Consecutive Affinity and Ion-Exchange Chromatography for AAV9 Vectors Purification
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. AAV9 Production and Purification
2.3. Transmission Electron Microscopy (TEM)
2.4. In Vitro Infectivity Assay
3. Results
3.1. Affinity Chromatography-Based Capture of AAV9 Vectors
3.2. Anion Exchange Chromatography Purification of AAV9 Vectors
3.3. TEM-Based Determination of DNA-Containing Capsid Ratio Showed the Effectiveness of Different Anion Exchange Columns
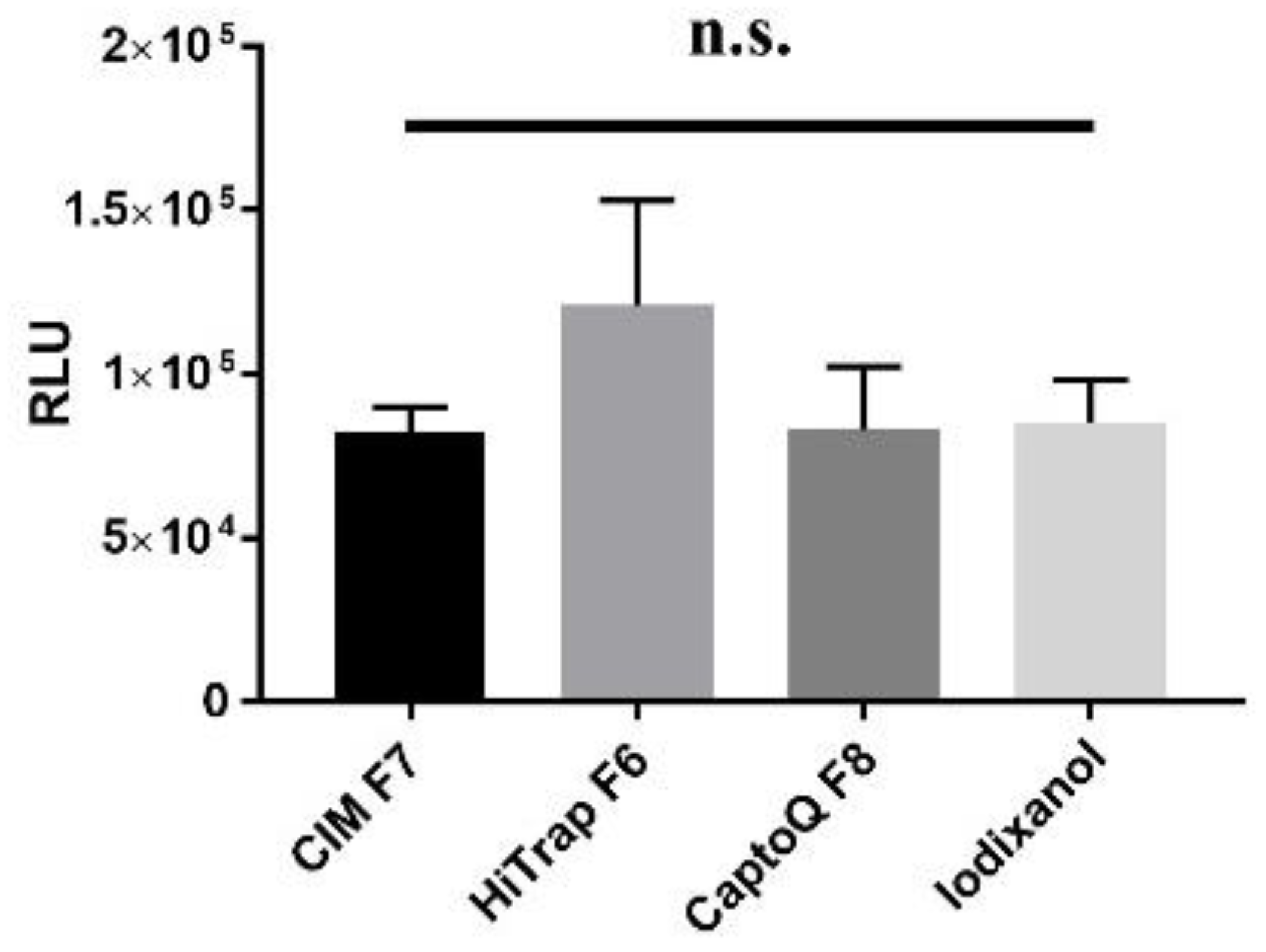
3.4. In Vitro Infectivity of AAV Does Not Depend on Purification Columns
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Au, H.K.E.; Isalan, M.; Mielcarek, M. Gene Therapy Advances: A Meta-Analysis of AAV Usage in Clinical Settings. Front. Med. 2022, 8, 809118. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, A.; Piglowska, N.; Ciulla, T.; Pitluck, S.; Johnson, S.; Buessing, M.; O’Connell, T. Estimation of impact of RPE65-mediated inherited retinal disease on quality of life and the potential benefits of gene therapy. Br. J. Ophthalmol. 2019, 103, 1610–1614. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Samulski, R.J. Engineering adeno-associated virus vectors for gene therapy. Nat. Rev. Genet. 2020, 21, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, S.E.; Pradeep, S.P.; Kasina, V.; Laddha, A.P.; Manautou, J.E.; Bahal, R. Casimersen for the treatment of Duchenne muscular dystrophy. Trends Pharmacol. Sci. 2022, 43, 607–608. [Google Scholar] [CrossRef]
- Philippidis, A. BioMarin’s ROCTAVIAN Wins Food and Drug Administration Approval As First Gene Therapy for Severe Hemophilia A. Hum. Gene Ther. 2023, 34, 665–668. [Google Scholar] [CrossRef]
- Abramowicz, M.; Pflomm, J.M.; Daron, S.M.; Faucard, A.; Viscusi, M.P.; Esterow, J.; Sacks, M.; Shah, B.M.; Swanson, F.P.; Bazil, C.W.; et al. Hemgenix-A gene therapy for hemophilia B. Med. Lett. Drugs Ther. 2023, 65, 9–10. [Google Scholar] [CrossRef]
- Canadian Agency for Drugs and Technologies in Health. Fidanacogene Elaparvovec (Beqvez): CADTH Reimbursement Recommendation: Indication: For the Treatment of Adults (Aged 18 Years or Older) with Moderately Severe to Severe Hemophilia B (Congenital Factor IX Deficiency) Who Are Negative for Neutralizing Antibodies to Variant AAV Serotype Rh74; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2024. [Google Scholar]
- FDA. FDA Approves First Gene Therapy for Treatment of Aromatic L-Amino Acid Decarboxylase Deficiency. 2024. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-gene-therapy-treatment-aromatic-l-amino-acid-decarboxylase-deficiency (accessed on 2 December 2024).
- Kotin, R.M.; Snyder, R.O. Manufacturing Clinical Grade Recombinant Adeno-Associated Virus Using Invertebrate Cell Lines. Hum. Gene Ther. 2017, 28, 350–360. [Google Scholar] [CrossRef]
- Adamson-Small, L.; Potter, M.; Falk, D.J.; Cleaver, B.; Byrne, B.J.; Clement, N. A scalable method for the production of high-titer and high-quality adeno-associated type 9 vectors using the HSV platform. Mol. Ther. Methods Clin. Dev. 2016, 3, 16031. [Google Scholar] [CrossRef]
- Chahal, P.S.; Schulze, E.; Tran, R.; Montes, J.; Kamen, A.A. Production of adeno-associated virus (AAV) serotypes by transient transfection of HEK293 cell suspension cultures for gene delivery. J. Virol. Methods 2014, 196, 163–173. [Google Scholar] [CrossRef]
- Tran, N.T.; Lecomte, E.; Saleun, S.; Namkung, S.; Robin, C.; Weber, K.; Devine, E.; Blouin, V.; Adjali, O.; Ayuso, E. Human and insect cell-produced recombinant adeno-associated viruses show differences in genome heterogeneity. Hum. Gene Ther. 2022, 33, 371–388. [Google Scholar] [CrossRef]
- Heldt, C.L.; Areo, O.; Joshi, P.U.; Mi, X.; Ivanova, Y.; Berrill, A. Empty and Full AAV Capsid Charge and Hydrophobicity Differences Measured with Single-Particle AFM. Langmuir 2023, 39, 5641–5648. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.F. Product-related impurities in clinical-grade recombinant AAV vectors: Characterization and risk assessment. Biomedicines 2014, 2, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Flotte, T.R. Empty Adeno-Associated Virus Capsids: Contaminant or Natural Decoy? Mary Ann Liebert, Inc.: New Rochelle, NY, USA, 2017; pp. 147–148. [Google Scholar]
- Manno, C.S.; Pierce, G.F.; Arruda, V.R.; Glader, B.; Ragni, M.; Rasko, J.J.; Ozelo, M.C.; Hoots, K.; Blatt, P.; Konkle, B. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 2006, 12, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Mingozzi, F.; Maus, M.V.; Hui, D.J.; Sabatino, D.E.; Murphy, S.L.; Rasko, J.E.; Ragni, M.V.; Manno, C.S.; Sommer, J.; Jiang, H. CD8+ T-cell responses to adeno-associated virus capsid in humans. Nat. Med. 2007, 13, 419–422. [Google Scholar] [CrossRef]
- Mingozzi, F.; High, K.A. Immune responses to AAV in clinical trials. Curr. Gene Ther. 2007, 7, 316–324. [Google Scholar] [CrossRef]
- Jarvi, N.; Hofman, K.; Venkatesh, A.; Gorecki, E.; Balu-Iyer, S.V. Immunogenicity risk assessment of empty capsids present in adeno-associated viral vectors using predictive innate immune responses. J. Pharm. Sci. 2024, 113, 3457–3469. [Google Scholar] [CrossRef]
- Gao, K.; Li, M.; Zhong, L.; Su, Q.; Li, J.; Li, S.; He, R.; Zhang, Y.; Hendricks, G.; Wang, J. Empty virions in AAV8 vector preparations reduce transduction efficiency and may cause total viral particle dose-limiting side effects. Mol. Ther.-Methods Clin. Dev. 2014, 1, 9. [Google Scholar] [CrossRef]
- Hitchcock, T. Manufacturing of AAV vectors: Translational challenges from development to industrialisation. Emerg. Top. Life Sci. 2021, 5, 725–728. [Google Scholar] [CrossRef]
- Mietzsch, M.; Eddington, C.; Jose, A.; Hsi, J.; Chipman, P.; Henley, T.; Choudhry, M.; McKenna, R.; Agbandje-McKenna, M. Improved Genome Packaging Efficiency of Adeno-associated Virus Vectors Using Rep Hybrids. J. Virol. 2021, 95, e0077321. [Google Scholar] [CrossRef]
- Tejero, M.; Duzenli, O.F.; Caine, C.; Kuoch, H.; Aslanidi, G. Bioengineered Hybrid Rep 2/6 Gene Improves Encapsulation of a Single-Stranded Expression Cassette into AAV6 Vectors. Genes 2023, 14, 1866. [Google Scholar] [CrossRef]
- Jain, N.K.; Ogden, P.J.; Church, G.M. Comprehensive mutagenesis maps the effect of all single-codon mutations in the AAV2 rep gene on AAV production. elife 2024, 12, RP87730. [Google Scholar] [CrossRef] [PubMed]
- Scarrott, J.M.; Johari, Y.B.; Pohle, T.H.; Liu, P.; Mayer, A.; James, D.C. Increased recombinant adeno-associated virus production by HEK293 cells using small molecule chemical additives. Biotechnol. J. 2023, 18, 2200450. [Google Scholar] [CrossRef] [PubMed]
- Moreno, F.; Lip, F.; Rojas, H.; Anggakusuma. Development of an insect cell-based adeno-associated virus packaging cell line employing advanced Rep gene expression control system. Mol. Ther. Methods Clin. Dev. 2022, 27, 391–403. [Google Scholar] [CrossRef] [PubMed]
- van Lieshout, L.P.; Rubin, M.; Costa-Grant, K.; Ota, S.; Golebiowski, D.; Panico, T.; Wiberg, E.; Szymczak, K.; Gilmore, R.; Stanvick, M.; et al. A novel dual-plasmid platform provides scalable transfection yielding improved productivity and packaging across multiple AAV serotypes and genomes. Mol. Ther. Methods Clin. Dev. 2023, 29, 426–436. [Google Scholar] [CrossRef]
- Aslanidi, G.; Lamb, K.; Zolotukhin, S. An inducible system for highly efficient production of recombinant adeno-associated virus (rAAV) vectors in insect Sf9 cells. Proc. Natl. Acad. Sci. USA 2009, 106, 5059–5064. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, S.; Lee, W.; Walsh, N.; Iozza, K.; Huang, N.; Preston, G.; Drouin, L.M.; Jia, N.; Deng, J.; et al. A Comprehensive Study of the Effects by Sequence Truncation within Inverted Terminal Repeats (ITRs) on the Productivity, Genome Packaging, and Potency of AAV Vectors. Microorganisms 2024, 12, 310. [Google Scholar] [CrossRef]
- Mingozzi, F.; High, K.A. Immune responses to AAV vectors: Overcoming barriers to successful gene therapy. Blood J. Am. Soc. Hematol. 2013, 122, 23–36. [Google Scholar] [CrossRef]
- Tomono, T.; Hirai, Y.; Okada, H.; Adachi, K.; Ishii, A.; Shimada, T.; Onodera, M.; Tamaoka, A.; Okada, T. Ultracentrifugation-free chromatography-mediated large-scale purification of recombinant adeno-associated virus serotype 1 (rAAV1). Mol. Ther.-Methods Clin. Dev. 2016, 3, 15058. [Google Scholar] [CrossRef]
- Wang, C.; Mulagapati, S.H.R.; Chen, Z.; Du, J.; Zhao, X.; Xi, G.; Chen, L.; Linke, T.; Gao, C.; Schmelzer, A.E. Developing an anion exchange chromatography assay for determining empty and DNA-containing capsid contents in AAV6. 2. Mol. Ther.-Methods Clin. Dev. 2019, 15, 257–263. [Google Scholar] [CrossRef]
- Venkatakrishnan, B.; Yarbrough, J.; Domsic, J.; Bennett, A.; Bothner, B.; Kozyreva, O.G.; Samulski, R.J.; Muzyczka, N.; McKenna, R.; Agbandje-McKenna, M. Structure and dynamics of adeno-associated virus serotype 1 VP1-unique N-terminal domain and its role in capsid trafficking. J. Virol. 2013, 87, 4974–4984. [Google Scholar] [CrossRef]
- Dickerson, R.; Argento, C.; Pieracci, J.; Bakhshayeshi, M. Separating Empty and DNA-containing Recombinant Adeno-Associated Virus Particles Using Isocratic Anion Exchange Chromatography. Biotechnol. J. 2021, 16, 2000015. [Google Scholar] [CrossRef] [PubMed]
- Huato Hernandez, J.; Boenning, K.; Kavara, A.; Schofield, M. Membrane chromatography for AAV DNA-containing capsid enrichment: Process development to scale up. J. Chromatogr. B 2024, 1244, 124258. [Google Scholar] [CrossRef] [PubMed]
- Rieser, R.; Koch, J.; Faccioli, G.; Richter, K.; Menzen, T.; Biel, M.; Winter, G.; Michalakis, S. Comparison of different liquid chromatography-based purification strategies for adeno-associated virus vectors. Pharmaceutics 2021, 13, 748. [Google Scholar] [CrossRef] [PubMed]
- Kurth, S.; Li, T.; Hausker, A.; Evans, W.; Dabre, R.; Muller, E.; Kervinen, J. Separation of full and empty adeno-associated virus capsids by anion-exchange chromatography using choline-type salts. Anal. Biochem. 2024, 686, 115421. [Google Scholar] [CrossRef]
- Chen, D.P.; Wei, J.Y.; Warren, J.C.; Huang, C. Tuning mobile phase properties to improve empty and DNA-containing particle separation in adeno-associated virus productions by anion exchange chromatography. Biotechnol. J. 2024, 19, 2300063. [Google Scholar] [CrossRef]
- Mietzsch, M.; Liu, W.; Ma, K.; Bennett, A.; Nelson, A.R.; Gliwa, K.; Chipman, P.; Fu, X.; Bechler, S.; McKenna, R.; et al. Production and characterization of an AAV1-VP3-only capsid: An analytical benchmark standard. Mol. Ther. Methods Clin. Dev. 2023, 29, 460–472. [Google Scholar] [CrossRef]
- Lavoie, R.A.; Zugates, J.T.; Cheeseman, A.T.; Teten, M.A.; Ramesh, S.; Freeman, J.M.; Swango, S.; Fitzpatrick, J.; Joshi, A.; Hollers, B.; et al. Enrichment of adeno-associated virus serotype 5 DNA-containing capsids by anion exchange chromatography with dual salt elution gradients. Biotechnol. Bioeng. 2023, 120, 2953–2968. [Google Scholar] [CrossRef]
- Jandera, P. Liquid Chromatography|Normal Phase. In Encyclopedia of Analytical Science, 3rd ed.; Worsfold, P., Poole, C., Townshend, A., Miró, M., Eds.; Academic Press: Oxford, UK, 2013; pp. 162–173. [Google Scholar] [CrossRef]
- Nass, S.A.; Mattingly, M.A.; Woodcock, D.A.; Burnham, B.L.; Ardinger, J.A.; Osmond, S.E.; Frederick, A.M.; Scaria, A.; Cheng, S.H.; O’Riordan, C.R. Universal method for the purification of recombinant AAV vectors of differing serotypes. Mol. Ther. Methods Clin. Dev. 2018, 9, 33–46. [Google Scholar] [CrossRef]
- Florea, M.; Nicolaou, F.; Pacouret, S.; Zinn, E.M.; Sanmiguel, J.; Andres-Mateos, E.; Unzu, C.; Wagers, A.J.; Vandenberghe, L.H. High-efficiency purification of divergent AAV serotypes using AAVX affinity chromatography. Mol. Ther. Methods Clin. Dev. 2023, 28, 146–159. [Google Scholar] [CrossRef]
- Tomono, T.; Hirai, Y.; Okada, H.; Miyagawa, Y.; Adachi, K.; Sakamoto, S.; Kawano, Y.; Chono, H.; Mineno, J.; Ishii, A. Highly efficient ultracentrifugation-free chromatographic purification of recombinant AAV serotype 9. Mol. Ther.-Methods Clin. Dev. 2018, 11, 180–190. [Google Scholar] [CrossRef]
- Khanal, O.; Kumar, V.; Jin, M. Adeno-associated viral capsid stability on anion exchange chromatography column and its impact on empty and DNA-containing capsid separation. Mol. Ther.-Methods Clin. Dev. 2023, 31, 101112. [Google Scholar] [CrossRef] [PubMed]
- Colon-Cortes, Y.; Hasan, M.A.; Aslanidi, G. Intra-tracheal delivery of AAV6 vectors results in sustained transduction in murine lungs without genomic integration. Gene 2020, 763, 100037. [Google Scholar] [CrossRef] [PubMed]
- Krotova, K.; Aslanidi, G. Modifiers of Adeno-Associated Virus-Mediated Gene Expression in Implication for Serotype-Universal Neutralizing Antibody Assay. Hum. Gene Ther. 2020, 31, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Pepligen. AVIPure® AAV Affinity Resins. 2024. Available online: https://www.repligen.com/products/chromatography/affinity-resins/avipure-aav#bioz-citation-listing (accessed on 23 April 2024).
- U.S. Department of Health and Human Services Food and Drug Administration Center for Biologics Evaluation and Research. Chemistry, Manufacturing, and Control (CMC) Information for Human Gene Therapy Investigational New Drug Applications (INDs). 2020. Available online: https://www.fda.gov/media/113760/download (accessed on 6 February 2024).
- Joshi, P.R.; Bernier, A.; Moço, P.D.; Schrag, J.; Chahal, P.S.; Kamen, A. Development of a scalable and robust AEX method for enriched rAAV preparations in genome-containing VCs of serotypes 5, 6, 8, and 9. Mol. Ther.-Methods Clin. Dev. 2021, 21, 341–356. [Google Scholar] [CrossRef]
- Urh, M.; Simpson, D.; Zhao, K. Chapter 26 Affinity Chromatography: General Methods. In Methods in Enzymology; Burgess, R.R., Deutscher, M.P., Eds.; Academic Press: Cambridge, MA, USA, 2009; pp. 417–438. [Google Scholar]
- Qu, W.; Wang, M.; Wu, Y.; Xu, R. Scalable downstream strategies for purification of recombinant adeno-associated virus vectors in light of the properties. Curr. Pharm. Biotechnol. 2015, 16, 684–695. [Google Scholar] [CrossRef]
- Challener, C. Choosing the Right Resins for Viral Vector Affinity Chromatography. BioPharm Int. 2023, 36, 19–21. [Google Scholar]
- Cummins, P.M.; Rochfort, K.D.; O’Connor, B.F. Ion-Exchange Chromatography: Basic Principles and Application. Methods Mol. Biol. 2017, 1485, 209–223. [Google Scholar] [CrossRef]
- Lock, M.; Alvira, M.R.; Wilson, J.M. Analysis of particle content of recombinant adeno-associated virus serotype 8 vectors by ion-exchange chromatography. Hum. Gene Ther. Part B Methods 2012, 23, 56–64. [Google Scholar] [CrossRef]
- Aebischer, M.K.; Gizardin-Fredon, H.; Lardeux, H.; Kochardt, D.; Elger, C.; Haindl, M.; Ruppert, R.; Guillarme, D.; D’Atri, V. Anion-exchange chromatography at the service of gene therapy: Baseline separation of DNA-containing/empty adeno-associated virus capsids by screening of conditions and step gradient elution mode. Int. J. Mol. Sci. 2022, 23, 12332. [Google Scholar] [CrossRef]
- Khatwani, S.L.; Pavlova, A.; Pirot, Z. Anion-exchange HPLC assay for separation and quantification of empty and DNA-containing capsids in multiple adeno-associated virus serotypes. Mol. Ther.-Methods Clin. Dev. 2021, 21, 548–558. [Google Scholar] [CrossRef]
- El Andari, J.; Grimm, D. Production, processing, and characterization of synthetic AAV gene therapy vectors. Biotechnol. J. 2021, 16, 2000025. [Google Scholar] [CrossRef]


| Column | Procedure | Column Volume, x | Flow Rate (mL/min) | Buffer Composition |
|---|---|---|---|---|
| AVIPure®-AAV9 | 1. Equilibration | 20 | 2 | 50 mM Tris, 400 mM NaCl, pH 7.5 |
| 2. Sample Application | 100 | 1.5 | HEK293 lysate with AAV9 in 50 mM Tris, 400 mM NaCl, pH 7.5 | |
| 3. Column Wash | 20 | 2 | 50 mM Tris, 400 mM NaCl, pH 7.5 | |
| 4. Elution | 10 | 2 | 50 mM Glycine, 150 mM NaCl, pH 2 | |
| 5. CIP | 10 10 20 | 2 2(30 min hold) 2 | a. 50 mM Glycine, 150 mM NaCl, pH 1 b. 0.5 M NaOH, c. 50 mM Tris, 400 mM NaCl, pH 7.5 | |
| CIMmultusTM QA | Equilibration | 20 | 1.5 | 10 mM Bis-Tris Propane (BTP), pH 9 |
| Sample Application | 10 | 1.5 | Post-affinity AAV9 eluate diluted into 10 mM BTP, pH 9 | |
| Column Wash | 30 | 1.5 | 10 mM Bis-Tris Propane (BTP), pH 9 | |
| Elution (Step Gradients) | 5 | 1.5 | A: 10 mM BTP pH 9+ B:10 mM BTP, 150 mM MgSO4 pH 9 Step 1 = 40% B + 60% A, Step 2 = 50% B + 50% A, Step 3 = 60% B + 40% A, Step 4 = 85% B + 15% A, Step 5 = 100% B | |
| CIP | 20 | 1.5 | a. 10 mM BTP, pH 9 | |
| 10 | 10 | b. 1 M NaCl | ||
| 20 | 1.5 | c. 10 mM BTP, pH 9 | ||
| Cytiva HiTapTM Q HP column | Equilibration | 10 | 5 | 10 mM BTP, pH 9 |
| Sample Application | 50 | 3 | Post-affinity AAV9 eluate diluted into 10 mM BTP, pH 9 | |
| Column Wash | 10 | 5 | 10 mM BTP, pH 9 | |
| Elution | 2 | 5 | A: 10 mM BTP, pH 9+ B: 10 mM BTP, 100 mM MgSO4, pH 9 Step 1 = 25% B + 75% A, Step 2 = 50% B + 50% A, Step 3 = 100% B | |
| CIP | 5 | 5 | a. 2 M NaCl | |
| 10 | 5 | b. 10 mM BTP, pH 9 | ||
| Cytiva HiTapTM CaptoQTM | Equilibration | 10 | 5 | 20 mM BTP (pH 9) |
| Sample Application | 50 | 1 | Post-affinity AAV9 eluate diluted into 10 mM BTP, pH 9 | |
| Column Wash | 10 | 5 | 20 mM BTP (pH 9) | |
| Elution | 4 | 5 | A: 20 mM BTP, pH 9+ B: 20 mM BTP, 2 mM MgCl2, 250 mM NaOAc (pH 9) Step 1 = 25% B + 75% A, Step 2 = 35% B + 65% A, Step 3 = 45% B + 55% A, Step 4 = 60% B + 40% A, Step 5 = 75% B + 25% A, Step 6 = 100% B | |
| Strip | 5 | 5 | 2 M NaCl | |
| CIP | 5 | 5 | a. 2 M NaCl | |
| 10 | 5 | b. 1 M NaOH | ||
| 5 | 5 | c. 2 M NaCl | ||
| 10 | 5 | d. 20 mM BTP, pH 9 |
| Sample | Column Binding Efficiency | Vector Recovery Within the Fraction |
|---|---|---|
| CiMmultus F7 | 99.60% | 67.50% |
| HiTrap F6 | 99.60% | 18.70% |
| CaptoQ F8 | 99% | 80.90% |
| Problem | Solution |
|---|---|
| Flow rate fluctuates during sample application. | After cell harvesting and disruption, the content of the sample includes not only AAV vectors but also cellular components, such as membrane lipids, proteins, genomic biomolecules, etc. Centrifugation steps for the removal of cellular debris is not sufficient for clarification. Therefore, filtering samples with different mesh sizes, or application of specific columns allowing the removal of such impurities by using flow-through mode is highly recommended. Additionally, uncut gDNA can promote column plugging, since the DNA double helix has a large surface area and high viscosity, which can lead to the accumulation of the sample with high viscosity inside the column. Ensure sufficient time and concentration of Benzonase treatment. |
| AAV capsid does not bind to the affinity resin. | Any modification within the AAV capsid creates new AAV variants which can show less or no binding efficacy to the affinity column material. Ensure that the binding site of the antibody in the resin has not an affinity to region mutated/modified within AAV capsid. |
| Loss of AAV infectivity after affinity column purification. | The elution from affinity columns generally requires low pH conditions. Since the acidic environment can trigger the spatial conformational change in AAV capsid, which may lead to the loss of infectivity. To prevent the loss of infectivity, pH of environment should be adjusted to a pH value which is tolerable for the AAV vector. You can add a buffer to ensure that the eluted fraction will be maintained at an optimal pH for stability. |
| The co-purification of DNA-containing and empty capsids. | To increase the efficiency of the separation in AEX column, the composition of the buffers, the purification mode (linear or step gradient), and incubation time should be optimized depending on AAV capsid serotype. Additionally, the column resins or materials, regardless of the column type (affinity or ion exchange), have a lifetime so that the degradation of resin material can result in the loss of resolution, binding, and recovery capacity. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duzenli, O.F.; Aslanidi, G. Consecutive Affinity and Ion-Exchange Chromatography for AAV9 Vectors Purification. Biomedicines 2025, 13, 361. https://doi.org/10.3390/biomedicines13020361
Duzenli OF, Aslanidi G. Consecutive Affinity and Ion-Exchange Chromatography for AAV9 Vectors Purification. Biomedicines. 2025; 13(2):361. https://doi.org/10.3390/biomedicines13020361
Chicago/Turabian StyleDuzenli, Ozgun Firat, and George Aslanidi. 2025. "Consecutive Affinity and Ion-Exchange Chromatography for AAV9 Vectors Purification" Biomedicines 13, no. 2: 361. https://doi.org/10.3390/biomedicines13020361
APA StyleDuzenli, O. F., & Aslanidi, G. (2025). Consecutive Affinity and Ion-Exchange Chromatography for AAV9 Vectors Purification. Biomedicines, 13(2), 361. https://doi.org/10.3390/biomedicines13020361








