Identifying Biomarkers for Remyelination and Recovery in Multiple Sclerosis: A Measure of Progress
Abstract
1. Introduction
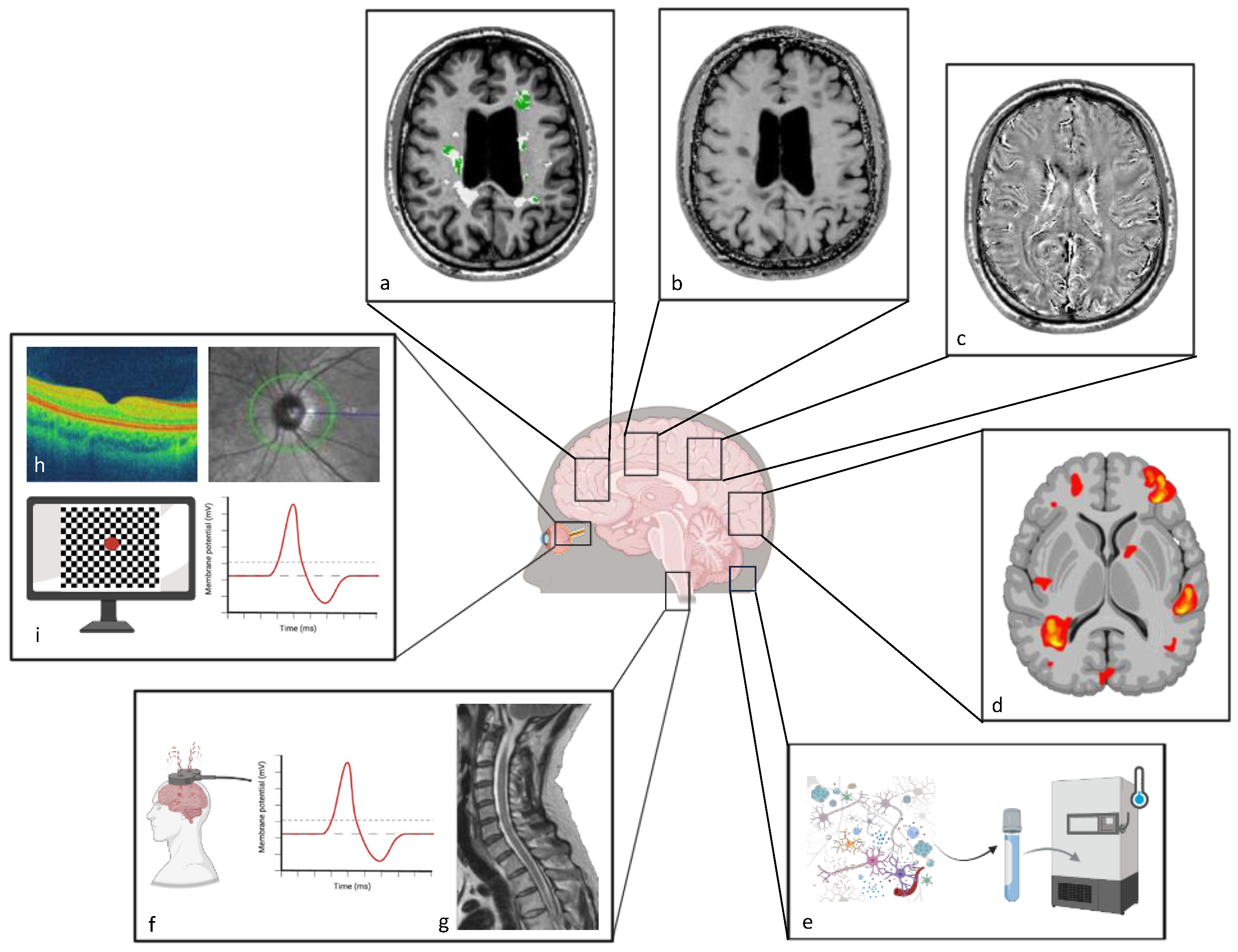
2. Techniques to Study Recovery
2.1. Magnetic Resonance Imaging (MRI)
2.2. Positron Emission Tomography (PET)
2.3. Functional Metrics (fMRI, EPs, OCT, and Digital Markers)
3. Outcome Biomarkers for Therapeutic Interventions to Enhance Recovery
3.1. Vision
3.2. Brain
3.3. Spinal Cord
4. Fluid-Derived Biomarkers to Track Neuroprotection and Repair
5. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Santos, E.N.; Fields, R.D. Regulation of myelination by microglia. Sci. Adv. 2021, 7, eabk1131. [Google Scholar] [CrossRef]
- Marton, R.M.; Miura, Y.; Sloan, S.A.; Li, Q.; Revah, O.; Levy, R.J.; Huguenard, J.R.; Pașca, S.P. Differentiation and maturation of oligodendrocytes in human three-dimensional neural cultures. Nat. Neurosci. 2019, 22, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.J.M.; Frisén, J.; Lyons, D.A. Revisiting remyelination: Towards a consensus on the regeneration of CNS myelin. Semin. Cell Dev. Biol. 2021, 116, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Kent, S.A.; Miron, V.E. Microglia regulation of central nervous system myelin health and regeneration. Nat. Rev. Immunol. 2024, 24, 49–63. [Google Scholar] [CrossRef]
- Klotz, L.; Antel, J.; Kuhlmann, T. Inflammation in multiple sclerosis: Consequences for remyelination and disease progression. Nat. Rev. Neurol. 2023, 19, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Lubetzki, C.; Zalc, B.; Williams, A.; Stadelmann, C.; Stankoff, B. Remyelination in multiple sclerosis: From basic science to clinical translation. Lancet Neurol. 2020, 19, 678–688. [Google Scholar] [CrossRef]
- Heß, K.; Starost, L.; Kieran, N.W.; Thomas, C.; Vincenten, M.C.J.; Antel, J.; Martino, G.; Huitinga, I.; Healy, L.; Kuhlmann, T. Lesion stage-dependent causes for impaired remyelination in MS. Acta Neuropathol. 2020, 140, 359–375. [Google Scholar] [CrossRef]
- Bramow, S.; Frischer, J.M.; Lassmann, H.; Koch-Henriksen, N.; Lucchinetti, C.F.; Sørensen, P.S.; Laursen, H. Demyelination versus remyelination in progressive multiple sclerosis. Brain 2010, 133, 2983–2998. [Google Scholar] [CrossRef]
- Brown, R.A.; Narayanan, S.; Arnold, D.L. Imaging of repeated episodes of demyelination and remyelination in multiple sclerosis. Neuroimage Clin. 2014, 6, 20–25. [Google Scholar] [CrossRef]
- Qin, C.; Yang, S.; Chen, M.; Dong, M.H.; Zhou, L.Q.; Chu, Y.H.; Shen, Z.X.; Bosco, D.B.; Wu, L.J.; Tian, D.S.; et al. Modulation of microglial metabolism facilitates regeneration in demyelination. iScience 2023, 26, 106588. [Google Scholar] [CrossRef]
- Ronzano, R.; Roux, T.; Thetiot, M.; Aigrot, M.S.; Richard, L.; Lejeune, F.X.; Mazuir, E.; Vallat, J.M.; Lubetzki, C.; Desmazières, A. Microglia-neuron interaction at nodes of Ranvier depends on neuronal activity through potassium release and contributes to remyelination. Nat. Commun. 2021, 12, 5219. [Google Scholar] [CrossRef]
- Tan, Y.L.; Yuan, Y.; Tian, L. Microglial regional heterogeneity and its role in the brain. Mol. Psychiatry 2020, 25, 351–367. [Google Scholar] [CrossRef]
- das Neves, S.P.; Delivanoglou, N.; Ren, Y.; Cucuzza, C.S.; Makuch, M.; Almeida, F.; Sanchez, G.; Barber, M.J.; Rego, S.; Schrader, R.; et al. Meningeal lymphatic function promotes oligodendrocyte survival and brain myelination. Immunity 2024, 57, 2328–2343.e8. [Google Scholar] [CrossRef]
- Prineas, J.W.; Kwon, E.E.; Cho, E.S.; Sharer, L.R. Continual breakdown and regeneration of myelin in progressive multiple sclerosis plaques. Ann. N. Y. Acad. Sci. 1984, 436, 11–32. [Google Scholar] [CrossRef]
- Patrikios, P.; Stadelmann, C.; Kutzelnigg, A.; Rauschka, H.; Schmidbauer, M.; Laursen, H.; Sorensen, P.S.; Brück, W.; Lucchinetti, C.; Lassmann, H. Remyelination is extensive in a subset of multiple sclerosis patients. Brain 2006, 129, 3165–3172. [Google Scholar] [CrossRef]
- Neumann, B.; Foerster, S.; Zhao, C.; Bodini, B.; Reich, D.S.; Bergles, D.E.; Káradóttir, R.T.; Lubetzki, C.; Lairson, L.L.; Zalc, B.; et al. Problems and Pitfalls of Identifying Remyelination in Multiple Sclerosis. Cell Stem Cell 2020, 26, 617–619. [Google Scholar] [CrossRef]
- Xing, Y.L.; Röth, P.T.; Stratton, J.A.; Chuang, B.H.; Danne, J.; Ellis, S.L.; Ng, S.W.; Kilpatrick, T.J.; Merson, T.D. Adult neural precursor cells from the subventricular zone contribute significantly to oligodendrocyte regeneration and remyelination. J. Neurosci. 2014, 34, 14128–14146. [Google Scholar] [CrossRef]
- Huré, J.-B.; Foucault, L.; Ghayad, L.M.; Marie, C.; Vachoud, N.; Baudouin, L.; Azmani, R.; Ivljanin, N.; Arevalo-Nuevo, A.; Pigache, M.; et al. Pharmacogenomic screening identifies and repurposes leucovorin and dyclonine as pro-oligodendrogenic compounds in brain repair. Nat. Commun. 2024, 15, 9837. [Google Scholar] [CrossRef]
- Cunniffe, N.; Coles, A. Promoting remyelination in multiple sclerosis. J. Neurol. 2021, 268, 30–44. [Google Scholar] [CrossRef]
- Sim, F.J.; Zhao, C.; Penderis, J.; Franklin, R.J. The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J. Neurosci. 2002, 22, 2451–2459. [Google Scholar] [CrossRef]
- Smith, K.J.; Blakemore, W.F.; McDonald, W.I. The restoration of conduction by central remyelination. Brain 1981, 104, 383–404. [Google Scholar] [CrossRef]
- Irvine, K.A.; Blakemore, W.F. Remyelination protects axons from demyelination-associated axon degeneration. Brain 2008, 131, 1464–1477. [Google Scholar] [CrossRef]
- Koch, M.W.; Moral, E.; Brieva, L.; Mostert, J.; Strijbis, E.M.; Comtois, J.; Repovic, P.; Bowen, J.D.; Wolinsky, J.S.; Lublin, F.D.; et al. Relapse recovery in relapsing-remitting multiple sclerosis: An analysis of the CombiRx dataset. Mult. Scler. 2023, 29, 1776–1785. [Google Scholar] [CrossRef]
- Lee, J.; Hyun, J.W.; Lee, J.; Choi, E.J.; Shin, H.G.; Min, K.; Nam, Y.; Kim, H.J.; Oh, S.H. So You Want to Image Myelin Using MRI: An Overview and Practical Guide for Myelin Water Imaging. J. Magn. Reson. Imaging 2021, 53, 360–373. [Google Scholar] [CrossRef]
- Laule, C.; Vavasour, I.M.; Moore, G.R.W.; Oger, J.; Li, D.K.B.; Paty, D.W.; MacKay, A.L. Water content and myelin water fraction in multiple sclerosis. A T2 relaxation study. J. Neurol. 2004, 251, 284–293. [Google Scholar] [CrossRef]
- Sommer, R.C.; Hata, J.; Rimkus, C.d.M.; Klein da Costa, B.; Nakahara, J.; Sato, D.K. Mechanisms of myelin repair, MRI techniques and therapeutic opportunities in multiple sclerosis. Mult. Scler. Relat. Disord. 2022, 58, 103407. [Google Scholar] [CrossRef]
- Baliyan, V.; Das, C.J.; Sharma, R.; Gupta, A.K. Diffusion weighted imaging: Technique and applications. World J. Radiol. 2016, 8, 785–798. [Google Scholar] [CrossRef]
- Heath, F.; Hurley, S.A.; Johansen-Berg, H.; Sampaio-Baptista, C. Advances in noninvasive myelin imaging. Dev. Neurobiol. 2018, 78, 136–151. [Google Scholar] [CrossRef]
- MacKay, A.L.; Laule, C. Magnetic Resonance of Myelin Water: An in vivo Marker for Myelin. Brain Plast. 2016, 2, 71–91. [Google Scholar] [CrossRef]
- Alonso-Ortiz, E.; Levesque, I.R.; Pike, G.B. MRI-based myelin water imaging: A technical review. Magn. Reson. Med. 2015, 73, 70–81. [Google Scholar] [CrossRef]
- Wheeler-Kingshott, C.A.; Cercignani, M. About “axial” and “radial” diffusivities. Magn. Reson. Med. 2009, 61, 1255–1260. [Google Scholar] [CrossRef]
- Winklewski, P.J.; Sabisz, A.; Naumczyk, P.; Jodzio, K.; Szurowska, E.; Szarmach, A. Understanding the Physiopathology Behind Axial and Radial Diffusivity Changes-What Do We Know? Front. Neurol. 2018, 9, 92. [Google Scholar] [CrossRef]
- Aung, W.Y.; Mar, S.; Benzinger, T.L. Diffusion tensor MRI as a biomarker in axonal and myelin damage. Imaging Med. 2013, 5, 427. [Google Scholar] [CrossRef]
- York, E.N.; Thrippleton, M.J.; Meijboom, R.; Hunt, D.P.J.; Waldman, A.D. Quantitative magnetization transfer imaging in relapsing-remitting multiple sclerosis: A systematic review and meta-analysis. Brain Commun. 2022, 4, fcac088. [Google Scholar] [CrossRef]
- Müller, J.; Lu, P.J.; Cagol, A.; Ruberte, E.; Shin, H.G.; Ocampo-Pineda, M.; Chen, X.; Tsagkas, C.; Barakovic, M.; Galbusera, R.; et al. Quantifying Remyelination Using χ-Separation in White Matter and Cortical Multiple Sclerosis Lesions. Neurology 2024, 103, e209604. [Google Scholar] [CrossRef]
- Mallik, S.; Samson, R.S.; Wheeler-Kingshott, C.A.; Miller, D.H. Imaging outcomes for trials of remyelination in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2014, 85, 1396–1404. [Google Scholar] [CrossRef]
- Jespersen, S.N.; Bjarkam, C.R.; Nyengaard, J.R.; Chakravarty, M.M.; Hansen, B.; Vosegaard, T.; Østergaard, L.; Yablonskiy, D.; Nielsen, N.C.; Vestergaard-Poulsen, P. Neurite density from magnetic resonance diffusion measurements at ultrahigh field: Comparison with light microscopy and electron microscopy. Neuroimage 2010, 49, 205–216. [Google Scholar] [CrossRef]
- Chen, J.T.; Kuhlmann, T.; Jansen, G.H.; Collins, D.L.; Atkins, H.L.; Freedman, M.S.; O’Connor, P.W.; Arnold, D.L. Voxel-based analysis of the evolution of magnetization transfer ratio to quantify remyelination and demyelination with histopathological validation in a multiple sclerosis lesion. Neuroimage 2007, 36, 1152–1158. [Google Scholar] [CrossRef]
- Wisnieff, C.; Ramanan, S.; Olesik, J.; Gauthier, S.; Wang, Y.; Pitt, D. Quantitative susceptibility mapping (QSM) of white matter multiple sclerosis lesions: Interpreting positive susceptibility and the presence of iron. Magn. Reson. Med. 2015, 74, 564–570. [Google Scholar] [CrossRef]
- Chen, W.; Gauthier, S.A.; Gupta, A.; Comunale, J.; Liu, T.; Wang, S.; Pei, M.; Pitt, D.; Wang, Y. Quantitative susceptibility mapping of multiple sclerosis lesions at various ages. Radiology 2014, 271, 183–192. [Google Scholar] [CrossRef]
- Rahmanzadeh, R.; Galbusera, R.; Lu, P.J.; Bahn, E.; Weigel, M.; Barakovic, M.; Franz, J.; Nguyen, T.D.; Spincemaille, P.; Schiavi, S.; et al. A New Advanced MRI Biomarker for Remyelinated Lesions in Multiple Sclerosis. Ann. Neurol. 2022, 92, 486–502. [Google Scholar] [CrossRef]
- Bodini, B.; Tonietto, M.; Airas, L.; Stankoff, B. Positron emission tomography in multiple sclerosis—Straight to the target. Nat. Rev. Neurol. 2021, 17, 663–675. [Google Scholar] [CrossRef]
- Stankoff, B.; Wang, Y.; Bottlaender, M.; Aigrot, M.S.; Dolle, F.; Wu, C.; Feinstein, D.; Huang, G.F.; Semah, F.; Mathis, C.A.; et al. Imaging of CNS myelin by positron-emission tomography. Proc. Natl. Acad. Sci. USA 2006, 103, 9304–9309. [Google Scholar] [CrossRef]
- Bodini, B.; Veronese, M.; García-Lorenzo, D.; Battaglini, M.; Poirion, E.; Chardain, A.; Freeman, L.; Louapre, C.; Tchikviladze, M.; Papeix, C.; et al. Dynamic Imaging of Individual Remyelination Profiles in Multiple Sclerosis. Ann. Neurol. 2016, 79, 726–738. [Google Scholar] [CrossRef]
- Auvity, S.; Tonietto, M.; Caillé, F.; Bodini, B.; Bottlaender, M.; Tournier, N.; Kuhnast, B.; Stankoff, B. Repurposing radiotracers for myelin imaging: A study comparing 18F-florbetaben, 18F-florbetapir, 18F-flutemetamol,11C-MeDAS, and 11C-PiB. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 490–501. [Google Scholar] [CrossRef]
- Carotenuto, A.; Giordano, B.; Dervenoulas, G.; Wilson, H.; Veronese, M.; Chappell, Z.; Polychronis, S.; Pagano, G.; Mackewn, J.; Turkheimer, F.E.; et al. [(18)F]Florbetapir PET/MR imaging to assess demyelination in multiple sclerosis. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 366–378. [Google Scholar] [CrossRef]
- Brugarolas, P.; Sánchez-Rodríguez, J.E.; Tsai, H.M.; Basuli, F.; Cheng, S.H.; Zhang, X.; Caprariello, A.V.; Lacroix, J.J.; Freifelder, R.; Murali, D.; et al. Development of a PET radioligand for potassium channels to image CNS demyelination. Sci. Rep. 2018, 8, 607. [Google Scholar] [CrossRef]
- Brugarolas, P.; Wilks, M.Q.; Noel, J.; Kaiser, J.A.; Vesper, D.R.; Ramos-Torres, K.M.; Guehl, N.J.; Macdonald-Soccorso, M.T.; Sun, Y.; Rice, P.A.; et al. Human biodistribution and radiation dosimetry of the demyelination tracer [(18)F]3F4AP. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 344–351. [Google Scholar] [CrossRef]
- Guehl, N.J.; Ramos-Torres, K.M.; Linnman, C.; Moon, S.H.; Dhaynaut, M.; Wilks, M.Q.; Han, P.K.; Ma, C.; Neelamegam, R.; Zhou, Y.P.; et al. Evaluation of the potassium channel tracer [(18)F]3F4AP in rhesus macaques. J. Cereb. Blood Flow Metab. 2021, 41, 1721–1733. [Google Scholar] [CrossRef]
- Schäffner, E.; Bosch-Queralt, M.; Edgar, J.M.; Lehning, M.; Strauß, J.; Fleischer, N.; Kungl, T.; Wieghofer, P.; Berghoff, S.A.; Reinert, T.; et al. Myelin insulation as a risk factor for axonal degeneration in autoimmune demyelinating disease. Nat. Neurosci. 2023, 26, 1218–1228. [Google Scholar] [CrossRef]
- Freeman, L.; Garcia-Lorenzo, D.; Bottin, L.; Leroy, C.; Louapre, C.; Bodini, B.; Papeix, C.; Assouad, R.; Granger, B.; Tourbah, A.; et al. The neuronal component of gray matter damage in multiple sclerosis: A [(11) C]flumazenil positron emission tomography study. Ann. Neurol. 2015, 78, 554–567. [Google Scholar] [CrossRef]
- Mansur, A.; Rabiner, E.A.; Comley, R.A.; Lewis, Y.; Middleton, L.T.; Huiban, M.; Passchier, J.; Tsukada, H.; Gunn, R.N. Characterization of 3 PET Tracers for Quantification of Mitochondrial and Synaptic Function in Healthy Human Brain: (18)F-BCPP-EF, (11)C-SA-4503, and (11)C-UCB-J. J. Nucl. Med. 2020, 61, 96–103. [Google Scholar] [CrossRef]
- Hagens, M.H.J.; Golla, S.S.V.; Janssen, B.; Vugts, D.J.; Beaino, W.; Windhorst, A.D.; O’Brien-Brown, J.; Kassiou, M.; Schuit, R.C.; Schwarte, L.A.; et al. The P2X(7) receptor tracer [(11)C]SMW139 as an in vivo marker of neuroinflammation in multiple sclerosis: A first-in man study. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 379–389. [Google Scholar] [CrossRef]
- Wei, W.; Poirion, E.; Bodini, B.; Durrleman, S.; Ayache, N.; Stankoff, B.; Colliot, O. Predicting PET-derived demyelination from multimodal MRI using sketcher-refiner adversarial training for multiple sclerosis. Med. Image Anal. 2019, 58, 101546. [Google Scholar] [CrossRef]
- Gore, J.C. Principles and practice of functional MRI of the human brain. J. Clin. Investig. 2003, 112, 4–9. [Google Scholar] [CrossRef]
- Backner, Y.; Kuchling, J.; Massarwa, S.; Oberwahrenbrock, T.; Finke, C.; Bellmann-Strobl, J.; Ruprecht, K.; Brandt, A.U.; Zimmermann, H.; Raz, N.; et al. Anatomical Wiring and Functional Networking Changes in the Visual System Following Optic Neuritis. JAMA Neurol. 2018, 75, 287–295. [Google Scholar] [CrossRef]
- Villoslada, P.; Solana, E.; Alba-Arbalat, S.; Martinez-Heras, E.; Vivo, F.; Lopez-Soley, E.; Calvi, A.; Camos-Carreras, A.; Dotti-Boada, M.; Bailac, R.A.; et al. Retinal Damage and Visual Network Reconfiguration Defines Visual Function Recovery in Optic Neuritis. Neurol. Neuroimmunol. Neuroinflamm. 2024, 11, e200288. [Google Scholar] [CrossRef]
- Rocca, M.A.; Schoonheim, M.M.; Valsasina, P.; Geurts, J.J.G.; Filippi, M. Task- and resting-state fMRI studies in multiple sclerosis: From regions to systems and time-varying analysis. Current status and future perspective. Neuroimage Clin. 2022, 35, 103076. [Google Scholar] [CrossRef]
- Huntenburg, J.M.; Bazin, P.L.; Goulas, A.; Tardif, C.L.; Villringer, A.; Margulies, D.S. A Systematic Relationship Between Functional Connectivity and Intracortical Myelin in the Human Cerebral Cortex. Cereb. Cortex 2017, 27, 981–997. [Google Scholar] [CrossRef]
- You, Y.; Gupta, V.K.; Chitranshi, N.; Reedman, B.; Klistorner, A.; Graham, S.L. Visual Evoked Potential Recording in a Rat Model of Experimental Optic Nerve Demyelination. J. Vis. Exp. 2015, 101, e52934. [Google Scholar] [CrossRef]
- Castoldi, V.; Marenna, S.; d’Isa, R.; Huang, S.C.; De Battista, D.; Chirizzi, C.; Chaabane, L.; Kumar, D.; Boschert, U.; Comi, G.; et al. Non-invasive visual evoked potentials to assess optic nerve involvement in the dark agouti rat model of experimental autoimmune encephalomyelitis induced by myelin oligodendrocyte glycoprotein. Brain Pathol. 2020, 30, 137–150. [Google Scholar] [CrossRef]
- Marenna, S.; Huang, S.C.; Dalla Costa, G.; d’Isa, R.; Castoldi, V.; Rossi, E.; Comi, G.; Leocani, L. Visual Evoked Potentials to Monitor Myelin Cuprizone-Induced Functional Changes. Front. Neurosci. 2022, 16, 820155. [Google Scholar] [CrossRef]
- Marenna, S.; Huang, S.C.; Rossi, E.; Castoldi, V.; Comi, G.; Leocani, L. Transcranial direct current stimulation as a preventive treatment in multiple sclerosis? Preclinical evidence. Exp. Neurol. 2022, 357, 114201. [Google Scholar] [CrossRef]
- Bejarano, B.; Bianco, M.; Gonzalez-Moron, D.; Sepulcre, J.; Goñi, J.; Arcocha, J.; Soto, O.; Del Carro, U.; Comi, G.; Leocani, L.; et al. Computational classifiers for predicting the short-term course of Multiple sclerosis. BMC Neurol. 2011, 11, 67. [Google Scholar] [CrossRef]
- Hardmeier, M.; Leocani, L.; Fuhr, P. A new role for evoked potentials in MS? Repurposing evoked potentials as biomarkers for clinical trials in MS. Mult. Scler. 2017, 23, 1309–1319. [Google Scholar] [CrossRef]
- Leocani, L.; Rovaris, M.; Boneschi, F.M.; Medaglini, S.; Rossi, P.; Martinelli, V.; Amadio, S.; Comi, G. Multimodal evoked potentials to assess the evolution of multiple sclerosis: A longitudinal study. J. Neurol. Neurosurg. Psychiatry 2006, 77, 1030–1035. [Google Scholar] [CrossRef]
- Nuwer, M.R.; Packwood, J.W.; Myers, L.W.; Ellison, G.W. Evoked potentials predict the clinical changes in a multiple sclerosis drug study. Neurology 1987, 37, 1754. [Google Scholar] [CrossRef]
- Pisa, M.; Chieffo, R.; Giordano, A.; Gelibter, S.; Comola, M.; Comi, G.; Leocani, L. Upper limb motor evoked potentials as outcome measure in progressive multiple sclerosis. Clin. Neurophysiol. 2020, 131, 401–405. [Google Scholar] [CrossRef]
- Dalla Costa, G.; Pisa, M.; Fabbella, L.; Furlan, R.; Comi, G.; Leocani, L. Serum neurofilaments predict recovery after acute optic neuritis. In Proceedings of the 28th Annual Meeting of the European Charcot Foundation, Baveno, Italy, 15–19 November 2020. Digital Edition. [Google Scholar]
- Cadavid, D.; Balcer, L.; Galetta, S.; Aktas, O.; Ziemssen, T.; Vanopdenbosch, L.; Frederiksen, J.; Skeen, M.; Jaffe, G.J.; Butzkueven, H.; et al. Safety and efficacy of opicinumab in acute optic neuritis (RENEW): A randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2017, 16, 189–199. [Google Scholar] [CrossRef]
- Green, A.J.; Gelfand, J.M.; Cree, B.A.; Bevan, C.; Boscardin, W.J.; Mei, F.; Inman, J.; Arnow, S.; Devereux, M.; Abounasr, A.; et al. Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): A randomised, controlled, double-blind, crossover trial. Lancet 2017, 390, 2481–2489. [Google Scholar] [CrossRef]
- Brown, J.W.L.; Cunniffe, N.G.; Prados, F.; Kanber, B.; Connick, P.; lin, R.F.; Chandran, S.; Altmann, D.; Chard, D.T.; Coles, A.J. Retinoid-X receptor agonism promotes remyelination in relapsing-remitting multiple sclerosis: A phase 2 clinical trial. J. Neurol. Neurosurg. Psychiatry 2022, 93, A92. [Google Scholar] [CrossRef]
- Jenkins, T.M.; Toosy, A.T.; Ciccarelli, O.; Miszkiel, K.A.; Wheeler-Kingshott, C.A.; Henderson, A.P.; Kallis, C.; Mancini, L.; Plant, G.T.; Miller, D.H.; et al. Neuroplasticity predicts outcome of optic neuritis independent of tissue damage. Ann. Neurol. 2010, 67, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Pisa, M.; Guerrieri, S.; Di Maggio, G.; Medaglini, S.; Moiola, L.; Martinelli, V.; Comi, G.; Leocani, L. No evidence of disease activity is associated with reduced rate of axonal retinal atrophy in MS. Neurology 2017, 89, 2469–2475. [Google Scholar] [CrossRef]
- Pisa, M.; Croese, T.; Dalla Costa, G.; Guerrieri, S.; Huang, S.C.; Finardi, A.; Fabbella, L.; Sangalli, F.; Colombo, B.; Moiola, L.; et al. Subclinical anterior optic pathway involvement in early multiple sclerosis and clinically isolated syndromes. Brain 2021, 144, 848–862. [Google Scholar] [CrossRef]
- Knier, B.; Schmidt, P.; Aly, L.; Buck, D.; Berthele, A.; Mühlau, M.; Zimmer, C.; Hemmer, B.; Korn, T. Retinal inner nuclear layer volume reflects response to immunotherapy in multiple sclerosis. Brain 2016, 139, 2855–2863. [Google Scholar] [CrossRef]
- Montalban, X.; Graves, J.; Midaglia, L.; Mulero, P.; Julian, L.; Baker, M.; Schadrack, J.; Gossens, C.; Ganzetti, M.; Scotland, A.; et al. A smartphone sensor-based digital outcome assessment of multiple sclerosis. Mult. Scler. 2022, 28, 654–664. [Google Scholar] [CrossRef]
- Shema-Shiratzky, S.; Hillel, I.; Mirelman, A.; Regev, K.; Hsieh, K.L.; Karni, A.; Devos, H.; Sosnoff, J.J.; Hausdorff, J.M. A wearable sensor identifies alterations in community ambulation in multiple sclerosis: Contributors to real-world gait quality and physical activity. J. Neurol. 2020, 267, 1912–1921. [Google Scholar] [CrossRef]
- Pratap, A.; Grant, D.; Vegesna, A.; Tummalacherla, M.; Cohan, S.; Deshpande, C.; Mangravite, L.; Omberg, L. Evaluating the Utility of Smartphone-Based Sensor Assessments in Persons With Multiple Sclerosis in the Real-World Using an App (elevateMS): Observational, Prospective Pilot Digital Health Study. JMIR Mhealth Uhealth 2020, 8, e22108. [Google Scholar] [CrossRef]
- Block, V.J.; Bove, R.; Zhao, C.; Garcha, P.; Graves, J.; Romeo, A.R.; Green, A.J.; Allen, D.D.; Hollenbach, J.A.; Olgin, J.E.; et al. Association of Continuous Assessment of Step Count by Remote Monitoring With Disability Progression Among Adults With Multiple Sclerosis. JAMA Netw. Open 2019, 2, e190570. [Google Scholar] [CrossRef]
- Zheng, P.; Jeng, B.; Huynh, T.L.T.; Aguiar, E.J.; Motl, R.W. Free-Living Peak Cadence in Multiple Sclerosis: A New Measure of Real-World Walking? Neurorehabilit. Neural Repair 2023, 37, 716–726. [Google Scholar] [CrossRef]
- Sehic, A.; Guo, S.; Cho, K.S.; Corraya, R.M.; Chen, D.F.; Utheim, T.P. Electrical Stimulation as a Means for Improving Vision. Am. J. Pathol. 2016, 186, 2783–2797. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Kim, S.; Kwon, Y.W.; Seo, H.; Kim, M.; Chung, W.G.; Park, W.; Song, H.; Lee, D.H.; Lee, J.; et al. Electrical stimulation for therapeutic approach. Interdiscip. Med. 2023, 1, e20230003. [Google Scholar] [CrossRef]
- Li, D.C.; Li, Q. Electrical stimulation of cortical neurons promotes oligodendrocyte development and remyelination in the injured spinal cord. Neural Regen. Res. 2017, 12, 1613–1615. [Google Scholar] [CrossRef] [PubMed]
- Frühbeis, C.; Kuo-Elsner, W.P.; Müller, C.; Barth, K.; Peris, L.; Tenzer, S.; Möbius, W.; Werner, H.B.; Nave, K.A.; Fröhlich, D.; et al. Oligodendrocytes support axonal transport and maintenance via exosome secretion. PLoS Biol. 2020, 18, e3000621. [Google Scholar] [CrossRef]
- Hood, D.C.; Odel, J.G.; Zhang, X. Tracking the recovery of local optic nerve function after optic neuritis: A multifocal VEP study. Investig. Ophthalmol. Vis. Sci. 2000, 41, 4032–4038. [Google Scholar]
- Klistorner, A.; Graham, S.L. Role of Multifocal Visually Evoked Potential as a Biomarker of Demyelination, Spontaneous Remyelination, and Myelin Repair in Multiple Sclerosis. Front. Neurosci. 2021, 15, 725187. [Google Scholar] [CrossRef]
- Schmidt, M.F.; Pihl-Jensen, G.; Bille, M.B.; Frederiksen, J.L. Anti-myelin oligodendrocyte glycoprotein antibodies in a girl with good recovery after five episodes of prior idiopathic optic neuritis. Am. J. Ophthalmol. Case Rep. 2021, 22, 101060. [Google Scholar] [CrossRef]
- Meuth, S.G.; Bittner, S.; Seiler, C.; Göbel, K.; Wiendl, H. Natalizumab restores evoked potential abnormalities in patients with relapsing-remitting multiple sclerosis. Mult. Scler. 2011, 17, 198–203. [Google Scholar] [CrossRef]
- Pfeuffer, S.; Kerschke, L.; Ruck, T.; Rolfes, L.; Pawlitzki, M.; Albrecht, P.; Wiendl, H.; Meuth, S.G. Teriflunomide treatment is associated with optic nerve recovery in early multiple sclerosis. Ther. Adv. Neurol. Disord. 2021, 14, 1756286421997372. [Google Scholar] [CrossRef]
- Wang, C.; Barton, J.; Kyle, K.; Ly, L.; Barnett, Y.; Hartung, H.P.; Reddel, S.W.; Beadnall, H.; Taha, M.; Klistorner, A.; et al. Multiple sclerosis: Structural and functional integrity of the visual system following alemtuzumab therapy. J. Neurol. Neurosurg. Psychiatry 2021, 92, 1319–1324. [Google Scholar] [CrossRef]
- Nij Bijvank, J.A.; Hof, S.N.; Prouskas, S.E.; Schoonheim, M.M.; Uitdehaag, B.M.J.; van Rijn, L.J.; Petzold, A. A novel eye-movement impairment in multiple sclerosis indicating widespread cortical damage. Brain 2023, 146, 2476–2488. [Google Scholar] [CrossRef]
- Brown, J.W.L.; Cunniffe, N.G.; Prados, F.; Kanber, B.; Jones, J.L.; Needham, E.; Georgieva, Z.; Rog, D.; Pearson, O.R.; Overell, J.; et al. Safety and efficacy of bexarotene in patients with relapsing-remitting multiple sclerosis (CCMR One): A randomised, double-blind, placebo-controlled, parallel-group, phase 2a study. Lancet Neurol. 2021, 20, 709–720. [Google Scholar] [CrossRef]
- Hof, S.N.; Loonstra, F.C.; de Ruiter, L.R.J.; van Rijn, L.J.; Petzold, A.; Uitdehaag, B.M.J.; Nij Bijvank, J.A. The prevalence of internuclear ophthalmoparesis in a population-based cohort of individuals with multiple sclerosis. Mult. Scler. Relat. Disord. 2022, 63, 103824. [Google Scholar] [CrossRef]
- Kanhai, K.M.S.; Nij Bijvank, J.A.; Wagenaar, Y.L.; Klaassen, E.S.; Lim, K.; Bergheanu, S.C.; Petzold, A.; Verma, A.; Hesterman, J.; Wattjes, M.P.; et al. Treatment of internuclear ophthalmoparesis in multiple sclerosis with fampridine: A randomized double-blind, placebo-controlled cross-over trial. CNS Neurosci. Ther. 2019, 25, 697–703. [Google Scholar] [CrossRef]
- Arnold, D.L.; Piani-Meier, D.; Bar-Or, A.; Benedict, R.H.B.; Cree, B.A.C.; Giovannoni, G.; Gold, R.; Vermersch, P.; Arnould, S.; Dahlke, F.; et al. Effect of siponimod on magnetic resonance imaging measures of neurodegeneration and myelination in secondary progressive multiple sclerosis: Gray matter atrophy and magnetization transfer ratio analyses from the EXPAND phase 3 trial. Mult. Scler. (Houndmills Basingstoke Engl.) 2022, 28, 1526. [Google Scholar] [CrossRef]
- Caverzasi, E.; Papinutto, N.; Cordano, C.; Kirkish, G.; Gundel, T.J.; Zhu, A.; Akula, A.V.; John Boscardin, W.; Neeb, H.; Henry, R.G.; et al. MWF of the corpus callosum is a robust measure of remyelination: Results from the ReBUILD trial. Proc. Natl. Acad. Sci. USA 2023, 120, e2217635120. [Google Scholar] [CrossRef]
- Trapp, B.D.; Nave, K.-A. Multiple Sclerosis: An Immune or Neurodegenerative Disorder? Annu. Rev. Neurosci. 2008, 31, 247–269. [Google Scholar] [CrossRef]
- Chen, J.T.; Easley, K.; Schneider, C.; Nakamura, K.; Kidd, G.J.; Chang, A.; Staugaitis, S.M.; Fox, R.J.; Fisher, E.; Arnold, D.L.; et al. Clinically feasible MTR is sensitive to cortical demyelination in MS. Neurology 2013, 80, 246–252. [Google Scholar] [CrossRef]
- Lazzarotto, A.; Hamzaoui, M.; Tonietto, M.; Dubessy, A.L.; Khalil, M.; Pirpamer, L.; Ropele, S.; Enzinger, C.; Battaglini, M.; Stromillo, M.L.; et al. Time is myelin: Early cortical myelin repair prevents atrophy and clinical progression in multiple sclerosis. Brain 2024, 147, 1331–1343. [Google Scholar] [CrossRef]
- Kornek, B.; Storch, M.K.; Weissert, R.; Wallstroem, E.; Stefferl, A.; Olsson, T.; Linington, C.; Schmidbauer, M.; Lassmann, H. Multiple sclerosis and chronic autoimmune encephalomyelitis: A comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. Am. J. Pathol. 2000, 157, 267–276. [Google Scholar] [CrossRef]
- Fyfe, I. Remyelination stops degeneration in MS. Nat. Rev. Neurol. 2022, 18, 187. [Google Scholar] [CrossRef]
- Ricigliano, V.A.G.; Tonietto, M.; Hamzaoui, M.; Poirion, É.; Lazzarotto, A.; Bottlaender, M.; Gervais, P.; Maillart, E.; Stankoff, B.; Bodini, B. Spontaneous remyelination in lesions protects the integrity of surrounding tissues over time in multiple sclerosis. Eur. J. Neurol. 2022, 29, 1719–1729. [Google Scholar] [CrossRef]
- Tonietto, M.; Poirion, E.; Lazzarotto, A.; Ricigliano, V.; Papeix, C.; Bottlaender, M.; Bodini, B.; Stankoff, B. Periventricular remyelination failure in multiple sclerosis: A substrate for neurodegeneration. Brain 2023, 146, 182–194. [Google Scholar] [CrossRef]
- Miron, V.E.; Boyd, A.; Zhao, J.W.; Yuen, T.J.; Ruckh, J.M.; Shadrach, J.L.; van Wijngaarden, P.; Wagers, A.J.; Williams, A.; Franklin, R.J.M.; et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat. Neurosci. 2013, 16, 1211–1218. [Google Scholar] [CrossRef]
- Hamzaoui, M.; Garcia, J.; Boffa, G.; Lazzarotto, A.; Absinta, M.; Ricigliano, V.A.G.; Soulier, T.; Tonietto, M.; Gervais, P.; Bissery, A.; et al. Positron Emission Tomography with [18F]-DPA-714 Unveils a Smoldering Component in Most Multiple Sclerosis Lesions which Drives Disease Progression. Ann. Neurol. 2023, 94, 366–383. [Google Scholar] [CrossRef]
- Poirion, E.; Tonietto, M.; Lejeune, F.X.; Ricigliano, V.A.G.; Boudot de la Motte, M.; Benoit, C.; Bera, G.; Kuhnast, B.; Bottlaender, M.; Bodini, B.; et al. Structural and Clinical Correlates of a Periventricular Gradient of Neuroinflammation in Multiple Sclerosis. Neurology 2021, 96, e1865–e1875. [Google Scholar] [CrossRef]
- Ricigliano, V.A.G.; Morena, E.; Colombi, A.; Tonietto, M.; Hamzaoui, M.; Poirion, E.; Bottlaender, M.; Gervais, P.; Louapre, C.; Bodini, B.; et al. Choroid Plexus Enlargement in Inflammatory Multiple Sclerosis: 3.0-T MRI and Translocator Protein PET Evaluation. Radiology 2021, 301, 166–177. [Google Scholar] [CrossRef]
- Ricigliano, V.A.G.; Louapre, C.; Poirion, E.; Colombi, A.; Yazdan Panah, A.; Lazzarotto, A.; Morena, E.; Martin, E.; Bottlaender, M.; Bodini, B.; et al. Imaging Characteristics of Choroid Plexuses in Presymptomatic Multiple Sclerosis: A Retrospective Study. Neurol. Neuroimmunol. Neuroinflamm. 2022, 9, e200026. [Google Scholar] [CrossRef]
- Ricigliano, V.A.G.; Stankoff, B. Choroid plexuses at the interface of peripheral immunity and tissue repair in multiple sclerosis. Curr. Opin. Neurol. 2023, 36, 214–221. [Google Scholar] [CrossRef]
- Stellmann, J.P.; Maarouf, A.; Schulz, K.H.; Baquet, L.; Pöttgen, J.; Patra, S.; Penner, I.K.; Gellißen, S.; Ketels, G.; Besson, P.; et al. Aerobic Exercise Induces Functional and Structural Reorganization of CNS Networks in Multiple Sclerosis: A Randomized Controlled Trial. Front. Hum. Neurosci. 2020, 14, 255. [Google Scholar] [CrossRef]
- Bučková, B.; Kopal, J.; Řasová, K.; Tintěra, J.; Hlinka, J. Open Access: The Effect of Neurorehabilitation on Multiple Sclerosis-Unlocking the Resting-State fMRI Data. Front. Neurosci. 2021, 15, 662784. [Google Scholar] [CrossRef] [PubMed]
- Sîrbu, C.A.; Thompson, D.C.; Plesa, F.C.; Vasile, T.M.; Jianu, D.C.; Mitrica, M.; Anghel, D.; Stefani, C. Neurorehabilitation in Multiple Sclerosis—A Review of Present Approaches and Future Considerations. J. Clin. Med. 2022, 11, 7003. [Google Scholar] [CrossRef] [PubMed]
- Chard, D.T.; Alahmadi, A.A.S.; Audoin, B.; Charalambous, T.; Enzinger, C.; Hulst, H.E.; Rocca, M.A.; Rovira, À.; Sastre-Garriga, J.; Schoonheim, M.M.; et al. Mind the gap: From neurons to networks to outcomes in multiple sclerosis. Nat. Rev. Neurol. 2021, 17, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Ciccarelli, O.; Cohen, J.A.; Reingold, S.C.; Weinshenker, B.G. Spinal cord involvement in multiple sclerosis and neuromyelitis optica spectrum disorders. Lancet Neurol. 2019, 18, 185–197. [Google Scholar] [CrossRef]
- Combes, A.J.E.; Clarke, M.A.; O’Grady, K.P.; Schilling, K.G.; Smith, S.A. Advanced spinal cord MRI in multiple sclerosis: Current techniques and future directions. Neuroimage Clin. 2022, 36, 103244. [Google Scholar] [CrossRef]
- Brownlee, W.J.; Altmann, D.R.; Prados, F.; Miszkiel, K.A.; Eshaghi, A.; Gandini Wheeler-Kingshott, C.A.M.; Barkhof, F.; Ciccarelli, O. Early imaging predictors of long-term outcomes in relapse-onset multiple sclerosis. Brain 2019, 142, 2276–2287. [Google Scholar] [CrossRef]
- Sacks, D.; Baxter, B.; Campbell, B.C.V.; Carpenter, J.S.; Cognard, C.; Dippel, D.; Eesa, M.; Fischer, U.; Hausegger, K.; Hirsch, J.A.; et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int. J. Stroke 2018, 13, 612–632. [Google Scholar] [CrossRef]
- Dvorak, A.V.; Ljungberg, E.; Vavasour, I.M.; Liu, H.; Johnson, P.; Rauscher, A.; Kramer, J.L.K.; Tam, R.; Li, D.K.B.; Laule, C.; et al. Rapid myelin water imaging for the assessment of cervical spinal cord myelin damage. Neuroimage Clin. 2019, 23, 101896. [Google Scholar] [CrossRef]
- Granziera, C.; Wuerfel, J.; Barkhof, F.; Calabrese, M.; De Stefano, N.; Enzinger, C.; Evangelou, N.; Filippi, M.; Geurts, J.J.G.; Reich, D.S.; et al. Quantitative magnetic resonance imaging towards clinical application in multiple sclerosis. Brain 2021, 144, 1296–1311. [Google Scholar] [CrossRef]
- Lévy, S.; Guertin, M.C.; Khatibi, A.; Mezer, A.; Martinu, K.; Chen, J.I.; Stikov, N.; Rainville, P.; Cohen-Adad, J. Test-retest reliability of myelin imaging in the human spinal cord: Measurement errors versus region- and aging-induced variations. PLoS ONE 2018, 13, e0189944. [Google Scholar] [CrossRef]
- Combès, B.; Monteau, L.; Bannier, E.; Callot, V.; Labauge, P.; Ayrignac, X.; Carra Dallière, C.; Pelletier, J.; Maarouf, A.; de Seze, J.; et al. Measurement of magnetization transfer ratio (MTR) from cervical spinal cord: Multicenter reproducibility and variability. J. Magn. Reson. Imaging 2019, 49, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.A.; Narayanan, S.; Arnold, D.L. Segmentation of magnetization transfer ratio lesions for longitudinal analysis of demyelination and remyelination in multiple sclerosis. Neuroimage 2013, 66, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Gaubert, M.; Combès, B.; Bannier, E.; Masson, A.; Caron, V.; Baudron, G.; Ferré, J.C.; Michel, L.; Le Page, E.; Stankoff, B.; et al. Microstructural Damage and Repair in the Spinal Cord of Patients With Early Multiple Sclerosis and Association With Disability at 5 Years. Neurol. Neuroimmunol. Neuroinflamm. 2025, 12, e200333. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.A.; Pareto, D.; Pessini-Ferreira, L.; Arrambide, G.; Alberich, M.; Crescenzo, F.; Cappelle, S.; Tintoré, M.; Sastre-Garriga, J.; Auger, C.; et al. Value of 3T Susceptibility-Weighted Imaging in the Diagnosis of Multiple Sclerosis. AJNR Am. J. Neuroradiol. 2020, 41, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Gros, C.; De Leener, B.; Badji, A.; Maranzano, J.; Eden, D.; Dupont, S.M.; Talbott, J.; Zhuoquiong, R.; Liu, Y.; Granberg, T.; et al. Automatic segmentation of the spinal cord and intramedullary multiple sclerosis lesions with convolutional neural networks. Neuroimage 2019, 184, 901–915. [Google Scholar] [CrossRef]
- Heidari, M.; Radcliff, A.B.; McLellan, G.J.; Ver Hoeve, J.N.; Chan, K.; Kiland, J.A.; Keuler, N.S.; August, B.K.; Sebo, D.; Field, A.S.; et al. Evoked potentials as a biomarker of remyelination. Proc. Natl. Acad. Sci. USA 2019, 116, 27074–27083. [Google Scholar] [CrossRef]
- Kerbrat, A.; Gros, C.; Badji, A.; Bannier, E.; Galassi, F.; Combès, B.; Chouteau, R.; Labauge, P.; Ayrignac, X.; Carra-Dalliere, C.; et al. Multiple sclerosis lesions in motor tracts from brain to cervical cord: Spatial distribution and correlation with disability. Brain 2020, 143, 2089–2105. [Google Scholar] [CrossRef]
- Pallix-Guyot, M.; Guennoc, A.M.; Blasco, H.; de Toffol, B.; Corcia, P.; Praline, J. Predictive value of motor evoked potentials in clinically isolated syndrome. Acta Neurol. Scand. 2011, 124, 410–416. [Google Scholar] [CrossRef]
- Schlaeger, R.; D’Souza, M.; Schindler, C.; Grize, L.; Kappos, L.; Fuhr, P. Prediction of MS disability by multimodal evoked potentials: Investigation during relapse or in the relapse-free interval? Clin. Neurophysiol. 2014, 125, 1889–1892. [Google Scholar] [CrossRef]
- Hardmeier, M.; Jacques, F.; Albrecht, P.; Bousleiman, H.; Schindler, C.; Leocani, L.; Fuhr, P. Multicentre assessment of motor and sensory evoked potentials in multiple sclerosis: Reliability and implications for clinical trials. Mult. Scler. J. Exp. Transl. Clin. 2019, 5, 2055217319844796. [Google Scholar] [CrossRef]
- Wang, Y.; Kyauk, R.V.; Shen, Y.A.; Xie, L.; Reichelt, M.; Lin, H.; Jiang, Z.; Ngu, H.; Shen, K.; Greene, J.J.; et al. TREM2-dependent microglial function is essential for remyelination and subsequent neuroprotection. Glia 2023, 71, 1247–1258. [Google Scholar] [CrossRef]
- Cignarella, F.; Filipello, F.; Bollman, B.; Cantoni, C.; Locca, A.; Mikesell, R.; Manis, M.; Ibrahim, A.; Deng, L.; Benitez, B.A.; et al. TREM2 activation on microglia promotes myelin debris clearance and remyelination in a model of multiple sclerosis. Acta Neuropathol. 2020, 140, 513–534. [Google Scholar] [CrossRef] [PubMed]
- Azzolini, F.; Gilio, L.; Pavone, L.; Iezzi, E.; Dolcetti, E.; Bruno, A.; Buttari, F.; Musella, A.; Mandolesi, G.; Guadalupi, L.; et al. Neuroinflammation Is Associated with GFAP and sTREM2 Levels in Multiple Sclerosis. Biomolecules 2022, 12, 222. [Google Scholar] [CrossRef] [PubMed]
- Cross, A.H.; Gelfand, J.M.; Thebault, S.; Bennett, J.L.; von Büdingen, H.C.; Cameron, B.; Carruthers, R.; Edwards, K.; Fallis, R.; Gerstein, R.; et al. Emerging Cerebrospinal Fluid Biomarkers of Disease Activity and Progression in Multiple Sclerosis. JAMA Neurol. 2024, 81, 373–383. [Google Scholar] [CrossRef]
- Öhrfelt, A.; Axelsson, M.; Malmeström, C.; Novakova, L.; Heslegrave, A.; Blennow, K.; Lycke, J.; Zetterberg, H. Soluble TREM-2 in cerebrospinal fluid from patients with multiple sclerosis treated with natalizumab or mitoxantrone. Mult. Scler. 2016, 22, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Burman, J.; Zetterberg, H.; Fransson, M.; Loskog, A.S.; Raininko, R.; Fagius, J. Assessing tissue damage in multiple sclerosis: A biomarker approach. Acta Neurol. Scand. 2014, 130, 81–89. [Google Scholar] [CrossRef]
- Zjukovskaja, C.; Larsson, A.; Cherif, H.; Kultima, K.; Burman, J. Biomarkers of demyelination and axonal damage are decreased after autologous hematopoietic stem cell transplantation for multiple sclerosis. Mult. Scler. Relat. Disord. 2022, 68, 104210. [Google Scholar] [CrossRef]
- Péter, M.; Török, W.; Petrovics-Balog, A.; Vígh, L.; Vécsei, L.; Balogh, G. Cerebrospinal fluid lipidomic biomarker signatures of demyelination for multiple sclerosis and Guillain-Barré syndrome. Sci. Rep. 2020, 10, 18380. [Google Scholar] [CrossRef]
- Maciak, K.; Dziedzic, A.; Saluk, J. Remyelination in multiple sclerosis from the miRNA perspective. Front. Mol. Neurosci. 2023, 16, 1199313. [Google Scholar] [CrossRef]
- Kornfeld, S.F.; Cummings, S.E.; Yaworski, R.; De Repentigny, Y.; Gagnon, S.; Zandee, S.; Fathi, S.; Prat, A.; Kothary, R. Loss of miR-145 promotes remyelination and functional recovery in a model of chronic central demyelination. Commun. Biol. 2024, 7, 813. [Google Scholar] [CrossRef]
- Gross, C.C.; Schulte-Mecklenbeck, A.; Steinberg, O.V.; Wirth, T.; Lauks, S.; Bittner, S.; Schindler, P.; Baranzini, S.E.; Groppa, S.; Bellmann-Strobl, J.; et al. Multiple sclerosis endophenotypes identified by high-dimensional blood signatures are associated with distinct disease trajectories. Sci. Transl. Med. 2024, 16, eade8560. [Google Scholar] [CrossRef] [PubMed]
- Barbour, C.; Kosa, P.; Komori, M.; Tanigawa, M.; Masvekar, R.; Wu, T.; Johnson, K.; Douvaras, P.; Fossati, V.; Herbst, R.; et al. Molecular-based diagnosis of multiple sclerosis and its progressive stage. Ann. Neurol. 2017, 82, 795–812. [Google Scholar] [CrossRef] [PubMed]
- Boulant, N.; Mauconduit, F.; Gras, V.; Amadon, A.; Le Ster, C.; Luong, M.; Massire, A.; Pallier, C.; Sabatier, L.; Bottlaender, M.; et al. In vivo imaging of the human brain with the Iseult 11.7-T MRI scanner. Nat. Methods 2024, 21, 2013–2016. [Google Scholar] [CrossRef] [PubMed]
| Explored Organ/ Compartment | Tool | Biomarker |
|---|---|---|
| Brain | MRI | MWF, NODDI, RD, MTR, QSM, functional connectivity (matrix, distant and local connectivity density) |
| PET | Voxel-based maps of myelin content change | |
| Brain and spinal cord | MEP/SSEP | latency, amplitude |
| Biosensors | Digital markers (e.g., walking speed, manual dexterity, balance) | |
| Spinal cord | MRI | MTR |
| Visual system | VEP | latency, amplitude |
| OCT | RNFL, GCL, IPL thickness | |
| Oculography | versional dysconjugacy index | |
| CSF | ELISA, proteomic analysis, mass spectrometry, quantitative PCR | sTREM2, MBP, lipidome, miR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricigliano, V.A.G.; Marenna, S.; Borrelli, S.; Camera, V.; Carnero Contentti, E.; Szejko, N.; Bakirtzis, C.; Gluscevic, S.; Samadzadeh, S.; Hartung, H.-P.; et al. Identifying Biomarkers for Remyelination and Recovery in Multiple Sclerosis: A Measure of Progress. Biomedicines 2025, 13, 357. https://doi.org/10.3390/biomedicines13020357
Ricigliano VAG, Marenna S, Borrelli S, Camera V, Carnero Contentti E, Szejko N, Bakirtzis C, Gluscevic S, Samadzadeh S, Hartung H-P, et al. Identifying Biomarkers for Remyelination and Recovery in Multiple Sclerosis: A Measure of Progress. Biomedicines. 2025; 13(2):357. https://doi.org/10.3390/biomedicines13020357
Chicago/Turabian StyleRicigliano, Vito A. G., Silvia Marenna, Serena Borrelli, Valentina Camera, Edgar Carnero Contentti, Natalia Szejko, Christos Bakirtzis, Sanja Gluscevic, Sara Samadzadeh, Hans-Peter Hartung, and et al. 2025. "Identifying Biomarkers for Remyelination and Recovery in Multiple Sclerosis: A Measure of Progress" Biomedicines 13, no. 2: 357. https://doi.org/10.3390/biomedicines13020357
APA StyleRicigliano, V. A. G., Marenna, S., Borrelli, S., Camera, V., Carnero Contentti, E., Szejko, N., Bakirtzis, C., Gluscevic, S., Samadzadeh, S., Hartung, H.-P., Selmaj, K., Stankoff, B., Comi, G., & ECF Young Investigators/Fellows Initiative. (2025). Identifying Biomarkers for Remyelination and Recovery in Multiple Sclerosis: A Measure of Progress. Biomedicines, 13(2), 357. https://doi.org/10.3390/biomedicines13020357








