Mechanisms Underlying the Stimulation of DUSP10/MKP5 Expression in Chondrocytes by High Molecular Weight Hyaluronic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
2.2. Cell Culture
2.3. Western Blotting
2.4. Real-Time Quantitative Reverse Transcription PCR (RT-qPCR)
2.5. RT-qPCR for miRNA
2.6. Transfection with miRNA Inhibitors
2.7. Transfection with miRNA Mimics
2.8. Pull-Down Assay for Rho Activity
2.9. Statistical Analysis
3. Results
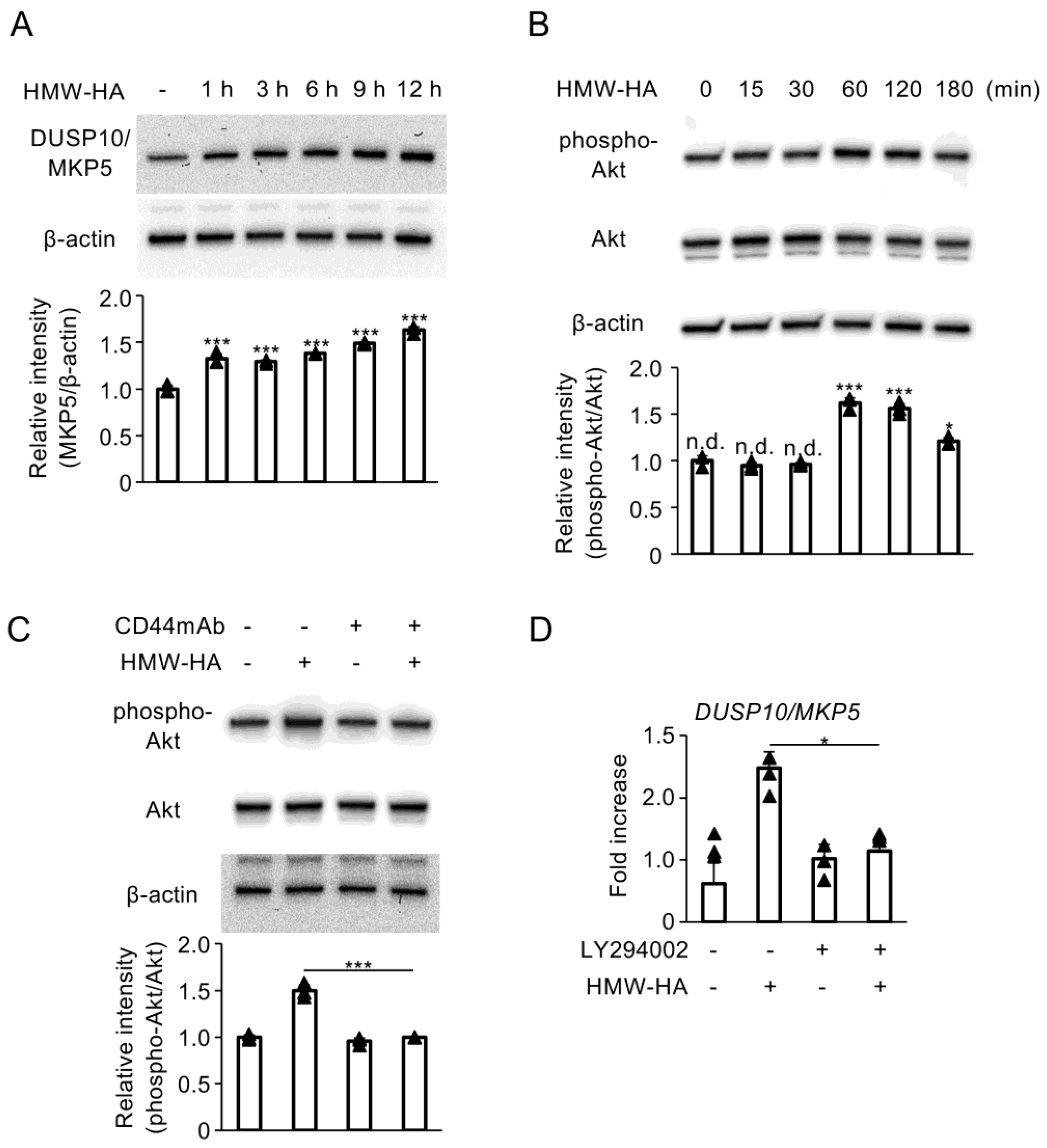
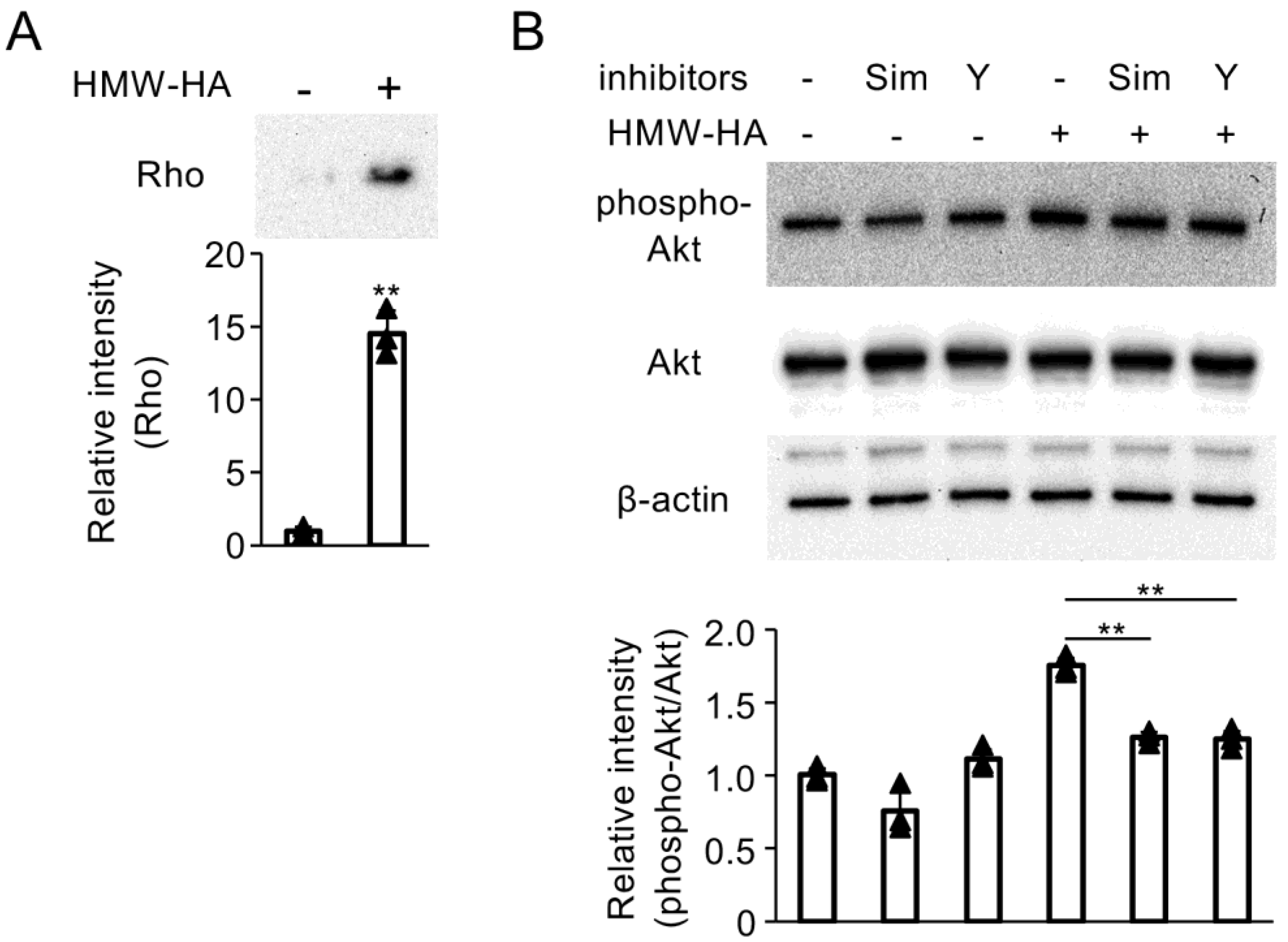
3.1. Interaction of HMW-HA with CD44 Enhances DUSP10/MKP5 Expression via Activation of PI3K/Akt Signaling
3.2. HMW-HA Inhibits the Biosynthesis of miR-92a, miR-181a, and miR-181d
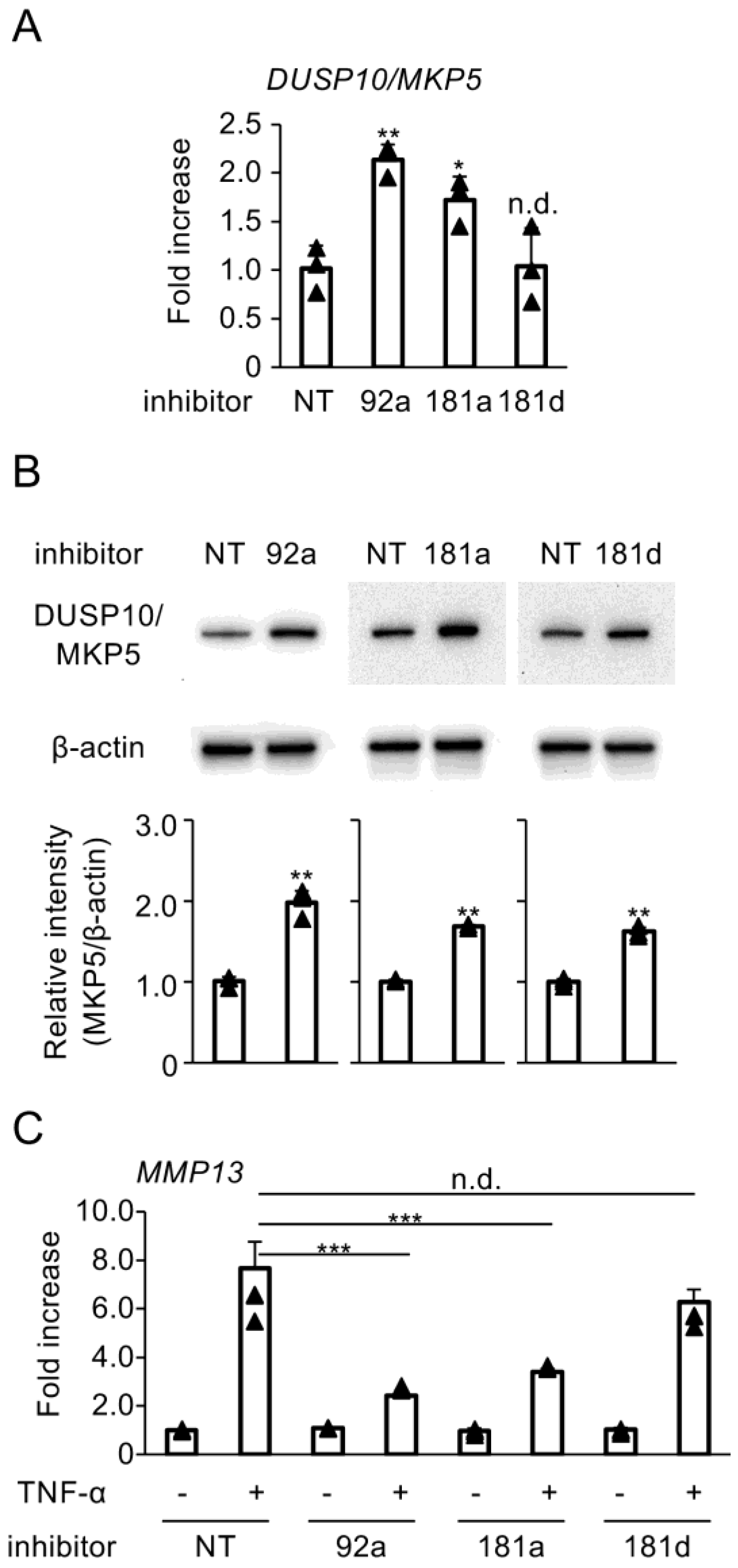
3.3. Blockade of Hybridization Activity of miR-92a, miR-181a, and miR-181d Enhances DUSP10/MKP5 Expression
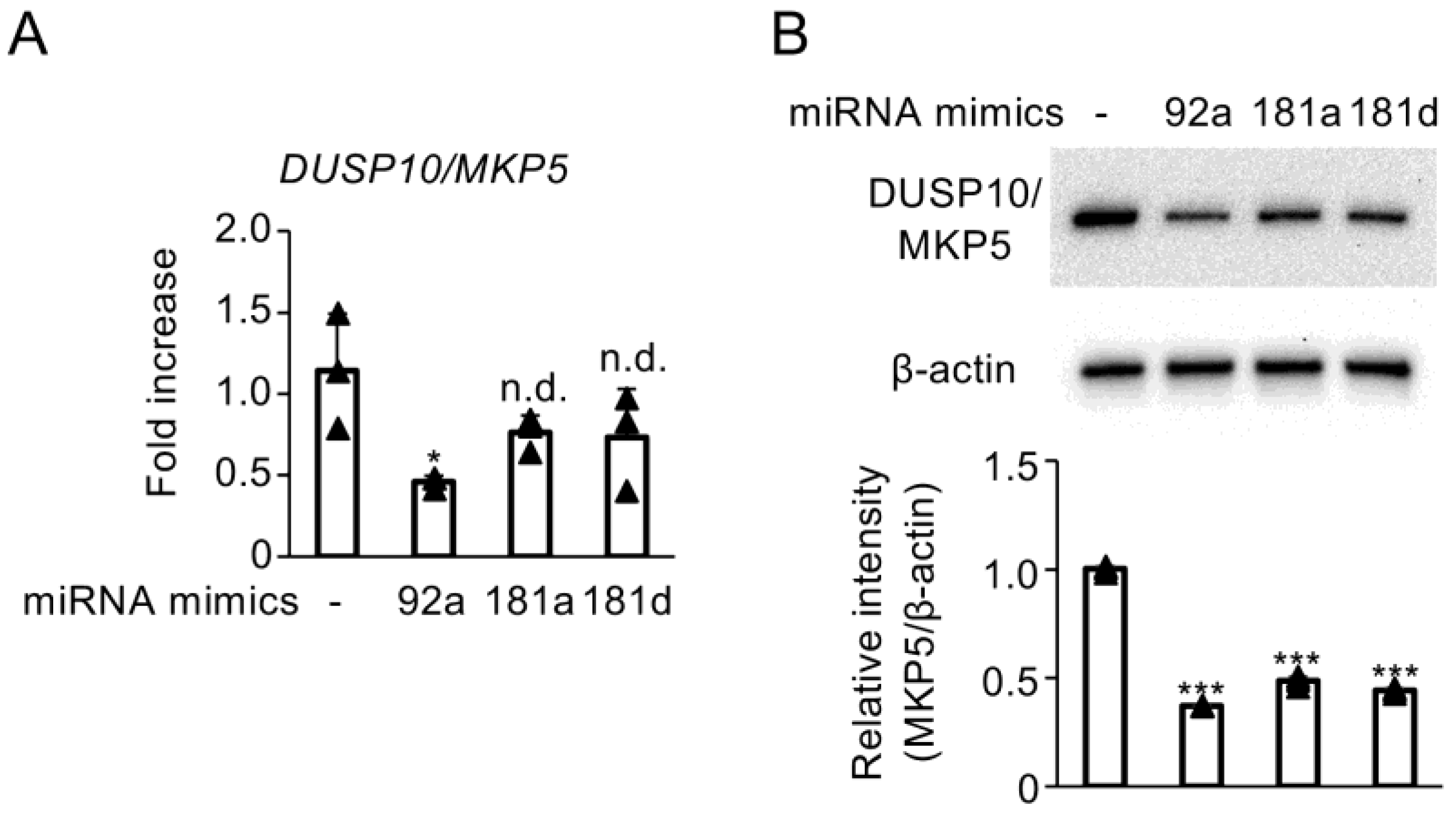
3.4. Enhanced Activity of miR-92a, miR-181a, and miR-181d Suppresses DUSP10/MKP5 Expression
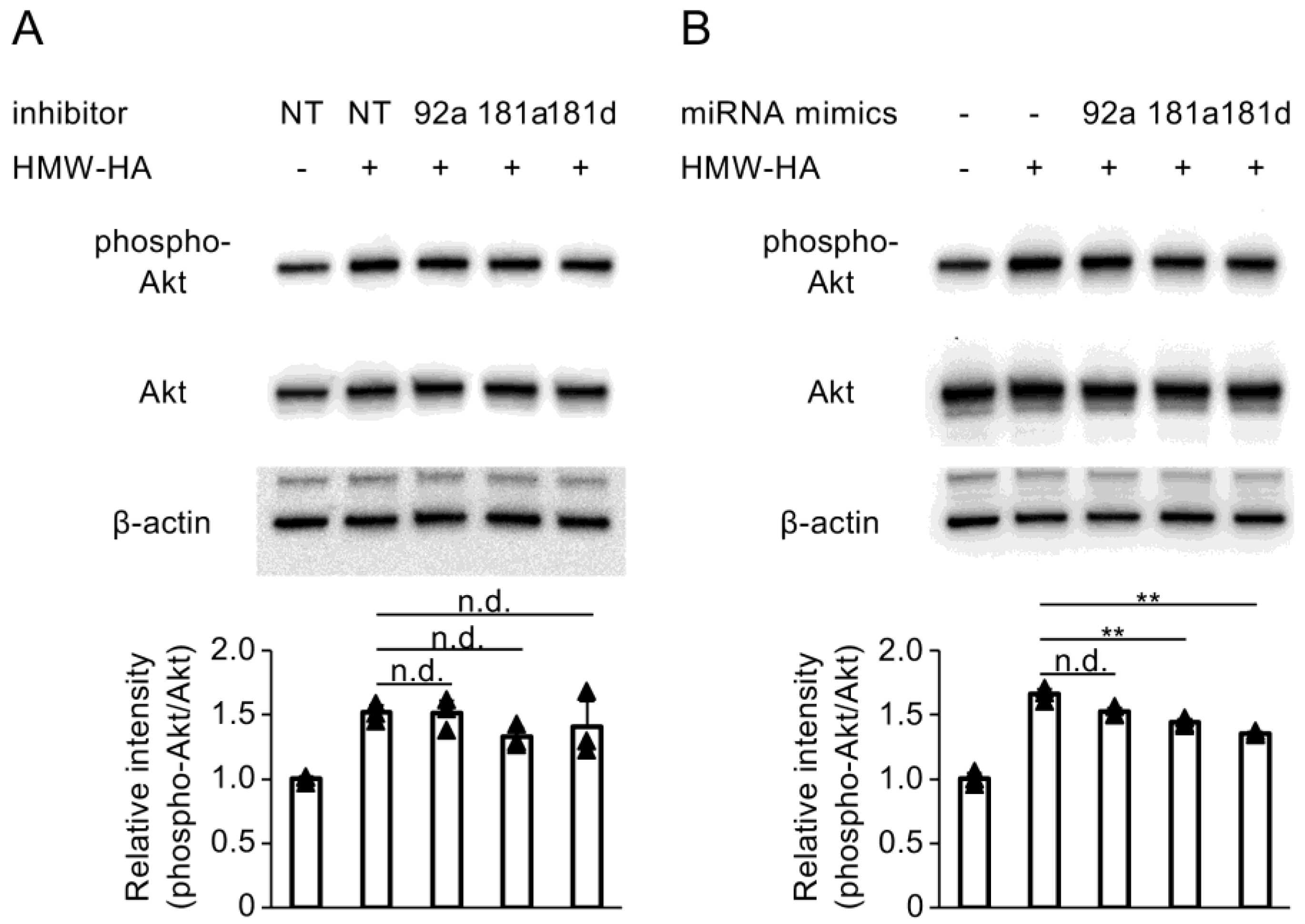
3.5. Enhancement of miR-181a and miR-181d Activity Inhibits HMW-HA-Induced Activation of PI3K/Akt Signaling
3.6. RhoA and RhoA-Associated Protein Kinase (ROK) Inhibitors Attenuate PI3K/Akt Activation Mediated by HMW-HA
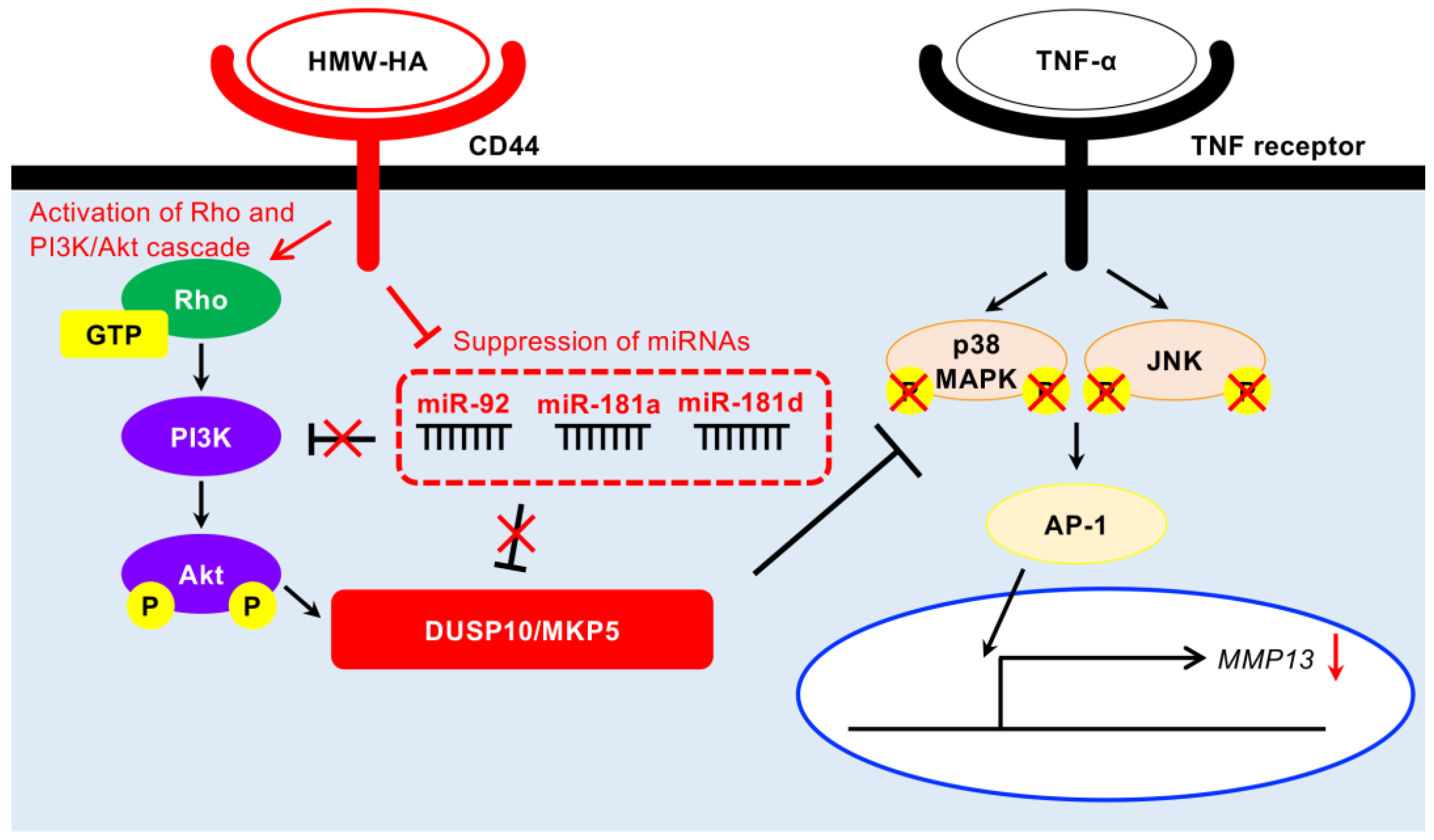
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kiani, C.; Chen, L.; Wu, Y.J.; Yee, A.J.; Yang, B.B. Structure and function of aggrecan. Cell. Res. 2002, 12, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Dovedytis, M.; Liu, Z.J.; Bartlett, S. Hyaluronic acid and its biomedical applications: A review. Eng. Regen. 2020, 1, 102–113. [Google Scholar] [CrossRef]
- Ohnishi, T.; Novais, E.J.; Risbud, M.V. Alterations in ECM signature underscore multiple sub-phenotypes of intervertebral disc degeneration. Matrix Biol. Plus 2020, 6, 100036. [Google Scholar] [CrossRef] [PubMed]
- Iaconisi, G.N.; Lunetti, P.; Gallo, N.; Cappello, A.R.; Fiermonte, G.; Dolce, V.; Capobianco, L. Hyaluronic Acid: A Powerful Biomolecule with Wide-Ranging Applications-A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 10296. [Google Scholar] [CrossRef]
- Ragan, C.; Meyer, K. The Hyaluronic Acid of Synovial Fluid in Rheumatoid Arthritis. J. Clin. Investig. 1949, 28, 56–59. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, Z.; Yang, H.; Zhong, H.; Peng, W.; Xie, R. Recent Advances in Understanding the Role of Cartilage Lubrication in Osteoarthritis. Molecules 2021, 26, 6122. [Google Scholar] [CrossRef] [PubMed]
- Kamada, H.; Masuda, K.; D’Souza, A.L.; Lenz, M.E.; Pietryla, D.; Otten, L.; Thonar, E.J. Age-related differences in the accumulation and size of hyaluronan in alginate culture. Arch. Biochem. Biophys. 2002, 408, 192–199. [Google Scholar] [CrossRef]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic Acid: Molecular Mechanisms and Therapeutic Trajectory. Front. Vet. Sci. 2019, 6, 192. [Google Scholar] [CrossRef]
- Thorne, R.F.; Legg, J.W.; Isacke, C.M. The role of the CD44 transmembrane and cytoplasmic domains in co-ordinating adhesive and signalling events. J. Cell. Sci. 2004, 117, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Hassn Mesrati, M.; Syafruddin, S.E.; Mohtar, M.A.; Syahir, A. CD44: A Multifunctional Mediator of Cancer Progression. Biomolecules 2021, 11, 1850. [Google Scholar] [CrossRef]
- Chernos, M.; Grecov, D.; Kwok, E.; Bebe, S.; Babsola, O.; Anastassiades, T. Rheological study of hyaluronic acid derivatives. Biomed. Eng. Lett. 2017, 7, 17–24. [Google Scholar] [CrossRef]
- Masuko, K.; Murata, M.; Yudoh, K.; Kato, T.; Nakamura, H. Anti-inflammatory effects of hyaluronan in arthritis therapy: Not just for viscosity. Int. J. Gen. Med. 2009, 2, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Guidolin, D. Potential mechanism of action of intra-articular hyaluronan therapy in osteoarthritis: Are the effects molecular weight dependent? Semin. Arthritis. Rheum. 2002, 32, 10–37. [Google Scholar] [CrossRef]
- de la Peña, E.; Sala, S.; Rovira, J.C.; Schmidt, R.F.; Belmonte, C. Elastoviscous substances with analgesic effects on joint pain reduce stretch-activated ion channel activity in vitro. Pain 2002, 99, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Ariyoshi, W.; Okinaga, T.; Knudson, C.B.; Knudson, W.; Nishihara, T. High molecular weight hyaluronic acid regulates osteoclast formation by inhibiting receptor activator of NF-κB ligand through Rho kinase. Osteoarthr. Cartil. 2014, 22, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Nakao, Y.; Hikiji, H.; Okinaga, T.; Takeuchi, J.; Habu, M.; Yoshiga, D.; Yoshioka, I.; Nishihara, T.; Ariyoshi, W. Accumulation of hyaluronic acid in stromal cells modulates osteoclast formation by regulation of receptor activator of nuclear factor kappa-B ligand expression. Biochem. Biophys. Res. Commun. 2019, 512, 537–543. [Google Scholar] [CrossRef]
- Furuta, J.; Ariyoshi, W.; Okinaga, T.; Takeuchi, J.; Mitsugi, S.; Tominaga, K.; Nishihara, T. High molecular weight hyaluronic acid regulates MMP13 expression in chondrocytes via DUSP10/MKP5. J. Orthop. Res. 2017, 35, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Tan, T.H. DUSPs, to MAP kinases and beyond. Cell. Biosci. 2012, 2, 24. [Google Scholar] [CrossRef] [PubMed]
- Skeens, E.; Maschietto, F.; Manjula, R.; Shillingford, S.; Lolis, E.J.; Batista, V.S.; Bennett, A.M.; Lisi, G.P. Dynamic and structural insights into allosteric regulation on MKP5 a dual-specificity phosphatase. bioRxiv 2024. [Google Scholar] [CrossRef]
- Keyse, S.M. Dual-specificity MAP kinase phosphatases (MKPs) and cancer. Cancer Metastasis Rev. 2008, 27, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B. Immortalization of human articular chondrocytes for generation of stable, differentiated cell lines. Methods Mol. Med. 2004, 100, 23–36. [Google Scholar]
- Claassen, H.; Schicht, M.; Brandt, J.; Reuse, K.; Schädlich, R.; Goldring, M.B.; Guddat, S.S.; Thate, A.; Paulsen, F. C-28/I2 and T/C-28a2 chondrocytes as well as human primary articular chondrocytes express sex hormone and insulin receptors--Useful cells in study of cartilage metabolism. Ann. Anat. 2011, 193, 23–29. [Google Scholar] [CrossRef]
- Koga, A.; Thongsiri, C.; Kudo, D.; Phuong, D.N.D.; Iwamoto, Y.; Fujii, W.; Nagai-Yoshioka, Y.; Yamasaki, R.; Ariyoshi, W. Mechanisms Underlying the Suppression of IL-1β Expression by Magnesium Hydroxide Nanoparticles. Biomedicines 2023, 11, 1291. [Google Scholar] [CrossRef] [PubMed]
- Ariyoshi, W.; Usui, M.; Sano, K.; Kawano, A.; Okinaga, T.; Nakashima, K.; Nakazawa, K.; Nishihara, T. 3D spheroid culture models for chondrocytes using polyethylene glycol-coated microfabricated chip. Biomed. Res. 2020, 41, 187–197. [Google Scholar] [CrossRef]
- Little, C.B.; Barai, A.; Burkhardt, D.; Smith, S.M.; Fosang, A.J.; Werb, Z.; Shah, M.; Thompson, E.W. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 2009, 60, 3723–3733. [Google Scholar] [CrossRef] [PubMed]
- Neuhold, L.A.; Killar, L.; Zhao, W.; Sung, M.L.; Warner, L.; Kulik, J.; Turner, J.; Wu, W.; Billinghurst, C.; Meijers, T.; et al. Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J. Clin. Investig. 2001, 107, 35–44. [Google Scholar] [CrossRef]
- Chen, D.; Li, Y.; Dai, X.; Zhou, X.; Tian, W.; Zhou, Y.; Zou, X.; Zhang, C. 1,25-Dihydroxyvitamin D3 activates MMP13 gene expression in chondrocytes through p38 MARK pathway. Int. J. Biol. Sci. 2013, 9, 649–655. [Google Scholar] [CrossRef]
- Ge, H.X.; Zou, F.M.; Li, Y.; Liu, A.M.; Tu, M. JNK pathway in osteoarthritis: Pathological and therapeutic aspects. J. Recept. Signal Transduct. Res. 2017, 37, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Boyd, D.D. Regulation of matrix metalloproteinase gene expression. J. Cell Physiol. 2007, 211, 19–26. [Google Scholar] [CrossRef]
- Humar, M.; Loop, T.; Schmidt, R.; Hoetzel, A.; Roesslein, M.; Andriopoulos, N.; Pahl, H.L.; Geiger, K.K.; Pannen, B.H. The mitogen-activated protein kinase p38 regulates activator protein 1 by direct phosphorylation of c-Jun. Int. J. Biochem. Cell Biol. 2007, 39, 2278–2288. [Google Scholar] [CrossRef]
- Hess, J.; Angel, P.; Schorpp-Kistner, M. AP-1 subunits: Quarrel and harmony among siblings. J. Cell Sci. 2004, 117, 5965–5973. [Google Scholar] [CrossRef]
- Schmitz, I.; Ariyoshi, W.; Takahashi, N.; Knudson, C.B.; Knudson, W. Hyaluronan oligosaccharide treatment of chondrocytes stimulates expression of both HAS-2 and MMP-3, but by different signaling pathways. Osteoarthr. Cartil. 2010, 18, 447–454. [Google Scholar] [CrossRef]
- Benavides-Serrato, A.; Anderson, L.; Holmes, B.; Cloninger, C.; Artinian, N.; Bashir, T.; Gera, J. mTORC2 modulates feedback regulation of p38 MAPK activity via DUSP10/MKP5 to confer differential responses to PP242 in glioblastoma. Genes Cancer 2014, 5, 393–406. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Liu, L.; Yagasaki, L.; Inotsume, M.; Chiba, T.; Asahara, H. Cartilage Homeostasis and Osteoarthritis. Int. J. Mol. Sci. 2022, 23, 6316. [Google Scholar] [CrossRef] [PubMed]
- Swingler, T.E.; Niu, L.; Smith, P.; Paddy, P.; Le, L.; Barter, M.J.; Young, D.A.; Clark, I.M. The function of microRNAs in cartilage and osteoarthritis. Clin. Exp. Rheumatol. 2019, 37, 40–47. [Google Scholar]
- Goldring, M.B.; Marcu, K.B. Epigenomic and microRNA-mediated regulation in cartilage development, homeostasis, and osteoarthritis. Trends Mol. Med. 2012, 18, 109–118. [Google Scholar] [CrossRef]
- Peng, X.; Wang, Q.; Li, W.; Ge, G.; Peng, J.; Xu, Y.; Yang, H.; Bai, J.; Geng, D. Comprehensive overview of microRNA function in rheumatoid arthritis. Bone Res. 2023, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Eom, S.; Park, D.; Kim, H.; Jeoung, D. The Hyaluronic Acid-HDAC3-miRNA Network in Allergic Inflammation. Front. Immunol. 2015, 6, 210. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Zhang, L.; Li, Q.; Yang, L. miR-92a/DUSP10/JNK signalling axis promotes human pancreatic cancer cells proliferation. Biomed. Pharmacother. 2014, 68, 25–30. [Google Scholar] [CrossRef]
- Rezaei, N.; Talebi, F.; Ghorbani, S.; Rezaei, A.; Esmaeili, A.; Noorbakhsh, F.; Hakemi, M.G. MicroRNA-92a Drives Th1 Responses in the Experimental Autoimmune Encephalomyelitis. Inflammation 2019, 42, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Song, M.K.; Park, Y.K.; Ryu, J.C. Polycyclic aromatic hydrocarbon (PAH)-mediated upregulation of hepatic microRNA-181 family promotes cancer cell migration by targeting MAPK phosphatase-5, regulating the activation of p38 MAPK. Toxicol. Appl. Pharmacol. 2013, 273, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Scuruchi, M.; Avenoso, A.; Aliquò, F.; Pantano, A.; Campo, G.M.; Campo, S.; D’Ascola, A. miR-21 attenuated inflammation targeting MyD88 in human chondrocytes stimulated with Hyaluronan oligosaccharides. Arch. Biochem. Biophys. 2024, 759, 110112. [Google Scholar] [CrossRef]
- Avenoso, A.; D’Ascola, A.; Scuruchi, M.; Mandraffino, G.; Campo, S.; Campo, G.M. miR146a up-regulation is involved in small HA oligosaccharides-induced pro-inflammatory response in human chondrocytes. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129731. [Google Scholar] [CrossRef]
- Abatangelo, G.; Vindigni, V.; Avruscio, G.; Pandis, L.; Brun, P. Hyaluronic Acid: Redefining Its Role. Cells 2020, 9, 1743. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Fang, X.; Chen, M.; Wang, Y.; Zhang, L. Hyaluronan-CD44 Interaction Regulates Mouse Retinal Progenitor Cells Migration, Proliferation and Neuronal Differentiation. Stem Cell Rev. Rep. 2023, 19, 2929–2942. [Google Scholar] [CrossRef] [PubMed]
- Lee-Sayer, S.S.M.; Maeshima, N.; Dougan, M.N.; Dahiya, A.; Arif, A.A.; Dosanjh, M.; Maxwell, C.A.; Johnson, P. Hyaluronan-binding by CD44 reduces the memory potential of activated murine CD8 T cells. Eur. J. Immunol. 2018, 48, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Umemura, N.; Ohkoshi, E.; Tajima, M.; Kikuchi, H.; Katayama, T.; Sakagami, H. Hyaluronan induces odontoblastic differentiation of dental pulp stem cells via CD44. Stem Cell Res. Ther. 2016, 7, 135. [Google Scholar] [CrossRef]
- Bourguignon, L.Y.W. Matrix Hyaluronan-CD44 Interaction Activates MicroRNA and LncRNA Signaling Associated With Chemoresistance, Invasion, and Tumor Progression. Front. Oncol. 2019, 9, 492. [Google Scholar] [CrossRef]
- Bourguignon, L.Y.; Shiina, M.; Li, J.J. Hyaluronan-CD44 interaction promotes oncogenic signaling, microRNA functions, chemoresistance, and radiation resistance in cancer stem cells leading to tumor progression. Adv. Cancer Res. 2014, 123, 255–275. [Google Scholar]
- Van Meter, E.N.; Onyango, J.A.; Teske, K.A. A review of currently identified small molecule modulators of microRNA function. Eur. J. Med. Chem. 2020, 188, 112008. [Google Scholar] [CrossRef]
- Ji, C.Y.; Yuan, M.X.; Chen, L.J.; Gao, H.Q.; Zhang, T.; Yin, Q. miR-92a represses the viability and migration of nerve cells in Hirschsprung’s disease by regulating the KLF4/PI3K/AKT pathway. Acta Neurobiol. Exp. (Wars) 2022, 82, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Wang, Z.; Cheng, Y.; Ma, C.; Zhong, Y.; Xiao, Y.; Gao, X.; Li, Z. M2 macrophage-derived exosomal microRNAs inhibit cell migration and invasion in gliomas through PI3K/AKT/mTOR signaling pathway. J. Transl. Med. 2021, 19, 99. [Google Scholar] [CrossRef]
- Xu, F.; Zhou, F. Inhibition of microRNA-92a ameliorates lipopolysaccharide-induced endothelial barrier dysfunction by targeting ITGA5 through the PI3K/Akt signaling pathway in human pulmonary microvascular endothelial cells. Int. Immunopharmacol. 2020, 78, 106060. [Google Scholar] [CrossRef]
- Cai, G.; Wang, Y.; Houda, T.; Yang, C.; Wang, L.; Gu, M.; Mueck, A.; Croteau, S.; Ruan, X.; Hardy, P. MicroRNA-181a suppresses norethisterone-promoted tumorigenesis of breast epithelial MCF10A cells through the PGRMC1/EGFR-PI3K/Akt/mTOR signaling pathway. Transl. Oncol. 2021, 14, 101068. [Google Scholar] [CrossRef]
- Jiang, K.; Xie, L.F.; Xiao, T.Z.; Qiu, M.Y.; Wang, W.L. MiR-181d inhibits cell proliferation and metastasis through PI3K/AKT pathway in gastric cancer. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8861–8869. [Google Scholar] [PubMed]
- Bourguignon, L.Y. Matrix hyaluronan-activated CD44 signaling promotes keratinocyte activities and improves abnormal epidermal functions. Am. J. Pathol. 2014, 184, 1912–1919. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Bourguignon, L.Y. Role of hyaluronan-mediated CD44 signaling in head and neck squamous cell carcinoma progression and chemoresistance. Am. J. Pathol. 2011, 178, 956–963. [Google Scholar] [CrossRef]
- Senoo, H.; Murata, D.; Wai, M.; Arai, K.; Iwata, W.; Sesaki, H.; Iijima, M. KARATE: PKA-induced KRAS4B-RHOA-mTORC2 supercomplex phosphorylates AKT in insulin signaling and glucose homeostasis. Mol. Cell 2021, 81, 4622–4634. [Google Scholar] [CrossRef] [PubMed]
- Vainchenker, W.; Arkoun, B.; Basso-Valentina, F.; Lordier, L.; Debili, N.; Raslova, H. Role of Rho-GTPases in megakaryopoiesis. Small GTPases 2021, 12, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Fromigué, O.; Haÿ, E.; Modrowski, D.; Bouvet, S.; Jacquel, A.; Auberger, P.; Marie, P.J. RhoA GTPase inactivation by statins induces osteosarcoma cell apoptosis by inhibiting p42/p44-MAPKs-Bcl-2 signaling independently of BMP-2 and cell differentiation. Cell Death Differ. 2006, 13, 1845–1856. [Google Scholar] [CrossRef]
- Uehata, M.; Ishizaki, T.; Satoh, H.; Ono, T.; Kawahara, T.; Morishita, T.; Tamakawa, H.; Yamagami, K.; Inui, J.; Maekawa, M.; et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 1997, 389, 990–994. [Google Scholar] [CrossRef]
- Chen, Y.L.; Yan, D.Y.; Wu, C.Y.; Xuan, J.W.; Jin, C.Q.; Hu, X.L.; Bao, G.D.; Bian, Y.J.; Hu, Z.C.; Shen, Z.H.; et al. Maslinic acid prevents IL-1β-induced inflammatory response in osteoarthritis via PI3K/AKT/NF-κB pathways. J. Cell Physiol. 2021, 236, 1939–1949. [Google Scholar] [CrossRef] [PubMed]
- Edouard, P.; Rannou, F.; Coudeyre, E. Animal evidence for hyaluronic acid efficacy in knee trauma injuries. Review of animal-model studies. Phys. Ther. Sport. 2013, 14, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Avenoso, A.; D’Ascola, A.; Scuruchi, M.; Mandraffino, G.; Calatroni, A.; Saitta, A.; Campo, S.; Campo, G.M. Hyaluronan in experimental injured/inflamed cartilage: In vivo studies. Life Sci. 2018, 193, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Manley, G.C.A.; Stokes, C.A.; Marsh, E.K.; Sabroe, I.; Parker, L.C. DUSP10 Negatively Regulates the Inflammatory Response to Rhinovirus through Interleukin-1β Signaling. J. Virol. 2019, 93, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Ma, J.; Zhao, J.; Song, Z.; Zhou, C.; Liu, X.; Teng, W.; Wang, W.; Zhang, Q.; Yan, W.; et al. The Role of MKP-5 in Adipocyte-Macrophage Interactions during Obesity. Obes. Facts 2020, 13, 86–101. [Google Scholar] [CrossRef]

| Predicted Consequential Pairing of Target Region (Top) and miRNA (Bottom) | |
|---|---|
| Position 449–456 of DUSP10 3′-UTR hsa-miR-92a-5p | 5′-…CACAGAAAACAAAAACCCAACCA… | | | | | | | | | | 3′-UCGUAACGUUGGCUA- GGGUUGGA |
| Position 876–882 of DUSP10 3′-UTR hsa-miR-181a-5p | 5′-…UACAUAUGUAUAUCAGAAUGUAA… | | | | | | 3′-UGAGUGGCUGUCGCAACUUACAA |
| Position 876–882 of DUSP10 3′-UTR hsa-miR-181b-5p | 5′-…UACAUAUGUAUAUCAGAAUGUAA… | | | | | | 3′-UGGGUGGCUGUCGUUACUUACAA |
| Position 876–882 of DUSP10 3′-UTR hsa-miR-181d-5p | 5′-…UACAUAUGUAUAUCAGAAUGUAA… | | | | | | 3′-UGGGUGGCUGUUGUUACUUACAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ariyoshi, W.; Takeuchi, J.; Mitsugi, S.; Koga, A.; Nagai-Yoshioka, Y.; Yamasaki, R. Mechanisms Underlying the Stimulation of DUSP10/MKP5 Expression in Chondrocytes by High Molecular Weight Hyaluronic Acid. Biomedicines 2025, 13, 376. https://doi.org/10.3390/biomedicines13020376
Ariyoshi W, Takeuchi J, Mitsugi S, Koga A, Nagai-Yoshioka Y, Yamasaki R. Mechanisms Underlying the Stimulation of DUSP10/MKP5 Expression in Chondrocytes by High Molecular Weight Hyaluronic Acid. Biomedicines. 2025; 13(2):376. https://doi.org/10.3390/biomedicines13020376
Chicago/Turabian StyleAriyoshi, Wataru, Jun Takeuchi, Sho Mitsugi, Ayaka Koga, Yoshie Nagai-Yoshioka, and Ryota Yamasaki. 2025. "Mechanisms Underlying the Stimulation of DUSP10/MKP5 Expression in Chondrocytes by High Molecular Weight Hyaluronic Acid" Biomedicines 13, no. 2: 376. https://doi.org/10.3390/biomedicines13020376
APA StyleAriyoshi, W., Takeuchi, J., Mitsugi, S., Koga, A., Nagai-Yoshioka, Y., & Yamasaki, R. (2025). Mechanisms Underlying the Stimulation of DUSP10/MKP5 Expression in Chondrocytes by High Molecular Weight Hyaluronic Acid. Biomedicines, 13(2), 376. https://doi.org/10.3390/biomedicines13020376







