Abstract
Mast cells (MCs) are essential components of the immune system that enter the circulation as immature bone marrow progenitors and differentiate in peripheral organs under the influence of microenvironment factors. As tissue-resident secretory immune cells, MCs rapidly detect the presence of bacteria and parasites because they harbor many surface receptors, which enable their activation via a multitude of stimuli. MC activation has been traditionally linked to IgE-mediated allergic reactions, but MCs play a pivotal role in different physiological and pathological processes. In gut, MCs are essential for the maintenance of gastrointestinal (GI) barrier function, and their interactions with neurons, immune cells, and epithelial cells have been related to various GI disorders. This review recapitulates intestinal MC roles in diseases with a main focus on inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS). Emerging therapies targeting MCs and their mediators in clinical practices will also be discussed.
1. Introduction
It has been known for decades that mast cells (MCs) are key players in immunoglobulin E (IgE)-dependent allergic disorders, including asthma and systemic anaphylaxis [1]. However, their participation in inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS) is less appreciated. One reason is that a clear link between MC-restricted genes and IBD has not been revealed by genome-wide association studies [2]. Another reason for the lack of appreciation is that cationic stains (e.g., toluidine blue, safranin, and methylene blue) routinely performed on biopsies of connective tissues to quantitate MCs are not particularly effective for the identification of these cells in the gastrointestinal tract [3].
Despite these difficulties, an essential role for mouse and human MCs and their proteases in promoting barrier dysfunctions has been documented either in acute experimental colitis and in gastrointestinal disorders including IBD and IBS [4,5,6]. Moreover, the use of different antibody combinations able to detect proteins restricted to human MCs (e.g., the granule proteases tryptase and chymase and the surface receptor c-kit) has finally permitted the characterization of the distribution and phenotype of MCs in gut biopsies of patients with IBD [7].
Thus, it is now becoming clear that MCs play a major role in the regulation of intestinal mucosal permeability, in the initiation and maintenance of neuro-immune interaction and inflammatory response in the gut, as well as in tissue remodeling after resolution of the acute inflammatory phase.
In this review, we first describe the biological properties of MCs in the gastrointestinal tract and their essential role in preserving homeostatic conditions. We will then discuss the contribution of MCs and their selective mediators in the inflammation that occurs in the gut of patients with IBD and IBS. The review also highlights the potential clinical applications of recent findings.
2. Phenotypic and Functional Heterogeneity of Intestinal MCs
MCs are myeloid cells that originate from poorly granulated CD34+/c-Kit+ bone marrow progenitors that enter the circulation and complete their maturation into peripheral tissues under the influence of the local microenvironment [8,9]. Notably, in the mouse, the greatest number of MC-committed progenitors (MCp) are found in the gut compared to other peripheral tissues [10].
The homing of murine MCp in the intestine is driven by the expression of the α4β7 integrin and the chemokine receptor CXCR2 on the surface of the progenitors and the expression of Madcam1 and VCAM1 on the intestinal endothelium [11,12]. However, the most important signaling pathway that controls the retention and viability of MCp in the GI tract is that between the tyrosine kinase receptor c-Kit (CD117) on MCs and c-Kit ligand (stem cell factor, SCF) on the plasma membranes of fibroblasts, endothelial cells, and other stromal cells [13,14]. Additionally, several other cytokines [e.g., interleukin (IL)-3, IL-4, IL-6, IL-9, IL-10, IL-33, nerve growth factor, and transforming growth factor-β] are needed for the final stages of differentiation and for the development of phenotypically distinct populations of mouse and human MCs [15,16,17,18,19,20].
Mature MCs express an extensive array of receptors that allow them to recognize invading pathogens and respond to different stimuli coming from the microenvironment [21,22]. Triggering of these receptors leads to exocytosis of granule-stored mediators or the generation of newly biologically active lipid mediators (e.g., leukotrienes, prostaglandins, thromboxane, and platelet-activating factor) [23,24,25]. Moreover, activated MCs can release numerous cytokines and chemokines orchestrating the delayed phase of inflammation that occurs several hours after their initial stimulation.
The high-affinity receptor for IgE (FcεRI) binds monomeric IgE and is the main activating receptor constitutively expressed on the surface of MCs. Multivalent antigen crosslinking of the receptor-bound IgE leads to activation of signaling pathways that are the primary cause of MC-dependent hypersensitivity reactions in vivo, including systemic anaphylaxis [26,27].
Mouse and human MCs can also be activated in many non-IgE-dependent manners [25]. For instance, MCs can be induced to degranulate by thrombin via protease-activated receptor-1 (Par-1) [28,29], by IgG complexes via FcγRIIa or FcγRIIIa [30], by ATP via P2X, P2Y, and adenosine receptors [31,32,33], and by complement-derived anaphylatoxins via the C3a and C5a receptors [34].
The number of mature MCs varies throughout the body, and a great number of mature MCs is present in the gastrointestinal (GI) tract, in which they are implicated in host defense. In particular, intestinal MCs are involved in the maintenance of homeostasis as well as in orchestrating local inflammation leading to the development of disease [35].
Based on the expression of different proteases stored in cytoplasmic granules, two main subsets of murine intestinal MCs have been identified [36]: MCs that are positive for mMCP-1 and mMCP-2 proteases are mainly located in the lamina propria close to the epithelium and for this reason are called mucosal MCs (MMCs); MCs that preferentially express in their granules the chymase mMCP-4, the elastase mMCP-5, and the tryptases mMCP-6 and 7 are found in the lower submucosa and are named connective tissue-type MCs (CTMCs). A third MC subtype, the interepithelial mucosal MCs (ieMMC), has been identified in mice [37]. The two mucosal subtypes, MMCs and ieMMCs, are rare in normal mouse GI, but increased during immune responses to intestinal helminth infections and in food allergies.
MC heterogeneity was also reported in the human GI tract, where two main subsets have been identified, mainly based on their differential expression of tryptase-β and chymase-1 [38]. Of note, 2 highly polymorphic genes (designated as hTPSAB1 and hTPSB2) give rise to different isoforms of tryptase-β [39], but their functional significance has not been evaluated experimentally. MCs that only express tryptase (MCT) are mainly present in the mucosal layer of the GI tract, while the dominant MC subset in the submucosa of the gut is characterized by the expression of tryptase, chymase, and carboxypeptidase A3 (CPA3) (MCTCA) [40,41]. A rare population of MCs exclusively expressing tryptase and CPA3, but not chymase, has recently been identified in the bronchial and esophageal mucosa of patients with asthma and eosinophilic esophagitis, respectively [42,43]. However, whether and how this subset contributes to human disease remains unknown.
These classifications are simplistic since they do not reflect the high level of intestinal MC plasticity due to the constant change in the local microenvironment. For instance, in respect to the small intestine, MCs that reside in the large intestine express higher amounts of toll-like receptors (TLRs) that are important for host defense against the abundant bacteria in the colon [44], demonstrating that regional diversity in microbiota composition can differently affect MC phenotype and function.
The advent of single-cell RNA sequencing (scRNAseq) technologies provides the great opportunity to analyze tissue-resident MCs, supporting the conclusion that MCs’ classification might be extended beyond the mucosal versus connective-like dichotomy across organs, including the gut [45,46,47].
3. Role of Intestinal MCs in Immune Homeostasis
Intestinal MCs play important homeostatic roles in the gut, controlling physiological processes such as the integrity and the baseline permeability of the gut’s epithelium mainly through the release of different granule proteases [35].
In humans, tryptase disturbs endocytic traffic and degradation of internalized antigens, allowing them to be easily transported across a damaged epithelial barrier [48].
A crucial role for the MC-released proteases in epithelial integrity was demonstrated by studies carried out on mMCP-4-null and mMCP-5-null B6 mice: these proteases mediate ischemia–reperfusion injury of skeletal muscle and thermal injury of skin mainly through the proteolytic disruption of tight junctions [49,50,51].
In the gut, the lack of mMCP-4 chymase increased crypt depth and decreased expression of the tight-junction protein claudin-3 on the lateral membranes of the epithelium with respect to a WT phenotype, revealing an important role for this chymase in the homeostatic regulation of intestinal epithelial anatomy and function [52].
Crosstalk Between Microbiota and MCs in the Maintenance Gut Homeostasis
Emerging evidence supports the existence of a mutual crosstalk between intestinal MCs and the gut microbiota, which could have a strong impact on intestinal homeostasis [53,54].
Commensal bacteria promote the expression of CXCR2 ligands by intestinal epithelial cells, which, in turn, is responsible for MC migration into the intestine [55]. Accordingly, germ-free mice exhibit an impaired homing of gut MCs and do not develop food allergy upon oral sensitization [56].
Intestinal MCs respond to microbial antigens thanks to the expression on their membrane surface of TLR2 and TLR4 [57]. TLR2-mediated response consists of degranulation followed by cytokine release, while TLR4 activates the cytokine release without degranulation [58].
However, although some microorganisms can elicit an MC-driven pro-inflammatory response, other microorganisms are able to reduce MC activation, thus limiting inflammation and favoring homeostatic conditions.
For instance, in vitro coculture of MCs with some bacteria strains induces exocytosis of enzymes and proteases stored in MC granules and the release of pro-inflammatory cytokines and chemokines [59,60,61]. Moreover, human MC cultured with Listeria monocytogenes are able to reduce bacterial growth through the production of ROS and the consequent release of extracellular traps (MCETs) [62]. Similarly, upon Candida albicans recognition, human MCs were transiently able to release tryptase-containing MCETs to kill the opportunistic pathogen [63].
On the other hand, several commensal bacteria, such as Enterococcus fecalis, Lactobacillus paracaseii, and nonpathogenic Escherichia coli, utilize distinct inhibitory mechanisms to impair in vitro murine MC degranulation induced by IgE/antigen triggering [64,65]. Of note, in a model of murine atopic dermatitis, oral administration of Enterococcus faecalis reduced MC infiltration and serum IgE levels, ameliorating the pathology [66], supporting the ability of gut microbiota to limit in vivo MC functions.
Thus, it is likely that during dysbiosis, intestinal MCs rapidly respond to abnormal gut flora by releasing preformed and newly synthesized mediators, which contribute to promoting a local hyperinflammatory response [67].
Accordingly, supplementation with specific probiotic strains is considered a preventive/therapeutic strategy for dysbiosis management and immune homeostasis [68] and contributes to MC stabilization [69].
In recent years, several studies demonstrated that a microbial dysbiosis can be associated with many human inflammatory disorders [70,71].
4. Gut Microenvironment and MC Activation During Inflammation
MC ability to rapidly sense a changing environment and consequently adapt to the specific received triggers can explain the influence of the gut cytokines, growth factors, and microbial components in shaping the phenotype and functions of intestinal MCs observed in inflammatory disorders and during parasitic infections [70,71,72,73,74,75].
Following infection by Trichinella spiralis and Trichuris muris, the total number of MCs increases, and in the acute phase, a shift from a connective tissue-like phenotype to a mucosal phenotype is occurring with a consequent elevated expression of the protease MCP-1 [72,73,74]. MCP-1 appeared to be responsible for the degradation of occludin, thus increasing intestinal permeability and facilitating worm expulsion [73,74]. On the other hand, in the chronic phase of inflammation, Shin and co-authors underline a selective role of connective tissue-like MCs. Indeed, the tryptase MCP-6 was shown to be required for eosinophil recruitment and for the eradication of Trichinella spiralis [75].
Many studies have well established that MCs in the intestinal mucosa are the major effector cells in IgE-mediated food-induced disorders, including food allergies [76,77].
Several excellent articles summarize critical knowledge on the immune mechanisms of MC sensitization in detail [77,78,79,80]; thus, we touch only on some aspects responsible for the food induction of a TH2 cell-mediated inflammatory response in the gut.
Exposure to a certain food in the context of concomitant external and internal trigger(s) inducing tissue damage produces an alarmin signature [epithelium-derived interleukin-25 (IL-25), IL-33, and thymic stromal lymphopoietin (TSLP)] that converges on IL-12 inhibition on DCs and upregulation of Th2 polarizing co-stimulatory molecules [81].
Th2 cells activate food allergen-specific B cells, promoting the production of allergen-specific IgE.
Allergens induce cross-linking of IgE bound to FcεRI on the surface of MCs, promoting the release of pro-inflammatory mediators able to induce both local and systemic responses. In addition to controlling type I hypersensitivity reactions, in the gastrointestinal mucosa, MCs orchestrate the recruitment of tissue-infiltrating leukocytes that amplify type 2 tissue inflammation [77].
Several studies have also demonstrated an increased number of activated MCs in the inflamed intestinal mucosa of patients affected by IBD and IBS, often associated with a concomitant stress response [6,82]. The implication of MCs and their selective mediators in disease progression is depicted in Figure 1 and will be discussed in the following paragraphs.
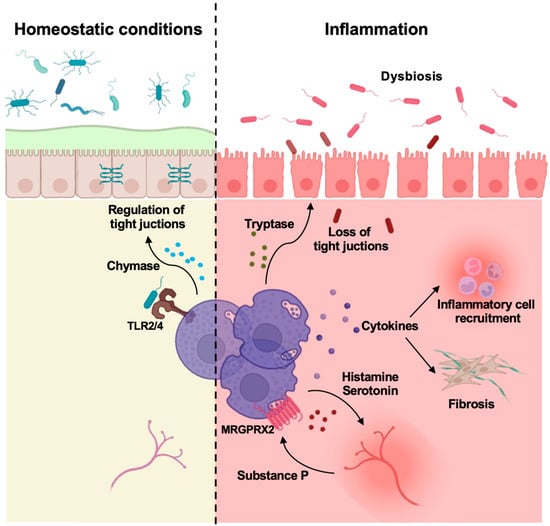
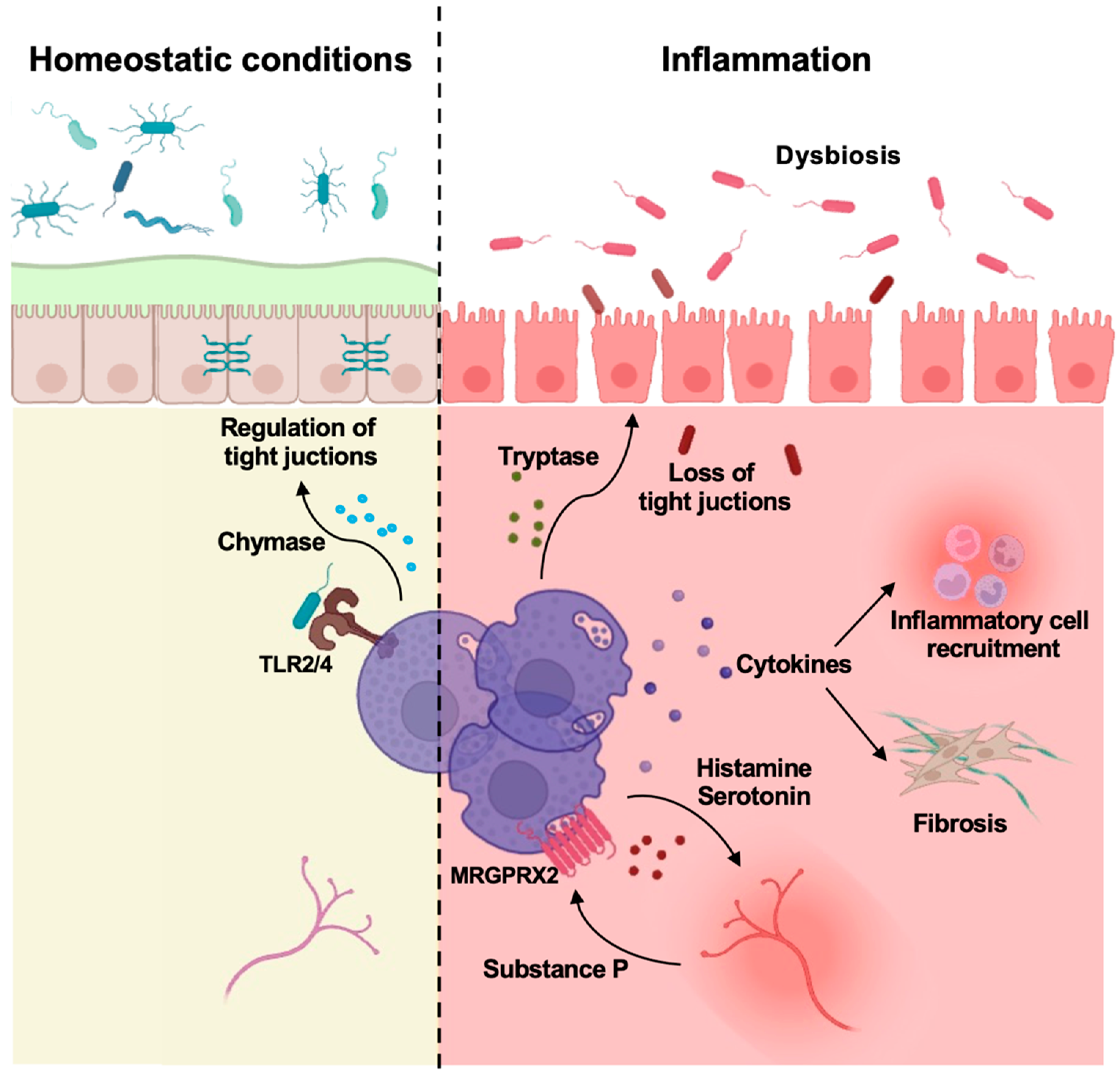
Figure 1.
Mast cells control gut homeostasis and inflammation. Intestinal MCs are involved in the maintenance of homeostasis (left side) as well as in orchestrating local inflammation leading to the development of IBD (right side). The main effects of mast cell mediators are depicted. Modified from Van Remoortel S et al., Cell Mol Gastroenterol Hepatol. 2024, 18, 101391 [83] and created in https://BioRender.com.
Of note, the presence of activated and degranulated MCs in the colon during the transition from gut inflammation to transformation supports MC implication in colon cancer (CRC) development [6]. However, the precise role of the different intestinal MC subsets in the diverse phases of tumor development is still a matter of debate [84,85].
Thus, intestinal MCs acquire a different behavior when faced with normal, damaged, or transformed epithelial cells in the gut, and eventually they can orchestrate deviated immune responses.
5. Characterization of Intestinal MCs in Patients with IBD
IBD, including ulcerative colitis (UC) and Crohn’s disease (CD), are complex multifactorial diseases of the gastrointestinal tract, triggered by environmental factors in genetically susceptible individuals [6]. Current therapies based on the use of monoclonal antibodies directed against cytokines offer amelioration and prolonged periods of remission but have important limitations. Indeed, during ulcerative colitis, more than 30% of patients do not initially respond to therapy, while others lose response over time [86]. Thus, new treatment strategies are needed.
Several studies have been performed to characterize the distribution and phenotype of MCs within the various intestinal segments of patients with IBD [7].
Initially, histological studies in humans performed by Bischoff and coauthors detected a reduced number of toluidine blue+ MCs in the involved intestinal segments of patients with IBD [87]. However, upon immunohistochemistry performed to evaluate the presence of hTryptase-β and hChymase-1, the same study discovered that a large portion of the immunoreactive proteases resided near but outside the MCs, suggesting that the apparent decreased number of MCs was due to MC degranulation.
Later, several authors provided convincing evidence of increased MC numbers in inflammatory bowel diseases, including UC and CD [88,89,90,91]. Nolte and coauthors found that MCs were increased in patients with UC compared with control subjects [88], while Nishida and colleagues found increased numbers of MCs in the upper part of lamina propria in patients with IBD [90]. A greater number of MCs was also observed in the hypertrophied and fibrotic tissue sites of CD patients compared with normal gut [89], suggesting a role for MCs in regulating intestinal fibrosis. Relevant to this, tryptases secreted from human MCs activate fibroblasts to differentiate into myofibroblasts, able to release more extracellular matrix (ECM) proteins during fibrosis changes revealed in IBD patients [92]. These findings have important clinical implications, providing support for the use of MC drugs to prevent IBD-induced intestinal fibrosis.
Not only the number of MCs was elevated, but also the expression levels of several MC mediators were greatly changed in IBD in comparison with normal subjects [82,86,87,88,89,90,91,92,93,94,95,96,97,98]. Early studies support MC contribution to mucosal inflammation demonstrating an increased level of MC-derived pro-inflammatory mediators in intestinal biopsies of IBD patients [93,94]. To further assess the degree of MC activation in patients with IBD, mucosa biopsies were placed in an oxygenation system for 4 h and the levels of tryptase-β and histamine were then measured in the supernatants: their exocytosis was more pronounced in inflamed tissue compared with noninflamed colon [95]. An increased secretion of tryptase and histamine has also been documented in biopsies from the duodenum, colon, and rectum of IBD patients [96,97]. These results are in line with a previous study revealing a positive correlation between the level of the histamine metabolite in the urine of IBD patients with the severity and the extent of disease [98].
Both histamine and tryptase can trigger nociceptive receptors leading to hypersensitivity of visceral sensory nerves [99]. Moreover, mouse and human tryptases can favor barrier dysfunctions, as revealed in acute experimental colitis and IBD [4,5,6].
Interestingly, many populations of degranulating MCs also release substantial amounts of TNF-α, IL-16, and substance P in the mucosa of the ileum and colon of patients with IBD [100,101], supporting a role for those MC-released mediators in the pathogenesis of IBD.
Regarding the specific MC subset(s) involved in IBD, a recent paper supported a pivotal role for a large subset of connective-like murine MCs expressing the Mas-related G protein-coupled receptor b2 (Mrgprb2) receptor in the development of colitis [83]. Mrgprb2-expressing MCs increased in the inflamed colon of WT-type mice and were found in proximity with nerve fibers, whereas Mrgprb2-/- mice showed a reduction in the influx of neutrophils and acute colitis progression [83].
Accordingly, by single-cell RNA technology, Chen and coauthors demonstrated the presence of a human MC subset expressing MRGPRX2 that plays a role in the development of UC through the establishment of a positive feedback inflammatory loop [102].
These results suggest the targeting of MRGPRX2 as a novel potential therapeutic strategy in UC. However, signals regulating the expansion/recruitment of this connective-like MC subset during colonic inflammation remain undefined.
Different IgE-independent mechanisms have been proposed as triggers for the activation of intestinal MCs in patients with IBD.
Stress factors can enhance mucosal MC degranulation, resulting in GI barrier impairment and intestinal inflammation [103,104,105]. In humans, the release of MC proteases into the lumen of the small intestine occurs in response to cold pain stress, while the release of tryptase and histamine, but not PGD2, is more pronounced in food-allergic patients than in healthy volunteers [106].
Ig-free light chains are also able to activate MCs and play a role in murine MC-dependent colitis and possibly also in human IBDs [107]. Although this finding is intriguing, the mechanism of MC activation remains largely unclear.
A high number of MCs that express both FcγRI and TLR4 has been reported in IBD patients [108], suggesting that in vivo a synergistic action of IgG and LPS may account for MC activation. Apart from TLR4, the intracellular bacterial receptor NOD2 was also found to be up-regulated in intestinal MCs of CD patients [109], suggesting its contribution in the recruitment and/or activation of MCs.
The amounts of neuropeptide(s) increased in IBD patients and their capability to support activation of both murine and human intestinal MCs has been reported [110], suggesting a possible role for MC/intestinal neuron cooperation in the pathogenesis of the disease (Figure 2).
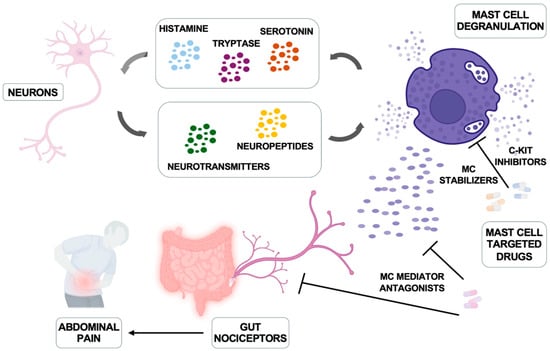
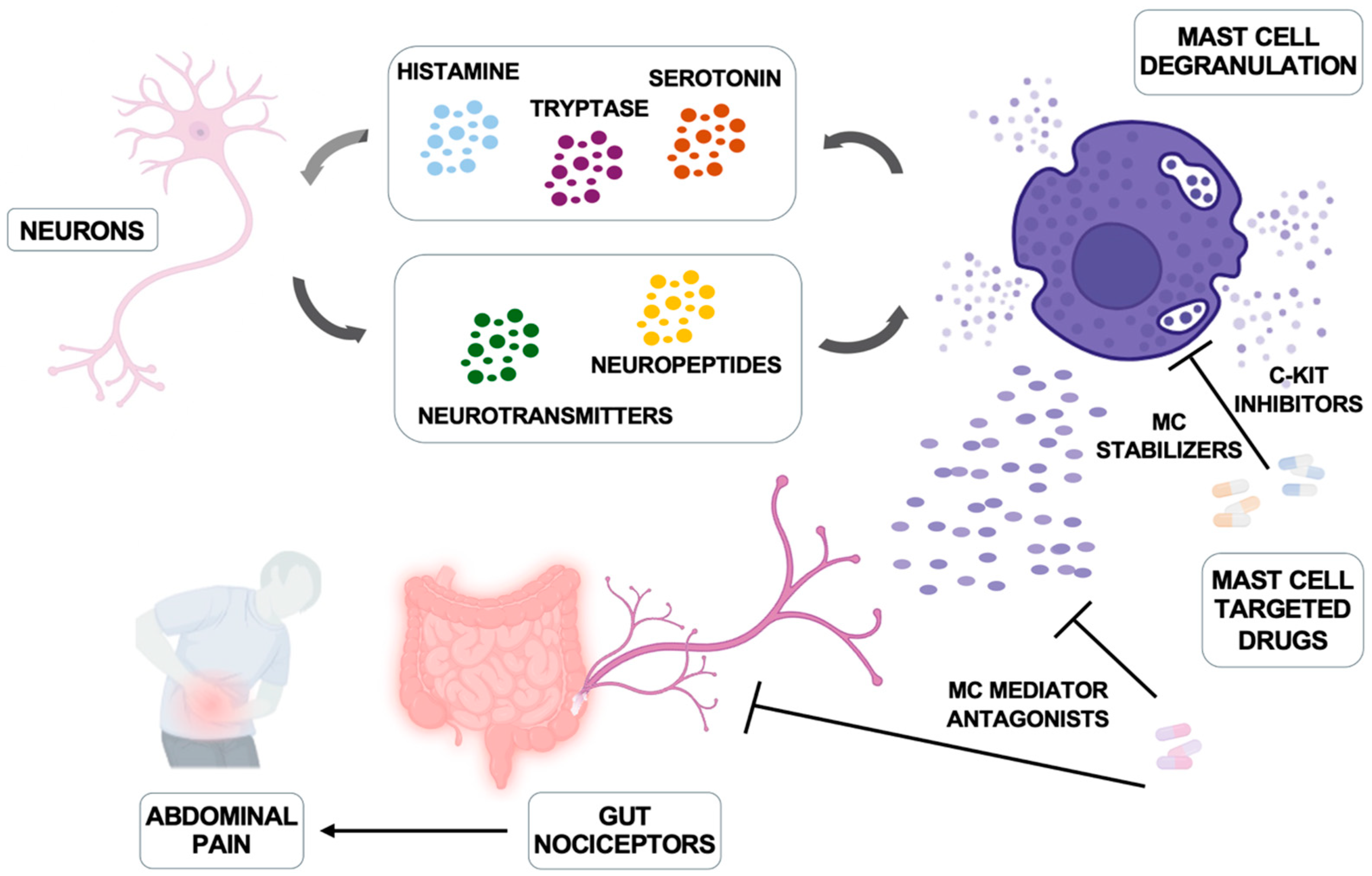
Figure 2.
Mast cells and nerve bidirectional communication and therapeutic interventions. Neurons produce neuropeptides and hormones that trigger mast cell activation and degranulation; in turn, mast cells produce histamine, serotonin, and tryptase that can regulate neuronal function. The main inhibitors of mast cells and their mediators are illustrated. Modified from Jacobson A et al. Mucosal Immunology 2021, 14, 555–565 [111] and created in https://BioRender.com.
6. Mast Cells and Irritable Bowel Syndrome
IBD and irritable bowel syndrome (IBS) are for the most part overlapping in terms of several symptoms, including abdominal pain and diarrhea. However, IBS is a functional gut disorder characterized by neuroinflammation and irregular digestive problems resulting from several non-pharmacological or pathological stimuli and by emotional feelings [112].
A first link between MCs and IBS was demonstrated by Weston and coauthors, reporting increased numbers of mucosal MCs in the terminal ileum of IBS patients [113]. Along the same line, Barbara and coauthors showed that patients suffering from IBS had more MCs in the left colon and rectum than those of normal individuals, and many of them resided near to nerve endings [114].
Once activated, those MCs release histamine and tryptase that may act on intestinal neurons through histamine receptors and proteinase-activated receptor (PAR-2) [115,116], thus explaining not only the pain but also gut sensorimotor dysfunction and related diarrhea in patients with IBS [117,118]. Thus, a bi-directional MC-nerve-interaction occurs, as illustrated in Figure 2: MCs trigger neurons for activation, and neuronal factors promote/amplify MC activation [6,111,119].
Interestingly, Aguillera-Lizarraga and coauthors described an IgE-dependent mechanism of MC activation caused by a brake in oral tolerance to dietary antigens induced by an inflammatory environment and resulted in food-induced abdominal pain [76]. However, the main MC subset implicated has not been characterized.
Upon MC activation, enhanced tryptase activity has been shown in ex vivo colonic biopsies obtained by patients with diarrhea-predominant IBS (IBS-D) [120]. On the contrary, patients with constipation-predominant IBS (IBS-C) show high levels of MC cysteine protease activity that positively correlates with disease severity and abdominal pain scoring [121].
Of note, upregulation of the gene encoding for the MRGPRX2 receptor has been observed in colonic biopsies of some IBS patients that also show high levels of IL-1b and prostaglandin synthase PTGS2 gene expression [122], supporting the presence in those patients of a connective-like MC subset that may contribute to the development of abdominal pain. However, to what extent this subgroup of patients could benefit from mast cell-targeted therapy remains to be investigated.
Thus, different environmental stimuli cause the onset of IBS-D or IBS-C symptoms, in which the involvement of specific MC-released mediators may be hypothesized. However, further investigations are needed to identify the main MC subset(s) involved.
7. Targeting MCs and Their Mediators in IBD and IBS
MCs and their derived mediators may represent targets for therapeutic options in patients with IBD and IBS.
The candidate drugs include MC stabilizers (ketotifen or cromoglycate), antagonists of MC mediators, and inhibitors of MC proteases [112], some of which show benefit to IBD and IBS patients (Table 1).
Treatment with the MC stabilizer ketotifen had shown beneficial effects in IBD and IBS patients [123,124,125,126]. Similarly, disodium cromoglycate (DSCG) administration resulted in a clinical improvement of symptoms in IBS-D patients by decreasing the release of tryptase but also in IBD [127,128,129].
Second-generation anti-histaminergic drugs, such as ebastine, prevent histamine-mediated signaling by blocking the histamine receptor H1 and represent the first-line treatment option for IgE-mediated MC disorders such as allergic rhinitis and chronic urticaria [130].
The effect of ebastine has been evaluated in a first pilot study on patients with IBS: up to 46% of patients receiving ebastine for 12 weeks reported significantly improved symptom relief and reduced abdominal pain, compared to 13% in the placebo-treated group [131]. More recently, a phase 2 randomized, placebo-controlled study further confirmed the potential of ebastine in improving global relief of hypersensitivity symptoms and abdominal pain intensity in IBS-D patients [132].
Blockade of serotonin may also represent another interesting approach to treat abdominal pain. Indeed, 5-HT3 antagonists, such as alosetron and ramosetron, have been repeatedly shown to be effective in IBS-D patients with reduction in abdominal pain and discomfort [133,134].
Serine protease inhibitors have proven efficacy as a treatment for visceral hypersensitivity in preclinical models of IBS [135,136]. Moreover, in a phase II study on UC patients, the tryptase inhibitor APC 2059 showed symptom improvement in more than 50% of the treated patients [137].

Table 1.
Mast cell-targeted therapies in IBD and IBS.
Table 1.
Mast cell-targeted therapies in IBD and IBS.
| Targets | Drugs | Mechanism of Action | Clinical Outcomes | References |
MC (activation and degranulation) | Ketotifen Disodium Cromoglycate | Antagonize H1R and stabilize MCs Inhibit MC degranulation | Improve bowel functions of IBD (UC) and IBS-D patients Improve symptoms of IBD (UC) and IBS-D patients | [123,124,125,126] [127,128,129] |
| MC mediators: Histamine Serotonin Tryptase Serine proteases | Ebastin Ramosetron Alosetron APC2059 | H1R antagonist 5-HT3 antagonists PAR2 antagonist | Improve visceral pain of IBS-D and IBS-NC patients Reduction of abdominal pain and discomfort in IBS-D and IBS-M patients Inhibit hypersensitivity and improve symptoms of IBD (UC) and IBS patients | [131,132] [133,134] [135,136,137] |
H1R, histamine H1 receptor; 5-HT3, serotonin receptor subtype 3; IBD, irritable bowel disease; IBS, irritable bowel syndrome; IBS-D, diarrhea-predominant IBS; IBS-M, mixed IBS; IBS-NC, non-constipated IBS; MC, mast cells; PAR, protease-activated receptor; SCF, stem cell factor; UC, ulcerative colitis.
Considering the ability of specific bacterial or fungal strains to selectively modulate MC functions [69], the use of probiotics may also represent a therapeutic option to modulate MCs in patients with IBD and IBS. Indeed, the administration of probiotics displayed positive effects on the treatment of pouchitis [138], in the induction of remission in patients with UC [139,140], and in the reduction in symptoms and inflammation in patients suffering from IBS [141].
However, at present no recommendations regarding individual species, strains, or mixed compositions can be made because of the limited data available. Moreover, the mechanisms by which probiotics exert their effect are highly complex, and a better characterization of the main MC subset(s) involved is needed to identify more effective and safe therapies for patients.
Several studies have identified pro-inflammatory dietary patterns, such as those high in processed foods and low in fiber, as contributors to gastrointestinal symptoms in IBS [142,143]. Of note, injection of certain food antigens (gluten, wheat, soy, and milk) into the rectosigmoid mucosa of patients with IBS may promote MC activation [76].
However, further studies are required to reveal whether and how dietary components contribute to IBS to define the right diet for the prevention and/or the management of the gut syndrome.
8. Conclusions and Future Directions
MCs can carry out diverse functions in the gut. Indeed, they can exert a protective role that contributes to intestinal homeostasis, but they can also release potent pro-inflammatory mediators that exacerbate the many features of IBD and IBS. This shift in function is likely due to intestinal MC plasticity driven by cues from the altered tissue microenvironments. Furthermore, it is possible that a shift in the composition of the microbiota may affect the phenotype and the functions of MCs and may contribute to the increased prevalence of IBD in the last decade [70,71].
The release of pro-inflammatory mediators from the activated MCs in the gut of patients with IBD and IBS likely contributes to the inability of the epithelium to act as an effective barrier to pathogens.
The possibilities to suppress MC functions are quite limited since available drugs are restricted to MC stabilizers or antagonists of MC mediators. MC stabilizers have an effect on some IBD and IBS patients; however, the effects are rather weak and inconsistent. Although a reduction in abdominal pain and symptoms has been reported in IBS patients upon treatment with the histamine receptor H1 antagonist ebastine, histamine antagonists turned out to have limited effect in IBD patients, possibly because MC mediators other than histamine play a major role. Regarding the c-kit inhibitors, both Midostaurin and Avapritinib are only approved for the treatment of advanced systemic mastocytosis [144].
In conclusion, a better characterization of the main MC subset(s) involved in IBD and IBS is needed to identify more effective and safe therapies for patients.
Author Contributions
All authors contributed to writing the manuscript and preparing the figures. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by grants from the Italian Association for Cancer Research (AIRC IG-24955) and Istituto Pasteur Italia-Fondazione Cenci Bolognetti (2020-366).
Acknowledgments
We apologize to all our colleagues whose important work could not be cited directly. Most of these references can be found in the review articles cited in the manuscript.
Conflicts of Interest
The authors declare no commercial or financial conflicts of interest.
References
- Kinet, J.P. The high-affinity IgE receptor (Fc epsilon RI): From physiology to pathology. Annu. Rev. Immunol. 1999, 17, 931–972. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Brant, S.R. Recent insights into the genetics of inflammatory bowel disease. Gastroenterology 2011, 140, 1704–1712. [Google Scholar] [CrossRef] [PubMed]
- Stevens, R.L.; Lee, T.D.; Seldin, D.C.; Austen, K.F.; Befus, A.D.; Bienenstock, J. Intestinal mucosal mast cells from rats infected with Nippostrongylus brasiliensis contain protease-resistant chondroitin sulfate di-B proteoglycans. J. Immunol. 1986, 137, 291–295. [Google Scholar] [CrossRef]
- Hamilton, M.J.; Sinnamon, M.J.; Lyng, G.D.; Glickman, J.N.; Wang, X.; Xing, W.; Krilis, S.A.; Blumberg, R.S.; Adachi, R.; Lee, D.M.; et al. Essential role for mast cell tryptase in acute experimental colitis. Proc. Natl. Acad. Sci. USA 2011, 108, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Wilcz-Villega, E.M.; McClean, S.; O’Sullivan, M.A. Mast cell tryptase reduces junctional adhesion molecule-A (JAM-A) expression in intestinal epithelial cells: Implications for the mechanisms of barrier dysfunction in irritable bowel syndrome. Am. J. Gastroenterol. 2013, 108, 1140–1151. [Google Scholar] [CrossRef]
- Bischoff, S.C. Mast cells in gastrointestinal disorders. Eur. J. Pharmacol. 2016, 778, 139–145. [Google Scholar] [CrossRef]
- Hamilton, M.J.; Frei, S.M.; Stevens, R.L. The multifaceted mast cell in inflammatory bowel disease. Inflamm. Bowel Dis. 2014, 20, 2364–2378. [Google Scholar] [CrossRef]
- Chen, C.C.; Grimbaldeston, M.A.; Tsai, M.; Weissman, I.L.; Galli, S.J. Identification of mast cell progenitors in adult mice. Proc. Natl. Acad. Sci. USA 2005, 102, 11408–11413. [Google Scholar] [CrossRef] [PubMed]
- Arinobu, Y.; Iwasaki, H.; Gurish, M.F.; Mizuno, S.; Shigematsu, H.; Ozawa, H.; Tenen, D.G.; Austen, K.F.; Akashi, K. Developmental checkpoints of the basophil/mast cell lineages in adult murine hematopoiesis. Proc. Natl. Acad. Sci. USA 2005, 102, 18105–18110. [Google Scholar] [CrossRef] [PubMed]
- Crapper, R.M.; Schrader, J.W. Frequency of mast cell precursors in normal tissues determined by an in vitro assay: Antigen induces parallel increases in the frequency of P cell precursors and mast cells. J. Immunol. 1983, 131, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Gurish, M.F.; Tao, H.; Abonia, J.P.; Arya, A.; Friend, D.S.; Parker, C.M.; Austen, K.F. Intestinal mast cell progenitors require CD49dbeta7 (alpha4beta7 integrin) for tissue-specific homing. J. Exp. Med. 2001, 194, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Abonia, J.P.; Austen, K.F.; Rollins, B.J.; Joshi, S.K.; Flavell, R.A.; Kuziel, W.A.; Koni, P.A.; Gurish, M.F. Constitutive homing of mast cell progenitors to the intestine depends on autologous expression of the chemokine receptor CXCR2. Blood 2005, 105, 4308–4313. [Google Scholar] [CrossRef]
- Geissler, E.N.; Ryan, M.A.; Housman, D.E. The dominant-white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell 1988, 55, 185–192. [Google Scholar] [CrossRef]
- Flanagan, J.G.; Leder, P. The kit ligand: A cell surface molecule altered in steel mutant fibroblasts. Cell 1990, 63, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Levi-Schaffer, F.; Austen, K.F.; Gravallese, P.M.; Stevens, R.L. Coculture of interleukin 3-dependent mouse mast cells with fibroblasts results in a phenotypic change of the mast cells. Proc. Natl. Acad. Sci. USA 1986, 83, 6485–6488. [Google Scholar] [CrossRef]
- Karimi, K.; Redegeld, F.A.; Blom, R.; Nijkamp, F.P. Stem cell factor and interleukin-4 increase responsiveness of mast cells to substance P. Exp. Hematol. 2000, 28, 626–634. [Google Scholar] [CrossRef]
- Ochi, H.; De Jesus, N.H.; Hsieh, F.H.; Austen, K.F.; Boyce, J.A. IL-4 and -5 prime human mast cells for different profiles of IgE-dependent cytokine production. Proc. Natl. Acad. Sci. USA 2000, 97, 10509–10513. [Google Scholar] [CrossRef] [PubMed]
- Kaieda, S.; Shin, K.; Nigrovic, P.A.; Seki, K.; Lee, R.T.; Stevens, R.L.; Lee, D.M. Synovial fibroblasts promote the expression and granule accumulation of tryptase via interleukin-33 and its receptor ST-2 (IL1RL1). J. Biol. Chem. 2010, 285, 21478–21486. [Google Scholar] [CrossRef] [PubMed]
- Caslin, H.L.; Kiwanuka, K.N.; Haque, T.T.; Taruselli, M.T.; MacKnight, H.P.; Paranjape, A.; Ryan, J.J. Controlling Mast Cell Activation and Homeostasis: Work Influenced by Bill Paul That Continues Today. Front. Immunol. 2018, 9, 868. [Google Scholar] [CrossRef]
- Molfetta, R.; Lecce, M.; Milito, N.D.; Putro, E.; Pietropaolo, G.; Marangio, C.; Scarno, G.; Moretti, M.; De Smaele, E.; Santini, T.; et al. SCF and IL-33 regulate mouse mast cell phenotypic and functional plasticity supporting a pro-inflammatory microenvironment. Cell Death Dis. 2023, 14, 616. [Google Scholar] [CrossRef]
- Kawakami, T.; Galli, S.J. Regulation of mast-cell and basophil function and survival by IgE. Nat. Rev. Immunol. 2002, 2, 773–786. [Google Scholar] [CrossRef]
- Marone, G.; Galli, S.J.; Kitamura, Y. Probing the roles of mast cells and basophils in natural and acquired immunity, physiology and disease. Trends Immunol. 2002, 23, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Frossi, B.; Mion, F.; Tripodo, C.; Colombo, M.P.; Pucillo, C.E. Rheostatic Functions of Mast Cells in the Control of Innate and Adaptive Immune Responses. Trends Immunol. 2017, 38, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Mukai, K.; Tsai, M.; Saito, H.; Galli, S.J. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol. Rev. 2018, 282, 121–150. [Google Scholar] [CrossRef] [PubMed]
- Redegeld, F.A.; Yu, Y.; Kumari, S.; Charles, N.; Blank, U. Non-IgE mediated mast cell activation. Immunol. Rev. 2018, 282, 87–113. [Google Scholar] [CrossRef]
- Kraft, S.; Kinet, J.P. New developments in FcepsilonRI regulation, function and inhibition. Nat. Rev. Immunol. 2007, 7, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Lecce, M.; Molfetta, R.; Milito, N.D.; Santoni, A.; Paolini, R. FcεRI Signaling in the Modulation of Allergic Response: Role of Mast Cell-Derived Exosomes. Int. J. Mol. Sci. 2020, 21, 5464. [Google Scholar] [CrossRef]
- Razin, E.; Marx, G. Thrombin-induced degranulation of cultured bone marrow-derived mast cells. J. Immunol. 1984, 133, 3282–3285. [Google Scholar] [CrossRef]
- Vliagoftis, H. Thrombin induces mast cell adhesion to fibronectin: Evidence for involvement of protease-activated receptor-1. J. Immunol. 2002, 169, 4551–4558. [Google Scholar] [CrossRef] [PubMed]
- Malbec, O.; Daëron, M. The mast cell IgG receptors and their roles in tissue inflammation. Immunol. Rev. 2007, 217, 206–221. [Google Scholar] [CrossRef] [PubMed]
- Sudo, N.; Tanaka, K.; Koga, Y.; Okumura, Y.; Kubo, C.; Nomoto, K. Extracellular ATP activates mast cells via a mechanism that is different from the activation induced by the cross-linking of Fc receptors. J. Immunol. 1996, 156, 3970–3979. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, P.; Ennis, M. Adenosine, mast cells and asthma. Inflamm. Res. 1999, 48, 301–307. [Google Scholar] [CrossRef]
- Kurashima, Y.; Amiya, T.; Nochi, T.; Fujisawa, K.; Haraguchi, T.; Iba, H.; Tsutsui, H.; Sato, S.; Nakajima, S.; Iijima, H.; et al. Extracellular ATP mediates mast cell-dependent intestinal inflammation through P2X7 purinoceptors. Nat. Commun. 2012, 3, 1034. [Google Scholar] [CrossRef] [PubMed]
- Erdei, A.; Pecht, I. Complement peptides and mast cell triggering. Immunol. Lett. 1996, 54, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Kurashima, Y.; Goto, Y.; Kiyono, H. Mucosal innate immune cells regulate both gut homeostasis and intestinal inflammation. Eur. J. Immunol. 2013, 43, 3108–3115. [Google Scholar] [CrossRef] [PubMed]
- Collington, S.J.; Williams, T.J.; Weller, C.L. Mechanisms underlying the localisation of mast cells in tissues. Trends Immunol. 2011, 32, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; He, H.; Guo, X.K.; Wang, J.; Wang, W.; Li, D.; Liang, S.; Shao, F.; Liu, W.; Hu, X. Intraepithelial mast cells drive gasdermin C-mediated type 2 immunity. Immunity 2024, 57, 1056–1070.e5. [Google Scholar] [CrossRef]
- Irani, A.A.; Schechter, N.M.; Craig, S.S.; DeBlois, G.; Schwartz, L.B. Two types of human mast cells that have distinct neutral protease compositions. Proc. Natl. Acad. Sci. USA 1986, 83, 4464–4468. [Google Scholar] [CrossRef]
- Pallaoro, M.; Fejzo, M.S.; Shayesteh, L.; Blount, J.L.; Caughey, G.H. Characterization of genes encoding known and novel human mast cell tryptases on chromosome 16p13.3. J. Biol. Chem. 1999, 274, 3355–3362. [Google Scholar] [CrossRef]
- Weidner, N.; Austen, K.F. Heterogeneity of mast cells at multiple body sites. Fluorescent determination of avidin binding and immunofluorescent determination of chymase, tryptase, and carboxypeptidase content. Pathol. Res. Pract. 1993, 189, 156–162. [Google Scholar] [CrossRef]
- Gurish, M.F.; Austen, K.F. Developmental origin and functional specialization of mast cell subsets. Immunity 2012, 37, 25–33. [Google Scholar] [CrossRef]
- Dougherty, R.H.; Sidhu, S.S.; Raman, K.; Solon, M.; Solberg, O.D.; Caughey, G.H.; Woodruff, P.G.; Fahy, J.V. Accumulation of intraepithelial mast cells with a unique protease phenotype in T(H)2-high asthma. J. Allergy Clin. Immunol. 2010, 125, 1046–1053.e8. [Google Scholar] [CrossRef]
- Abonia, J.P.; Blanchard, C.; Butz, B.B.; Rainey, H.F.; Collins, M.H.; Stringer, K.; Putnam, P.E.; Rothenberg, M.E. Involvement of mast cells in eosinophilic esophagitis. J. Allergy Clin. Immunol. 2010, 126, 140–149. [Google Scholar] [CrossRef]
- Shea-Donohue, T.; Stiltz, J.; Zhao, A.; Notari, L. Mast cells. Curr. Gastroenterol. Rep. 2010, 12, 349–357. [Google Scholar] [CrossRef]
- Tauber, M.; Basso, L.; Martin, J.; Bostan, L.; Pinto, M.M.; Thierry, G.R.; Houmadi, R.; Serhan, N.; Loste, A.; Blériot, C.; et al. Landscape of mast cell populations across organs in mice and humans. J. Exp. Med. 2023, 220, e20230570, Erratum in J. Exp. Med. 2024, 221, e2023057001172024c. [Google Scholar] [CrossRef]
- Putro, E.; Carnevale, A.; Marangio, C.; Fulci, V.; Paolini, R.; Molfetta, R. New Insight into Intestinal Mast Cells Revealed by Single-Cell RNA Sequencing. Int. J. Mol. Sci. 2024, 25, 5594. [Google Scholar] [CrossRef]
- Liu, L.; Davidorf, B.; Dong, P.; Peng, A.; Song, Q.; He, Z. Decoding the mosaic of inflammatory bowel disease: Illuminating insights with single-cell RNA technology. Comput. Struct. Biotechnol. J. 2024, 23, 2911–2923. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Y.; Zhu, M.; Zhu, B.; Wang, Z.Q. Tryptase disturbs endocytic allergen degradation in intestinal epithelial cells. Anal. Biochem. 2013, 434, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Abonia, J.P.; Friend, D.S.; Austen, W.G., Jr.; Moore, F.D., Jr.; Carroll, M.C.; Chan, R.; Afnan, J.; Humbles, A.; Gerard, C.; Knight, P.; et al. Mast cell protease 5 mediates ischemia-reperfusion injury of mouse skeletal muscle. J. Immunol. 2005, 174, 7285–7291. [Google Scholar] [CrossRef]
- Younan, G.; Suber, F.; Xing, W.; Shi, T.; Kunori, Y.; Abrink, M.; Pejler, G.; Schlenner, S.M.; Rodewald, H.R.; Moore, F.D., Jr.; et al. The inflammatory response after an epidermal burn depends on the activities of mouse mast cell proteases 4 and 5. J. Immunol. 2010, 185, 7681–7690. [Google Scholar] [CrossRef] [PubMed]
- Bankova, L.G.; Lezcano, C.; Pejler, G.; Stevens, R.L.; Murphy, G.F.; Austen, K.F.; Gurish, M.F. Mouse mast cell proteases 4 and 5 mediate epidermal injury through disruption of tight junctions. J. Immunol. 2014, 192, 2812–2820. [Google Scholar] [CrossRef]
- Groschwitz, K.R.; Ahrens, R.; Osterfeld, H.; Gurish, M.F.; Han, X.; Abrink, M.; Finkelman, F.D.; Pejler, G.; Hogan, S.P. Mast cells regulate homeostatic intestinal epithelial migration and barrier function by a chymase/Mcpt4-dependent mechanism. Proc. Natl. Acad. Sci. USA 2009, 106, 22381–22386. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Zmora, N.; Levy, M.; Elinav, E. The microbiome and innate immunity. Nature 2016, 535, 65–74. [Google Scholar] [CrossRef] [PubMed]
- De Zuani, M.; Dal Secco, C.; Frossi, B. Mast cells at the crossroads of microbiota and IBD. Eur. J. Immunol. 2018, 48, 1929–1937. [Google Scholar] [CrossRef] [PubMed]
- Kunii, J.; Takahashi, K.; Kasakura, K.; Tsuda, M.; Nakano, K.; Hosono, A.; Kaminogawa, S. Commensal bacteria promote migration of mast cells into the intestine. Immunobiology 2011, 216, 692–697. [Google Scholar] [CrossRef]
- Schwarzer, M.; Hermanova, P.; Srutkova, D.; Golias, J.; Hudcovic, T.; Zwicker, C.; Sinkora, M.; Akgün, J.; Wiedermann, U.; Tuckova, L.; et al. Germ-Free Mice Exhibit Mast Cells With Impaired Functionality and Gut Homing and Do Not Develop Food Allergy. Front. Immunol. 2019, 10, 205. [Google Scholar] [CrossRef]
- Abraham, S.N.; St John, A.L. Mast cell-orchestrated immunity to pathogens. Nat. Rev. Immunol. 2010, 10, 440–452. [Google Scholar] [CrossRef]
- Supajatura, V.; Ushio, H.; Nakao, A.; Akira, S.; Okumura, K.; Ra, C.; Ogawa, H. Differential responses of mast cell Toll-like receptors 2 and 4 in allergy and innate immunity. J. Clin. Investig. 2002, 109, 1351–1359. [Google Scholar] [CrossRef]
- Wesolowski, J.; Paumet, F. The impact of bacterial infection on mast cell degranulation. Immunol. Res. 2011, 51, 215–226. [Google Scholar] [CrossRef]
- Rönnberg, E.; Johnzon, C.F.; Calounova, G.; Garcia Faroldi, G.; Grujic, M.; Hartmann, K.; Roers, A.; Guss, B.; Lundequist, A.; Pejler, G. Mast cells are activated by Staphylococcus aureus in vitro but do not influence the outcome of intraperitoneal S. aureus infection in vivo. Immunology 2014, 143, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Gendrin, C.; Shubin, N.J.; Boldenow, E.; Merillat, S.; Clauson, M.; Power, D.; Doran, K.S.; Abrink, M.; Pejler, G.; Rajagopal, L.; et al. Mast cell chymase decreases the severity of group B Streptococcus infections. J. Allergy Clin. Immunol. 2018, 142, 120–129.e6. [Google Scholar] [CrossRef]
- Campillo-Navarro, M.; Leyva-Paredes, K.; Donis-Maturano, L.; González-Jiménez, M.; Paredes-Vivas, Y.; Cerbulo-Vázquez, A.; Serafín-López, J.; García-Pérez, B.; Ullrich, S.E.; Flores-Romo, L.; et al. Listeria monocytogenes induces mast cell extracellular traps. Immunobiology 2017, 222, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.P.; Stylianou, M.; Nilsson, G.; Urban, C.F. Opportunistic pathogen Candida albicans elicits a temporal response in primary human mast cells. Sci. Rep. 2015, 5, 12287. [Google Scholar] [CrossRef] [PubMed]
- Kasakura, K.; Takahashi, K.; Itoh, T.; Hosono, A.; Momose, Y.; Itoh, K.; Nishiyama, C.; Kaminogawa, S. Commensal bacteria directly suppress in vitro degranulation of mast cells in a MyD88-independent manner. Biosci. Biotechnol. Biochem. 2014, 78, 1669–1676. [Google Scholar] [CrossRef] [PubMed]
- Cassard, L.; Lalanne, A.I.; Garault, P.; Cotillard, A.; Chervaux, C.; Wels, M.; Smokvina, T.; Daëron, M.; Bourdet-Sicard, R. Individual strains of Lactobacillus paracasei differentially inhibit human basophil and mouse mast cell activation. Immun. Inflamm. Dis. 2016, 4, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Iwasa, M.; Han, K.I.; Kim, W.J.; Tang, Y.; Hwang, Y.J.; Chae, J.R.; Han, W.C.; Shin, Y.S.; Kim, E.K. Heat-Killed Enterococcus faecalis EF-2001 Ameliorates Atopic Dermatitis in a Murine Model. Nutrients 2016, 8, 146. [Google Scholar] [CrossRef] [PubMed]
- Toor, D.; Wsson, M.K.; Kumar, P.; Karthikeyan, G.; Kaushik, N.K.; Goel, C.; Singh, S.; Kumar, A.; Prakash, H. Dysbiosis Disrupts Gut Immune Homeostasis and Promotes Gastric Diseases. Int. J. Mol. Sci. 2019, 20, 2432. [Google Scholar] [CrossRef]
- Bäckhed, F.; Fraser, C.M.; Ringel, Y.; Sanders, M.E.; Sartor, R.B.; Sherman, P.M.; Versalovic, J.; Young, V.; Finlay, B.B. Defining a healthy human gut microbiome: Current concepts, future directions, and clinical applications. Cell Host Microbe 2012, 12, 611–622. [Google Scholar] [CrossRef] [PubMed]
- di Vito, R.; Di Mezza, A.; Conte, C.; Traina, G. The Crosstalk between Intestinal Epithelial Cells and Mast Cells Is Modulated by the Probiotic Supplementation in Co-Culture Models. Int. J. Mol. Sci. 2023, 24, 4157. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.N.; St Amand, A.L.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef]
- Halfvarson, J.; Brislawn, C.J.; Lamendella, R.; Vázquez-Baeza, Y.; Walters, W.A.; Bramer, L.M.; D’Amato, M.; Bonfiglio, F.; McDonald, D.; Gonzalez, A.; et al. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat. Microbiol. 2017, 2, 17004. [Google Scholar] [CrossRef] [PubMed]
- Knight, P.A.; Wright, S.H.; Lawrence, C.E.; Paterson, Y.Y.; Miller, H.R. Delayed expulsion of the nematode Trichinella spiralis in mice lacking the mucosal mast cell-specific granule chymase, mouse mast cell protease-1. J. Exp. Med. 2000, 192, 1849–1856. [Google Scholar] [CrossRef]
- McDermott, J.R.; Bartram, R.E.; Knight, P.A.; Miller, H.R.; Garrod, D.R.; Grencis, R.K. Mast cells disrupt epithelial barrier function during enteric nematode infection. Proc. Natl. Acad. Sci. USA 2003, 100, 7761–7766. [Google Scholar] [CrossRef] [PubMed]
- Sorobetea, D.; Holm, J.B.; Henningsson, H.; Kristiansen, K.; Svensson-Frej, M. Acute infection with the intestinal parasite Trichuris muris has long-term consequences on mucosal mast cell homeostasis and epithelial integrity. Eur. J. Immunol. 2017, 47, 257–268. [Google Scholar] [CrossRef]
- Shin, K.; Watts, G.F.; Oettgen, H.C.; Friend, D.S.; Pemberton, A.D.; Gurish, M.F.; Lee, D.M. Mouse mast cell tryptase mMCP-6 is a critical link between adaptive and innate immunity in the chronic phase of Trichinella spiralis infection. J. Immunol. 2008, 180, 4885–4891. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Lizarraga, J.; Florens, M.V.; Viola, M.F.; Jain, P.; Decraecker, L.; Appeltans, I.; Cuende-Estevez, M.; Fabre, N.; Van Beek, K.; Perna, E.; et al. Local immune response to food antigens drives meal-induced abdominal pain. Nature 2021, 590, 151–156. [Google Scholar] [CrossRef]
- Oettgen, H.C. Mast cells in food allergy: Inducing immediate reactions and shaping long-term immunity. J. Allergy Clin. Immunol. 2023, 151, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Oyoshi, M.K.; Oettgen, H.C.; Chatila, T.A.; Geha, R.S.; Bryce, P.J. Food allergy: Insights into etiology, prevention, and treatment provided by murine models. J. Allergy Clin. Immunol. 2014, 133, 309–317. [Google Scholar] [CrossRef]
- Kanagaratham, C.; El Ansari, Y.S.; Lewis, O.L.; Oettgen, H.C. IgE and IgG Antibodies as Regulators of Mast Cell and Basophil Functions in Food Allergy. Front. Immunol. 2020, 11, 603050. [Google Scholar] [CrossRef] [PubMed]
- Dispenza, M.C.; Bochner, B.S.; MacGlashan, D.W., Jr. Targeting the FcεRI Pathway as a Potential Strategy to Prevent Food-Induced Anaphylaxis. Front. Immunol. 2020, 17, 614402. [Google Scholar] [CrossRef] [PubMed]
- Divekar, R.; Kita, H. Recent advances in epithelium-derived cytokines (IL-33, IL-25, and thymic stromal lymphopoietin) and allergic inflammation. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 98–103. [Google Scholar] [CrossRef]
- Theoharides, T.C. Mast cells in irritable bowel syndrome and ulcerative colitis: Function not numbers is what makes all the difference. Dig. Dis. Sci. 2014, 59, 897–898. [Google Scholar] [CrossRef][Green Version]
- Van Remoortel, S.; Lambeets, L.; De Winter, B.; Dong, X.; Rodriguez Ruiz, J.P.; Kumar-Singh, S.; Martinez, S.I.; Timmermans, J.P. Mrgprb2-dependent Mast Cell Activation Plays a Crucial Role in Acute Colitis. Cell Mol. Gastroenterol. Hepatol. 2024, 18, 101391. [Google Scholar] [CrossRef] [PubMed]
- Heijmans, J.; Büller, N.V.; Muncan, V.; van den Brink, G.R. Role of mast cells in colorectal cancer development, the jury is still out. Biochim. Biophys. Acta 2012, 1822, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Molfetta, R.; Paolini, R. The Controversial Role of Intestinal Mast Cells in Colon Cancer. Cells 2023, 12, 459. [Google Scholar] [CrossRef]
- Smillie, C.S.; Biton, M.; Ordovas-Montanes, J.; Sullivan, K.M.; Burgin, G.; Graham, D.B.; Herbst, R.H.; Rogel, N.; Slyper, M.; Waldman, J.; et al. Intra- and Inter-cellular Rewiring of the Human Colon during Ulcerative Colitis. Cell 2019, 178, 714–730.e22. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Wedemeyer, J.; Herrmann, A.; Meier, P.N.; Trautwein, C.; Cetin, Y.; Maschek, H.; Stolte, M.; Gebel, M.; Manns, M.P. Quantitative assessment of intestinal eosinophils and mast cells in inflammatory bowel disease. Histopathology 1996, 28, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Nolte, H.; Spjeldnaes, N.; Kruse, A.; Windelborg, B. Histamine release from gut mast cells from patients with inflammatory bowel diseases. Gut 1990, 31, 791–794. [Google Scholar] [CrossRef]
- Gelbmann, C.M.; Mestermann, S.; Gross, V.; Köllinger, M.; Schölmerich, J.; Falk, W. Strictures in Crohn’s disease are characterised by an accumulation of mast cells colocalised with laminin but not with fibronectin or vitronectin. Gut 1999, 45, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Murase, K.; Isomoto, H.; Furusu, H.; Mizuta, Y.; Riddell, R.H.; Kohno, S. Different distribution of mast cells and macrophages in colonic mucosa of patients with collagenous colitis and inflammatory bowel disease. Hepatogastroenterology 2002, 49, 678–682. [Google Scholar] [PubMed]
- Sasaki, Y.; Tanaka, M.; Kudo, H. Differentiation between ulcerative colitis and Crohn’s disease by a quantitative immunohistochemical evaluation of T lymphocytes, neutrophils, histiocytes and mast cells. Pathol. Int. 2002, 52, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yang, M.Q.; Yu, T.Y.; Yin, Y.Y.; Liu, Y.; Wang, X.D.; He, Z.G.; Yin, L.; Chen, C.Q.; Li, J.Y. Mast Cell Tryptase Promotes Inflammatory Bowel Disease-Induced Intestinal Fibrosis. Inflamm. Bowel Dis. 2021, 27, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.C.; Lazenby, A.J.; Moore, W.C.; Yardley, J.H.; Bayless, T.M.; Lichtenstein, L.M. Enhancement of human intestinal mast cell mediator release in active ulcerative colitis. Gastroenterology 1990, 99, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Knutson, L.; Ahrenstedt, O.; Odlind, B.; Hällgren, R. The jejunal secretion of histamine is increased in active Crohn’s disease. Gastroenterology 1990, 98, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Raithel, M.; Winterkamp, S.; Pacurar, A.; Ulrich, P.; Hochberger, J.; Hahn, E.G. Release of mast cell tryptase from human colorectal mucosa in inflammatory bowel disease. Scand. J. Gastroenterol. 2001, 36, 174–179. [Google Scholar] [CrossRef]
- De Winter, B.Y.; van den Wijngaard, R.M.; de Jonge, W.J. Intestinal mast cells in gut inflammation and motility disturbances. Biochim. Biophys. Acta 2012, 1822, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.Y.; Lee, K.H.; Choi, C.H.; Kim, J.W.; Lee, H.W.; Kim, J.W.; Kim, M.K.; Kwon, G.Y.; Han, S.; Kim, S.E.; et al. Colonic mucosal immune activity in irritable bowel syndrome: Comparison with healthy controls and patients with ulcerative colitis. Dig. Dis. Sci. 2014, 59, 1001–1011. [Google Scholar] [CrossRef]
- Winterkamp, S.; Weidenhiller, M.; Otte, P.; Stolper, J.; Schwab, D.; Hahn, E.G.; Raithel, M. Urinary excretion of N-methylhistamine as a marker of disease activity in inflammatory bowel disease. Am. J. Gastroenterol. 2002, 97, 3071–3077. [Google Scholar] [CrossRef]
- He, S.H. Key role of mast cells and their major secretory products in inflammatory bowel disease. World J. Gastroenterol. 2004, 10, 309–318. [Google Scholar] [CrossRef]
- Middel, P.; Reich, K.; Polzien, F.; Blaschke, V.; Hemmerlein, B.; Herms, J.; Korabiowska, M.; Radzun, H.J. Interleukin 16 expression and phenotype of interleukin 16 producing cells in Crohn’s disease. Gut 2001, 49, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Stoyanova, I.I.; Gulubova, M.V. Mast cells and inflammatory mediators in chronic ulcerative colitis. Acta Histochem. 2002, 104, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.; Chuang, L.S.; Giri, M.; Villaverde, N.; Hsu, N.Y.; Sabic, K.; Joshowitz, S.; Gettler, K.; Nayar, S.; Chai, Z.; et al. Inflamed Ulcerative Colitis Regions Associated With MRGPRX2-Mediated Mast Cell Degranulation and Cell Activation Modules, Defining a New Therapeutic Target. Gastroenterology 2021, 160, 1709–1724. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, A.; Keshavarzian, A.; Fields, J.Z.; Jakate, S.; Shaikh, M.; Banan, A. Reduced immunostaining for c-kit receptors in mucosal mast cells in inflammatory bowel disease. J. Gastroenterol. Hepatol. 2007, 22, 2338–2343. [Google Scholar] [CrossRef]
- Overman, E.L.; Rivier, J.E.; Moeser, A.J. CRF induces intestinal epithelial barrier injury via the release of mast cell proteases and TNF-α. PLoS ONE 2012, 7, e39935. [Google Scholar] [CrossRef]
- Vanuytsel, T.; van Wanrooy, S.; Vanheel, H.; Vanormelingen, C.; Verschueren, S.; Houben, E.; Salim Rasoel, S.; Tόth, J.; Holvoet, L.; Farré, R.; et al. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut 2014, 63, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.; Saperas, E.; Nogueiras, C.; Mourelle, M.; Antolín, M.; Cadahia, A.; Malagelada, J.R. Release of mast cell mediators into the jejunum by cold pain stress in humans. Gastroenterology 1998, 114, 640–648. [Google Scholar] [CrossRef]
- Rijnierse, A.; Redegeld, F.A.; Blokhuis, B.R.; Van der Heijden, M.W.; Te Velde, A.A.; Pronk, I.; Hommes, D.W.; Nijkamp, F.P.; Koster, A.S.; Kraneveld, A.D. Ig-free light chains play a crucial role in murine mast cell-dependent colitis and are associated with human inflammatory bowel diseases. J. Immunol. 2010, 185, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, R.; Okamura, S.; Ohno, T.; Saito, H.; Mori, M.; Ra, C.; Okayama, Y. Hyperexpression of FcgammaRI and Toll-like receptor 4 in the intestinal mast cells of Crohn’s disease patients. Clin. Immunol. 2007, 125, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Okumura, S.; Yuki, K.; Kobayashi, R.; Okamura, S.; Ohmori, K.; Saito, H.; Ra, C.; Okayama, Y. Hyperexpression of NOD2 in intestinal mast cells of Crohn’s disease patients: Preferential expression of inflammatory cell-recruiting molecules via NOD2 in mast cells. Clin. Immunol. 2009, 130, 175–185. [Google Scholar] [CrossRef]
- Van Nassauw, L.; Adriaensen, D.; Timmermans, J.P. The bidirectional communication between neurons and mast cells within the gastrointestinal tract. Auton. Neurosci. 2007, 133, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, A.; Yang, D.; Vella, M.; Chiu, I.M. The intestinal neuro-immune axis: Crosstalk between neurons, immune cells, and microbes. Mucosal Immunol. 2021, 14, 555–565. [Google Scholar] [CrossRef]
- Zhang, L.; Song, J.; Hou, X. Mast Cells and Irritable Bowel Syndrome: From the Bench to the Bedside. J. Neurogastroenterol. Motil. 2016, 22, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Weston, A.P.; Biddle, W.L.; Bhatia, P.S.; Miner, P.B., Jr. Terminal ileal mucosal mast cells in irritable bowel syndrome. Dig. Dis. Sci. 1993, 38, 1590–1595. [Google Scholar] [CrossRef] [PubMed]
- Barbara, G.; Stanghellini, V.; De Giorgio, R.; Cremon, C.; Cottrell, G.S.; Santini, D.; Pasquinelli, G.; Morselli-Labate, A.M.; Grady, E.F.; Bunnett, N.W.; et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology 2004, 126, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Cenac, N.; Andrews, C.N.; Holzhausen, M.; Chapman, K.; Cottrell, G.; Andrade-Gordon, P.; Steinhoff, M.; Barbara, G.; Beck, P.; Bunnett, N.W.; et al. Role for protease activity in visceral pain in irritable bowel syndrome. J. Clin. Investig. 2007, 117, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Buhner, S.; Li, Q.; Vignali, S.; Barbara, G.; De Giorgio, R.; Stanghellini, V.; Cremon, C.; Zeller, F.; Langer, R.; Daniel, H.; et al. Activation of human enteric neurons by supernatants of colonic biopsy specimens from patients with irritable bowel syndrome. Gastroenterology 2009, 137, 1425–1434. [Google Scholar] [CrossRef]
- Cremon, C.; Carini, G.; Wang, B.; Vasina, V.; Cogliandro, R.F.; De Giorgio, R.; Stanghellini, V.; Grundy, D.; Tonini, M.; De Ponti, F.; et al. Intestinal serotonin release, sensory neuron activation, and abdominal pain in irritable bowel syndrome. Am. J. Gastroenterol. 2011, 106, 1290–1298. [Google Scholar] [CrossRef]
- Buhner, S.; Braak, B.; Li, Q.; Kugler, E.M.; Klooker, T.; Wouters, M.; Donovan, J.; Vignali, S.; Mazzuoli-Weber, G.; Grundy, D.; et al. Neuronal activation by mucosal biopsy supernatants from irritable bowel syndrome patients is linked to visceral sensitivity. Exp. Physiol. 2014, 99, 1299–1311. [Google Scholar] [CrossRef]
- Forsythe, P.; Bienenstock, J. The mast cell-nerve functional unit: A key component of physiologic and pathophysiologic responses. Chem. Immunol. Allergy 2012, 98, 196–221. [Google Scholar] [CrossRef]
- Lee, J.W.; Park, J.H.; Park, D.I.; Park, J.H.; Kim, H.J.; Cho, Y.K.; Sohn, C.I.; Jeon, W.K.; Kim, B.I. Subjects with diarrhea-predominant IBS have increased rectal permeability responsive to tryptase. Dig. Dis. Sci. 2010, 55, 2922–2928. [Google Scholar] [CrossRef]
- Annaházi, A.; Ferrier, L.; Bézirard, V.; Lévêque, M.; Eutamène, H.; Ait-Belgnaoui, A.; Coëffier, M.; Ducrotté, P.; Róka, R.; Inczefi, O.; et al. Luminal cysteine-proteases degrade colonic tight junction structure and are responsible for abdominal pain in constipation-predominant IBS. Am. J. Gastroenterol. 2013, 108, 1322–1331. [Google Scholar] [CrossRef]
- Aguilera-Lizarraga, J.; Florens, M.V.; Van Brussel, T.; Clevers, E.; Van Oudenhove, L.; Lambrechts, D.; Wouters, M.M.; Boeckxstaens, G.E. Expression of immune-related genes in rectum and colon descendens of Irritable Bowel Syndrome patients is unrelated to clinical symptoms. Neurogastroenterol. Motil. 2019, 31, e13579. [Google Scholar] [CrossRef]
- Marshall, J.K.; Irvine, E.J. Ketotifen treatment of active colitis in patients with 5-aminosalicylate intolerance. Can. J. Gastroenterol. 1998, 12, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.L.; Roifman, C.M.; Griffiths, A.M.; Sherman, P. Ketotifen therapy for acute ulcerative colitis in children: A pilot study. Dig. Dis. Sci. 1998, 43, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Klooker, T.K.; Braak, B.; Koopman, K.E.; Welting, O.; Wouters, M.M.; van der Heide, S.; Schemann, M.; Bischoff, S.C.; van den Wijngaard, R.M.; Boeckxstaens, G.E. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut 2010, 59, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Y.; Zhou, H.; Gu, W.; Wang, X.; Yang, J. Clinical efficacy and safety of ketotifen in treating irritable bowel syndrome with diarrhea. Eur. J. Gastroenterol. Hepatol. 2020, 32, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Stefanini, G.F.; Saggioro, A.; Alvisi, V.; Angelini, G.; Capurso, L.; di Lorenzo, G.; Dobrilla, G.; Dodero, M.; Galimberti, M.; Gasbarrini, G.; et al. Oral cromolyn sodium in comparison with elimination diet in the irritable bowel syndrome, diarrheic type. Multicenter study of 428 patients. Scand. J. Gastroenterol. 1995, 30, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Leri, O.; Tubili, S.; De Rosa, F.G.; Addessi, M.A.; Scopelliti, G.; Lucenti, W.; De Luca, D. Management of diarrhoeic type of irritable bowel syndrome with exclusion diet and disodium cromoglycate. Inflammopharmacology 1997, 5, 153–158. [Google Scholar] [CrossRef]
- Lobo, B.; Ramos, L.; Martínez, C.; Guilarte, M.; González-Castro, A.M.; Alonso-Cotoner, C.; Pigrau, M.; de Torres, I.; Rodiño-Janeiro, B.K.; Salvo-Romero, E.; et al. Downregulation of mucosal mast cell activation and immune response in diarrhoea-irritable bowel syndrome by oral disodium cromoglycate: A pilot study. United European Gastroenterol. J. 2017, 5, 887–897. [Google Scholar] [CrossRef]
- Sastre, J. Ebastine in allergic rhinitis and chronic idiopathic urticaria. Allergy 2008, 63 (Suppl. S89), 1–20. [Google Scholar] [CrossRef] [PubMed]
- Wouters, M.M.; Balemans, D.; Van Wanrooy, S.; Dooley, J.; Cibert-Goton, V.; Alpizar, Y.A.; Valdez-Morales, E.E.; Nasser, Y.; Van Veldhoven, P.P.; Vanbrabant, W.; et al. Histamine Receptor H1-Mediated Sensitization of TRPV1 Mediates Visceral Hypersensitivity and Symptoms in Patients With Irritable Bowel Syndrome. Gastroenterology 2016, 150, 875–887.e9. [Google Scholar] [CrossRef]
- Decraecker, L.; De Looze, D.; Hirsch, D.P.; De Schepper, H.; Arts, J.; Caenepeel, P.; Bredenoord, A.J.; Kolkman, J.; Bellens, K.; Van Beek, K.; et al. Treatment of non-constipated irritable bowel syndrome with the histamine 1 receptor antagonist ebastine: A randomised, double-blind, placebo-controlled trial. Gut 2024, 73, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Fukudo, S.; Kinoshita, Y.; Okumura, T.; Ida, M.; Akiho, H.; Nakashima, Y.; Nishida, A.; Haruma, K. Ramosetron Reduces Symptoms of Irritable Bowel Syndrome With Diarrhea and Improves Quality of Life in Women. Gastroenterology 2016, 150, 358–366.e8. [Google Scholar] [CrossRef] [PubMed]
- Black, C.J.; Burr, N.E.; Camilleri, M.; Earnest, D.L.; Quigley, E.M.M.; Moayyedi, P.; Houghton, L.A.; Ford, A.C. Efficacy of pharmacological therapies in patients with IBS with diarrhoea or mixed stool pattern: Systematic review and network meta-analysis. Gut 2020, 69, 74–82. [Google Scholar] [CrossRef]
- Ceuleers, H.; Hanning, N.; Heirbaut, J.; Van Remoortel, S.; Joossens, J.; Van Der Veken, P.; Francque, S.M.; De bruyn, M.; Lambeir, A.-M.; De Man, J.G.; et al. Newly developed serine protease inhibitors decrease visceral hypersensitivity in a post-inflammatory rat model for irritable bowel syndrome. Br. J. Pharmacol. 2018, 175, 3516–3533. [Google Scholar] [CrossRef] [PubMed]
- Decraecker, L.; Boeckxstaens, G.; Denadai-Souza, A. Inhibition of Serine Proteases as a Novel Therapeutic Strategy for Abdominal Pain in IBS. Front. Physiol. 2022, 13, 880422. [Google Scholar] [CrossRef] [PubMed]
- Tremaine, W.J.; Brzezinski, A.; Katz, J.A.; Wolf, D.C.; Fleming, T.J.; Mordenti, J.; Strenkoski-Nix, L.C.; Kurth, M.C.; AXYS Ulcerative Colitis Study Group. Treatment of mildly to moderately active ulcerative colitis with a tryptase inhibitor (APC 2059): An open-label pilot study. Aliment. Pharmacol. Ther. 2002, 16, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Gionchetti, P.; Rizzello, F.; Venturi, A.; Campieri, M. Probiotics in infective diarrhoea and inflammatory bowel diseases. J. Gastroenterol. Hepatol. 2000, 15, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Kruis, W.; Fric, P.; Pokrotnieks, J.; Lukás, M.; Fixa, B.; Kascák, M.; Kamm, M.A.; Weismueller, J.; Beglinger, C.; Stolte, M.; et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut 2004, 53, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Bibiloni, R.; Fedorak, R.N.; Tannock, G.W.; Madsen, K.L.; Gionchetti, P.; Campieri, M.; De Simone, C.; Sartor, R.B. VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. Am. J. Gastroenterol. 2005, 100, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ma, C.; Zhao, F.; Chen, P.; Liu, Y.; Sun, Z.; Cui, L.; Kwok, L.Y.; Zhang, H. Adjunctive treatment with probiotics partially alleviates symptoms and reduces inflammation in patients with irritable bowel syndrome. Eur. J. Nutr. 2021, 60, 2553–2565. [Google Scholar] [CrossRef]
- Salari-Moghaddam, A.; Keshteli, A.H.; Esmaillzadeh, A.; Adibi, P. Adherence to the pro-inflammatory diet in relation to prevalence of irritable bowel syndrome. Nutr. J. 2019, 18, 72. [Google Scholar] [CrossRef]
- Uranga, J.A.; Martínez, V.; Abalo, R. Mast Cell Regulation and Irritable Bowel Syndrome: Effects of Food Components with Potential Nutraceutical Use. Molecules 2020, 25, 4314. [Google Scholar] [CrossRef]
- Sabato, V.; Beyens, M.; Toscano, A.; Van Gasse, A.; Ebo, D.G. Mast Cell-Targeting Therapies in Mast Cell Activation Syndromes. Curr. Allergy Asthma Rep. 2024, 24, 63–71. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).