Effects of Sildenafil on Cognitive Function Recovery and Neuronal Cell Death Protection after Transient Global Cerebral Ischemia in Gerbils
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Induction of Transient Global Cerebral Ischemia
2.3. Drug Administration
2.4. Behavioral Tests
2.4.1. Open-Field Test
2.4.2. Novel Object Recognition
2.4.3. Barnes Maze
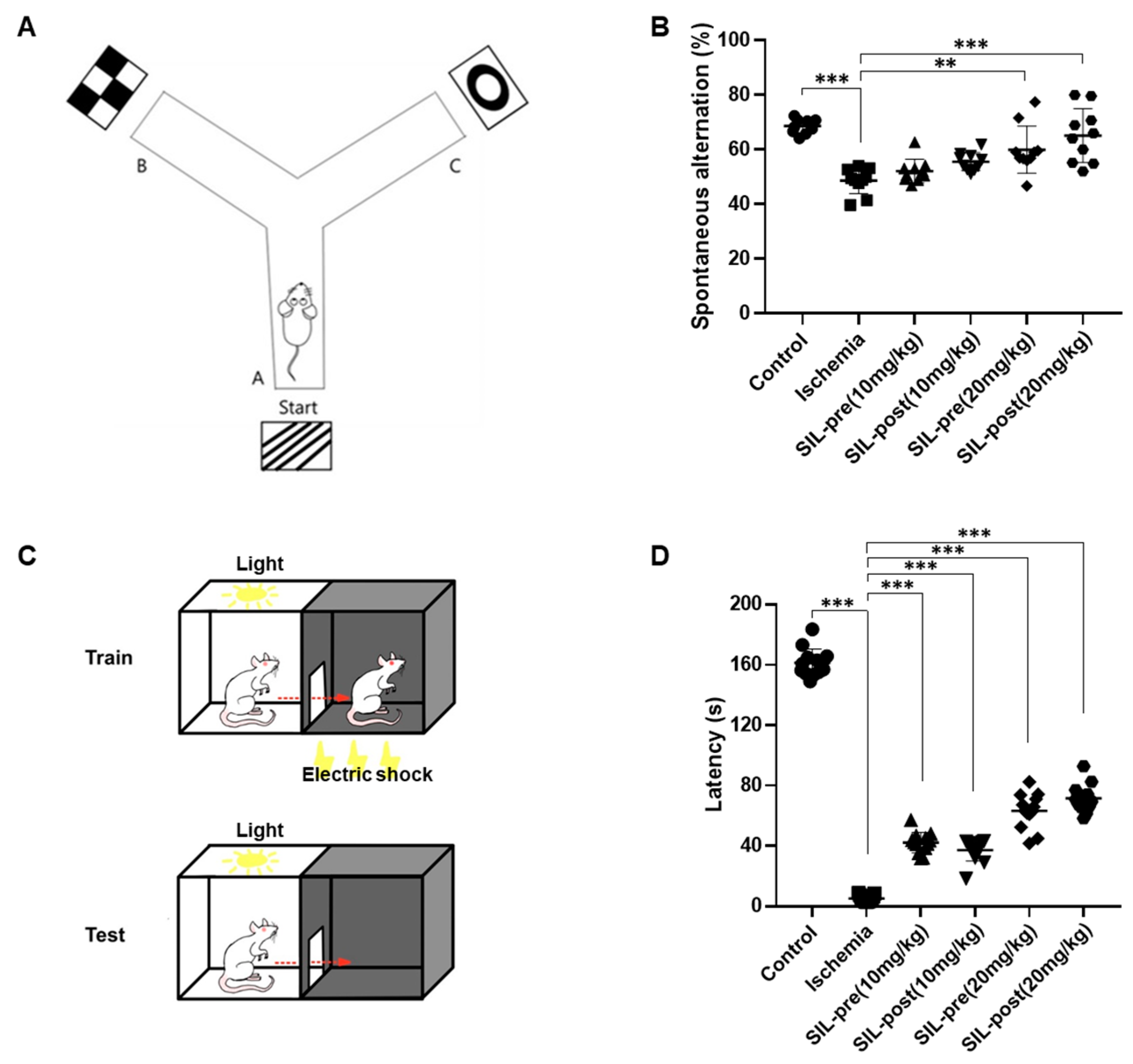
2.4.4. Y-Maze
2.4.5. Passive Avoidance
2.5. Tissue Processing and Cresyl Violet Staining
2.6. Double Immunofluorescence
2.7. Quantification of Data and Statistical Analysis
3. Results
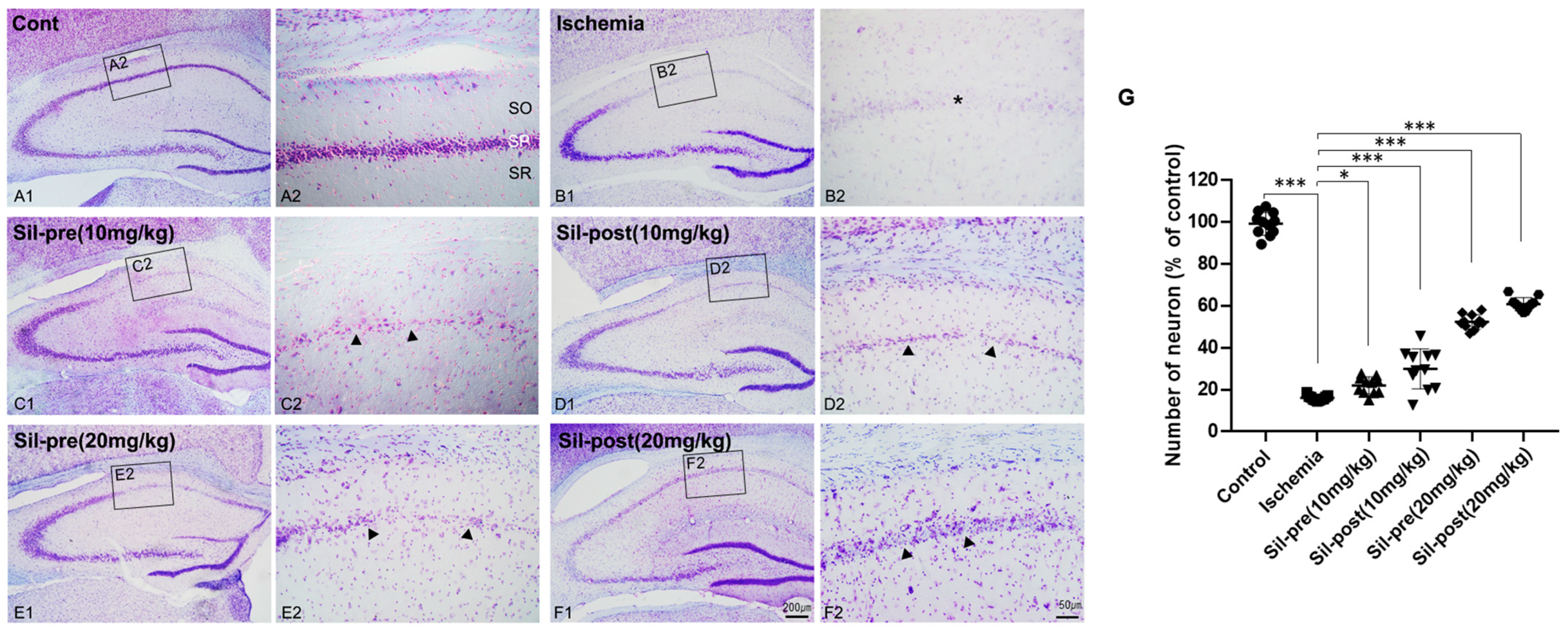
3.1. Neuroprotective Effects of Sildenafil on Neuronal Cell Death Following Transient Global Cerebral Ischemia-Reperfusion
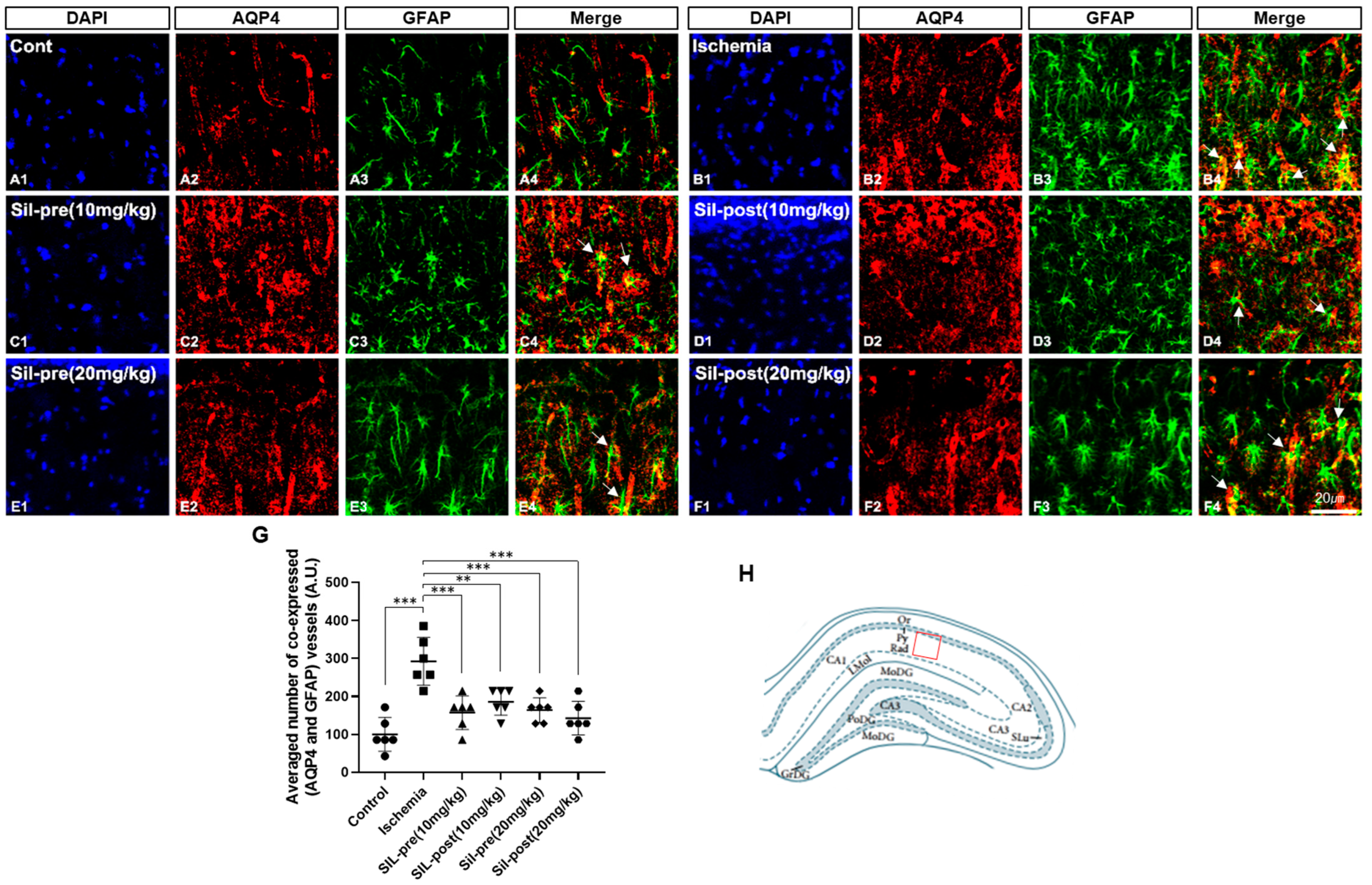
3.2. Effects of Sildenafil on AQP4 and GFAP Expression Following Transient Global Cerebral Ischemia-Reperfusion
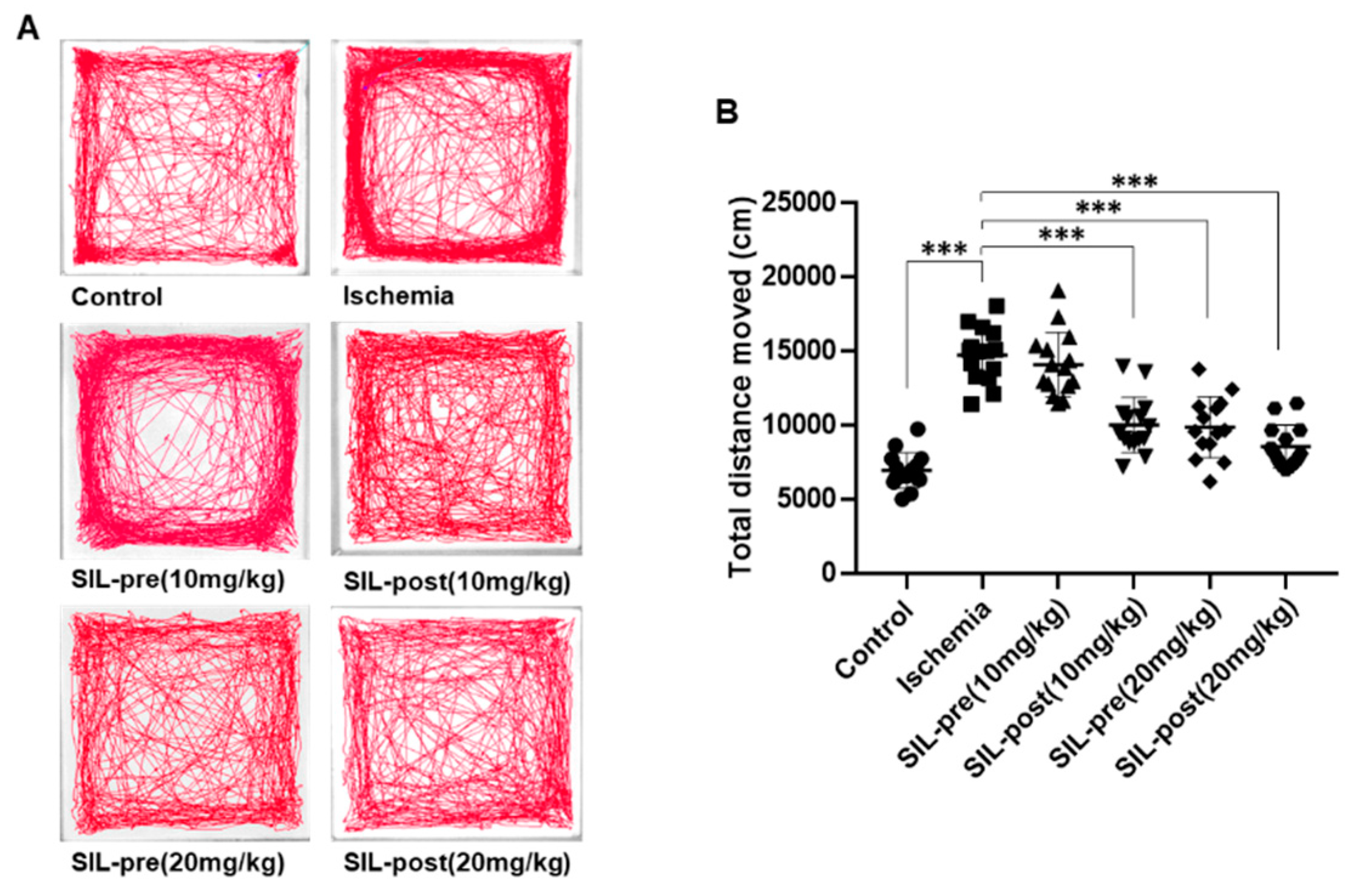
3.3. Hyper-Locomotion Activity Following isChemia in Gerbils Treated with Sildenafil
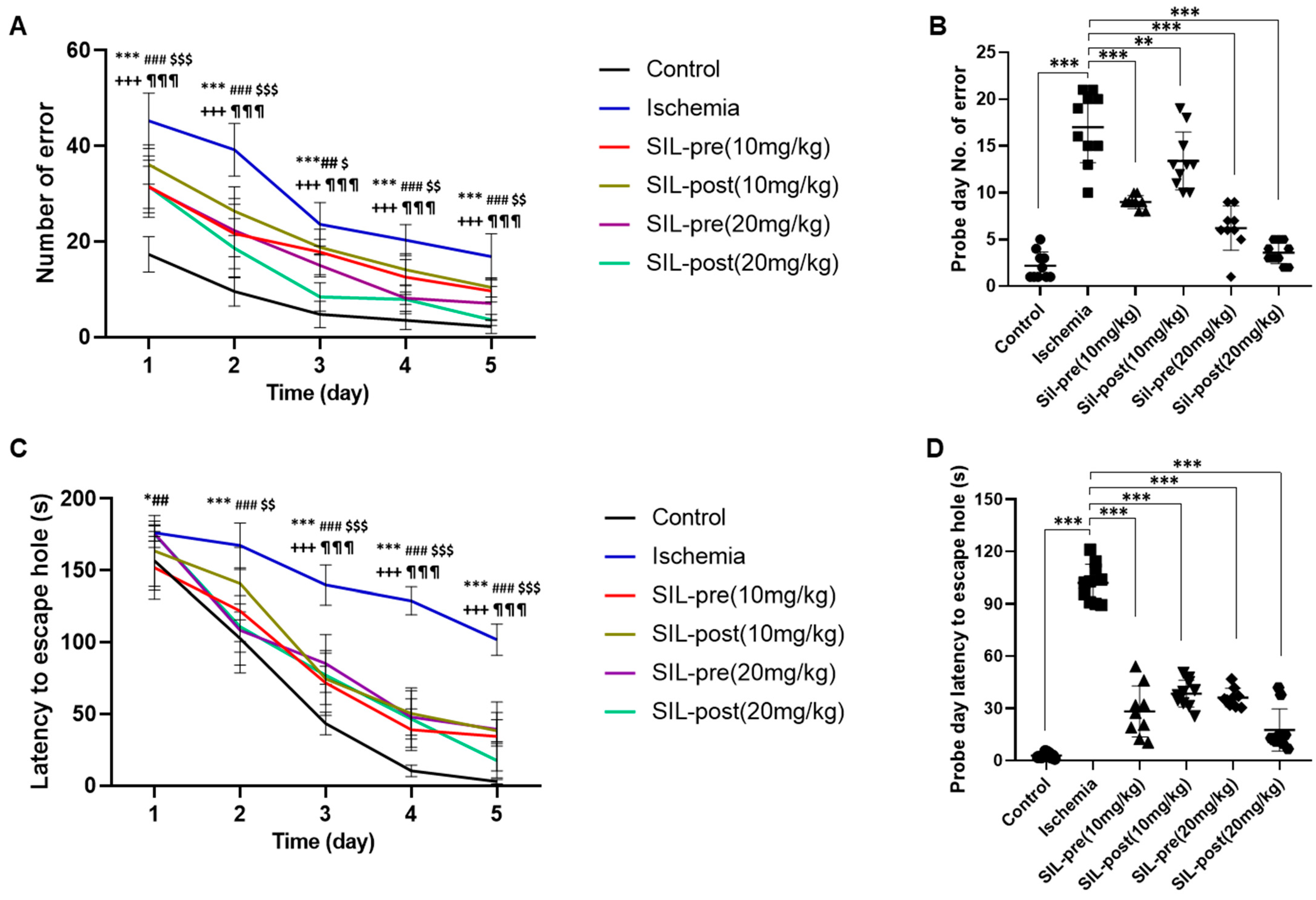
3.4. Sildenafil Treatment Alleviates Deficits in Cognitive Behavior Following Ischemia in Gerbils
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Katan, M.; Luft, A. Global Burden of Stroke. Semin. Neurol. 2018, 38, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, M.; Reddy, P.H. Peripheral Biomarkers of Stroke: Focus on Circulatory MicroRNAs. Biochim. Biophys. Acta-Mol. Basis Dis. 2016, 1862, 1984–1993. [Google Scholar] [CrossRef] [PubMed]
- Motawi, T.K.; Sadik, N.A.H.; Shaker, O.G.; Ghaleb, M.M.H.; Elbaz, E.M. Expression, Functional Polymorphism, and Diagnostic Values of MIAT Rs2331291 and H19 Rs217727 Long Non-Coding RNAs in Cerebral Ischemic Stroke Egyptian Patients. Int. J. Mol. Sci. 2024, 25, 842. [Google Scholar] [CrossRef]
- Nagakannan, P.; Shivasharan, B.D.; Thippeswamy, B.S.; Veerapur, V.P.; Bansal, P. Protective Effect of Hydroalcoholic Extract of Mimusops Elengi Linn. Flowers against Middle Cerebral Artery Occlusion Induced Brain Injury in Rats. J. Ethnopharmacol. 2012, 140, 247–254. [Google Scholar] [CrossRef]
- Leys, D.; Hénon, H.; Mackowiak-Cordoliani, M.A.; Pasquier, F. Poststroke Dementia. Lancet Neurol. 2005, 4, 752–759. [Google Scholar] [CrossRef]
- Román, G.C.; Tatemichi, T.K.; Erkinjuntti, T.; Cummings, J.L.; Masdeu, J.C.; Garcia, J.H.; Amaducci, L.; Orgogozo, J.M.; Brun, A.; Hofman, A.; et al. Vascular Dementia: Diagnostic Criteria for Research Studies—Report of the NINDS-AIREN International Workshop. Neurology 1993, 43, 250–260. [Google Scholar] [CrossRef]
- Rost, N.S.; Brodtmann, A.; Pase, M.P.; van Veluw, S.J.; Biffi, A.; Duering, M.; Hinman, J.D.; Dichgans, M. Post-Stroke Cognitive Impairment and Dementia. Circ. Res. 2022, 130, 1252–1271. [Google Scholar] [CrossRef]
- Miller, E.L.; Murray, L.; Richards, L.; Zorowitz, R.D.; Bakas, T.; Clark, P.; Billinger, S.A. Comprehensive Overview of Nursing and Interdisciplinary Rehabilitation Care of the Stroke Patient: A Scientific Statement from the American Heart Association. Stroke 2010, 41, 2402–2448. [Google Scholar] [CrossRef] [PubMed]
- Teasell, R.; Hussein, N. Clinical Consequences of Stroke. In Evidence Reviews; Evidence-Based Review of Stroke Rehabilitation: London, ON, Canada, 2018; Volume 1, pp. 1–29. [Google Scholar]
- Aqueveque, P.; Ortega, P.; Pino, E.; Saavedra, F.; Germany, E.; Gómez, B. After Stroke Movement Impairments: A Review of Current Technologies for Rehabilitation. In Physical Disabilities—Therapeutic Implications; Intechopen: Rijeka, Croatia, 2017. [Google Scholar]
- Hewetson, R.; Cornwell, P.; Shum, D. Social Participation Following Right Hemisphere Stroke: Influence of a Cognitive-Communication Disorder. Aphasiology 2018, 32, 164–182. [Google Scholar] [CrossRef]
- Sun, J.H.; Tan, L.; Yu, J.T. Post-Stroke Cognitive Impairment: Epidemiology, Mechanisms and Management. Ann. Transl. Med. 2014, 2, 80. [Google Scholar]
- Fure, B.; Wyller, T.B.; Engedal, K.; Thommessen, B. Emotional Symptoms in Acute Ischemic Stroke. Int. J. Geriatr. Psychiatry 2006, 21, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Ferro, J.M.; Caeiro, L.; Figueira, M.L. Neuropsychiatric Sequelae of Stroke. Nat. Rev. Neurol. 2016, 12, 269–280. [Google Scholar] [CrossRef]
- Wechsler, L.R. Intravenous Thrombolytic Therapy for Acute Ischemic Stroke. N. Engl. J. Med. 2011, 364, 2138–2146. [Google Scholar] [CrossRef]
- O’Collins, V.E.; Macleod, M.R.; Donnan, G.A.; Horky, L.L.; Van Der Worp, B.H.; Howells, D.W. 1,026 Experimental Treatments in Acute Stroke. Ann. Neurol. 2006, 59, 467–477. [Google Scholar] [CrossRef]
- Andersson, K.E. PDE5 Inhibitors—Pharmacology and Clinical Applications 20 Years after Sildenafil Discovery. Br. J. Pharmacol. 2018, 175, 2554–2565. [Google Scholar] [CrossRef]
- Rutten, K.; De Vente, J.; Şik, A.; Markerink-Van Ittersum, M.; Prickaerts, J.; Blokland, A. The Selective PDE5 Inhibitor, Sildenafil, Improves Object Memory in Swiss Mice and Increases CGMP Levels in Hippocampal Slices. Behav. Brain Res. 2005, 164, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Moretti, R.; Leger, P.L.; Besson, V.C.; Csaba, Z.; Pansiot, J.; Di Criscio, L.; Gentili, A.; Titomanlio, L.; Bonnin, P.; Baud, O.; et al. Sildenafil, a Cyclic GMP Phosphodiesterase Inhibitor, Induces Microglial Modulation after Focal Ischemia in the Neonatal Mouse Brain. J. Neuroinflamm. 2016, 13, 95. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jiang, Q.; Zhang, L.; Ding, G.; Zhang, Z.G.; Li, Q.; Ewing, J.R.; Lu, M.; Panda, S.; Ledbetter, K.A.; et al. Angiogenesis and Improved Cerebral Blood Flow in the Ischemic Boundary Area Detected by MRI after Administration of Sildenafil to Rats with Embolic Stroke. Brain Res. 2007, 1132, 185–192. [Google Scholar] [CrossRef][Green Version]
- de Visser, Y.P.; Walther, F.J.; Laghmani, E.H.; Boersma, H.; van der Laarse, A.; Wagenaar, G.T.M. Sildenafil Attenuates Pulmonary Inflammation and Fibrin Deposition, Mortality and Right Ventricular Hypertrophy in Neonatal Hyperoxic Lung Injury. Respir. Res. 2009, 10, 30. [Google Scholar] [CrossRef]
- Cuadrado-Tejedor, M.; Hervias, I.; Ricobaraza, A.; Puerta, E.; Pérez-Roldán, J.M.; García-Barroso, C.; Franco, R.; Aguirre, N.; García-Osta, A. Sildenafil Restores Cognitive Function without Affecting β-Amyloid Burden in a Mouse Model of Alzheimer’s Disease. Br. J. Pharmacol. 2011, 164, 2029–2041. [Google Scholar] [CrossRef]
- Xiong, Y.; Wintermark, P. The Role of Sildenafil in Treating Brain Injuries in Adults and Neonates. Front. Cell. Neurosci. 2022, 16, 879649. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.H.; Kim, S.W.; Kang, J.; Song, Y.; Im, H.; Kim, S.J.; Yoo, D.Y.; Lee, M.R.; Park, D.K.; Oh, J.S.; et al. Phosphodiesterase-5 Inhibitor Attenuates Anxious Phenotypes and Movement Disorder Induced by Mild Ischemic Stroke in Rats. J. Korean Neurosurg. Soc. 2022, 65, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Martínez, N.S.; Machado, J.M.; Saad, H.P.; Antich, R.M.C.; Acosta, J.A.B.; Salgueiro, S.R.; Illera, G.G.; Alba, J.S.; del Barco Herrera, D.G. Global Brain Ischemia in Mongolian Gerbils: Assessing the Level of Anastomosis in the Cerebral Circle of Willis. Acta Neurobiol. Exp. 2012, 72, 377–384. [Google Scholar]
- Delbarre, G.; Delbarre, B.; Barrau, Y. A Suitable Method to Select Gerbils with Incomplete Circle of Willis: To the Editor. Stroke 1988, 19, 126. [Google Scholar] [CrossRef] [PubMed]
- Katsuta, K.; Umemura, K.; Ueyama, N.; Matsuoka, N. Pharmacological Evidence for a Correlation between Hippocampal CA1 Cell Damage and Hyperlocomotion Following Global Cerebral Ischemia in Gerbils. Eur. J. Pharmacol. 2003, 467, 103–109. [Google Scholar] [CrossRef]
- Charriaut-Marlangue, C.; Nguyen, T.; Bonnin, P.; Duy, A.P.; Leger, P.L.; Csaba, Z.; Pansiot, J.; Bourgeois, T.; Renolleau, S.; Baud, O. Sildenafil Mediates Blood-Flow Redistribution and Neuroprotection after Neonatal Hypoxia-Ischemia. Stroke 2014, 45, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Jacob, S. A Simple Practice Guide for Dose Conversion between Animals and Human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Ritzel, R.M.; Patel, A.R.; Spychala, M.; Verma, R.; Crapser, J.; Koellhoffer, E.C.; Schrecengost, A.; Jellison, E.R.; Zhu, L.; Venna, V.R.; et al. Multiparity Improves Outcomes after Cerebral Ischemia in Female Mice despite Features of Increased Metabovascular Risk. Proc. Natl. Acad. Sci. USA 2017, 114, E5673–E5682. [Google Scholar] [CrossRef]
- Tóthová, Ľ.; Bábíčková, J.; Borbélyová, V.; Filová, B.; Šebeková, K.; Hodosy, J. Chronic Renal Insufficiency Does Not Induce Behavioral and Cognitive Alteration in Rats. Physiol. Behav. 2015, 138, 133–140. [Google Scholar] [CrossRef]
- Gangadharan, G.; Shin, J.; Kim, S.W.; Kim, A.; Paydar, A.; Kim, D.S.; Miyazaki, T.; Watanabe, M.; Yanagawa, Y.; Kim, J.; et al. Medial Septal GABAergic Projection Neurons Promote Object Exploration Behavior and Type 2 Theta Rhythm. Proc. Natl. Acad. Sci. USA 2016, 113, 6550–6555. [Google Scholar] [CrossRef]
- Wappler, E.A.; Szilágyi, G.; Gál, A.; Skopál, J.; Nyakas, C.; Nagy, Z.; Felszeghy, K. Adopted Cognitive Tests for Gerbils: Validation by Studying Ageing and Ischemia. Physiol. Behav. 2009, 97, 107–114. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, T.P.; Brown, R.E. The Effects of Apparatus Design and Test Procedure on Learning and Memory Performance of C57BL/6J Mice on the Barnes Maze. J. Neurosci. Methods 2012, 203, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Parent, J.M.; Elliott, R.C.; Pleasure, S.J.; Barbaro, N.M.; Lowenstein, D.H. Aberrant Seizure-Induced Neurogenesis in Experimental Temporal Lobe Epilepsy. Ann. Neurol. 2006, 59, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Nasehi, M.; Piri, M.; Nouri, M.; Farzin, D.; Nayer-Nouri, T.; Zarrindast, M.R. Involvement of Dopamine D1/D2 Receptors on Harmane-Induced Amnesia in the Step-down Passive Avoidance Test. Eur. J. Pharmacol. 2010, 634, 77–83. [Google Scholar] [CrossRef]
- Yu, Y.H.; Kim, S.W.; Im, H.; Oh, S.W.; Cho, N.J.; Park, S.; Park, D.K.; Kim, D.S.; Gil, H.W. Cognitive Sequelae and Hippocampal Dysfunction in Chronic Kidney Disease Following 5/6 Nephrectomy. Brain Sci. 2022, 12, 905. [Google Scholar] [CrossRef]
- Adebayo, A.A.; Oboh, G.; Ademosun, A.O. Almond-Supplemented Diet Improves Sexual Functions beyond Phosphodiesterase-5 Inhibition in Diabetic Male Rats. Heliyon 2019, 5, e03035. [Google Scholar] [CrossRef]
- Wu, C.; Huang, L.; Liou, C.; Wang, T.; Tung, Y.; Hsu, H.-Y.; Lai, M.-C. Lithium-Pilocarpine-Induced Status Epilepticus in Immature Rats Result in Long-Term Deficits in Spatial Learning and Hippocampal Cell Loss. Neurosci. Lett. 2001, 312, 113–117. [Google Scholar] [CrossRef]
- Zinni, M.; Pansiot, J.; Léger, P.L.; El Kamouh, M.; Baud, O. Sildenafil-Mediated Neuroprotection from Adult to Neonatal Brain Injury: Evidence, Mechanisms, and Future Translation. Cells 2021, 10, 2766. [Google Scholar] [CrossRef] [PubMed]
- Vella, J.; Zammit, C.; Di Giovanni, G.; Muscat, R.; Valentino, M. The Central Role of Aquaporins in the Pathophysiology of Ischemic Stroke. Front. Cell. Neurosci. 2015, 9, 108. [Google Scholar] [CrossRef]
- Li, Y.K.; Wang, F.; Wang, W.; Luo, Y.; Wu, P.F.; Xiao, J.L.; Hu, Z.L.; Jin, Y.; Hu, G.; Chen, J.G. Aquaporin-4 Deficiency Impairs Synaptic Plasticity and Associative Fear Memory in the Lateral Amygdala: Involvement of Downregulation of Glutamate Transporter-1 Expression. Neuropsychopharmacology 2012, 37, 1867–1878. [Google Scholar] [CrossRef]
- Binder, D.K.; Yao, X.; Zador, Z.; Sick, T.J.; Verkman, A.S.; Manley, G.T. Increased Seizure Duration and Slowed Potassium Kinetics in Mice Lacking Aquaporin-4 Water Channels. Glia 2006, 53, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Szu, J.I.; Binder, D.K. The Role of Astrocytic Aquaporin-4 in Synaptic Plasticity and Learning and Memory. Front. Integr. Neurosci. 2016, 10, 8. [Google Scholar] [CrossRef]
- Hirt, L.; Fukuda, A.M.; Ambadipudi, K.; Rashid, F.; Binder, D.; Verkman, A.; Ashwal, S.; Obenaus, A.; Badaut, J. Improved Long-Term Outcome after Transient Cerebral Ischemia in Aquaporin-4 Knockout Mice. J. Cereb. Blood Flow Metab. 2017, 37, 277–290. [Google Scholar] [CrossRef]
- Pifarré, P.; Gutierrez-Mecinas, M.; Prado, J.; Usero, L.; Roura-Mir, C.; Giralt, M.; Hidalgo, J.; García, A. Phosphodiesterase 5 Inhibition at Disease Onset Prevents Experimental Autoimmune Encephalomyelitis Progression through Immunoregulatory and Neuroprotective Actions. Exp. Neurol. 2014, 251, 58–71. [Google Scholar] [CrossRef]
- de Santana Nunes, A.K.; Rapôso, C.; Björklund, U.; da Cruz-Höfling, M.A.; Peixoto, C.A.; Hansson, E. Sildenafil (Viagra®) Prevents and Restores LPS-Induced Inflammation in Astrocytes. Neurosci. Lett. 2016, 630, 59–65. [Google Scholar] [CrossRef]
- Araújo, S.M.d.R.; Duarte-Silva, E.; Marinho, C.G.d.S.; Oliveira, W.H.; de França, M.E.R.; Lós, D.; Peron, G.; Tomaz, L.; Bonfanti, A.P.; Verinaud, L.; et al. Effect of Sildenafil on Neuroinflammation and Synaptic Plasticity Pathways in Experimental Autoimmune Encephalomyelitis. Int. Immunopharmacol. 2020, 85, 106581. [Google Scholar] [CrossRef] [PubMed]
- Karasawa, Y.; Araki, H.; Otomo, S. Changes in Locomotor Activity and Passive Avoidance Task Performance Induced by Cerebral Ischemia in Mongolian Gerbils. Stroke 1994, 25, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Katsumata, N.; Kuroiwa, T.; Ishibashi, S.; Li, S.; Endo, S.; Ohno, K. Heterogeneous Hyperactivity and Distribution of Ischemic Lesions after Focal Cerebral Ischemia in Mongolian Gerbils. Neuropathology 2006, 26, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kang, H.; Lee, Y.B.; Lee, B.; Lee, D. A Quantitative Analysis of Spontaneous Alternation Behaviors on a Y-Maze Reveals Adverse Effects of Acute Social Isolation on Spatial Working Memory. Sci. Rep. 2023, 13, 14722. [Google Scholar] [CrossRef]
- Hazim, A.I.; Mustapha, M.; Mansor, S.M. The Effects on Motor Behaviour and Short-Term Memory Tasks in Mice Following an Acute Administration of Mitragyna Speciosa Alkaloid Extract and Mitragynine. J. Med. Plant Res. 2011, 5, 5810–5817. [Google Scholar]
- Chachua, T.; Goletiani, C.; Maglakelidze, G.; Sidyelyeva, G.; Daniel, M.; Morris, E.; Miller, J.; Shang, E.; Wolgemuth, D.J.; Greenberg, D.A.; et al. Sex-Specific Behavioral Traits in the Brd2 Mouse Model of Juvenile Myoclonic Epilepsy. Genes Brain Behav. 2014, 13, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Pitts, M. Barnes Maze Procedure for Spatial Learning and Memory in Mice. Bio-Protocol 2018, 8, e2744. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, O.; Roche, M.; McGuire, B.E.; Finn, D.P. Validation of an Air-Puff Passive-Avoidance Paradigm for Assessment of Aversive Learning and Memory in Rat Models of Chronic Pain. J. Neurosci. Methods 2012, 204, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mendl, M.; Burman, O.; Laughlin, K.; Paul, E. Animal Memory and Animal Welfare. Anim. Welf. 2001, 10, S141–S159. [Google Scholar] [CrossRef]
- Steele, R.J.; Stewart, M.G.; Rose, S.P.R. Increases in NMDA Receptor Binding Are Specifically Related to Memory Formation for a Passive Avoidance Task in the Chick: A Quantitative Autoradiographic Study. Brain Res. 1995, 674, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Squire, L.R.; Zola, S.M. Ischemic Brain Damage and Memory Impairment: A Commentary. Hippocampus 1996, 6, 546–552. [Google Scholar] [CrossRef]
- Lee, T.K.; Hong, J.; Lee, J.W.; Kim, S.S.; Sim, H.; Lee, J.C.; Kim, D.W.; Lim, S.S.; Kang, I.J.; Won, M.H. Ischemia-Induced Cognitive Impairment Is Improved via Remyelination and Restoration of Synaptic Density in the Hippocampus after Treatment with Cog-Up® in a Gerbil Model of Ischemic Stroke. Vet. Sci. 2021, 8, 321. [Google Scholar] [CrossRef]
- Bravo-Rivera, C.; Sotres-Bayon, F. From Isolated Emotional Memories to Their Competition During Conflict. Front. Behav. Neurosci. 2020, 14, 36. [Google Scholar] [CrossRef]
- Peixoto, C.A.; Nunes, A.K.S.; Garcia-Osta, A. Phosphodiesterase-5 Inhibitors: Action on the Signaling Pathways of Neuroinflammation, Neurodegeneration, and Cognition. Mediators Inflamm. 2015, 2015, 940207. [Google Scholar] [CrossRef]
- Yazdani, A.; Howidi, B.; Shi, M.Z.; Tugarinov, N.; Khoja, Z.; Wintermark, P. Sildenafil Improves Hippocampal Brain Injuries and Restores Neuronal Development after Neonatal Hypoxia–Ischemia in Male Rat Pups. Sci. Rep. 2021, 11, 22046. [Google Scholar] [CrossRef]
- Puzzo, D.; Staniszewski, A.; XianDeng, S.; Privitera, L.; Leznik, E.; Liu, S.; Zhang, H.; Feng, Y.; Palmeri, A.; Landry, D.W.; et al. Phosphodiesterase 5 Inhibition Improves Synaptic Function, Memory, and Amyloid-β Load in an Alzheimer’s Disease Mouse Model. J. Neurosci. 2009, 29, 8075–8086. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.M.; Wang, N.N.; Zhang, T.Y.; Wang, F.; Wu, C.F.; Yang, J.Y. Neuroprotection by Sildenafil: Neuronal Networks Potentiation in Acute Experimental Stroke. CNS Neurosci. Ther. 2014, 20, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Halassa, M.M.; Fellin, T.; Haydon, P.G. Tripartite Synapses: Roles for Astrocytic Purines in the Control of Synaptic Physiology and Behavior. Neuropharmacology 2009, 57, 343–346. [Google Scholar] [CrossRef]
- Barker, A.J.; Ullian, E.M. Astrocytes and Synaptic Plasticity. Neuroscientist 2010, 16, 40–50. [Google Scholar] [CrossRef]
- Perea, G.; Navarrete, M.; Araque, A. Tripartite Synapses: Astrocytes Process and Control Synaptic Information. Trends Neurosci. 2009, 32, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Ben Achour, S.; Pascual, O. Glia: The Many Ways to Modulate Synaptic Plasticity. Neurochem. Int. 2010, 57, 440–445. [Google Scholar] [CrossRef]
- Parpura, V.; Zorec, R. Gliotransmission: Exocytotic Release from Astrocytes. Brain Res. Rev. 2010, 63, 83–92. [Google Scholar] [CrossRef]
- Danbolt, N.C. Glutamate Uptake. Prog. Neurobiol. 2001, 65, 1–105. [Google Scholar] [PubMed]
- Yang, J.; Li, M.X.; Luo, Y.; Chen, T.; Liu, J.; Fang, P.; Jiang, B.; Hu, Z.L.; Jin, Y.; Chen, J.G.; et al. Chronic Ceftriaxone Treatment Rescues Hippocampal Memory Deficit in AQP4 Knockout Mice via Activation of GLT-1. Neuropharmacology 2013, 75, 213–222. [Google Scholar] [CrossRef]
- Zeng, X.N.; Sun, X.L.; Gao, L.; Fan, Y.; Ding, J.H.; Hu, G. Aquaporin-4 Deficiency down-Regulates Glutamate Uptake and GLT-1 Expression in Astrocytes. Mol. Cell. Neurosci. 2007, 34, 34–39. [Google Scholar] [CrossRef]
- Katagiri, H.; Tanaka, K.; Manabe, T. Requirement of Appropriate Glutamate Concentrations in the Synaptic Cleft for Hippocampal LTP Induction. Eur. J. Neurosci. 2001, 14, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.; Biala, G. The Novel Object Recognition Memory: Neurobiology, Test Procedure, and Its Modifications. Cogn. Process. 2012, 13, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Kinnavane, L.; Amin, E.; Olarte-Sánchez, C.M.; Aggleton, J.P. Detecting and Discriminating Novel Objects: The Impact of Perirhinal Cortex Disconnection on Hippocampal Activity Patterns. Hippocampus 2016, 26, 1393–1413. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Lozano-Soldevilla, D.; Gil-Nagel, A.; Toledano, R.; Oehrn, C.R.; Kunz, L.; Yebra, M.; Mendez-Bertolo, C.; Stieglitz, L.; Sarnthein, J.; et al. Aversive Memory Formation in Humans Involves an Amygdala-Hippocampus Phase Code. Nat. Commun. 2022, 13, 6403. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.H.; Kim, G.W.; Lee, Y.R.; Park, D.-K.; Song, B.; Kim, D.-S. Effects of Sildenafil on Cognitive Function Recovery and Neuronal Cell Death Protection after Transient Global Cerebral Ischemia in Gerbils. Biomedicines 2024, 12, 2077. https://doi.org/10.3390/biomedicines12092077
Yu YH, Kim GW, Lee YR, Park D-K, Song B, Kim D-S. Effects of Sildenafil on Cognitive Function Recovery and Neuronal Cell Death Protection after Transient Global Cerebral Ischemia in Gerbils. Biomedicines. 2024; 12(9):2077. https://doi.org/10.3390/biomedicines12092077
Chicago/Turabian StyleYu, Yeon Hee, Gun Woo Kim, Yu Ran Lee, Dae-Kyoon Park, Beomjong Song, and Duk-Soo Kim. 2024. "Effects of Sildenafil on Cognitive Function Recovery and Neuronal Cell Death Protection after Transient Global Cerebral Ischemia in Gerbils" Biomedicines 12, no. 9: 2077. https://doi.org/10.3390/biomedicines12092077
APA StyleYu, Y. H., Kim, G. W., Lee, Y. R., Park, D.-K., Song, B., & Kim, D.-S. (2024). Effects of Sildenafil on Cognitive Function Recovery and Neuronal Cell Death Protection after Transient Global Cerebral Ischemia in Gerbils. Biomedicines, 12(9), 2077. https://doi.org/10.3390/biomedicines12092077






