Abstract
Cardiovascular disease (CVD) remains a leading cause of mortality globally, underscoring the need for robust predictive biomarkers to enhance risk stratification. Soluble lectin-like oxidized low-density lipoprotein receptor-1 (sLOX-1) has emerged as a promising biomarker linked to oxidative stress and endothelial dysfunction, both critical mechanisms in atherogenesis and cardiovascular events. Objectives: This study aimed to evaluate the prognostic value of sLOX-1 in predicting major adverse cardiovascular and cerebrovascular events (MACCEs), myocardial infarction (MI), heart failure (HF), and stroke outcomes through a systematic review and meta-analysis. Methods: A systematic literature search was conducted across PubMed, Scopus, Web of Science, and Ovid databases for studies published between 2014 and October 2024. Eligible studies assessed the association between sLOX-1 levels and future CVD outcomes in adult populations. Meta-analysis pooled hazard ratios (HRs) were assessed using random- and fixed-effects models. Heterogeneity was evaluated using the I2 statistic, and study quality was assessed using the Newcastle–Ottawa Scale. Results: Fourteen studies were included, encompassing diverse populations with coronary artery disease (CAD), acute coronary syndrome (ACS), or stroke, with follow-up durations ranging from 30 days to 19.5 years. The meta-analysis of three studies on CAD patients demonstrated a significant association between elevated sLOX-1 levels and increased MACCE risk (HR: 2.3, 95% CI: 0.99–5.33, p = 0.05), albeit with high heterogeneity (I2 = 83%). The fixed-effects analysis yielded a more consistent HR of 1.47 (95% CI: 1.19–1.81, p < 0.01). Conclusions: sLOX-1 shows promising potential as a prognostic biomarker for CVD and is associated with an increased risk of MACCEs in CAD patients. However, the high heterogeneity among the included studies highlights the need for standardized protocols and larger, well-designed prospective studies to validate its clinical utility. The integration of sLOX-1 into risk prediction models could improve CVD management by identifying high-risk individuals for targeted interventions.
1. Introduction
Cardiovascular disease (CVD) remains the leading cause of morbidity and mortality worldwide, accounting for an estimated 17.9 million deaths each year, representing 31% of all global deaths [1]. CVD encompasses a broad spectrum of heart and vascular disorders, including coronary artery disease (CAD), stroke, and peripheral artery disease. In Malaysia, the burden of CVD is alarming, with ischemic heart disease identified as the leading cause of death, contributing to 17% of total fatalities in 2020 [2]. The rising prevalence of risk factors such as hypertension, diabetes, obesity, and smoking has further escalated the incidence of CVD in the country [3]. Given the substantial health and economic toll of CVD, enhancing early detection and prevention strategies is imperative. Identifying reliable biomarkers to predict future cardiovascular events is vital for mitigating the impact of CVD on individuals and healthcare systems.
In recent years, the search for reliable biomarkers has become an integral focus of cardiovascular research, aimed at improving the early diagnosis and prognosis of CVD. Biomarkers provide insight into underlying pathophysiological processes and can help identify individuals at high risk for cardiovascular events, even before clinical symptoms manifest [4]. Traditional markers, such as high-sensitivity C-reactive protein (hs-CRP) and troponins, have been widely used, yet their predictive value may be limited in certain populations [5]. This has led to the investigation of novel biomarkers with greater specificity and accuracy. One such promising marker is soluble lectin-like oxidized low-density lipoprotein receptor-1 (sLOX-1), a receptor involved in the binding and uptake of oxidized LDL (oxLDL), which plays a pivotal role in the development and progression of atherosclerosis [6]. Elevated levels of sLOX-1 have been associated with endothelial dysfunction, plaque instability, and an increased risk of acute coronary events, making it a potential candidate for improving CVD risk stratification [7].
sLOX-1 is a truncated, circulating form of the membrane-bound LOX-1 receptor, which is predominantly expressed on endothelial cells, macrophages, and smooth muscle cells within atherosclerotic plaques [8]. Structurally, LOX-1 belongs to the C-type lectin family and consists of four main domains: a short N-terminal cytoplasmic domain, a transmembrane domain, a neck region, and an extracellular C-type lectin-like domain [9]. The extracellular domain is responsible for binding oxLDL, a key driver of atherogenesis. Under conditions of oxidative stress and inflammation, oxLDL accumulates in the vascular endothelium, triggering LOX-1 activation [10]. This binding promotes endothelial dysfunction, foam cell formation, and plaque instability, all of which contribute to atherosclerosis progression.
The soluble form, sLOX-1, is released into circulation upon cleavage of the membrane-bound receptor, with elevated serum levels observed in patients with acute coronary syndrome (ACS) and other cardiovascular disorders. These findings suggest its potential as a non-invasive biomarker for CVD [11,12,13]. sLOX-1 has been linked to endothelial dysfunction, a critical early event in the development of atherosclerosis, driven by factors such as hypertension, diabetes, and hyperlipidemia [14]. Elevated sLOX-1 levels have also been associated with increased inflammatory markers such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), both of which exacerbate atherogenic processes [15]. Clinical investigations have demonstrated a correlation between sLOX-1 levels and the severity of atherosclerosis in patients with suspected CAD [16], reinforcing its relevance as a predictive biomarker in CVD management.
Beyond its association with acute events, growing evidence suggests that sLOX-1 could be a valuable predictor of future CVD events. Several longitudinal studies have investigated the relationship between baseline sLOX-1 levels and the incidence of cardiovascular outcomes such as myocardial infarction (MI), heart failure (HF), and stroke. For instance, baseline sLOX-1 concentrations have been shown to predict MI and heart failure in general populations [17]. Among patients with established CAD, higher sLOX-1 levels have been linked to increased long-term mortality and recurrent ischemic events [18]. However, despite the accumulating data, the overall consistency and strength of these associations remain unclear. To address this, a systematic review and meta-analysis were conducted to quantitatively assess the predictive value of sLOX-1 for future CVD events. This meta-analysis aimed to provide a comprehensive and reliable estimate of the association, explore potential sources of heterogeneity across studies, and further clarify sLOX-1′s role in cardiovascular risk stratification.
2. Methodology
This review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [19]. The PRISMA checklist is reported in Appendix A (Table A1 and Table A2). The review protocol has been registered with the International Platform of Registered Systematic Review Protocols (registration number INPLASY2024120078) [20].
2.1. Research Question and Search Strategy
The research question was formulated using the “PICO” framework. Patients with ACS, CAD, and acute ischemic stroke (AIS) or healthy subjects were identified as the “Population (P)”. Low sLOX-1 levels were defined as the “Comparison (C)”, while the occurrence of major adverse cardiocerebrovascular events (MACCEs), MI, HF, recurrent stroke, or unfavorable outcomes post stroke constituted the “Outcome (O)”. sLOX-1 levels were designated as the “Intervention (I)”. Hence, the research question was as follows: are increased sLOX-1 levels predictive of future CVD? A comprehensive literature search was carried out across four databases (Ovid, Scopus, Pubmed, and Google Scholar), focusing on studies published between 2014 and October 2024 (Accessed on 1 November 2024). The search employed the following keywords: (“LOX-1” OR “lectin-like oxidized LDL receptor-1”) AND (“Cardiovascular disease” OR “CVD” OR “Coronary artery disease” OR “CAD” OR “Myocardial infarction” OR “Acute coronary syndrome” OR “Atherosclerosis” OR “Peripheral vascular disease” OR “Cerebrovascular accident” OR “Stroke” OR “Major cardiovascular event” OR “MACE” OR “Mortality”).
2.2. Study Criteria
Two researchers (NS and AA) independently reviewed each article, ensuring compliance with predefined inclusion and exclusion criteria. The inclusion criteria included the following: (1) full-text peer-reviewed original articles published in English, (2) prospective and retrospective studies investigating the relationship between sLOX-1 and future coronary artery disease (CAD), cardiovascular disease (CVD), or major adverse cardiovascular events (MACEs), and (3) studies involving adult patients diagnosed with ACS, CAD, or AIS or healthy subjects, regardless of gender. The exclusion criteria consisted of (1) articles not published in English, (2) reviews, conference abstracts, editorials, newsletters, books, and book chapters, (3) in vitro studies, and (4) research involving animals.
2.3. Article Selection and Data Extraction
The article selection process comprised three stages. First, articles were screened according to their titles and types, with review or editorial articles excluded. Next, abstracts were examined to remove any articles that were irrelevant to sLOX-1 and CAD, CVD, or MACEs. Finally, the remaining articles underwent a detailed full-text review, and those that did not meet the inclusion criteria were eliminated. The article selection process was conducted independently by two reviewers (A.A. and N.S.). Similarly, data extraction was performed independently by two reviewers (A.A. and N.S.) using a standardized Excel form. Data extracted included study design, study duration, participant characteristics (e.g., age, gender, CVD diagnosis), methods of sLOX-1 measurement, and the relationship between sLOX-1 and atherosclerosis. Any disagreement was resolved by consensus or consultation with a third reviewer (A.U.) if necessary.
2.4. Risk of Bias Assessment
Two reviewers (N.S. and A.A.) independently assessed the risk of bias in the selected articles using the Newcastle–Ottawa Scale (NOS) [21]. For cohort and cross-sectional studies, the NOS evaluated the selection of study groups (exposed vs. non-exposed), their comparability, and outcome assessment. In contrast, for case–control studies, the NOS evaluated the selection of study groups (cases vs. controls), their comparability, and exposure assessment. Each of the eight NOS items was rated with one or two stars. Studies that received a total score of seven to nine stars were classified as high-quality, those with four to six stars as fair-quality, and those with one to three stars as low-quality.
2.5. Statistical Analysis
A meta-analysis was conducted using Review Manager (RevMan version 5.4 software, The Cochrane Collaboration 2000, Denmark) [22]. The hazard ratio (HR) or odds ratio (OR), with a 95% confidence interval (CI), derived from multivariate Cox proportional hazard analysis, were used as the effect estimate to evaluate s-LOX-1 as a predictor of MACEs in patients with ACS or AIS. The heterogeneity among studies was assessed using (1) the chi-squared test, with a p-value of less than 0.10 indicating statistical significance, and (2) the Higgin’s I2 statistic [23]. An I2 value of ˂25% indicated low heterogeneity, while an I2 value of ≥75% indicated high heterogeneity. Due to the limited number of studies available for meta-analysis and varying study population across studies, the random-effects (RE) model was employed. Statistical significance was set at p ˂ 0.05. Subgroup analysis was not performed due to the limited number of studies. In addition, funnel plots or the Egger test for publication bias were not conducted, as fewer than 10 studies were included.
3. Results
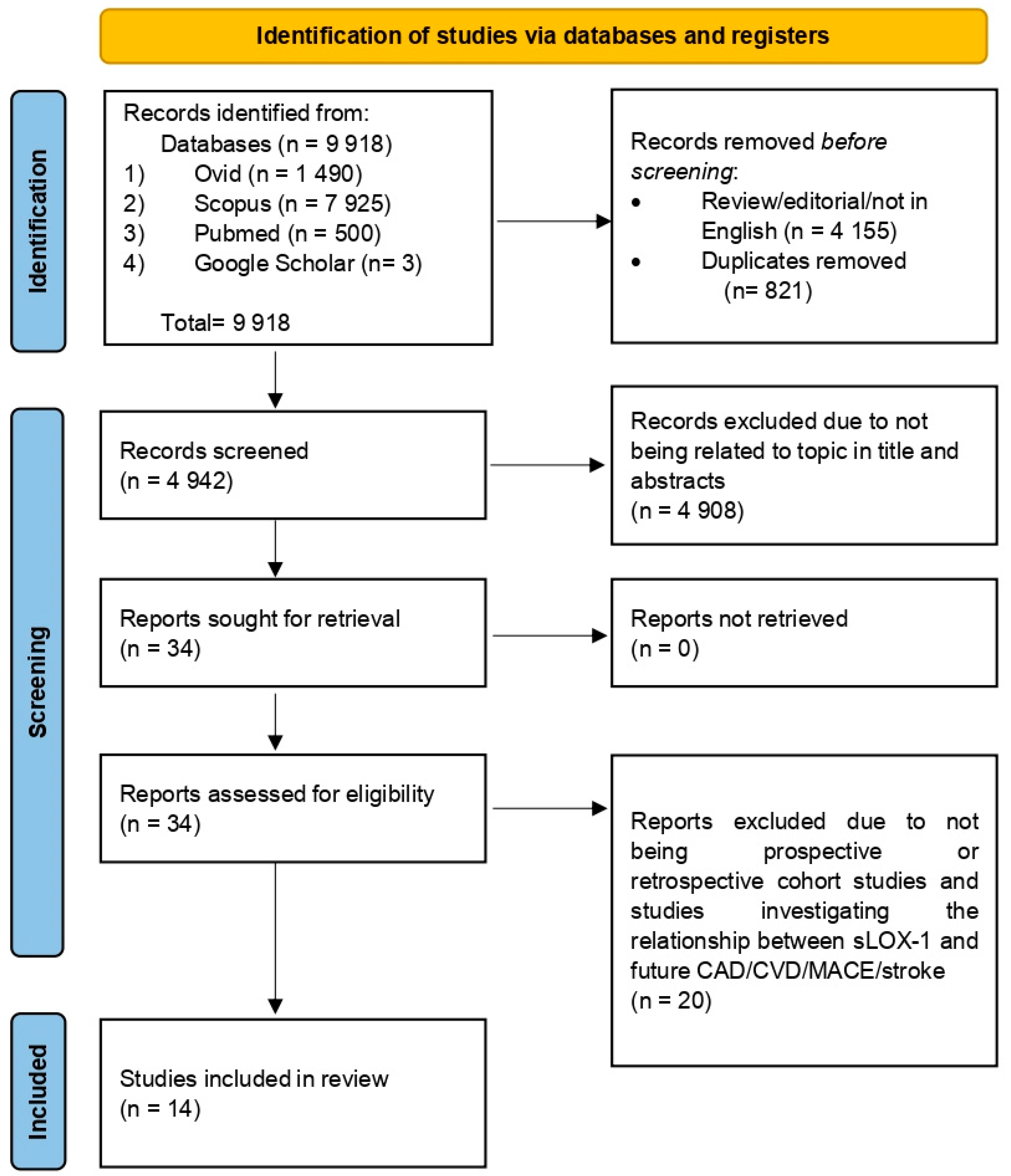
The search retrieved a total of 9918 articles from four databases: Ovid (1490), Scopus (7925), Pubmed (500), and Google Scholar (3). Articles were compiled using Mendeley Desktop Version 1.19.8 (Mendeley Ltd., London, UK) and 821 duplicates were removed. Additionally, 4155 reviews, editorials, and non-English-language articles were excluded. Title and abstract screening excluded 4908 articles unrelated to sLOX-1, CAD, or MACEs. The remaining 34 articles were read in full, with only 14 articles meeting the eligibility criteria for inclusion. The study selection process is summarized in Figure 1. The quality assessment of the 14 selected articles using the NOS scale is outlined in Table 1 and Table 2. The scores ranged from 6 to 8, reflecting fair to high quality. Specifically, 12 studies were categorized as high-quality [13,17,18,24,25,26,27,28,29,30,31,32], while 2 studies were classified as fair-quality [33,34]. Altogether, the risk of bias for all included studies in terms of the selection, comparator, and outcome reporting bias was considered low. Therefore, results synthesized using data from the included studies may contribute to a reliable source of information which could be used to inform clinical practice regarding the association between sLOX-1 and future CVD risk.
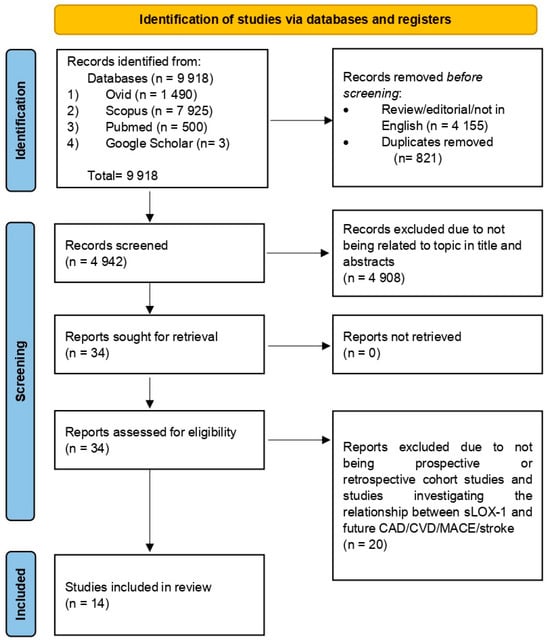
Figure 1.
Flow chart illustrating the process of selecting and screening based on PRISMA.

Table 1.
The Newcastle–Ottawa Scale (NOS) for assessing the quality of cohort studies (CAD studies).

Table 2.
The Newcastle–Ottawa Scale (NOS) for assessing the quality of cohort studies (stroke studies).
3.1. General Characteristics of the Included Studies
The 14 studies included in this study were published between 2014 and October 2024. Seven studies focused on the predictive value of sLOX-1 for CAD, while another seven focused on the role of sLOX-1 as predictor for stroke. All studies were prospective, except for the study by Ren et al. 2023 [28], which was retrospective. Two studies involved subjects without CAD or stroke at baseline [17,32], while the remaining studies involved subjects who already had CAD or ACS [18,24,25,26,27,33] or stroke [13,19,28,29,30,31]. The duration of follow-up ranged from 30 days to 19.5 years. Most of the subjects were male, with ages ranging from middle-aged to elderly. All studies assessed sLOX-1 levels from plasma or serum samples. For CAD or ACS patients, outcome measures included MACCEs, HF, or MI. For stroke patients, outcomes focused on functional recovery post stroke and stroke recurrence. The definition of MACCEs varied among studies. For instance, Zhao (a) et al. 2019 [33] defined MACCEs as all-cause death, readmission for ACS, unplanned repeat revascularization, definite stent thrombosis, and ischemic stroke, while Zhao (b) et al. 2019 [26] defined them as all-cause death, nonfatal acute MI, and readmission for Braunwald’s class IIIb unstable angina requiring treatment. In addition, Higuma et al. 2015 [27] defined MACCEs as cardiovascular mortality and recurrent nonfatal MI. Several studies used different cut-off values to define high sLOX-1 levels. For instance, Higuma et al. defined it as >71 pg/mL, while Zhao et al. [33] used ≥1.10 ng/mL, and Zhao et al. [26] applied >0.91 ng/mL. For sLOX-1 measurements, the ELISA technique was used in most of the studies [13,18,24,25,26,29,30,34]. However, there were other methods used, such as the PEA technique [17], sandwich chemiluminescent enzyme immunoassay [27], anti-analyzer [31], and multiplex kit (34).
3.2. Summary of Findings
3.2.1. sLOX-1 and Future MACCEs, MI and HF
For studies investigating the association between sLOX-1 and future MACCEs, MI, or HF, most subjects had ACS or CAD, with only one study involving a non-CAD population [17]. Among CAD or ACS patients, five studies reported a significant positive association (Table 3). For example, Higuma et al. 2015 [27] found that higher sLOX-1 levels predicted a higher risk of MACCEs, with a HR of 2.44 (95% CI: 1.07–5.57), while Zhao (a) and (b) et al. 2019 [26,33] reported HRs of 1.28 (95% CI: 1.02–1.60) and 4.73 (95% CI: 2.17–10.30), respectively. The authors of [24] also reported that elevated sLOX-1 levels were associated with a 2.3-fold increased risk of cardiovascular mortality within one year of follow-up (HR: 2.29, 95% CI: 1.19–5.34; p = 0.0148). In another study, sLOX-1 levels were associated with MACCE in ST-elevation MI (STEMI) and unstable angina (UA)/non-STEMI groups (Spearman correlation coefficients: 0.345, p < 0.001, and 0.189, p = 0.017, respectively) [18]. Among patients with recurrent cases of MI and stroke, the baseline post interventional sLOX-1 levels were higher compared to the non-recurrent cases [25]. In a study of non-CAD subjects, higher sLOX-1 levels were associated with an increased risk of MACCEs (HR: 1.76, 95% CI: 1.40–2.21) [17].

Table 3.
Association between circulating sLOX-1 and future cardiovascular events among CAD patients and healthy subjects.
3.2.2. sLOX-1 and Future Functional Outcomes Post Strokes or Recurrent Strokes
Among stroke patients, several studies identified significant associations between baseline sLOX-1 and unfavorable or favorable functional outcomes post strokes [13,29,30,34] (Table 4). Functional outcomes were measured using the Modified Rankin Score (mRS). Additionally, several studies reported a significant association between sLOX-1 levels and higher risk of recurrent stroke among patients with or without prior history of stroke [28,31,32].

Table 4.
Association between circulating s-LOX-1 and future recurrent stroke or unfavorable outcomes post stroke among stroke patients and healthy subjects.
3.2.3. Meta-Analysis
sLOX-1 and MACCEs
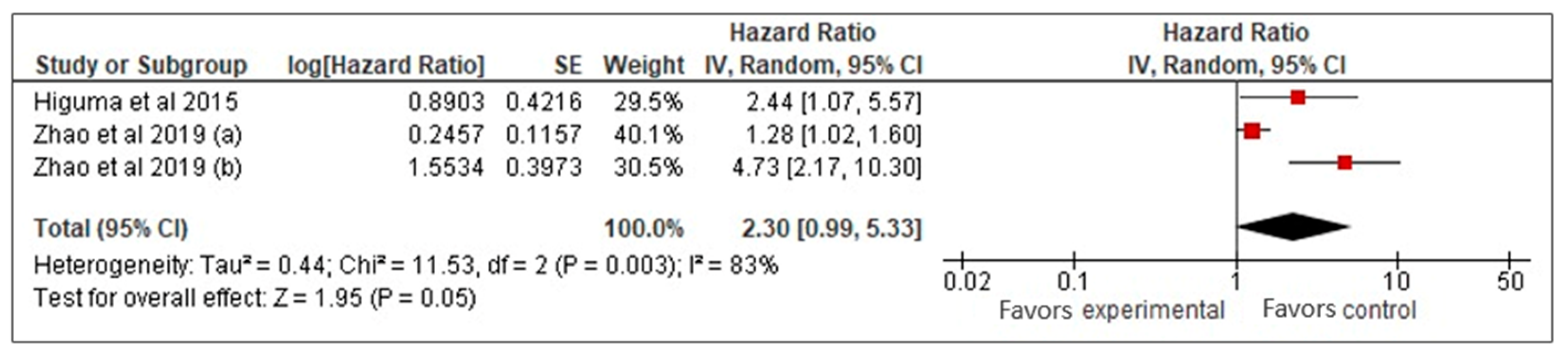
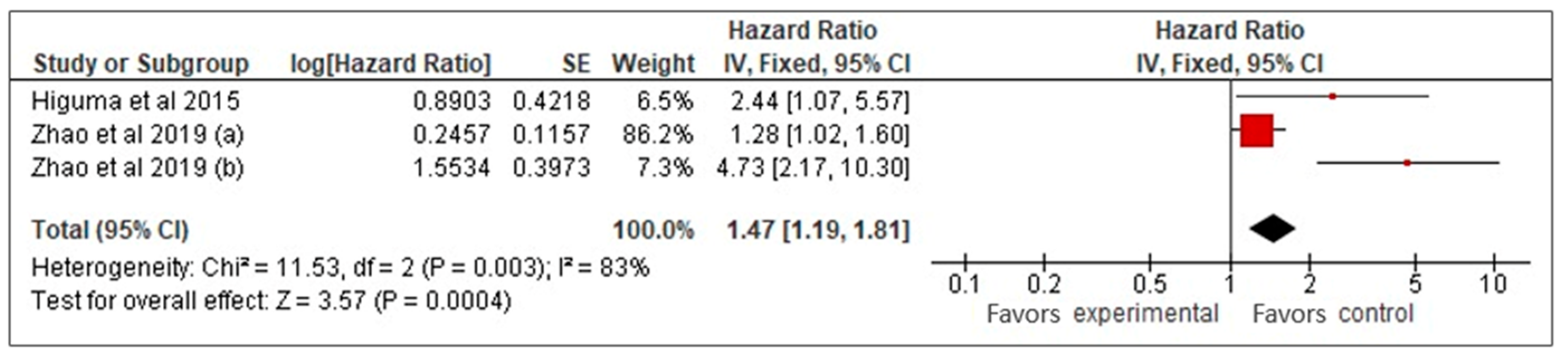
Our meta-analysis of three studies [26,27,33] examined the prognostic value of sLOX-1 for predicting MACCE in CAD patients. Using a random-effects model, it was found that higher sLOX-1 levels were associated with a 2.3-fold increased risk of MACCE (HR: 2.3, 95% CI: 0.99–5.33, p = 0.05) (Figure 2). However, the wide confidence interval and high heterogeneity (I2 = 83%) indicated variability across the studies. To address heterogeneity, a sensitivity analysis using a fixed-effects model was performed (Figure 3). This analysis yielded a more precise HR of 1.47 (95% CI: 1.19–1.81, p < 0.01), confirming a consistent and statistically significant association between elevated sLOX-1 levels and increased MACCE risk. There were several potential sources of variability. First, there were differences in study population, such as age and prevalence of comorbidities. Although the mean age of the subjects in the three studies was relatively similar (67–69 years old), the age range were different. For example, one study by Zhao et al. [26] involved subjects with age ranges between 32 and 87 years old, while the other study by Zhao et al. [33] involved subjects with age ranges between 59 and 76 years old. In addition, each study had different distributions of comorbidities. For example, in the study population in Higuma et al. [27], 75% had hypertension, 33% had diabetes, 63% had dyslipidemia, and 54% smoked. In Zhao et al. [33], among those with MACCEs, 50% smoked, 31.3% had diabetes, and 58.26% had hypertension. In the study population in Zhao et al. [26], 72% had hypertension, 45% smoked, and 45% had diabetes. Variations in comorbidities also contributed to variations in the medications used. Most of the populations in the three studies used statins, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and beta blockers; however, the percentages that used them were different [26,27,33]. Another possible contributing factor was the method used for sLOX-1 measurements. The studies by Zhao et al. ([26,33]) used ELISA, while the study by Higuma et al. [27] used a sandwich chemiluminescent enzyme immunoassay. Moreover, the definition of outcome was also differed across the studies, as mentioned above.
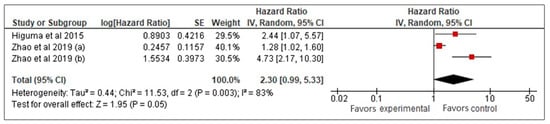
Figure 2.
sLOX-1 as a predictor of MACCEs (random-effects model). Red box indicates the effect size for individual study which in this model, the size of the boxes are fairly similar indicating equal weight distribution across all included studies. While, the black diamond, which is the visual representation of the pooled effect sizes, is wide with its right tip touches the line of no effect denoting borderline statistical significance with wide 95% CI.
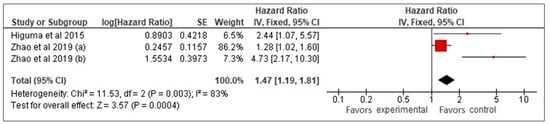
Figure 3.
sLOX-1 as a predictor of MACCEs (fixed-effects). Red box indicates the effect size for individual study while the black diamond is the visual representation of the pooled effect sizes. In this model, the most weight (86.2%) is allocated to the study with the biggest red square, thus influencing the pooled effect size (black diamond).
sLOX-1 and Stroke Outcomes
A meta-analysis for sLOX-1 and stroke outcomes could not be conducted due to high variation between the studies. Although there were three studies that investigated the association between baseline s-LOX-1 and functional outcomes post stroke [13,29,34], the patients included were not similar. For example, the study by Yan et al. [29] involved patients with first-ever stroke, while Zheng et al. [34] involved patients with first-ever stroke and recurrent ischemic stroke. In another study, this characteristic was not detailed [13]. In addition, there were differences in patient age, comorbidities, the medications used, and the duration of follow-up between studies. For example, the studies by Zheng et al. [34] and Yan et al. [29] assessed the patient condition at three months, while the study by Li et al. [13] reviewed the patient condition at one year. Because of these variation, the pooling of data was deemed inappropriate.
4. Discussion
Our meta-analyses involving CAD patients supported the role of sLOX-1 as a prognostic biomarker for MACCE. The stronger association observed in the fixed-effects model suggests that sLOX-1 may have consistent predictive value when study-level variations are accounted for. However, the high heterogeneity observed in both models necessitates caution when interpreting the pooled estimates. Possible contributors to this heterogeneity include differences in study populations (e.g., age, comorbidities, and treatment regimens), sLOX-1 measurement methods and threshold definitions, MACCE definitions, and follow-up durations. The variability in sLOX-1 cut-off values reported across studies may also reflect underlying differences in systemic inflammation, as inflammation is a known contributor to elevated sLOX-1 levels. However, most of the studies included in the meta-analysis did not report data on CRP, limiting our ability to normalize sLOX-1 levels based on inflammation. Despite this limitation, the robust association between sLOX-1 levels and cardiovascular outcomes observed in most of the studies suggests that sLOX-1 remains a valuable biomarker, even without adjustment for inflammatory status.
The integration of sLOX-1 into clinical practice offers several potential pathways to enhance cardiovascular care. As a biomarker associated with oxidative stress and endothelial dysfunction, sLOX-1 could serve as an adjunct to traditional risk scores, such as the Framingham Risk Score or GRACE score, to improve risk stratification, particularly for individuals at intermediate or high risk. Additionally, its role in the early detection of subclinical atherosclerosis or acute vascular events could be pivotal in identifying individuals with ambiguous symptoms or those requiring timely intervention. sLOX-1 levels may also inform personalized treatment decisions, such as guiding the use of statins, antioxidants, or anti-inflammatory therapies, and monitoring these levels could help assess treatment efficacy over time. Furthermore, in acute coronary syndrome patients, sLOX-1 could refine prognostic models, aiding in decisions regarding invasive procedures or extended monitoring during hospital admission and follow-up. Lastly, its utility in specific high-risk populations, such as individuals with diabetes, metabolic syndrome, or chronic inflammatory conditions, could enable targeted screening and earlier intervention to mitigate cardiovascular risk. However, integrating sLOX-1 into routine clinical practice faces several challenges. Firstly, the variability in sLOX-1 measurement techniques, including the use of different ELISA kits and assay methodologies, poses a significant challenge. The use of diverse kits, each employing antibodies with varying specificities and affinities, can substantially influence protein measurement [35]. Additionally, differences in detection limits and quantification thresholds among kits may lead to the underestimation of low protein concentrations when low-sensitivity kits are used [36]. These inconsistencies complicate result comparability and the establishment of universal reference ranges. Furthermore, there is no consensus on clinically meaningful sLOX-1 cut-off values for risk stratification. Many studies report associations between sLOX-1 levels and outcomes but fail to define threshold categories, such as low, moderate, or high risk [17]. Lastly, integrating sLOX-1 testing into routine clinical workflows requires the development of cost-effective, scalable methods and collaborative efforts between researchers and clinicians to establish a standardized interpretation of sLOX-1 levels for informed clinical decision-making.
Several mechanisms have been implicated in the association between sLOX-1, MACCEs, and post-stroke functional outcomes. First, sLOX-1 is associated with oxidative stress and endothelial dysfunction. Its ligand, oxLDL, disrupts eNOS activity in endothelial cells, reducing nitric oxide (NO) production. Lower NO levels lead to endothelial dysfunction, promoting damage, lipid buildup, and plaque formation [37]. Elevated sLOX-1 levels reflect oxidative stress, impair endothelial function, and contribute to atherosclerosis, plaque rupture, and thrombus formation [10]. High circulating sLOX-1 levels are linked to an increased likelihood of thrombotic events and worse outcomes in acute cardiovascular conditions [8]. Second, sLOX-1 release is associated with the activation of pro-inflammatory pathways, leading to the upregulation of adhesion molecules. Activation of mitogen-activated protein kinase (MAPK) by oxLDL promotes the transcription of adhesion molecules, including monocyte chemoattractant protein-1 (MCP-1), intracellular adhesion molecules-1 (ICAM-1), and vascular cell adhesion molecules (VCAM-1). Elevated MCP-1 attracts monocytes to the endothelium [38,39,40]. This inflammatory response exacerbates atherosclerosis and predisposes individuals to adverse cardiovascular and cerebrovascular events, impeding poor functional recovery after a stroke. Third, in atherosclerosis, sLOX-1 contributes to vascular remodeling, promoting the migration and proliferation of smooth muscle cells and the production of matrix metalloproteinases (MMPs) [41,42]. The binding of oxLDL to LOX-1 on endothelial cells activates the protein kinase C-beta (PKC-β) signaling cascade. PKC-β increases the expression of MMPs [41]. Excessive MMP activity degrades the extracellular matrix and contributes to endothelial dysfunction, vascular remodeling, and plaque instability. This process increases the vulnerability of plaques to rupture, thereby increasing the risk of acute cardiovascular events, including MI and stroke [41,42]. In stroke, elevated sLOX-1 levels are linked to increased vascular permeability and damage, correlating with poorer functional outcomes and recovery [43]. The detailed mechanisms underlying the role sLOX-1 in CVD pathophysiology have been extensively reviewed in previous studies [10,44].
5. Limitations and Future Directions
The exclusion of gray literature and unpublished data is a limitation of this review. While this decision was made to prioritize the quality and rigor of the evidence, it may have resulted in the omission of relevant studies, potentially introducing publication bias. In addition, a restriction of this meta-analysis lies in the limited number of available studies, which restricts the statistical power and the robustness of the pooled estimates. A small number of studies inherently increases the influence of individual study results on the overall meta-analysis, potentially biasing the conclusions. Furthermore, the wide confidence intervals observed in the random-effects model highlight imprecisions, necessitating caution in interpretation.
High heterogeneity across studies also raises concerns about the consistency and comparability of results. Variations in study design, population characteristics, sLOX-1 assay methodologies, and follow-up durations challenge the generalizability of the findings. Standardized protocols in future research are needed to address this issue. Publication bias and potential unmeasured confounders within the included studies cannot be excluded, further limiting the reliability of the results. Well-designed prospective studies are urgently needed to confirm the prognostic value of sLOX-1, particularly for MACCEs and functional outcomes post stroke. Such studies should recruit diverse populations to enhance the generalizability of findings across demographic and clinical subgroups. Efforts should also focus on standardizing sLOX-1 measurement protocols, including assay methods, sampling techniques, and risk stratification thresholds. A consensus on these parameters will facilitate the clinical integration of sLOX-1 and allow for meaningful comparisons between studies.
Additionally, further investigations into the biological mechanisms linking sLOX-1 and MACE are critical. Understanding sLOX-1′s role in endothelial dysfunction, inflammation, and plaque instability could validate its clinical relevance and uncover novel therapeutic targets for CVD. Exploring these mechanisms may also clarify sLOX-1′s potential utility in guiding treatment strategies or monitoring disease progression.
6. Conclusions
This systematic review highlights the potential of sLOX-1 as a prognostic biomarker for CVD, particularly in predicting MACCEs. Despite promising findings, limitations such as the small number of studies, wide confidence intervals, and significant heterogeneity across studies underscore the need for cautious interpretation. While sLOX-1 shows promise as a tool for cardiovascular risk stratification, more robust evidence is required before its integration into routine clinical practice. Future studies should focus on standardizing sLOX-1 measurement protocols and definitions of outcomes.
Author Contributions
Conceptualization and methodology, A.A.; methodology and data curation, N.S.; data curation and original-draft, A.A. and A.U.; formal analysis (meta-analysis), N.A.C.R.; writing—review and editing, S.F.M. and B.C.B.; formal analysis (risk bias) and investigation, A.A., N.S. and A.A.H.; writing—review, editing, validation and proofreading, A.A., N.S., A.A.H. and A.U. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a Research University Grant (GUP) with grant number (GUP-2024-030) provided by the Universiti Kebangsaan Malaysia.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
All authors would like to thank the Faculty of Medicine, UKM, for the resources used to complete this study.
Conflicts of Interest
The authors have declared no conflicts of interest.
Appendix A

Table A1.
PRISMA 2020 item checklist.
Table A1.
PRISMA 2020 item checklist.
| Section and Topic | Item | Checklist Item | Location Where Item is Reported |
|---|---|---|---|
| Title | 1 | Identify the report as a systematic review. | Title, line 3–4 |
| Abstract | 2 | See the PRISMA 2020 for Abstracts checklist. | Appendix A |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of existing knowledge. | Introduction, paragraphs 1–5 |
| Objectives | 4 | Provide an explicit statement of the objective(s) or question(s) the review addresses. | Introduction, paragraph 5, lines 97–101 |
| Methods | |||
| Eligibility criteria | 5 | Specify the inclusion and exclusion criteria for the review and how studies were grouped for the syntheses. | Methodology, see Section 2.2 |
| Information sources | 6 | Specify all databases, registers, websites, organisations, reference lists, and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted. | Methodology, see Section 2.1, line 118 |
| Search strategy | 7 | Present the full search strategies for all databases, registers and websites, including any filters and limits used. | Methodology, see Section 2.1 |
| Selection process | 8 | Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and if applicable, details of automation tools used in the process. | Methodology, see Section 2.3 |
| Data collection process | 9 | Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and if applicable, details or automation tools used in the process. | Methodology, see Section 2.3 |
| Data items | 10a | List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought (e.g., for all measures, time points, analyses), and if not, the methods used to decide which results to collect. | Methodology, see Section 2.3, line 144–147 |
| 10b | List and define all other variables for which data were sought (e.g., participant and intervention characteristics, funding sources). Describe any assumptions made about any missing or unclear information. | Not applicable | |
| Study risk of bias assessment | 11 | Specify the methods used to assess risk of bias in the included studies, including details of the tool(s) used, how many reviewers assessed each study and whether they worked independently, and if applicable, details of automation tools used in the process. | Methodology, see Section 2.4 |
| Effect measures | 12 | Specify for each outcome the effect measure(s) (e.g., risk ratio, mean difference) used in the synthesis or presentation of results. | Methodology, see Section 2.5, line 161 |
| Synthesis methods | 13a | Describe the processes used to decide which studies were eligible for each synthesis (e.g., tabulating the study intervention characteristics and comparing against the planned groups for each synthesis (item #5)) | Not applicable |
| 13b | Describe any methods required to prepare the data for presentation or synthesis, such as handling of missing summary statistics, or data conversions | Articles were exported from databases into Mendeley web as taught by Mrs. Norizam Salamat | |
| 13c | Describe any methods used to tabulate or visually display results of individual studies and syntheses. | Not applicable | |
| 13d | Describe any methods used to synthesise results and provide a rationale for the choice(s). If meta-analysis was performed, describe the model(s), method(s) to identify the presence and extent of statistical heterogeneity, and software package(s) used. | Methodology, see Section 2.5 | |
| 13e | Describe any methods used to explore possible causes of heterogeneity among study results (e.g., subgroup analysis, metaregression). | Methodology, see Section 2.5, line 163–166 | |
| 13f | Describe any sensitivity analyses conducted to assess robustness of the synthesised results. | Methodology, see Section 2.5, line 168 | |
| Reporting bias assessment | 14 | Describe any methods used to assess risk of bias due to missing results in a synthesis (arising from reporting biases). | Not applicable |
| Certainty assessment | 15 | Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome. | Not applicable |
| Results | |||
| Study selection | 16a | Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram (see Figure 1). | Results, see Figure 1, Section 3.1 and Section 3.2. |
| 16b | Cite studies that might appear to meet the inclusion criteria, but which were excluded, and explain why they were excluded | Not stated | |
| Study characteristics | 17 | Cite each included study and present its characteristics. | Results, see Table 3 and Table 4 |
| Risk of bias in studies | 18 | Present assessments of risk of bias for each included study. | Results, see Table 1 and Table 2 |
| Results of individual studies | 19 | For all outcomes, present, for each study: (a) summary statistics for each group (where appropriate) and (b) an effect estimate and its precision (e.g., confidence/credible interval), ideally using structured tables or plots. | Results, see Table 3 and Table 4, column “Key findings” and “Conclusion” |
| Results of syntheses | 20a | For each synthesis, briefly summarise the characteristics and risk of bias among contributing studies | Results, see Table 3 and Table 4, column “Population characteristic”. For risk of bias, see Table 1 and Table 2 |
| 20b | Present results of all statistical syntheses conducted. If meta-analysis was done, present for each the summary estimate and its precision (e.g., confidence/credible interval) and measures of statistical heterogeneity. If comparing groups, describe the direction of the effect. | Results, see Section 3.2.3 Meta-analysis | |
| 20c | Present results of all investigations of possible causes of heterogeneity among study results. | Results, see Figure 3 | |
| 20d | Present results of all sensitivity analyses conducted to assess the robustness of the synthesised results. | Results, see Figure 2 | |
| Reporting biases | 21 | Present assessments of risk of bias due to missing results (arising from reporting biases) for each synthesis assessed | Not applicable |
| Certainty of evidence | 22 | Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed. | Not applicable |
| Discussion | 23a | Provide a general interpretation of the results in the context of other evidence | Discussion, paragraph 1–3 |
| 23b | Discuss any limitations of the evidence included in the review. | Not applicable | |
| 23c | Discuss any limitations of the review processes used. | Limitations and Future Directions | |
| 23d | Discuss implications of the results for practice, policy, and future research. | Conclusion | |
| Other information | |||
| Registration and protocol | 24a | Provide registration information for the review, including register name and registration number, or state that the review was not registered. | See “Protocol Registration” |
| 24b | Indicate where the review protocol can be accessed, or state that a protocol was not prepared | See “Protocol Registration” | |
| 24c | Describe and explain any amendments to information provided at registration or in the protocol. | Not applicable | |
| Support | 25 | Describe sources of financial or non-financial support for the review, and the role of the funders or sponsors in the review. | See “Funding” |
| Competing interests | 26 | Declare any competing interests of review authors. | See “Conflict of Interest” |
| Availability of data, code, and other materials | 27 | Report which of the following are publicly available and where they can be found: template data collection forms; data extracted from included studies; data used for all analyses; analytic code; any other materials used in the review. | See “Data availability statement” |

Table A2.
PRISMA 2020 for Abstract checklist.
Table A2.
PRISMA 2020 for Abstract checklist.
| Section and Topic | Item | Checklist Item | Location Where Item is Reported |
|---|---|---|---|
| Title | 1 | Identify the report as a systematic review. | Abstract, line 22–23 |
| Background | |||
| Objectives | 2 | Provide an explicit statement of the main objective (s) or question (s) the review addresses. | Abstract, line 19–22 |
| Methods | |||
| Eligibility criteria | 3 | Specify the inclusion and exclusion criteria for the review. | Not stated in abstract |
| Information sources | 4 | Specify the information sources (e.g., databases, registers) used to identify studies and the date when each was last searched. | Abstract, line 22–27 |
| Risk of bias | 5 | Specify the methods used to assess risk of bias in the included studies. | Abstract, line 28 |
| Synthesis of results | 6 | Specify the methods used to present and synthesise results. | Abstract, line 22–28 |
| Results | |||
| Included studies | 7 | Give the total number of included studies and participants and summarise relevant characteristics of studies. | Abstract, line 28 |
| Synthesis of results | 8 | Present results for main outcomes, preferably indicating the number of included studies and participants for each. If meta-analysis was done, reports the summary estimate and confidence/credible interval. If comparing groups, indicate the direction of the effects (i.e., which group is favoured). | Abstract, line 28–34 |
| Discussion | |||
| Limitation of evidence | 9 | Provide a brief summary of the limitations of the evidence included in the review (e.g., study risk of bias, inconsistency and imprecision). | Not stated in abstracts |
| Interpretation | 10 | Provide a general interpretation of the results and important implications. | Abstract, line 34–39 |
| Other | |||
| Funding | 11 | Specify the primary source of funding for the review. | Not stated in abstract |
| Registration | 12 | Provide the register name and registration number | Not stated in abstract |
References
- Timmis, A.; Vardas, P.; Townsend, N.; Torbica, A.; Katus, H.; De Smedt, D.; Gale, C.P.; Maggioni, A.P.; Petersen, S.E.; Huculeci, R.; et al. European Society of Cardiology: Cardiovascular disease statistics 2021. Eur. Heart J. 2022, 43, 716–799. [Google Scholar] [CrossRef]
- Institute of Public Health. National Health and Morbidity Survey. 2020. Available online: https://iku.gov.my/images/IKU/Document/REPORT/2020/report/2_Report_NHMS2020_Vol-2.pdf (accessed on 19 October 2024).
- Institute of Public Health. National Health and Morbidity Survey. 2023. Available online: https://iku.gov.my/images/nhms2023/fact-sheet-nhms-2023.pdf (accessed on 19 October 2024).
- Ullah, A.; Sajid, S.; Qureshi, M.; Kamran, M.; Anwaar, M.A.; Naseem, M.A.; Zaman, M.U.; Mahmood, F.; Rehman, A.; Shehryar, A.; et al. Novel biomarkers and the multiple-marker approach in early detection, prognosis, and risk stratification of cardiac diseases: A narrative review. Cureus 2023, 15, e42081. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Xu, F.; Ma, Q.; Zhou, Y.; Liu, T. C-Reactive Protein Level Predicts Cardiovascular Risk in Chinese Young Female Population. Oxidative Med. Cell. Longev. 2021, 6538079. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.; Brunssen, C.; Wolk, S.; Reeps, C.; Morawietz, H. Soluble LOX-1: A novel biomarker in patients with coronary artery disease, stroke, and acute aortic dissection? J. Am. Heart Assoc. 2020, 9, e013803. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Cong, S. LOX-1 and atherosclerotic-related diseases. Clin. Chim. Acta 2009, 491, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Barreto, J.; Karathanasis, S.K.; Remaley, A.; Sposito, A.C. Role of LOX-1 (lectin-like oxidized low-density lipoprotein receptor 1) as a cardiovascular risk predictor: Mechanistic insight and potential clinical use. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Sawamura, T.; Kume, N.; Aoyama, T.; Moriwaki, H.; Hoshikawa, H.; Aiba, Y.; Tanaka, T.; Miwa, S.; Katsura, Y.; Kita, T.; et al. An endothelial receptor for oxidized low-density lipoprotein. Nature 1997, 386, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Kattoor, A.J.; Kanuri, S.H.; Mehta, J.L. Role of Ox-LDL and LOX-1 in Atherogenesis. Curr. Med. Chem. 2019, 26, 1693–1700. [Google Scholar] [CrossRef]
- Hussein, R.A.; Abdul-Rasheed, O.F.; Basheer, M. Evaluation of soluble lectin-like oxidized low-density lipoprotein receptor-1 (sLOX-1) and sLOX-1/oxidized LDL ratio as novel biomarkers of acute coronary syndrome. Acta Biochim. Pol. 2022, 69, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Kume, N.; Mitsuoka, H.; Hayashida, K.; Tanaka, M.; Kominami, G.; Kita, T. Soluble lectin-like oxidized LDL receptor-1 (sLOX-1) as a sensitive and specific biomarker for acute coronary syndrome—Comparison with other biomarkers. J. Cardiol. 2010, 56, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-M.; Jin, P.-P.; Xue, J.; Chen, J.; Chen, Q.-F.; Luan, X.-Q.; Zhang, Z.-R.; Yu, T.-E.; Cai, Z.-Y.; Zhao, K.; et al. Role of sLOX-1 in intracranial artery stenosis and in predicting long-term prognosis of acute ischemic stroke. Brain Behav. 2018, 8, e00879. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, Y.; Huang, Z.; Wu, F.; Li, N.; Liang, C. Association between impaired cutaneous microvascular endothelial function and lectin-like oxidized low-density lipoprotein receptor-1 in patients with coronary slow flow. Microvasc. Res. 2020, 129, 103984. [Google Scholar] [CrossRef] [PubMed]
- Florida, E.M.; Li, H.; Hong, C.G.; Ongstad, E.L.; Gaddipati, R.; Sitaula, S.; Varma, V.; Parel, P.M.; O’Hagan, R.; Chen, M.Y.; et al. Relationship of soluble lectin-like low-density lipoprotein receptor-1 (sLOX-1) with inflammation and coronary plaque progression in psoriasis. J. Am. Heart Assoc. 2023, 12, e031227. [Google Scholar] [CrossRef]
- Kott, K.A.; Genetzakis, E.; Gray, M.P.; Hansen, P.; McGuire, H.M.; Yang, J.Y.; Grieve, S.M.; Vernon, S.T.; Figtree, G.A. Serum soluble lectin-like oxidized low-density lipoprotein receptor-1 (sLOX-1) is associated with atherosclerosis severity in coronary artery disease. Biomolecules 2023, 13, 1187. [Google Scholar] [CrossRef] [PubMed]
- Schiopu, A.; Björkbacka, H.; Narasimhan, G.; Loong, B.J.; Engström, G.; Melander, O.; Orho-Melander, M.; Nilsson, J. Elevated soluble LOX-1 predicts risk of first-time myocardial infarction. Ann. Med. 2023, 55, 2296552. [Google Scholar] [CrossRef] [PubMed]
- Mashayekhi, S.; Ziaee, M.; Garjani, A.; Sarbakhsh, P.; Ghaffari, S. Prognostic value of sLOX-1 level in acute coronary syndromes based on thrombolysis in myocardial infarction risk score and clinical outcome. J. Emerg. Med. 2018, 55, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 39. [Google Scholar]
- Aminuddin, A.; Samah, N.; Che Roos, N.A.; Mohamad, S.F.; Beh, B.C.; Hamid, A.A.; Ugusman, A. Prognostic value of lectin-like oxidized LDL receptor-1 for future CVD risk and outcome: Systematic review and meta-analysis. INPLASY Protoc. 2024, 120078. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses. OHRI. 2021. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 25 October 2024).
- Cochrane Collaboration. Review Manager (RevMan) [Computer Software]. Cochrane. 2023. Available online: https://revman.cochrane.org/info (accessed on 4 November 2024).
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Kraler, S.; Wenzl, F.A.; Georgiopoulos, G.; Obeid, S.; Liberale, L.; von Eckardstein, A.; Muller, O.; Mach, F.; Räber, L.; Losdat, S.; et al. Soluble lectin-like oxidized low-density lipoprotein receptor-1 predicts premature death in acute coronary syndromes. Eur. Heart J. 2022, 43, 1849–1860. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Ali, W.; Mishra, S.; Pradhan, A.; Sethi, R.; Kushwaha, R.; Singh, U.S.; Perrone, M.A. Circulating soluble lectin-like oxidized low-density lipoprotein receptor-1: A diagnostic indicator across the spectrum of acute coronary syndrome. J. Clin. Med. 2021, 10, 5567. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Xu, Y.; Li, S.; Guo, J.; Sun, J.; Hong, J.; Chen, L. Baseline serum sLOX-1 concentrations are associated with 2-year major adverse cardiovascular and cerebrovascular events in patients after percutaneous coronary intervention. Dis. Markers 2019, 4925767. [Google Scholar] [CrossRef]
- Higuma, T.; Abe, N.; Tateyama, S.; Endo, T.; Shibutani, S.; Yokoyama, H.; Hanada, K.; Yamada, M.; Tomita, H.; Hanada, H.; et al. Plasma soluble lectin-like oxidized low-density lipoprotein receptor-1 as a novel prognostic biomarker in patients with ST-segment elevation acute myocardial infarction. Circ. J. 2015, 79, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Jiang, H.; Li, T.; Qian, C.; Zhu, L.; Wang, T. Correlation of sLOX-1 levels and MR characteristics of culprit plaques in intracranial arteries with stroke recurrence. Diagnostics 2023, 13, 804. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Cao, J.; Zhou, Y.; Zhou, X.; Sun, Z.; Zhu, X. Serum levels of sLOX-1 and Lp-PLA2 can predict the prognosis of acute cerebral infarction with high specificity. Physiol. Rep. 2022, 10, e15160. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Hou, D.; Liu, J.; Wang, T.; Luo, Y.; Sun, W.; Li, C.; Shen, L.; Liu, W.; Wu, D. Soluble lectin-like oxidized low-density lipoprotein receptor-1 level is related to clinical prognosis in patients with acute atherosclerosis-related ischemic stroke. Clin. Appl. Thromb./Hemost. 2021, 27, 10760296211059500. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Tian, X.; Xu, J.; Li, H.; Xu, Q.; Chen, P.; Meng, X.; Wang, Y. Soluble lectin-like oxidized low-density lipoprotein receptor-1 and recurrent stroke: A nested case–control study. CNS Neurosci. Ther. 2022, 28, 2001–2010. [Google Scholar] [CrossRef]
- Markstad, H.; Edsfeldt, A.; Mattison, I.Y.; Bengtsson, E.; Singh, P.; Cavalera, M.; Asciutto, G.; Björkbacka, H.; Fredrikson, G.N.; Dias, N.; et al. High levels of soluble lectin-like oxidized low-density lipoprotein receptor-1 are associated with carotid plaque inflammation and increased risk of ischemic stroke. J. Am. Heart Assoc. 2019, 8, e009874. [Google Scholar] [CrossRef]
- Zhao, Z.; Xu, Y.; Li, S.; Guo, J.; Chen, L. Higher serum lectin-like oxidized low-density lipoprotein receptor-1 in patients with stable coronary artery disease is associated with major adverse cardiovascular events: A multicenter pilot study. Biochem. Medica 2019, 29, 010705. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, Y.; Li, L.; Wang, P.; Wang, R.; Tao, Z.; Fan, J.; Han, Z.; Li, F.; Zhao, H.; et al. sLOX-1: A molecule for evaluating the prognosis of recurrent ischemic stroke. Neural Plast. 2021, 6718184. [Google Scholar] [CrossRef] [PubMed]
- Duncan, M.W. The importance of antibody validation for ELISA: Avoiding errors in biomarker quantification. J. Immunol. Methods 2013, 387, 1–13. [Google Scholar]
- Andreasson, U.; Perret-Liaudet, A.; van Waalwijk van Doorn, L.J.C.; Blennow, K.; Chiasserini, D.; Engelborghs, S.; Fladby, T.; Genc, S.; Kruse, N.; Kuiperij, H.B.; et al. A practical guide to immunoassay method validation. Front. Neurol. 2015, 6, 179. [Google Scholar] [CrossRef] [PubMed]
- Blair, A.; Shaul, P.W.; Yuhanna, I.S.; Conrad, P.A.; Smart, E.J. Oxidized low-density lipoprotein displaces endothelial nitric-oxide synthase (eNOS) from plasmalemmal caveolae and impairs eNOS activation. J. Boil. Chem. 1999, 274, 32512–32519. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Mehta, J.L. Théroux, Antisense to LOX-1 inhibits oxidized LDL–mediated upregulation of monocyte chemoattractant protein-1 and monocyte adhesion to human coronary artery endothelial cells. Circulation 2000, 101, 2889–2895. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, T.; Sawamura, T.; Furutani, Y.; Matsuoka, R.; Yoshida, M.C.; Fujiwara, H.; Masaki, T. Structure and chromosomal assignment of the human lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) gene. Biochem. J. 1999, 339, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.M.; Chen, H.; Kazzaz, N.; Li, D.; Liu, L.; Mehta, J.L. Oxidized-LDL through LOX-1 increases the expression of angiotensin-converting enzyme in human coronary artery endothelial cells. Cardiovasc. Res. 2003, 57, 238–243. [Google Scholar] [CrossRef]
- Li, D.; Liu, L.; Chen, H.; Sawamura, T.; Ranganathan, S.; Mehta, J.L. LOX-1 mediates oxidized low-density lipoprotein-induced expression of matrix metalloproteinases in human coronary artery endothelial cells. Circulation 2003, 107, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Renier, G. The oral anti-diabetic agent, gliclazide, inhibits oxidized LDL-mediated LOX-1 expression, metalloproteinase-9 secretion, and apoptosis in human aortic endothelial cells. Atherosclerosis 2009, 204, 40–46. [Google Scholar] [CrossRef]
- Xu, F.; Sun, Y.; Chen, Y.; Sun, Y.; Li, R.; Liu, C.; Zhang, C.; Wang, R.; Zhang, Y. Endothelial cell apoptosis is responsible for the formation of coronary thrombotic atherosclerotic plaques. Tohoku J. Exp. Med. 2009, 218, 25–33. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sánchez-León, M.E.; Loaeza-Reyes, K.J.; Matias-Cervantes, C.A.; Mayoral-Andrade, G.; Pérez-Campos, E.L.; Pérez-Campos-Mayoral, L.; Hernández-Huerta, M.T.; Zenteno, E.; Pérez-Cervera, Y.; Pina-Canseco, S. LOX-1 in cardiovascular disease: A comprehensive molecular and clinical review. Int. J. Mol. Sci. 2024, 25, 5276. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).