β-Catenin Regulates Glycolytic and Mitochondrial Function in T-Cell Acute Lymphoblastic Leukemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Plasmid Construction and Lentivirus Infection
2.3. Cell Proliferation Assay
2.4. Cell Apoptosis Assay
2.5. Glucose Measurement
2.6. Lactate Level Measurement
2.7. Detection of ROS Levels
2.8. Measurement of Mitochondria Membrane Potential
2.9. Intracellular Calcium Concentration Assay
2.10. Measurement of Mitochondria Content
2.11. Measurement of Adenosine Triphosphate (ATP) Levels
2.12. Western Blot
2.13. Xenotransplantation Experiments
2.14. Transcriptome Sequencing
2.15. Statistical Analyses
3. Results
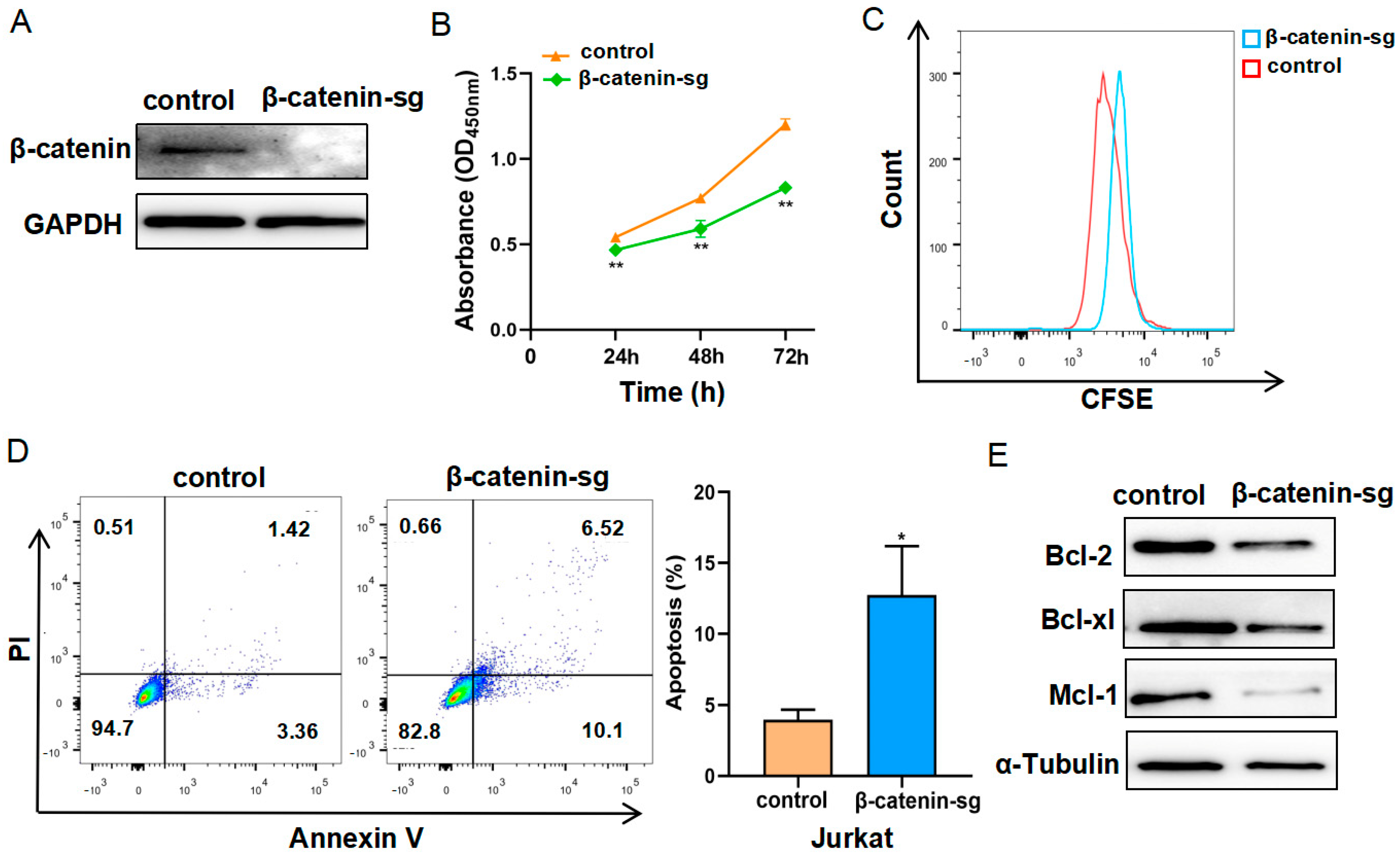
3.1. β-Catenin Deficiency Restrained Cell Proliferation and Promoted Apoptosis
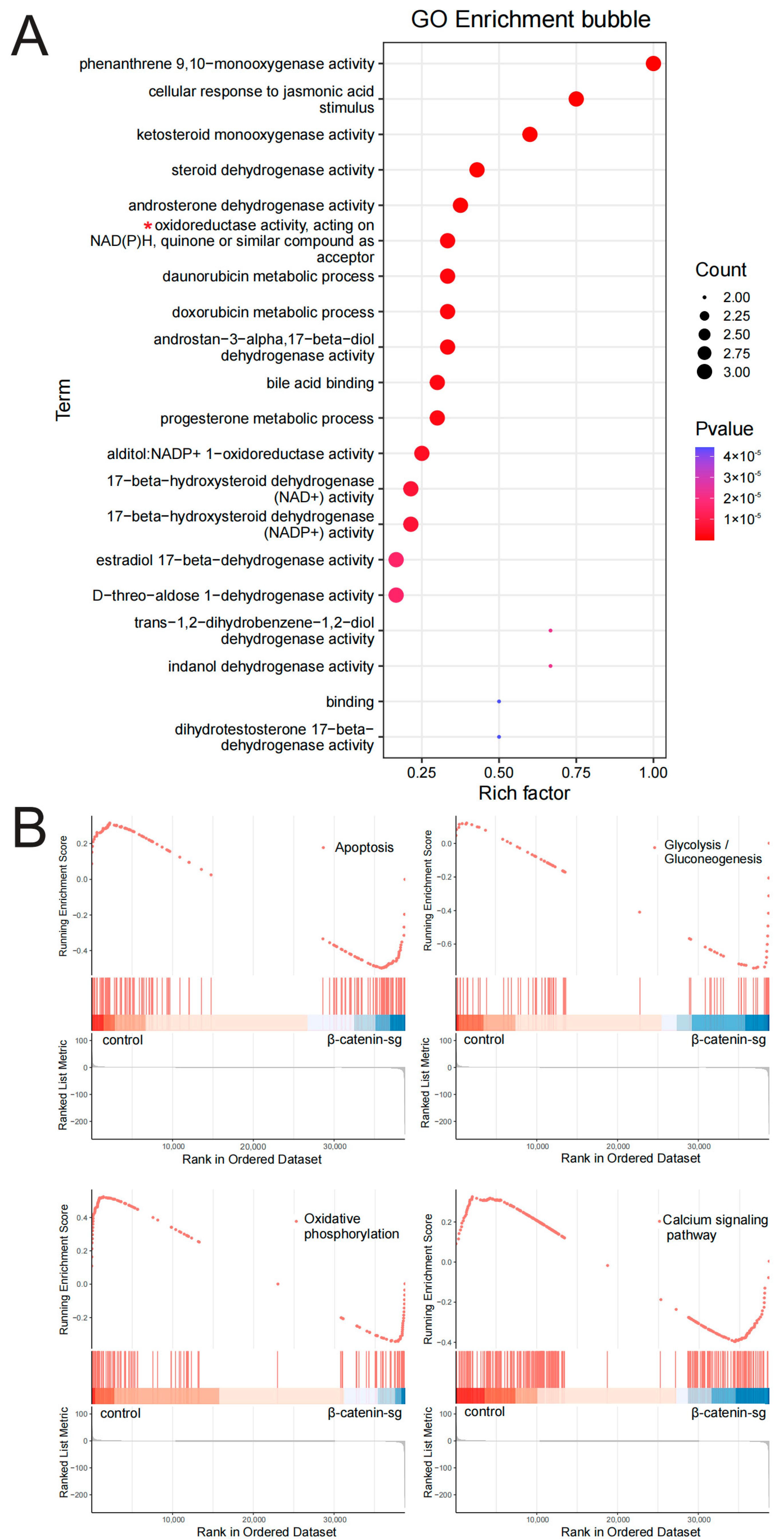
3.2. RNA-Seq Analysis Revealed That β-Catenin Might Regulate T-ALL Metabolism
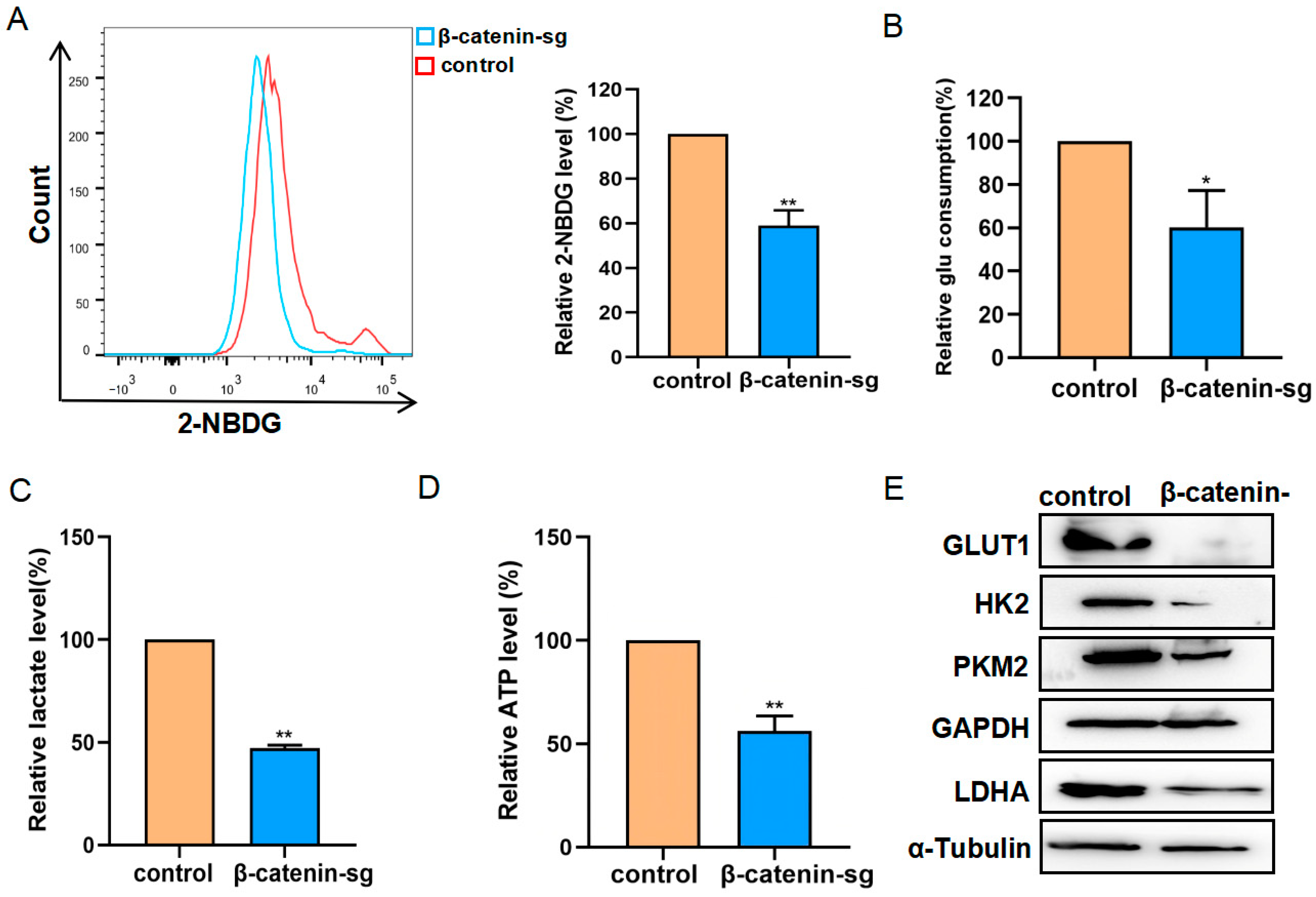
3.3. β-Catenin Deficiency Inhibited the Glycolysis of T-ALL Cells
3.4. β-Catenin Deficiency Induced Mitochondrial Impairment in T-ALL Cells
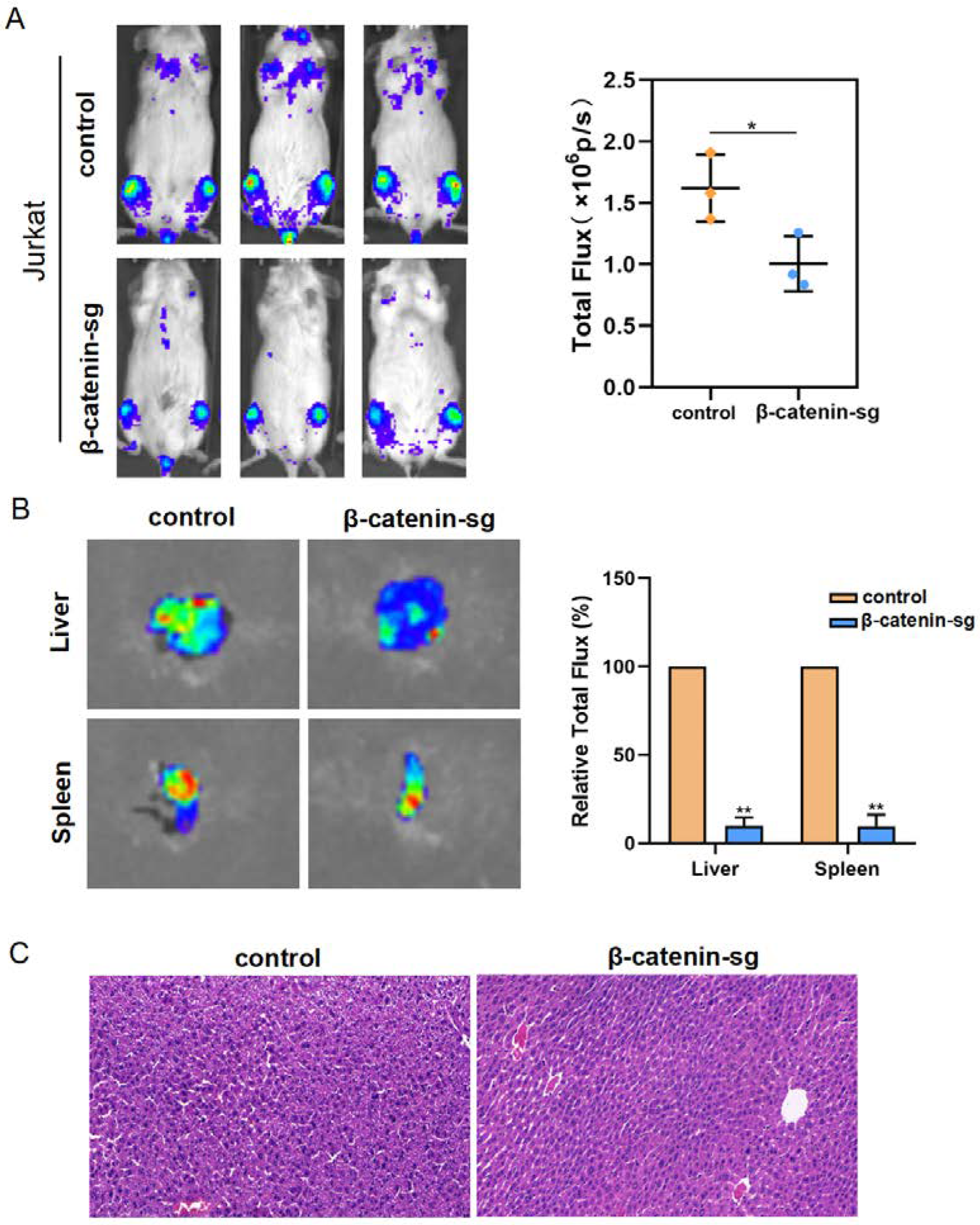
3.5. β-Catenin Promoted Organ Infiltration of T-ALL Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Bai, L.; Cheng, Y.; Lu, A.; Wang, Y.; Wu, J.; Zhang, X.; Zuo, Y.; Xu, L.; Jia, Y.; et al. Haploidentical hematopoietic stem cell transplantation may improve long-term survival for children with high-risk T-cell acute lymphoblastic leukemia in first complete remission. Chin. Med. J. 2022, 135, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Gianni, F.; Belver, L.; Ferrando, A. The Genetics and Mechanisms of T-Cell Acute Lymphoblastic Leukemia. Cold Spring Harb. Perspect. Med. 2020, 10, a035246. [Google Scholar] [CrossRef] [PubMed]
- Shiraz, P.; Jehangir, W.; Agrawal, V. T-Cell Acute Lymphoblastic Leukemia-Current Concepts in Molecular Biology and Management. Biomedicines 2021, 9, 1621. [Google Scholar] [CrossRef] [PubMed]
- Hunger, S.P.; Mullighan, C.G. Acute Lymphoblastic Leukemia in Children. N. Engl. J. Med. 2015, 373, 1541–1552. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X. Targeting the Wnt/β-catenin signaling pathway in cancer. J. Hematol. Oncol. 2020, 13, 165. [Google Scholar] [CrossRef] [PubMed]
- Giambra, V.; Jenkins, C.E.; Lam, S.H.; Hoofd, C.; Belmonte, M.; Wang, X.; Gusscott, S.; Gracias, D.; Weng, A.P. Leukemia stem cells in T-ALL require active Hif1α and Wnt signaling. Blood 2015, 125, 3917–3927. [Google Scholar] [CrossRef]
- Ng, O.H.; Erbilgin, Y.; Firtina, S.; Celkan, T.; Karakas, Z.; Aydogan, G.; Turkkan, E.; Yildirmak, Y.; Timur, C.; Zengin, E.; et al. Deregulated WNT signaling in childhood T-cell acute lymphoblastic leukemia. Blood Cancer J. 2014, 4, e192. [Google Scholar] [CrossRef] [PubMed]
- Evangelisti, C.; Chiarini, F.; Cappellini, A.; Paganelli, F.; Fini, M.; Santi, S.; Martelli, A.M.; Neri, L.M.; Evangelisti, C. Targeting Wnt/β-catenin and PI3K/Akt/mTOR pathways in T-cell acute lymphoblastic leukemia. J. Cell Physiol. 2020, 235, 5413–5428. [Google Scholar] [CrossRef]
- Ciccarese, F. Cancer Metabolism and Resistance to Cell Death: Novel Therapeutic Perspectives. Biomedicines 2022, 10, 1828. [Google Scholar] [CrossRef]
- Herbst, A.; Jurinovic, V.; Krebs, S.; Thieme, S.E.; Blum, H.; Göke, B.; Kolligs, F.T. Comprehensive analysis of β-catenin target genes in colorectal carcinoma cell lines with deregulated Wnt/β-catenin signaling. BMC Genom. 2014, 15, 74. [Google Scholar] [CrossRef]
- Vergara, D.; Stanca, E.; Guerra, F.; Priore, P.; Gaballo, A.; Franck, J.; Simeone, P.; Trerotola, M.; Domenico, S.D.; Fournier, I.; et al. β-Catenin Knockdown Affects Mitochondrial Biogenesis and Lipid Metabolism in Breast Cancer Cells. Front. Physiol. 2017, 8, 544. [Google Scholar] [CrossRef] [PubMed]
- Elesela, S.; Morris, S.B.; Narayanan, S.; Kumar, S.; Lombard, D.B.; Lukacs, N.W. Sirtuin 1 regulates mitochondrial function and immune homeostasis in respiratory syncytial virus infected dendritic cells. PLoS Pathog. 2020, 16, e1008319. [Google Scholar] [CrossRef] [PubMed]
- Harrington, J.S.; Ryter, S.W.; Plataki, M.; Price, D.R.; Choi, A.M.K. Mitochondria in health, disease, and aging. Physiol. Rev. 2023, 103, 2349–2422. [Google Scholar] [CrossRef] [PubMed]
- Arnovitz, S.; Mathur, P.; Tracy, M.; Mohsin, A.; Mondal, S.; Quandt, J.; Hernandez, K.M.; Khazaie, K.; Dose, M.; Emmanuel, A.O.; et al. Tcf-1 promotes genomic instability and T cell transformation in response to aberrant β-catenin activation.Proc. Natl. Acad. Sci. USA 2022, 119, e2201493119. [Google Scholar] [CrossRef]
- García-Hernández, V.; Arambilet, D.; Guillén, Y.; Lobo-Jarne, T.; Maqueda, M.; Gekas, C.; González, J.; Iglesias, A.; Vega-García, N.; Sentís, I.; et al. β-Catenin activity induces an RNA biosynthesis program promoting therapy resistance in T-cell acute lymphoblastic leukemia. EMBO Mol. Med. 2023, 15, e16554. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, J.; Feng, Y.; Khadka, B.; Fang, Z.; Gu, J.; Tang, B.; Xiao, R.; Pan, G.; Liu, J.J. A Regulatory Loop Involving Notch and Wnt Signaling Maintains Leukemia Stem Cells in T-Cell Acute Lymphoblastic Leukemia. Front. Cell Dev. Biol. 2021, 9, 678544. [Google Scholar] [CrossRef] [PubMed]
- Bhattacherjee, D.; Raina, K.; Mandal, T.K.; Thummer, R.P.; Bhabak, K.P. Targeting Wnt/β-catenin signaling pathway in triple-negative breast cancer by benzylic organotrisulfides: Contribution of the released hydrogen sulfide towards potent anti-cancer activity. Free Radic. Biol. Med. 2022, 191, 82–96. [Google Scholar] [CrossRef]
- Xu, Z.; Ran, J.; Gong, K.; Hou, Y.; Li, J.; Guo, Y. LncRNA SUMO1P3 regulates the invasion, migration and cell cycle of gastric cancer cells through Wnt/β-catenin signaling pathway. J. Recept. Signal Transduct. Res. 2021, 41, 574–581. [Google Scholar] [CrossRef]
- Schiliro, C.; Firestein, B.L. Mechanisms of Metabolic Reprogramming in Cancer Cells Supporting enhanced Growth and Proliferation. Cells 2021, 10, 1056. [Google Scholar] [CrossRef]
- Martínez-Reyes, I.; Chandel, N.S. Cancer metabolism: Looking forward. Nat. Rev. Cancer 2021, 21, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Kreitz, J.; Schönfeld, C.; Seibert, M.; Stolp, V.; Alshamleh, I.; Oellerich, T.; Steffen, B.; Schwalbe, H.; Schnütgen, F.; Kurrle, N.; et al. Metabolic plasticity of acute myeloid leukemia. Cells 2019, 8, 805. [Google Scholar] [CrossRef]
- Lapa, B.; Gonçalves, A.C.; Jorge, J.; Alves, R.; Pires, A.S.; Abrantes, A.M.; Coucelo, M.; Abrunhosa, A.; Botelho, M.F.; Nascimento-Costa, J.M.; et al. Acute myeloid leukemia sensitivity to metabolic inhibitors: Glycolysis showed to be a better therapeutic target. Med. Oncol. 2020, 37, 72. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, H.; Guo, W.; Zhou, X.; Shu, Y.; Liu, H.; Yang, L.; Tang, S.; Su, H.; Liu, Z.; et al. MiR-652-5p elevated glycolysis level by targeting TIGAR in T-cell acute lymphoblastic leukemia. Cell Death Dis. 2022, 13, 148. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, T.; Xu, J.; Wu, S.; Wang, L.; Su, H.; Jiang, J.; Yue, M.; Wang, J.; Wang, D.; et al. WEE1 inhibition induces glutamine addiction in T-cell acute lymphoblastic leukemia. Haematologica 2021, 106, 1816–1827. [Google Scholar] [CrossRef] [PubMed]
- Matthijssens, F.; Sharma, N.D.; Nysus, M.; Nickl, C.K.; Kang, H.; Perez, D.R.; Lintermans, B.; Van Loocke, W.; Roels, J.; Peirs, S.; et al. RUNX2 regulates leukemic cell metabolism and chemotaxis in high-risk T cell acute lymphoblastic leukemia. J. Clin. Investig. 2021, 131, e141566. [Google Scholar] [CrossRef] [PubMed]
- Balatskyi, V.V.; Vaskivskyi, V.O.; Myronova, A.; Avramets, D.; Nahia, A.K.; Macewicz, L.L.; Ruban, T.P.; Kucherenko, D.Y.; Soldatkin, O.O.; Lushnikova, I.V.; et al. Cardiac-specific β-catenin deletion dysregulates energetic metabolism and mitochondrial function in perinatal cardiomyocytes. Mitochondrion 2021, 60, 59–69. [Google Scholar] [CrossRef]
- Yeung, C.; Gibson, A.E.; Issaq, S.H.; Oshima, N.; Baumgart, J.T.; Edessa, L.D.; Rai, G.; Urban, D.J.; Johnson, M.S.; Benavides, G.A.; et al. Targeting glycolysis through inhibition of lactate dehydrogenase impairs tumor growth in preclinical models of ewing sarcoma. Cancer Res. 2019, 79, 5060–5073. [Google Scholar] [CrossRef]
- Schavemaker, P.E.; Muñoz-Gómez, S.A. The role of mitochondrial energetics in the origin and diversification of eukaryotes. Nat. Ecol. Evol. 2022, 6, 1307–1317. [Google Scholar] [CrossRef]
- Shen, K.; Pender, C.L.; Bar-Ziv, R.; Zhang, H.; Wickham, K.; Willey, E.; Durieux, J.; Ahmad, Q.; Dillin, A. Mitochondria as Cellular and Organismal Signaling Hubs. Annu. Rev. Cell Dev. Biol. 2022, 38, 179–218. [Google Scholar] [CrossRef] [PubMed]
- Ageeli, E.A. Alterations of Mitochondria and Related Metabolic Pathways in Leukemia: A Narrative Review. Saudi J. Med. Med. Sci. 2020, 8, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; di Fagagna, F.d. Cellular Senescence in Ageing: From Mechanisms to Therapeutic Opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Li, H.; Liao, P.; Chen, L.; Pan, Y.; Zheng, Y.; Zhang, C.; Liu, D.; Zheng, M.; Gao, J. Mitochondrial dysfunction: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 124. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Pu, X.; Zhou, S.; Huang, Z.; Chen, Q.; Zhang, Y.; Mao, Q.; Liang, Y.; Ding, G. Identification of exosomal miR-484 role in reprogramming mitochondrial metabolism in pancreatic cancer through Wnt/MAPK axis control. Pharmacol. Res. 2023, 197, 106980. [Google Scholar] [CrossRef]
- Li, H.; Leung, J.C.K.; Yiu, W.H.; Chan, L.Y.Y.; Li, B.; Lok, S.W.Y.; Xue, R.; Zou, Y.; Lai, K.N.; Tang, S.C.W. Tubular beta-catenin alleviates mitochondrial dysfunction and cell death in acute kidney injury. Cell Death Dis. 2022, 13, 1061. [Google Scholar] [CrossRef]
- Peng, W.; Wong, Y.C.; Krainc, D. Mitochondria-lysosome contacts regulate mitochondrial Ca2+ dynamics via lysosomal TRPML1. Proc. Natl. Acad. Sci. USA 2020, 117, 19266–19275. [Google Scholar] [CrossRef]
- Baran, N.; Lodi, A.; Dhungana, Y.; Herbrich, S.; Collins, M.; Sweeney, S.; Pandey, R.; Skwarska, A.; Patel, S.; Tremblay, M.; et al. Inhibition of mitochondrial complex I reverses NOTCH1-driven metabolic reprogramming in T-cell acute lymphoblastic leukemia. Nat. Commun. 2022, 13, 2801. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Krivtsov, A.V.; Sinha, A.U.; North, T.E.; Goessling, W.; Feng, Z.; Zon, L.I.; Armstrong, S.A. The Wnt/beta-catenin pathway is required for the development of leukemia stem cells in AML. Science 2010, 327, 1650–1653. [Google Scholar] [CrossRef]
- Panelli, P.; Santis, E.D.; Colucci, M.; Tamiro, F.; Sansico, F.; Miroballo, M.; Murgo, E.; Padovano, C.; Gusscott, S.; Ciavarella, M.; et al. Noncanonical β-catenin interactions promote leukemia-initiating activity in early T-cell acute lymphoblastic leukemia. Blood 2023, 141, 1597–1609. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Gao, Z.; Bao, Y.; Chen, L.; Huang, Y.; Liu, Y.; Dong, Q.; Wei, X. Wnt/β-catenin signaling pathway in carcinogenesis and cancer therapy. J. Hematol. Oncol. 2024, 17, 46. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Yu, C.; Li, F.; Zuo, Y.; Wang, Y.; Yao, L.; Wu, C.; Wang, C.; Ye, L. Wnt/β-catenin signaling in cancers and targeted therapies. Signal Transduct. Target. Ther. 2021, 6, 307. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Zhao, Y.; Wang, S.; Zhang, J.; Li, X.; Wang, S.; Huang, T.; Wang, J.; Liu, J. β-Catenin Regulates Glycolytic and Mitochondrial Function in T-Cell Acute Lymphoblastic Leukemia. Biomedicines 2025, 13, 292. https://doi.org/10.3390/biomedicines13020292
Zhang L, Zhao Y, Wang S, Zhang J, Li X, Wang S, Huang T, Wang J, Liu J. β-Catenin Regulates Glycolytic and Mitochondrial Function in T-Cell Acute Lymphoblastic Leukemia. Biomedicines. 2025; 13(2):292. https://doi.org/10.3390/biomedicines13020292
Chicago/Turabian StyleZhang, Ling, Yu Zhao, Shuoting Wang, Jian Zhang, Xiaohui Li, Shuangyin Wang, Taosheng Huang, Jinxing Wang, and Jiajun Liu. 2025. "β-Catenin Regulates Glycolytic and Mitochondrial Function in T-Cell Acute Lymphoblastic Leukemia" Biomedicines 13, no. 2: 292. https://doi.org/10.3390/biomedicines13020292
APA StyleZhang, L., Zhao, Y., Wang, S., Zhang, J., Li, X., Wang, S., Huang, T., Wang, J., & Liu, J. (2025). β-Catenin Regulates Glycolytic and Mitochondrial Function in T-Cell Acute Lymphoblastic Leukemia. Biomedicines, 13(2), 292. https://doi.org/10.3390/biomedicines13020292




