, 2615–2627. [Google Scholar] [CrossRef] [PubMed]
- Blázquez, R.; Sanchez-Margallo, F.M.; de la Rosa, O.; Dalemans, W.; Ãlvarez, V.; Tarazona, R.; Gasado, J. Immunomodulatory Potential of Human Adipose Mesenchymal Stem Cells Derived Exosomes on in vitro Stimulated T Cells. Front. Immunol. 2014, 5, 556. [Google Scholar] [CrossRef]
- Lopatina, T.; Favaro, E.; Grange, C.; Cedrino, M.; Ranghino, A.; Occhipinti, S.; Fallo, S.; Buffolo, F.; Gaykalova, D.A.; Zanone, M.M.; et al. PDGF enhances the protective effect of adipose stem cell-derived extracellular vesicles in a model of acute hindlimb ischemia. Sci. Rep. 2018, 8, 17458. [Google Scholar] [CrossRef] [PubMed]
- Carreras-Planella, L.; Monguió-Tortajada, M.; Borràs, F.E.; Franquesa, M. Immunomodulatory Effect of MSC on B Cells Is Independent of Secreted Extracellular Vesicles. Front. Immunol. 2019, 10, 1288. [Google Scholar] [CrossRef]
- Abumaree, M.H.; Abomaray, F.M.; Alshabibi, M.A.; AlAskar, A.S.; Kalionis, B. Immunomodulatory properties of human placental mesenchymal stem/stromal cells. Placenta 2017, 59, 87–95. [Google Scholar] [CrossRef]
- Silini, A.R.; Magatti, M.; Cargnoni, A.; Parolini, O. Is Immune Modulation the Mechanism Underlying the Beneficial Effects of Amniotic Cells and Their Derivatives in Regenerative Medicine? Cell Transplant. 2017, 26, 531–539. [Google Scholar] [CrossRef]
- Sandonà, M.; Esposito, F.; Cargnoni, A.; Silini, A.; Romele, P.; Parolini, O.; Saccone, V. Amniotic Membrane-Derived Stromal Cells Release Extracellular Vesicles That Favor Regeneration of Dystrophic Skeletal Muscles. Int. J. Mol. Sci. 2023, 24, 12457. [Google Scholar] [CrossRef] [PubMed]
- Bier, A.; Berenstein, P.; Kronfeld, N.; Morgoulis, D.; Ziv-Av, A.; Goldstein, H.; Kazimirsky, G.; Cazacu, S.; Meir, R.; Popovtzer, R.; et al. Placenta-derived mesenchymal stromal cells and their exosomes exert therapeutic effects in Duchenne muscular dystrophy. Biomaterials 2018, 174, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Magarotto, F.; Sgrò, A.; Dorigo Hochuli, A.H.; Andreetta, M.; Grassi, M.; Saggioro, M.; Nogara, L.; Tolomeo, A.M.; Francescato, R.; Collino, F.; et al. Muscle functional recovery is driven by extracellular vesicles combined with muscle extracellular matrix in a volumetric muscle loss murine model. Biomaterials 2021, 269, 120653. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Miyaki, S.; Ishitobi, H.; Matsuyama, S.; Nakasa, T.; Kamei, N.; Akimoto, T.; Higashi, Y.; Ochi, M. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett. 2015, 589, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Noonin, C.; Thongboonkerd, V. Exosome-inflammasome crosstalk and their roles in inflammatory responses. Theranostics 2021, 11, 4436–4451. [Google Scholar] [CrossRef]
- Li, J.; Csakai, A.; Jin, J.; Zhang, F.; Yin, H. Therapeutic Developments Targeting Toll-like Receptor-4-Mediated Neuroinflammation. ChemMedChem 2016, 11, 154–165. [Google Scholar] [CrossRef]
- Kwon, S.; Ki, S.M.; Park, S.E.; Kim, M.-J.; Hyung, B.; Lee, N.K.; Shim, S.; Choi, B.-O.; Na, D.L.; Lee, J.E.; et al. Anti-apoptotic Effects of Human Wharton’s Jelly-derived Mesenchymal Stem Cells on Skeletal Muscle Cells Mediated via Secretion of XCL1. Mol. Ther. 2016, 24, 1550–1560. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).