A VCG-Based Multiepitope Chlamydia Vaccine Incorporating the Cholera Toxin A1 Subunit (MECA) Confers Protective Immunity Against Transcervical Challenge
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statements
2.2. Chlamydia Stocks, Antigens and Mice
2.3. Construction of the Vaccine Vector, pCT-MECA, and Expression of MECA by Immunoblotting Analysis
2.4. Production of rVCG-MECA Vaccine
2.5. Immunization and Challenge of Immunized Mice
2.6. Determination of Antigen-Specific Humoral Immune Responses
2.7. Determination of Serum Antibody Avidity
2.8. Assessment of Antigen-Specific Cellular Immune Responses
2.9. Statistical Analysis
3. Results
3.1. Construction of Plasmid pCT-MECA and VCG Expression of rMECA Protein
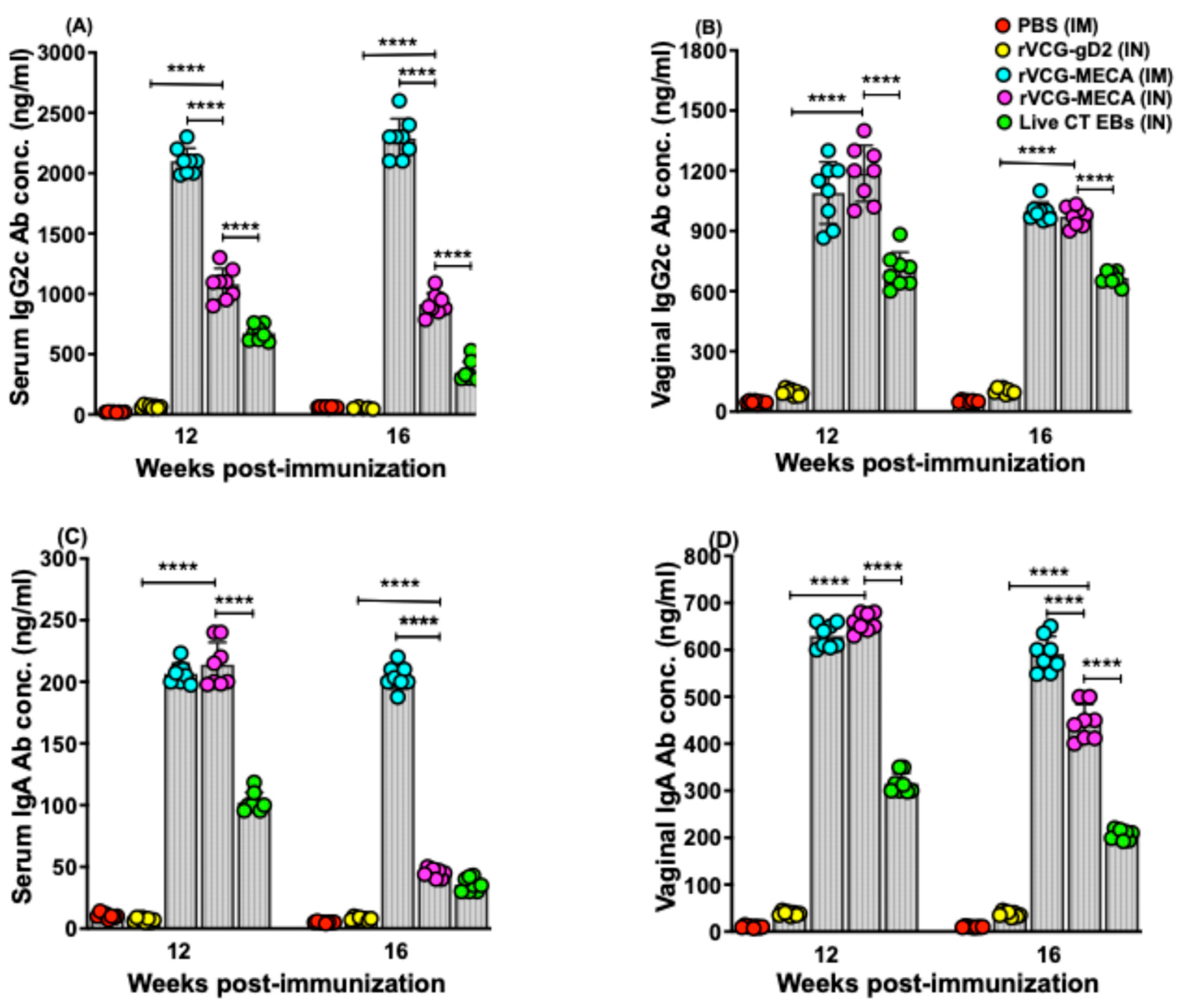
3.2. IM and IN Vaccination with rVCG-MECA Stimulated Robust Antigen-Specific Antibodies in Serum and Vaginal Secretions
3.3. Vaccine-Induced CT-Specific IgG2c and IgA Antibodies Persisted in Serum and Vaginal Secretions
3.4. Avidity of Antigen-Specific Serum IgG and IgG2c Antibodies
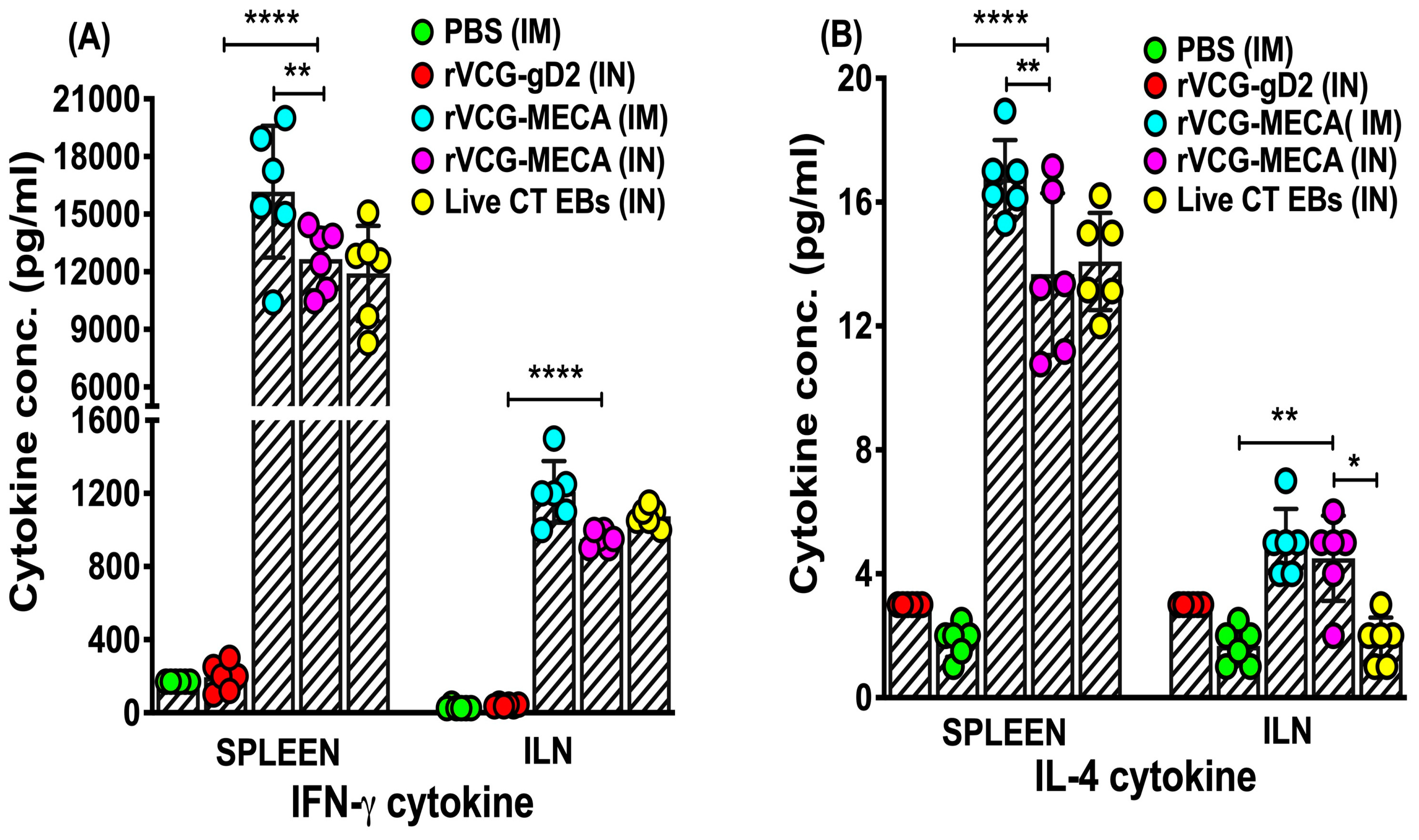
3.5. Magnitude of Antigen-Specific IFN-γ Were Induced in Mucosal and Systemic Tissues Following Immunization with rVCG-MECA
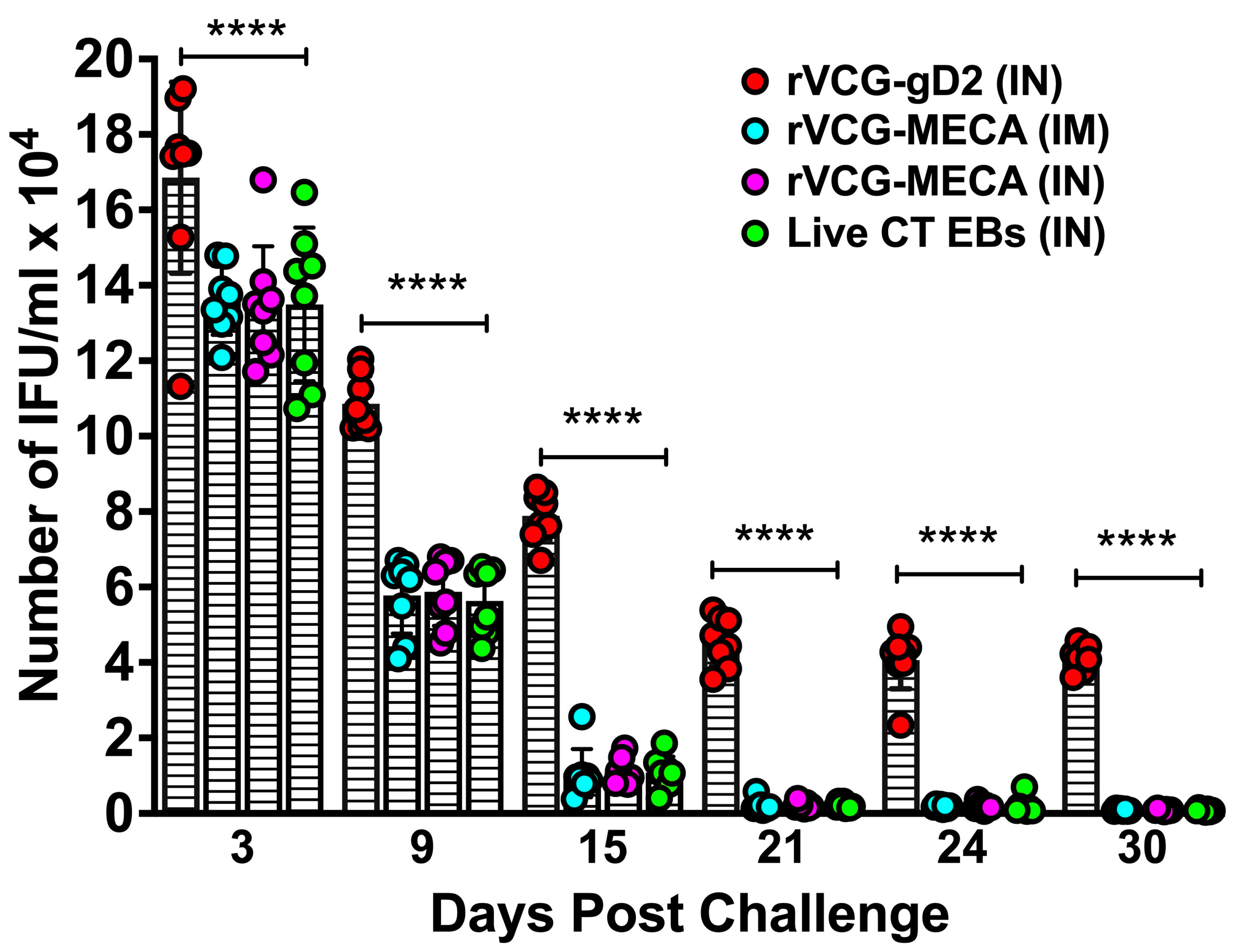
3.6. Immune Effectors Stimulated by Immunization with rVCG-MECA Protected Mice Against Transcervical Challenge with Live CT EBs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CDC: Centers for Disease Control and Prevention. National Overview of STIs; CDC: Atlanta, GA, USA, 2022. [Google Scholar]
- Farley, T.A.; Cohen, D.A.; Elkins, W. Asymptomatic sexually transmitted diseases: The case for screening. Prev. Med. 2003, 36, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Stamm, W.E. Chlamydia trachomatis infections: Progress and problems. J. Infect. Dis. 1999, 179 (Suppl. S2), S380–S383. [Google Scholar] [CrossRef]
- Yonke, N.; Aragón, M.; Phillips, J.K. Chlamydial and Gonococcal Infections: Screening, Diagnosis, and Treatment. Am. Fam. Physician 2022, 105, 388–396. [Google Scholar] [PubMed]
- Curry, A.; Williams, T.; Penny, M.L. Pelvic Inflammatory Disease: Diagnosis, Management, and Prevention. Am. Fam. Physician 2019, 100, 357–364. [Google Scholar]
- Haggerty, C.L.; Gottlieb, S.L.; Taylor, B.D.; Low, N.; Xu, F.; Ness, R.B. Risk of sequelae after Chlamydia trachomatis genital infection in women. J. Infect. Dis. 2010, 201 (Suppl. S2), S134–S155. [Google Scholar] [CrossRef]
- Chan, P.A.; Robinette, A.; Montgomery, M.; Almonte, A.; Cu-Uvin, S.; Lonks, J.R.; Chapin, K.C.; Kojic, E.M.; Hardy, E.J. Extragenital Infections Caused by Chlamydia trachomatis and Neisseria gonorrhoeae: A Review of the Literature. Infect. Dis. Obstet. Gynecol. 2016, 2016, 5758387. [Google Scholar] [CrossRef]
- Perrott, S.L.; Kar, S.P. Appraisal of the causal effect of Chlamydia trachomatis infection on epithelial ovarian cancer risk: A two-sample Mendelian randomisation study. medRxiv 2024. [Google Scholar] [CrossRef]
- Bryan, E.R.; Redgrove, K.A.; Mooney, A.R.; Mihalas, B.P.; Sutherland, J.M.; Carey, A.J.; Armitage, C.W.; Trim, L.K.; Kollipara, A.; Mulvey, P.B.M.; et al. Chronic testicular Chlamydia muridarum infection impairs mouse fertility and offspring developmentdagger. Biol. Reprod. 2020, 102, 888–901. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.H.; Follmann, F.; Dietrich, J. Chlamydia trachomatis vaccine development—A view on the current challenges and how to move forward. Expert. Rev. Vaccines 2022, 21, 1555–1567. [Google Scholar] [CrossRef]
- Murthy, A.K.; Li, W.; Ramsey, K.H. Immunopathogenesis of Chlamydial Infections. Curr. Top. Microbiol. Immunol. 2018, 412, 183–215. [Google Scholar] [CrossRef]
- Brunham, R.C. The genome, microbiome and evolutionary medicine. CMAJ 2018, 190, E162–E166. [Google Scholar] [CrossRef] [PubMed]
- Brunham, R.C.; Rey-Ladino, J. Immunology of Chlamydia infection: Implications for a Chlamydia trachomatis vaccine. Nat. Rev. Immunol. 2005, 5, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Poston, T.B.; Gottlieb, S.L.; Darville, T. Status of vaccine research and development of vaccines for Chlamydia trachomatis infection. Vaccine 2019, 37, 7289–7294. [Google Scholar] [CrossRef]
- Murray, S.M.; McKay, P.F. Chlamydia trachomatis: Cell biology, immunology and vaccination. Vaccine 2021, 39, 2965–2975. [Google Scholar] [CrossRef]
- Grayston, J.T.; Wang, S.-P.; Yeh, L.J.; Kuo, C.C. Importance of reinfection in the pathogenesis of trachoma. Rev. Infect. Dis. 1985, 7, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Schachter, J.; Dawson, C.R. Human Chlamydial Infections; PSG Publishing Company: Littleton, MA, USA, 1978. [Google Scholar]
- Eko, F.O.; Lubitz, W.; McMillan, L.; Ramey, K.; Moore, T.T.; Ananaba, G.A.; Lyn, D.; Black, C.M.; Igietseme, J.U. Recombinant Vibrio cholerae ghosts as a delivery vehicle for vaccinating against Chlamydia trachomatis. Vaccine 2003, 21, 1694–1703. [Google Scholar] [CrossRef] [PubMed]
- Eko, F.O.; Mayr, U.B.; Attridge, S.R.; Lubitz, W. Characterization and immunogenicity of Vibrio cholerae ghosts expressing toxin-coregulated pili. J. Biotechnol. 2000, 83, 115–123. [Google Scholar] [CrossRef]
- Eko, F.; Ekong, E.; Okenu, D.; He, Q.; Ananaba, G.; Black, C.; Igietseme, J. Induction of immune memory by a multisubunit chlamydial vaccine. Vaccine 2011, 29, 1472–1480. [Google Scholar] [CrossRef]
- Pais, R.; Omosun, Y.; Igietseme, J.U.; Fujihashi, K.; Eko, F.O. Route of Vaccine Administration Influences the Impact of Fms-Like Tyrosine Kinase 3 Ligand (Flt3L) on Chlamydial-Specific Protective Immune Responses. Front. Immunol. 2019, 10, 1577. [Google Scholar] [CrossRef] [PubMed]
- Redgrove, K.A.; McLaughlin, E.A. The Role of the Immune Response in Chlamydia trachomatis Infection of the Male Genital Tract: A Double-Edged Sword. Front. Immunol. 2014, 5, 534. [Google Scholar] [CrossRef]
- Helgeby, A.; Robson, N.C.; Donachie, A.M.; Beackock-Sharp, H.; Lövgren, K.; Schön, K.; Mowat, A.; Lycke, N.Y. The combined CTA1-DD/ISCOM adjuvant vector promotes priming of mucosal and systemic immunity to incorporated antigens by specific targeting of B cells. J. Immunol. 2006, 176, 3697–3706. [Google Scholar] [CrossRef]
- Agren, L.; Sverremark, E.; Ekman, L.; Schön, K.; Löwenadler, B.; Fernandez, C.; Lycke, N. The ADP-ribosylating CTA1-DD adjuvant enhances T cell-dependent and independent responses by direct action on B cells involving anti-apoptotic Bcl-2- and germinal center-promoting effects. J. Immunol. 2000, 164, 6276–6286. [Google Scholar] [CrossRef]
- Kim, J.C.; Choi, J.A.; Park, H.; Yang, E.; Noh, S.; Kim, J.S.; Kim, M.J.; Song, M.; Park, J.H. Pharmaceutical and Immunological Evaluation of Cholera Toxin A1 Subunit as an Adjuvant of Hepatitis B Vaccine Microneedles. Pharm. Res. 2023, 40, 3059–3071. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, A.M.; Schön, K.M.; Lycke, N.Y. The cholera toxin-derived CTA1-DD vaccine adjuvant administered intranasally does not cause inflammation or accumulate in the nervous tissues. J. Immunol. 2004, 173, 3310–3319. [Google Scholar] [CrossRef]
- Watanabe, H.; Numata, K.; Ito, T.; Takagi, K.; Matsukawa, A. Innate immune response in Th1- and Th2-dominant mouse strains. Shock 2004, 22, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.M.; Greenaway, S.; White, J.K.; Fuchs, H.; Gailus-Durner, V.; Wells, S.; Sorg, T.; Wong, K.; Bedu, E.; Cartwright, E.J.; et al. A comparative phenotypic and genomic analysis of C57BL/6J and C57BL/6N mouse strains. Genome Biol. 2013, 14, R82. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; He, Y.; Zhai, W.; Huang, Y.; Tao, C.; Pang, Z.; Wang, Z.; Zhang, D.; Li, H.; Jia, H. CpG adjuvant enhances humoral and cellular immunity against OVA in different degrees in BALB/c, C57BL/6J, and C57BL/6N mice. Int. Immunopharmacol. 2024, 138, 112593. [Google Scholar] [CrossRef]
- Li, W.; Murthy, A.K.; Guentzel, M.N.; Seshu, J.; Forsthuber, T.G.; Zhong, G.; Arulanandam, B.P. Antigen-Specific CD4+ T Cells Produce Sufficient IFN-γ to Mediate Robust Protective Immunity against Genital Chlamydia muridarum Infection. J. Immunol. 2008, 180, 3375–3382. [Google Scholar] [CrossRef] [PubMed]
- Kubo, A.; Stephens, R.S. Characterization and functional analysis of PorB, a Chlamydia porin and neutralizing target. Mol. Microbiol. 2000, 38, 772–780. [Google Scholar] [CrossRef]
- Brunham, R.C.; Peeling, R.W. Chlamydia trachomatis antigens: Role in immunity and pathogenesis. Infect. Agents Dis. 1994, 3, 218–233. [Google Scholar] [PubMed]
- Vasilevsky, S.; Stojanov, M.; Greub, G.; Baud, D. Chlamydial polymorphic membrane proteins: Regulation, function and potential vaccine candidates. Virulence 2016, 7, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Karunakaran, K.P.; Jiang, X.; Brunham, R.C. Evaluation of a multisubunit recombinant polymorphic membrane protein and major outer membrane protein T cell vaccine against Chlamydia muridarum genital infection in three strains of mice. Vaccine 2014, 32, 4672–4680. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Parnell, M.; Caldwell, H.D. Protective efficacy of a parenterally administered MOMP-derived synthetic oligopeptide vaccine in a murine model of Chlamydia trachomatis genital tract infection: Serum neutralizing IgG antibodies do not protect against chlamydial genital tract infection. Vaccine 1995, 13, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Macmillan, L.; Ifere, G.O.; He, Q.; Igietseme, J.U.; Kellar, K.L.; Okenu, D.M.; Eko, F.O. A recombinant multivalent combination vaccine protects against Chlamydia and genital herpes. FEMS Immunol. Med. Microbiol. 2007, 49, 46–55. [Google Scholar] [CrossRef]
- Almanzar, G.; Ottensmeier, B.; Liese, J.; Prelog, M. Assessment of IgG avidity against pertussis toxin and filamentous hemagglutinin via an adapted enzyme-linked immunosorbent assay (ELISA) using ammonium thiocyanate. J. Immunol. Methods 2013, 387, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Trzewikoswki de Lima, G.; Rodrigues, T.S.; Portilho, A.I.; Correa, V.A.; Gaspar, E.B.; De Gaspari, E. Immune responses of meningococcal B outer membrane vesicles in middle-aged mice. Pathog. Dis. 2020, 78, ftaa028. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Pais, R.; Ohandjo, A.; He, C.; He, Q.; Omosun, Y.; Igietseme, J.U.; Eko, F.O. Comparative evaluation of the protective efficacy of two formulations of a recombinant Chlamydia abortus subunit candidate vaccine in a mouse model. Vaccine 2015, 33, 1865–1872. [Google Scholar] [CrossRef] [PubMed]
- Eko, F.O.; Mania-Pramanik, J.; Pais, R.; Pan, Q.; Okenu, D.M.; Johnson, A.; Ibegbu, C.; He, C.; He, Q.; Russell, R.; et al. Vibrio cholerae ghosts (VCG) exert immunomodulatory effect on dendritic cells for enhanced antigen presentation and induction of protective immunity. BMC Immunol. 2014, 15, 584. [Google Scholar] [CrossRef]
- Pais, R.; Omosun, Y.; He, Q.; Blas-Machado, U.; Black, C.; Igietseme, J.U.; Fujihashi, K.; Eko, F.O. Rectal administration of a chlamydial subunit vaccine protects against genital infection and upper reproductive tract pathology in mice. PLoS ONE 2017, 12, e0178537. [Google Scholar] [CrossRef] [PubMed]
- Fujihashi, K.; Koga, T.; van Ginkel, F.W.; Hagiwara, Y.; McGhee, J.R. A dilemma for mucosal vaccination: Efficacy versus toxicity using enterotoxin-based adjuvants. Vaccine 2002, 20, 2431–2438. [Google Scholar] [CrossRef]
- Ekong, E.E.; Okenu, D.; Mania-Pramanik, J.; He, Q.; Igietseme, J.; Ananaba, G.; Lyn, D.; Black, C.; Eko, F. A Vibrio cholerae ghost-based subunit vaccine induces cross-protective chlamydial immunity that is enhanced by CTA2B, the nontoxic derivative of cholera toxin. FEMS Immunol. Med. Microbiol. 2009, 55, 280–291. [Google Scholar] [CrossRef]
- Bemark, M.; Bergqvist, P.; Stensson, A.; Holmberg, A.; Mattsson, J.; Lycke, N.Y. A unique role of the cholera toxin A1-DD adjuvant for long-term plasma and memory B cell development. J. Immunol. 2011, 186, 1399–1410. [Google Scholar] [CrossRef]
- Cunningham, K.A.; Carey, A.J.; Lycke, N.; Timms, P.; Beagley, K.W. CTA1-DD is an effective adjuvant for targeting anti-chlamydial immunity to the murine genital mucosa. J. Reprod. Immunol. 2009, 81, 34–38. [Google Scholar] [CrossRef]
- Schussek, S.; Bernasconi, V.; Mattsson, J.; Wenzel, U.A.; Strömberg, A.; Gribonika, I.; Schön, K.; Lycke, N.Y. The CTA1-DD adjuvant strongly potentiates follicular dendritic cell function and germinal center formation, which results in improved neonatal immunization. Mucosal Immunol. 2020, 13, 545–557. [Google Scholar] [CrossRef]
- Vasilevsky, S.; Greub, G.; Nardelli-Haefliger, D.; Baud, D. Genital Chlamydia trachomatis: Understanding the roles of innate and adaptive immunity in vaccine research. Clin. Microbiol. Rev. 2014, 27, 346–370. [Google Scholar] [CrossRef]
- Rixon, J.A.; Depew, C.E.; McSorley, S.J. Th1 cells are dispensable for primary clearance of Chlamydia from the female reproductive tract of mice. PLoS Pathog. 2022, 18, e1010333. [Google Scholar] [CrossRef]
- Tifrea, D.F.; Pal, S.; de la Maza, L.M. A Recombinant Chlamydia trachomatis MOMP Vaccine Elicits Cross-serogroup Protection in Mice Against Vaginal Shedding and Infertility. J. Infect. Dis. 2020, 221, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Murthy, A.K.; Chambers, J.P.; Meier, P.A.; Zhong, G.; Arulanandam, B.P. Intranasal Vaccination with a Secreted Chlamydial Protein Enhances Resolution of Genital Chlamydia muridarum Infection, Protects against Oviduct Pathology, and Is Highly Dependent upon Endogenous Gamma Interferon Production. Infect. Immun. 2007, 75, 666–676. [Google Scholar] [CrossRef]
- O’Meara, C.P.; Armitage, C.W.; Harvie, M.C.G.; Timms, P.; Lycke, N.Y.; Beagley, K.W. Immunization with a MOMP-Based Vaccine Protects Mice against a Pulmonary Chlamydia Challenge and Identifies a Disconnection between Infection and Pathology. PLoS ONE 2013, 8, e61962. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lewis, D.J.; Huo, Z.; Barnett, S.; Kromann, I.; Giemza, R.; Galiza, E.; Woodrow, M.; Thierry-Carstensen, B.; Andersen, P.; Novicki, D.; et al. Transient facial nerve paralysis (Bell’s palsy) following intranasal delivery of a genetically detoxified mutant of Escherichia coli heat labile toxin. PLoS ONE 2009, 4, e6999. [Google Scholar] [CrossRef]
- Carmichael, J.R.; Pal, S.; Tifrea, D.; de la Maza, L.M. Induction of protection against vaginal shedding and infertility by a recombinant Chlamydia vaccine. Vaccine 2011, 29, 5276–5283. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Lin, H.; Tang, L.; Chen, J.; Wu, Y.; Zhong, G. Oral Chlamydia vaccination induces transmucosal protection in the airway. Vaccine 2018, 36, 2061–2068. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, C.; Zhang, T.; Tian, Q.; Zhang, N.; Morrison, S.; Morrison, R.; Xue, M.; Zhong, G. Nonpathogenic Colonization with Chlamydia in the Gastrointestinal Tract as Oral Vaccination for Inducing Transmucosal Protection. Infect. Immun. 2018, 86, e00630-17. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.G.; Morrison, R.P. The protective effect of antibody in immunity to murine chlamydial genital tract reinfection is independent of immunoglobulin A. Infect. Immun. 2005, 73, 6183–6186. [Google Scholar] [CrossRef]
- Brunham, R.C.; Kuo, C.-C.; Cles, L.; Holmes, K.K. Correlation of host immune response with quantitative recovery of Chlamydia trachomatis from the human endocervix. Infect. Immun. 1983, 39, 1491–1494. [Google Scholar] [CrossRef]
- Morrison, R.P.; Feilzer, K.; Tumas, D.B. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect. Immun. 1995, 63, 4661–4668. [Google Scholar] [CrossRef] [PubMed]
- Shillova, N.; Howe, S.E.; Hyseni, B.; Ridgell, D.; Fisher, D.J.; Konjufca, V. Chlamydia-Specific IgA Secretion in the Female Reproductive Tract Induced via Per-Oral Immunization Confers Protection against Primary Chlamydia Challenge. Infect. Immun. 2020, 89, e00413-20. [Google Scholar] [CrossRef] [PubMed]
- Igietseme, J.U.; Eko, F.O.; Black, C.M. Contemporary approaches to designing and evaluating vaccines against Chlamydia. Expert. Rev. Vaccines 2003, 2, 129–146. [Google Scholar] [CrossRef] [PubMed]
- Morrison, R.; Caldwell, H. Immunity to murine chlamydial genital infection. Infect. Immun. 2002, 70, 2741–2751. [Google Scholar] [CrossRef]
- Nogueira, C.V.; Zhang, X.; Giovannone, N.; Sennott, E.L.; Starnbach, M.N. Protective immunity against Chlamydia trachomatis can engage both CD4+ and CD8+ T cells and bridge the respiratory and genital mucosae. J. Immunol. 2015, 194, 2319–2329. [Google Scholar] [CrossRef]
- Zhong, G.; de la Maza, L.M. Activation of mouse peritoneal macrophages in vitro or in vivo by recombinant murine gamma interferon inhibits the growth of Chlamydia trachomatis serovar L1. Infect. Immun. 1988, 56, 3322–3325. [Google Scholar] [CrossRef]
- Rank, R.G.; Whittum-Hudson, J.A. Protective immunity to chlamydial genital infection: Evidence from animal studies. J. Infect. Dis. 2010, 201 (Suppl. S2), S168–S177. [Google Scholar] [CrossRef]
- Vicetti Miguel, R.D.; Quispe Calla, N.E.; Pavelko, S.D.; Cherpes, T.L. Intravaginal Chlamydia trachomatis Challenge Infection Elicits TH1 and TH17 Immune Responses in Mice That Promote Pathogen Clearance and Genital Tract Damage. PLoS ONE 2016, 11, e0162445. [Google Scholar] [CrossRef] [PubMed]
- Sturdevant, G.; Caldwell, H. Innate immunity is sufficient for the clearance of Chlamydia trachomatis from the female mouse genital tract. Pathog. Dis. 2014, 72, 70–73. [Google Scholar] [CrossRef]
- Nogueira, A.T.; Braun, K.M.; Carabeo, R.A. Characterization of the Growth of Chlamydia trachomatis in In Vitro-Generated Stratified Epithelium. Front. Cell Infect. Microbiol. 2017, 7, 438. [Google Scholar] [CrossRef]
- Murthy, A.; Chaganty, B.; Li, W.; Guentzel, M.; Chambers, J.; Seshu, J.; Zhong, G.; Arulanandam, B. A limited role for antibody in protective immunity induced by rCPAF and CpG vaccination against primary genital Chlamydia muridarum challenge. FEMS Immunol. Med. Microbiol. 2009, 55, 271–279. [Google Scholar] [CrossRef]
- O’Meara, C.P.; Armitage, C.W.; Harvie, M.C.; Andrew, D.W.; Timms, P.; Lycke, N.Y.; Beagley, K.W. Immunity against a Chlamydia infection and disease may be determined by a balance of IL-17 signaling. Immunol. Cell Biol. 2014, 92, 287–297. [Google Scholar] [CrossRef]
- Pal, S.; Peterson, E.M.; Rappuoli, R.; Ratti, G.; de la Maza, L.M. Immunization with the Chlamydia trachomatis major outer membrane protein, using adjuvants developed for human vaccines, can induce partial protection in a mouse model against a genital challenge. Vaccine 2006, 24, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, K.; Tan, Y.; Wen, Y.; Wang, C.; Chen, Q.; Yu, J.; Xu, M.; Tan, M.; Wu, Y. A recombinant multi-epitope peptide vaccine based on MOMP and CPSIT_p6 protein protects against Chlamydia psittaci lung infection. Appl. Microbiol. Biotechnol. 2019, 103, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Xin, Q.; Niu, H.; Li, Z.; Zhang, G.; Hu, L.; Wang, B.; Li, J.; Yu, H.; Liu, W.; Wang, Y.; et al. Subunit vaccine consisting of multi-stage antigens has high protective efficacy against Mycobacterium tuberculosis infection in mice. PLoS ONE 2013, 8, e72745. [Google Scholar] [CrossRef] [PubMed]
- de la Maza, L.M.; Zhong, G.; Brunham, R.C. Update on Chlamydia trachomatis Vaccinology. Clin. Vaccine Immunol. 2017, 24, e00543-16. [Google Scholar] [CrossRef] [PubMed]
- Tanner, T.; Medhavi, F.N.U.; Richardson, S.; Omosun, Y.O.; Eko, F.O. In silico design and analysis of a multiepitope vaccine against Chlamydia. Pathog. Dis. 2024, 82, ftae015. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medhavi, F.; Tanner, T.; Richardson, S.; Lundy, S.; Omosun, Y.; Eko, F.O. A VCG-Based Multiepitope Chlamydia Vaccine Incorporating the Cholera Toxin A1 Subunit (MECA) Confers Protective Immunity Against Transcervical Challenge. Biomedicines 2025, 13, 288. https://doi.org/10.3390/biomedicines13020288
Medhavi F, Tanner T, Richardson S, Lundy S, Omosun Y, Eko FO. A VCG-Based Multiepitope Chlamydia Vaccine Incorporating the Cholera Toxin A1 Subunit (MECA) Confers Protective Immunity Against Transcervical Challenge. Biomedicines. 2025; 13(2):288. https://doi.org/10.3390/biomedicines13020288
Chicago/Turabian StyleMedhavi, Fnu, Tayhlor Tanner, Shakyra Richardson, Stephanie Lundy, Yusuf Omosun, and Francis O. Eko. 2025. "A VCG-Based Multiepitope Chlamydia Vaccine Incorporating the Cholera Toxin A1 Subunit (MECA) Confers Protective Immunity Against Transcervical Challenge" Biomedicines 13, no. 2: 288. https://doi.org/10.3390/biomedicines13020288
APA StyleMedhavi, F., Tanner, T., Richardson, S., Lundy, S., Omosun, Y., & Eko, F. O. (2025). A VCG-Based Multiepitope Chlamydia Vaccine Incorporating the Cholera Toxin A1 Subunit (MECA) Confers Protective Immunity Against Transcervical Challenge. Biomedicines, 13(2), 288. https://doi.org/10.3390/biomedicines13020288





