Lower Levels of TAZ Expression Associated with Post-Surgical Wound Healing Complications in Soft Tissue Sarcoma Patients Treated with Preoperative Radiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection Criteria
2.2. Histologic Analysis
2.3. Immunohistochemical Staining
2.4. Clinical Data Abstraction
2.5. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lawrence, W., Jr.; Donegan, W.L.; Natarajan, N.; Mettlin, C.; Beart, R.; Winchester, D. Adult soft tissue sarcomas. A pattern of care survey of the American College of Surgeons. Ann. Surg. 1987, 205, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Morrison, B. Soft tissue sarcomas of the extremities. Proc. (Bayl Univ. Med. Cent.) 2003, 16, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Matsumine, A.; Matsubara, T.; Asanuma, K.; Uchida, A.; Sudo, A. Clinical characteristics of patients with large and deep soft tissue sarcomas. Oncol. Lett. 2015, 10, 841–844. [Google Scholar] [CrossRef] [PubMed]
- Sanniec, K.J.; Swanson, S.; Casey, W.J., 3rd; Schwartz, A.; Bryant, L.; Rebecca, A.M. Predictive factors of wound complications after sarcoma resection requiring plastic surgeon involvement. Ann. Plast. Surg. 2013, 71, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Koshy, M.; Rich, S.E.; Mohiuddin, M.M. Improved survival with radiation therapy in high-grade soft tissue sarcomas of the extremities: A SEER analysis. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 203–209. [Google Scholar] [CrossRef]
- Hoefkens, F.; Dehandschutter, C.; Somville, J.; Meijnders, P.; Van Gestel, D. Soft tissue sarcoma of the extremities: Pending questions on surgery and radiotherapy. Radiat. Oncol. 2016, 11, 136. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Soft Tissue Sarcoma: Version 2.2021—28 April 2021. Available online: https://www.nccn.org/guidelines/guidelines-with-evidence-blocks (accessed on 15 December 2021).
- Ramey, S.J.; Yechieli, R.; Zhao, W.; Kodiyan, J.; Asher, D.; Chinea, F.M.; Patel, V.; Reis, I.M.; Wang, L.; Wilky, B.A.; et al. Limb-sparing surgery plus radiotherapy results in superior survival: An analysis of patients with high-grade, extremity soft-tissue sarcoma from the NCDB and SEER. Cancer Med. 2018, 7, 4228–4239. [Google Scholar] [CrossRef]
- O’Sullivan, B.; Davis, A.M.; Turcotte, R.; Bell, R.; Catton, C.; Chabot, P.; Wunder, J.; Kandel, R.; Goddard, K.; Sadura, A.; et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: A randomised trial. Lancet 2002, 359, 2235–2241. [Google Scholar] [CrossRef]
- Davis, A.M.; O’Sullivan, B.; Turcotte, R.; Bell, R.; Catton, C.; Chabot, P.; Wunder, J.; Hammond, A.; Benk, V.; Kandel, R.; et al. Canadian Sarcoma Group, NCI Canada Clinical Trial Group Randomized Trial: Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother. Oncol. 2005, 75, 48–53. [Google Scholar] [CrossRef]
- Bedi, M.; Ethun, C.G.; Charlson, J.; Tran, T.B.; Poultsides, G.; Grignol, V.; Howard, J.H.; Tseng, J.; Roggin, K.K.; Chouliaras, K.; et al. Is a Nomogram Able to Predict Postoperative Wound Complications in Localized Soft-tissue Sarcomas of the Extremity? Clin. Orthop. Relat. Res. 2020, 478, 550–559. [Google Scholar] [CrossRef]
- Baldini, E.H.; Lapidus, M.R.; Wang, Q.; Manola, J.; Orgill, D.P.; Pomahac, B.; Marcus, K.J.; Bertagnolli, M.M.; Devlin, P.M.; George, S.; et al. Predictors for major wound complications following preoperative radiotherapy and surgery for soft-tissue sarcoma of the extremities and trunk: Importance of tumor proximity to skin surface. Ann. Surg. Oncol. 2013, 20, 1494–1499. [Google Scholar] [CrossRef] [PubMed]
- Dadras, M.; Koepp, P.; Wagner, J.M.; Wallner, C.; Sogorski, A.; Lehnhardt, M.; Harati, K.; Behr, B. Antibiotic prophylaxis for prevention of wound infections after soft tissue sarcoma resection: A retrospective cohort study. J. Surg. Oncol. 2020, 122, 1685–1692. [Google Scholar] [CrossRef] [PubMed]
- Bedi, M.; King, D.M.; DeVries, J.; Hackbarth, D.A.; Neilson, J.C. Does Vacuum-assisted Closure Reduce the Risk of Wound Complications in Patients With Lower Extremity Sarcomas Treated With Preoperative Radiation? Clin. Orthop. Relat. Res. 2019, 477, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Nystrom, L.M.; Miller, B.J. Transcutaneous Oximetry May Predict Wound Healing Complications In Preoperatively Radiated Soft Tissue Sarcoma. Iowa Orthop. J. 2016, 36, 117–122. [Google Scholar]
- Griffin, A.M.; Dickie, C.I.; Catton, C.N.; Chung, P.W.M.; Ferguson, P.C.; Wunder, J.S.; O’Sullivan, B. The influence of time interval between preoperative radiation and surgical resection on the development of wound healing complications in extremity soft tissue sarcoma. Ann. Surg. Oncol. 2015, 22, 2824–2830. [Google Scholar] [CrossRef]
- Collier, C.D.; Su, C.A.; Reich, M.S.; Tu, L.A.; Getty, P.J. Six-Week Interval Between Preoperative Radiation and Surgery Is Associated with Fewer Major Wound Complications in Soft Tissue Sarcoma. Am. J. Clin. Oncol. 2020, 43, 491–495. [Google Scholar] [CrossRef]
- Ellis, S.; Lin, E.J.; Tartar, D. Immunology of Wound Healing. Curr. Dermatol. Rep. 2018, 7, 350–358. [Google Scholar] [CrossRef]
- Sanchez, M.C.; Lancel, S.; Boulanger, E.; Neviere, R. Targeting Oxidative Stress and Mitochondrial Dysfunction in the Treatment of Impaired Wound Healing: A Systematic Review. Antioxidants 2018, 7, 98. [Google Scholar] [CrossRef]
- LeBrun, D.G.; Guttmann, D.M.; Shabason, J.E.; Levin, W.P.; Kovach, S.J.; Weber, K.L. Predictors of Wound Complications following Radiation and Surgical Resection of Soft Tissue Sarcomas. Sarcoma 2017, 2017, 5465130. [Google Scholar] [CrossRef]
- Landen, N.X.; Li, D.; Stahle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef]
- Lee, M.J.; Byun, M.R.; Furutani-Seiki, M.; Hong, J.H.; Jung, H.S. YAP and TAZ regulate skin wound healing. J. Invest. Dermatol. 2014, 134, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Walko, G.; Woodhouse, S.; Pisco, A.O.; Rognoni, E.; Liakath-Ali, K.; Lichtenberger, B.M.; Mishra, A.; Telerman, S.B.; Viswanathan, P.; Logtenberg, M.; et al. A genome-wide screen identifies YAP/WBP2 interplay conferring growth advantage on human epidermal stem cells. Nat. Commun. 2017, 8, 14744. [Google Scholar] [CrossRef] [PubMed]
- Fullenkamp, C.A.; Hall, S.L.; Jaber, O.I.; Pakalniskis, B.L.; Savage, E.C.; Savage, J.M.; Ofori-Amanfo, G.K.; Lambertz, A.M.; Ivins, S.D.; Stipp, C.S.; et al. TAZ and YAP are frequently activated oncoproteins in sarcomas. Oncotarget 2016, 7, 30094–30108. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Chen, Q.; Gutkind, J.S. Oncotargeting G proteins: The Hippo in the room. Oncotarget 2014, 5, 10997–10999. [Google Scholar] [CrossRef]
- Taccioli, C.; Sorrentino, G.; Zannini, A.; Caroli, J.; Beneventano, D.; Anderlucci, L.; Lolli, M.; Bicciato, S.; Del Sal, G. MDP, a database linking drug response data to genomic information, identifies dasatinib and statins as a combinatorial strategy to inhibit YAP/TAZ in cancer cells. Oncotarget 2015, 6, 38854–38865. [Google Scholar] [CrossRef]
- Dey, A.; Varelas, X.; Guan, K.L. Targeting the Hippo pathway in cancer, fibrosis, wound healing and regenerative medicine. Nat. Rev. Drug Discov. 2020, 19, 480–494. [Google Scholar] [CrossRef]
- Isfort, I.; Elges, S.; Cyra, M.; Berthold, R.; Renner, M.; Mechtersheimer, G.; Aman, P.; Larsson, O.; Ratner, N.; Hafner, S.; et al. Prevalence of the Hippo Effectors YAP1/TAZ in Tumors of Soft Tissue and Bone. Sci. Rep. 2019, 9, 19704. [Google Scholar] [CrossRef]
- Sorrentino, G.; Ruggeri, N.; Specchia, V.; Cordenonsi, M.; Mano, M.; Dupont, S.; Manfrin, A.; Ingallina, E.; Sommaggio, R.; Piazza, S.; et al. Metabolic control of YAP and TAZ by the mevalonate pathway. Nat. Cell Biol. 2014, 16, 357–366. [Google Scholar] [CrossRef]
- Liu-Chittenden, Y.; Huang, B.; Shim, J.S.; Chen, Q.; Lee, S.J.; Anders, R.A.; Liu, J.O.; Pan, D. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012, 26, 1300–1305. [Google Scholar] [CrossRef]
- Rognoni, E.; Walko, G. The Roles of YAP/TAZ and the Hippo Pathway in Healthy and Diseased Skin. Cells 2019, 8, 411. [Google Scholar] [CrossRef]
- Kung, T.A.; Kidwell, K.M.; Speth, K.A.; Pang, J.C.; Jagsi, R.; Newman, L.A.; Wilkins, E.G.; Momoh, A.O. Radiation-Induced Skin Changes after Postmastectomy Radiation Therapy: A Pilot Study on Indicators for Timing of Delayed Breast Reconstruction. J. Reconstr. Microsurg. 2019, 35, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Schultz, G.S.; Chin, G.A.; Moldawer, L.; Diegelman, R.F. Principles of Wound Healing. In Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists; Fitridge, R., Thompson, M., Adelaide, A.U., Eds.; University of Adelaide Press: Adelaide, Australia, 2011. [Google Scholar]
- Koerdt, S.; Rohleder, N.H.; Rommel, N.; Nobis, C.; Stoeckelhuber, M.; Pigorsch, S.; Duma, M.N.; Wolff, K.D.; Kesting, M.R. An expression analysis of markers of radiation-induced skin fibrosis and angiogenesis in wound healing disorders of the head and neck. Radiat. Oncol. 2015, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, Y.H.; Kim, J.; Park, D.Y.; Bae, H.; Lee, D.H.; Kim, K.H.; Hong, S.P.; Jang, S.P.; Kubota, Y.; et al. YAP/TAZ regulates sprouting angiogenesis and vascular barrier maturation. J. Clin. Invest. 2017, 127, 3441–3461. [Google Scholar] [CrossRef] [PubMed]
- Mustoe, T.A.; O’Shaughnessy, K.; Kloeters, O. Chronic wound pathogenesis and current treatment strategies: A unifying hypothesis. Plast. Reconstr. Surg. 2006, 117, 35S–41S. [Google Scholar] [CrossRef]
- Sheng, X.; Zhou, Y.; Wang, H.; Shen, Y.; Liao, Q.; Rao, Z.; Deng, F.; Xie, L.; Yao, C.; Mao, H.; et al. Establishment and characterization of a radiation-induced dermatitis rat model. J. Cell. Mol. Med. 2019, 23, 3178–3189. [Google Scholar] [CrossRef]
- Rittie, L.; Sachs, D.L.; Orringer, J.S.; Voorhees, J.J.; Fisher, G.J. Eccrine sweat glands are major contributors to reepithelialization of human wounds. Am. J. Pathol. 2013, 182, 163–171. [Google Scholar] [CrossRef]
- Wilson, R.P.; McGettigan, S.E.; Dang, V.D.; Kumar, A.; Cancro, M.P.; Nikbakht, N.; Stohl, W.; Debes, G.F. IgM plasma cells reside in healthy skin and accumulate with chronic inflammation. J. Invest. Dermatol. 2019, 139, 2477–2487. [Google Scholar] [CrossRef]
- Walsh, N.M.; Kutzner, H.; Requena, L.; Cerroni, L. Plasmacytic cutaneous pathology: A review. J. Cutan. Pathol. 2019, 46, 698–708. [Google Scholar] [CrossRef]
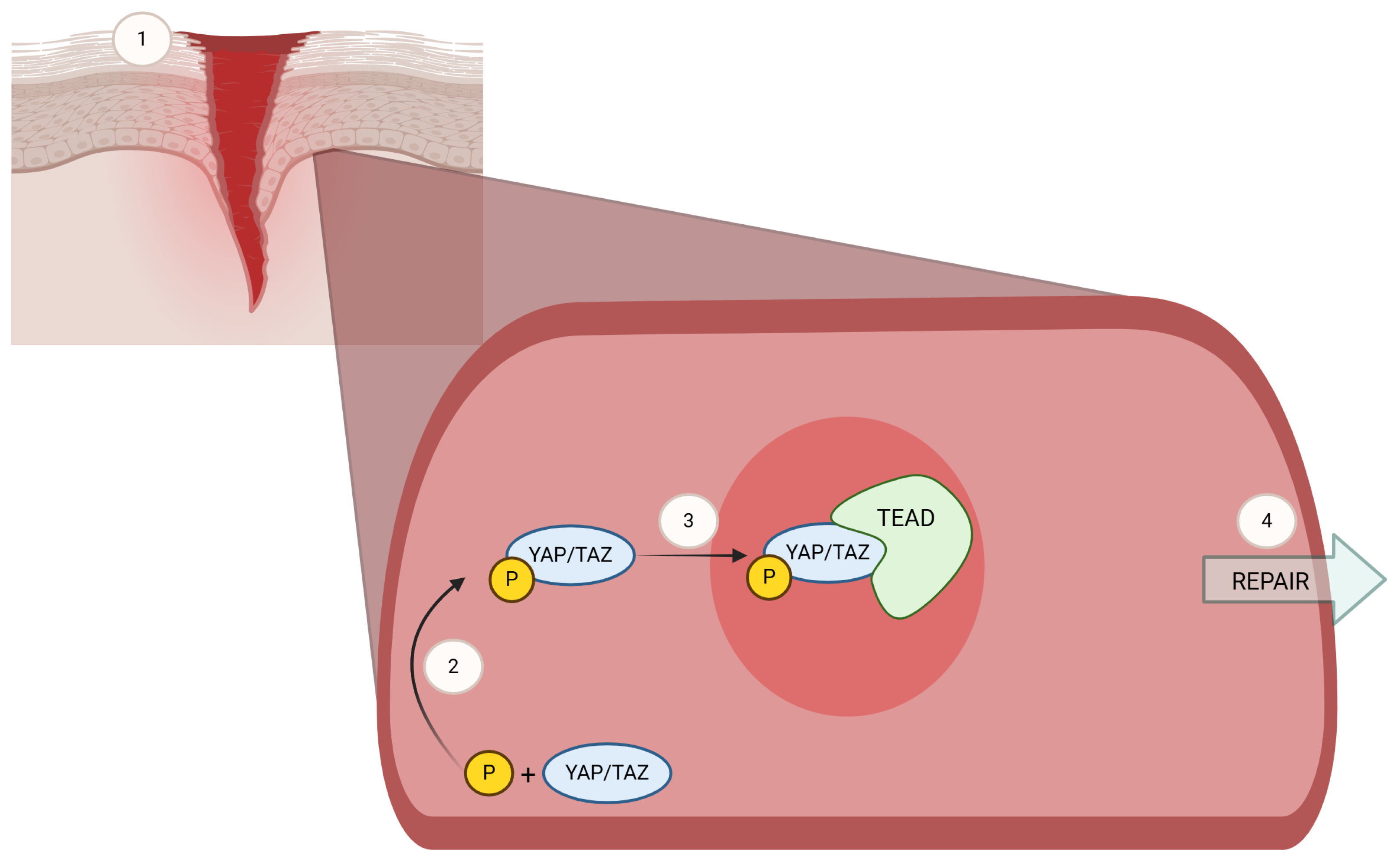

| Pre-RT (n = 30) | No Pre-RT (n = 24) | p-Value * | |
|---|---|---|---|
| Elastin Organization † | |||
| 0–1 | 23 (76.7%) | 15 (62.5%) | 0.257 |
| 3–4 | 7 (23.3%) | 9 (37.5%) | |
| Neutrophils ‡ | 7 (23.3%) | 1 (4.2%) | 0.0633 |
| Plasma Cells ‡ | 11 (36.7%) | 0 (0%) | 0.0255 |
| Inflammatory Cells § | |||
| >100/HPF | 13 (43.3%) | 8 (33.3%) | 0.4538 |
| ≤100/HPF | 17 (56.7%) | 16 (66.7%) | |
| Hair Follicles ‡ | 14 (46.7%) | 19 (79.2%) | 0.0149 |
| Eccrine Glands ‡ | 30 (100%) | 23 (95.8%) | 0.4444 |
| Sebaceous Glands ‡ | 1 (3.3%) | 7 (29.2%) | 0.0163 |
| Dermal Thickness (mm) | |||
| ≥4 | 6 | 7 | 0.4337 |
| <4 | 24 | 17 | |
| Vessels/10 HPF § | |||
| >30 | 2 | 7 | 0.0414 |
| ≤30 | 28 | 17 | |
| Total TAZ H-score¶ Mean ± SD | 276.5 ± 38.8 | 253.9 ± 48.5 | 0.017 |
| WHC (n = 8) | No WHC (n = 22) | p-Value * | |
|---|---|---|---|
| Elastin Organization † | |||
| 0–1 | 8 (100%) | 15 (68.2%) | 0.1434 |
| 3–4 | 0 (0%) | 7 (31.8%) | |
| Neutrophils ‡ | 4 (50.0%) | 3 (13.6%) | 0.0596 |
| Plasma Cells ‡ | 3 (37.5%) | 8 (36.4%) | 1.0000 |
| Inflammatory Cells § | |||
| >100/HPF | 2 (25.0%) | 11 (50.0%) | 0.4069 |
| ≤100/HPF | 6 (75.0%) | 11 (50.0%) | |
| Hair Follicles ‡ | 5 (62.5%) | 9 (40.9%) | 0.4171 |
| Eccrine Glands ‡ | 8 (100%) | 22 (100%) | – |
| Sebaceous Glands ‡ | 1 (12.5%) | 0 (0%) | 0.2667 |
| Dermal Thickness (mm) | |||
| ≥4 | 2 (25.0%) | 4 (18.2%) | 0.6452 |
| < 4 | 6 (75.0%) | 18 (81.8%) | |
| Vessels/10 HPF § | |||
| >30 | 1 (12.5%) | 1 (4.5%) | 0.4690 |
| ≤30 | 7 (87.5%) | 21 (95.5%) | |
| Total TAZ H-score¶ Mean ± SD | 260 ± 57.32 | 282.5 ± 29.02 | 0.0402 |
| Patient Characteristics | Total N (%) | WHC (n = 11) | No WHC (n = 43) | p-Value * |
|---|---|---|---|---|
| Sex | ||||
| Male | 30 (55.6%) | 5 (45.5%) | 25 (58.1%) | 0.5104 |
| Female | 24 (44.4%) | 6 (54.5%) | 18 (41.9%) | |
| Age | ||||
| Tumor Characteristics | ||||
| <65 | 26 (48.1%) | 5 (45.5%) | 21 (48.8%) | 0.8412 |
| ≥65 | 28 (51.9%) | 6 (54.5%) | 22 (51.2%) | |
| Size (cm) | ||||
| <5 | 4 (7.4%) | 0 (0%) | 4 (9.3%) | 0.3517 |
| 5–10 | 17 (31.5%) | 2 (18.1%) | 15 (34.9%) | |
| >10 | 33 (61.1%) | 9 (81.8%) | 24 (55.8%) | |
| Grade | ||||
| Low | 6 (11.1%) | 2 (18.1%) | 4 (9.3%) | 0.7075 |
| Intermediate | 24 (44.4%) | 4 (36.4%) | 20 (46.5%) | |
| High | 24 (44.4%) | 5 (45.5%) | 19 (44.2%) | |
| Location | ||||
| Upper Extremity | 11 (20.4%) | 0 (0%) | 11 (25.6%) | 0.0958 |
| Lower Extremity | 41 (75.9%) | 10 (90.9%) | 31 (72.1%) | |
| Axial | 2 (3.7%) | 1 (9.1%) | 1 (2.3%) | |
| Treatment Characteristics | ||||
| Radiation | ||||
| Preoperative | 30 (55.6%) | 8 (72.7%) | 22 (51.2%) | 0.6956 |
| Postoperative | 12 (22.2%) | 2 (18.2%) | 10 (23.2%) | |
| Neoadjuvant Chemotherapy | ||||
| Cytotoxic | 9 (16.7%) | 1 (9.1%) | 8 (18.6%) | 0.7334 |
| Immunotherapy | 1 (1.9%) | 0 (0%) | 1 (2.3%) | |
| None | 44 (81.5%) | 10 (90.9%) | 34 (79.1%) |
| WHC (n = 8) | No WHC (n = 22) | p-Value * | |
|---|---|---|---|
| Sex | |||
| Male | 4 | 14 | 0.6779 |
| Female | 4 | 8 | |
| Age | |||
| <65 | 5 | 12 | 1.0000 |
| ≥65 | 3 | 10 | |
| Tumor Size (cm) | |||
| <5 | 0 | 1 | 1.0000 |
| 5–10 | 2 | 7 | |
| ≥10 | 6 | 14 | |
| Tumor Grade | |||
| Low | 1 | 2 | 1.0000 |
| Intermediate | 4 | 13 | |
| High | 3 | 7 | |
| Location | |||
| Upper Extremity | 0 | 6 | 0.2560 |
| Lower Extremity | 7 | 15 | |
| Axial | 1 | 1 | |
| Neoadjuvant Chemotherapy | |||
| Cytotoxic | 0 | 4 | 0.6715 |
| Not Cytotoxic | 8 | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gylten, J.D.; Persons, J.E.; Miller, B.J.; An, Q.; Tanas, M.R.; Chen, S.J.T. Lower Levels of TAZ Expression Associated with Post-Surgical Wound Healing Complications in Soft Tissue Sarcoma Patients Treated with Preoperative Radiation. Biomedicines 2025, 13, 344. https://doi.org/10.3390/biomedicines13020344
Gylten JD, Persons JE, Miller BJ, An Q, Tanas MR, Chen SJT. Lower Levels of TAZ Expression Associated with Post-Surgical Wound Healing Complications in Soft Tissue Sarcoma Patients Treated with Preoperative Radiation. Biomedicines. 2025; 13(2):344. https://doi.org/10.3390/biomedicines13020344
Chicago/Turabian StyleGylten, Jacob D., Jane E. Persons, Benjamin J. Miller, Qiang An, Munir R. Tanas, and Stephanie J. T. Chen. 2025. "Lower Levels of TAZ Expression Associated with Post-Surgical Wound Healing Complications in Soft Tissue Sarcoma Patients Treated with Preoperative Radiation" Biomedicines 13, no. 2: 344. https://doi.org/10.3390/biomedicines13020344
APA StyleGylten, J. D., Persons, J. E., Miller, B. J., An, Q., Tanas, M. R., & Chen, S. J. T. (2025). Lower Levels of TAZ Expression Associated with Post-Surgical Wound Healing Complications in Soft Tissue Sarcoma Patients Treated with Preoperative Radiation. Biomedicines, 13(2), 344. https://doi.org/10.3390/biomedicines13020344






