Heatstroke-Induced Inflammatory Response and Therapeutic Biomarkers
Abstract
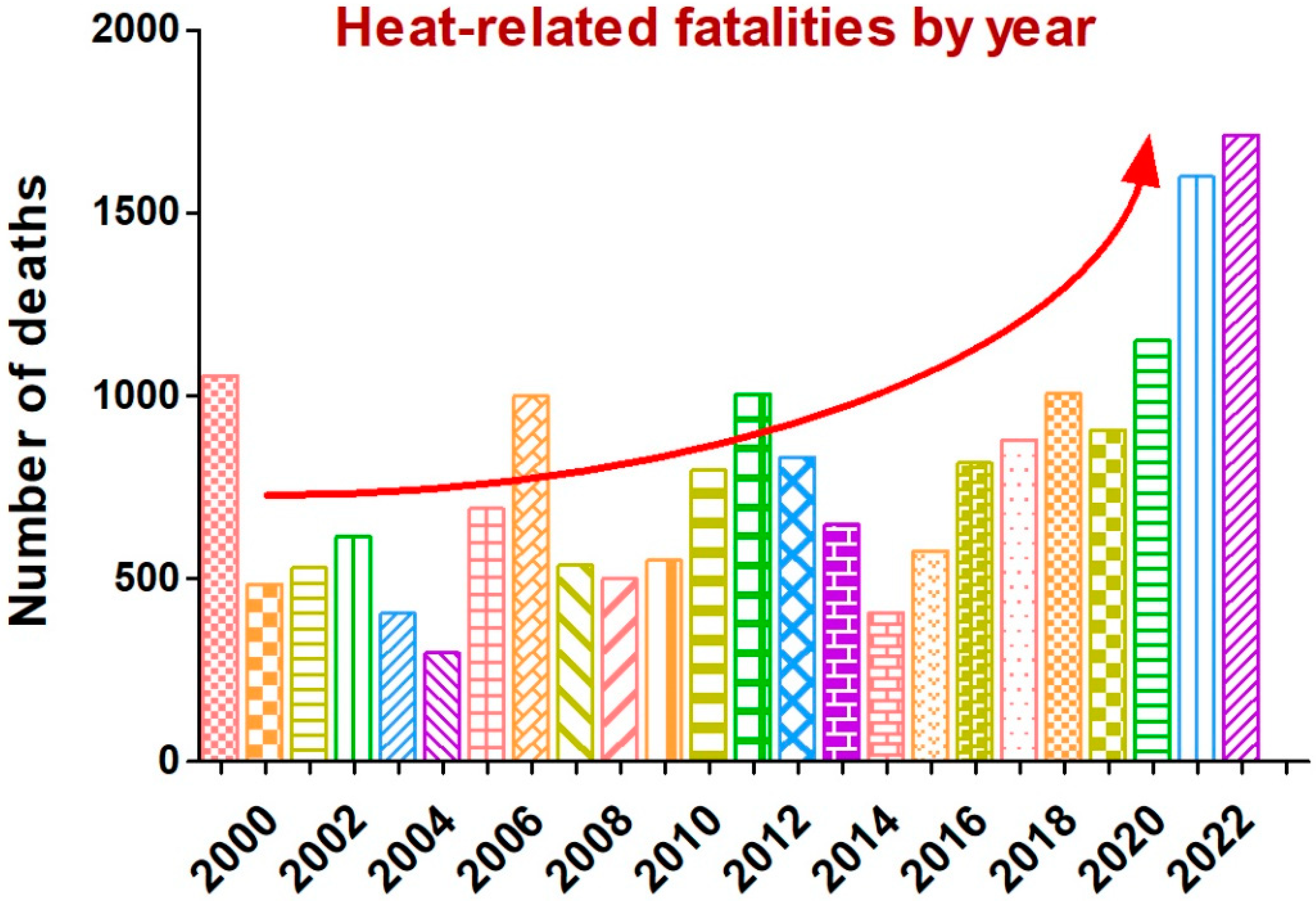
1. Introduction
2. Heatstroke Definition
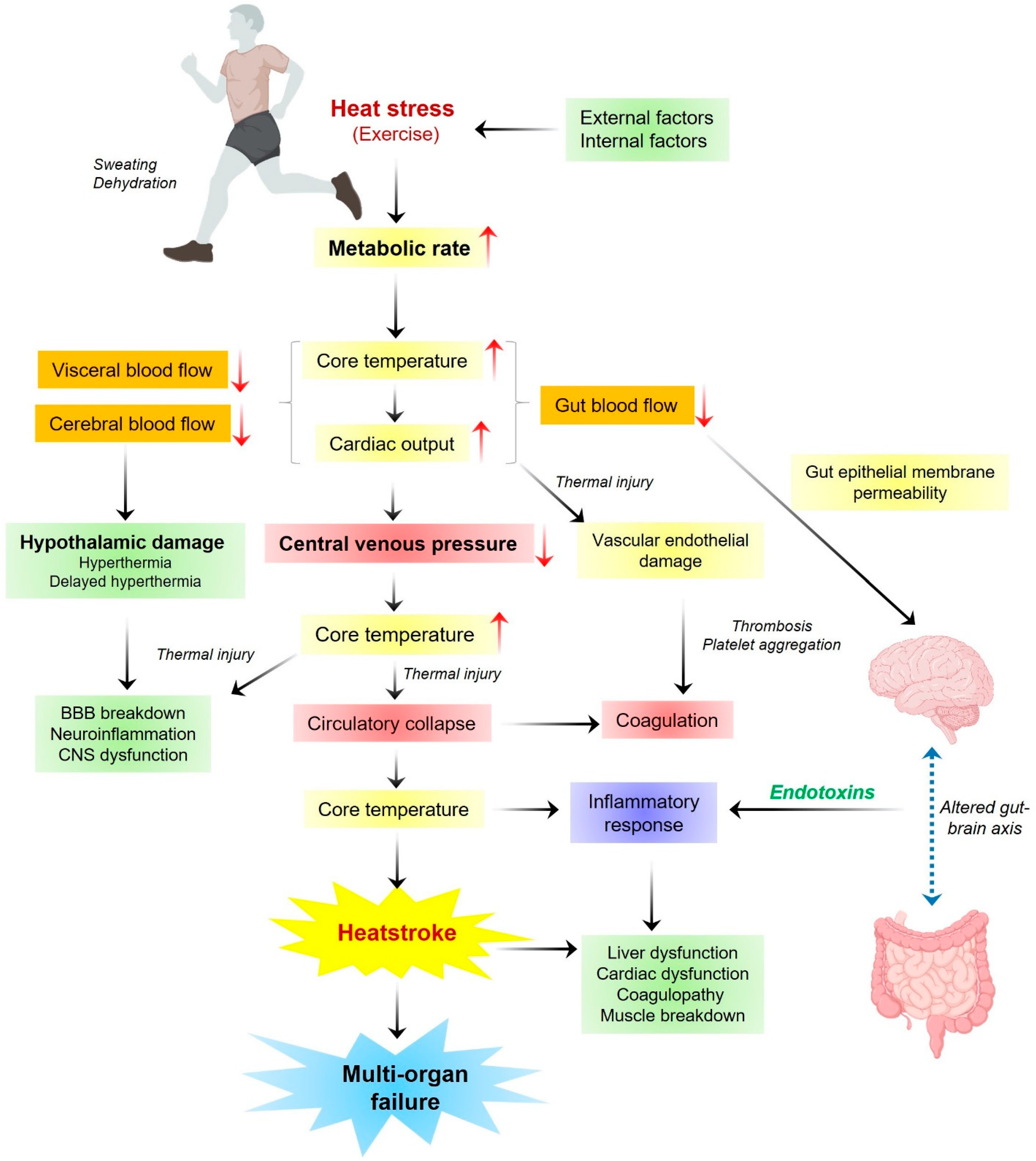
3. Pathogenesis of Heatstroke
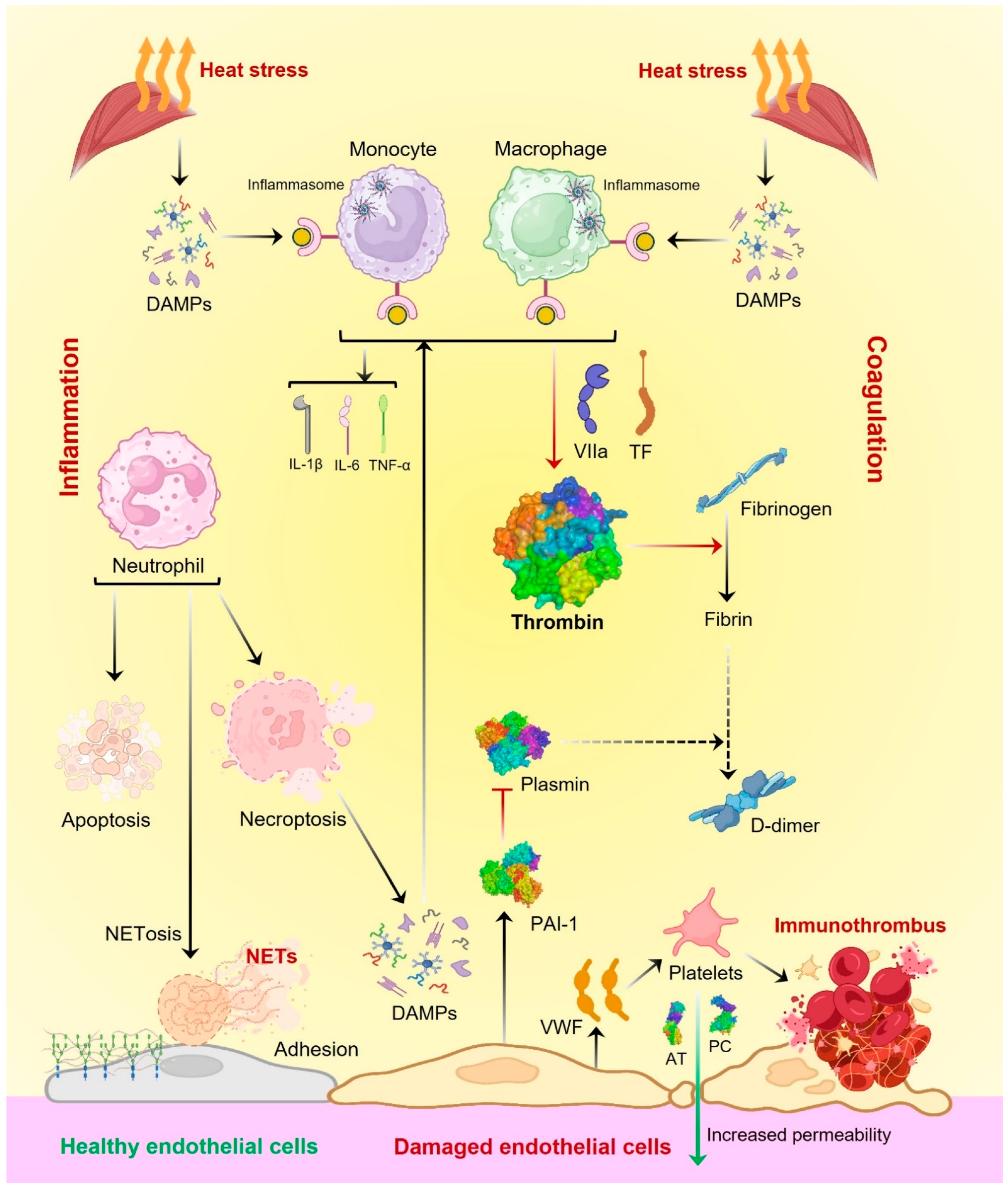
3.1. Inflammatory Immune Response in Heatstroke
3.2. Neuroinflammation Triggered by Heatstroke
4. Clinical Overview of Heatstroke and Associated Dysfunctions
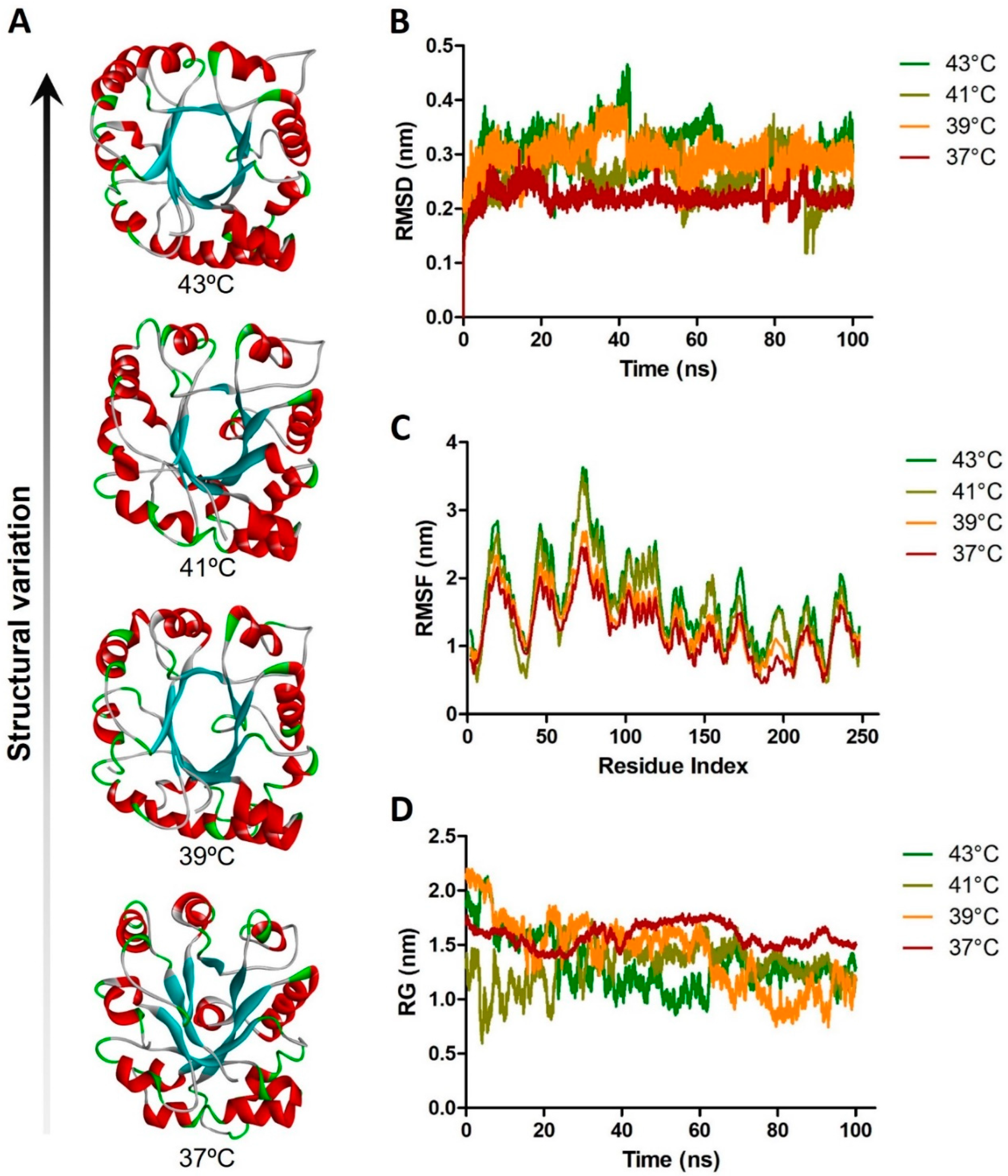
5. Heat-Associated Protein Misfolding and Aggregation
6. Heatstroke Biomarkers
7. Therapeutic Strategies for the Treatment of Heatstroke-Induced Injuries
8. Discussion and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hifumi, T.; Kondo, Y.; Shimizu, K.; Miyake, Y. Heat stroke. J. Intensive Care 2018, 6, 30. [Google Scholar] [CrossRef]
- Shapiro, Y.; Seidman, D.S. Field and clinical observations of exertional heat stroke patients. Med. Sci. Sports Exerc. 1990, 22, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Ballester, J.; Quijal-Zamorano, M.; Méndez Turrubiates, R.F.; Pegenaute, F.; Herrmann, F.R.; Robine, J.M.; Basagaña, X.; Tonne, C.; Antó, J.M.; Achebak, H. Heat-related mortality in Europe during the summer of 2022. Nat. Med. 2023, 29, 1857–1866. [Google Scholar] [CrossRef] [PubMed]
- Hajat, S.; Vardoulakis, S.; Heaviside, C.; Eggen, B. Climate change effects on human health: Projections of temperature-related mortality for the UK during the 2020s, 2050s and 2080s. J. Epidemiol. Community Health 2014, 68, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Misset, B.; De Jonghe, B.; Bastuji-Garin, S.; Gattolliat, O.; Boughrara, E.; Annane, D.; Hausfater, P.; Garrouste-Orgeas, M.; Carlet, J. Mortality of patients with heatstroke admitted to intensive care units during the 2003 heat wave in France: A national multiple-center risk-factor study. Crit. Care Med. 2006, 34, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Mahant, S. The evaluation and management of heat injuries in an intensive care unit. Indian J. Crit. Care Med. 2015, 19, 479–483. [Google Scholar] [CrossRef]
- Bouchama, A.; Knochel, J.P. Heat Stroke. N. Engl. J. Med. 2024, 346, 1978–1988. [Google Scholar] [CrossRef] [PubMed]
- Kravchenko, J.; Abernethy, A.P.; Fawzy, M.; Lyerly, H.K. Minimization of heatwave morbidity and mortality. Am. J. Prev. Med. 2013, 44, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Bouchama, A.; Dehbi, M.; Mohamed, G.; Matthies, F.; Shoukri, M.; Menne, B. Prognostic Factors in Heat Wave—Related Deaths. Arch. Intern. Med. 2012, 167, 2170–2176. [Google Scholar] [CrossRef]
- Rowland, T. Thermoregulation during exercise in the heat in children: Old concepts revisited. J. Appl. Physiol. 2008, 105, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Robertson, B.; Walter, E. “Cool runnings”: Heat stroke in cool conditions. Emerg. Med. J. 2010, 27, 387–388. [Google Scholar] [CrossRef] [PubMed]
- Bouchama, A.; Abuyassin, B.; Lehe, C.; Laitano, O.; Jay, O.; O’Connor, F.G.; Leon, L.R. Classic and exertional heatstroke. Nat. Rev. Dis. Prim. 2022, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.K.; Renteria, L.I.; Leite-Santos, G.; Leon, L.R.; Laitano, O. Exertional heat stroke: Pathophysiology and risk factors. BMJ Med. 2022, 1, e000239. [Google Scholar] [CrossRef]
- Lim, C.L. Heat sepsis precedes heat toxicity in the pathophysiology of heat stroke—A new paradigm on an ancient disease. Antioxidants 2018, 7, 149. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.E.; Zhao, Y.Q.; Wang, H.; Fan, M. Pathophysiological factors underlying heatstroke. Med. Hypotheses 2006, 67, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Dehbi, M.; Baturcam, E.; Eldali, A.; Ahmed, M.; Kwaasi, A.; Chishti, M.A.; Bouchama, A. Hsp-72, a candidate prognostic indicator of heatstroke. Cell Stress Chaperones 2010, 15, 593–603. [Google Scholar] [CrossRef]
- Leon, L.R.; Bouchama, A. Heat stroke. Compr. Physiol. 2015, 5, 611–647. [Google Scholar] [CrossRef] [PubMed]
- Bouchama, A.; Roberts, G.; Al Mohanna, F.; El-Sayed, R.; Lach, B.; Chollet-Martin, S.; Ollivier, V.; Al Baradei, R.; Loualich, A.; Nakeeb, S.; et al. Inflammatory, hemostatic, and clinical changes in a baboon experimental model for heatstroke. J. Appl. Physiol. 2005, 98, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Leon, L.R.; Helwig, B.G. Heat stroke: Role of the systemic inflammatory response. J. Appl. Physiol. 2010, 109, 1980–1988. [Google Scholar] [PubMed]
- Caserta, S.; Mengozzi, M.; Kern, F.; Newbury, S.F.; Ghezzi, P.; Llewelyn, M.J. Severity of Systemic Inflammatory Response Syndrome Affects the Blood Levels of Circulating Inflammatory-Relevant MicroRNAs. Front. Immunol. 2018, 8, 1977. [Google Scholar] [CrossRef]
- Liu, X.; Li, H.; Lu, A.; Zhong, Y.; Hou, X.; Wang, N.; Jia, D.; Zan, J.; Zhao, H.; Xu, J.; et al. Reduction of intestinal mucosal immune function in heat-stressed rats and bacterial translocation. Int. J. Hyperth. 2012, 28, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Heled, Y.; Fleischmann, C.; Epstein, Y. Cytokines and their role in hyperthermia and heat stroke. J. Basic Clin. Physiol. Pharmacol. 2013, 24, 85–96. [Google Scholar] [CrossRef]
- Walter, E.; Gibson, O.R.; Stacey, M.; Hill, N.; Parsons, I.T.; Woods, D. Changes in gastrointestinal cell integrity after marathon running and exercise-associated collapse. Eur. J. Appl. Physiol. 2021, 121, 1179–1187. [Google Scholar] [CrossRef]
- Walter, E.; W Watt, P.; Gibson, O.R.; Wilmott, A.G.B.; Mitchell, D.; Moreton, R.; Maxwell, N.S. Exercise hyperthermia induces greater changes in gastrointestinal permeability than equivalent passive hyperthermia. Physiol. Rep. 2021, 9, e14945. [Google Scholar] [CrossRef]
- Walter, E.J.; Carraretto, M. The neurological and cognitive consequences of hyperthermia. Crit. Care 2016, 20, 199. [Google Scholar] [CrossRef] [PubMed]
- Cremer, O.L.; Kalkman, C.J. Cerebral pathophysiology and clinical neurology of hyperthermia in humans. Prog. Brain Res. 2007, 162, 153–169. [Google Scholar]
- Zhang, Z.T.; Gu, X.L.; Zhao, X.; He, X.; Shi, H.W.; Zhang, K.; Zhang, Y.M.; Su, Y.N.; Zhu, J.B.; Li, Z.W.; et al. NLRP3 ablation enhances tolerance in heat stroke pathology by inhibiting IL-1β-mediated neuroinflammation. J. Neuroinflamm. 2021, 18, 128. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, Z.; Li, Y.; Yuan, R.; Yang, M.; Zhao, Y.; Wang, L.; Zhou, F.; Kang, H. Mesenchymal Stem Cells Provide Neuroprotection by Regulating Heat Stroke-Induced Brain Inflammation. Front. Neurol. 2020, 11, 372. [Google Scholar] [CrossRef]
- Tao, Z.; Cheng, M.; Wang, S.C.; Lv, W.; Hu, H.Q.; Li, C.F.; Cao, B.Z. JAK2/STAT3 pathway mediating inflammatory responses in heatstroke-induced rats. Int. J. Clin. Exp. Pathol. 2015, 8, 6732–6739. [Google Scholar]
- Malamud, N.; Haymaker, W.; Custer, R.P. Heat stroke; a clinico-pathologic study of 125 fatal cases. Mil. Surg. 1946, 99, 397–449. [Google Scholar] [PubMed]
- Maron, M.B.; Wagner, J.A.; Horvath, S.M. Thermoregulatory responses during competitive marathon running. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1977, 42, 909–914. [Google Scholar] [CrossRef]
- Wang, C.C.; Tsai, M.K.; Chen, I.H.; Hsueh, C.W.; Shiang, J.C. Heat stroke. J. Intern. Med. Taiwan 2008, 19, 136–147. [Google Scholar] [CrossRef]
- Yang, M.; Li, Z.; Zhao, Y.; Zhou, F.; Zhang, Y.; Gao, J.; Yin, T.; Hu, X.; Mao, Z.; Xiao, J.; et al. Outcome and risk factors associated with extent of central nervous system injury due to exertional heat stroke. Medicine 2017, 96, e8417. [Google Scholar] [CrossRef]
- Roberts, G.T.; Ghebeh, H.; Chishti, M.A.; Al-Mohaiina, F.; El-Sayed, R.; Al-Mohanna, F.; Bouchama, A. Microvascular injury, thrombosis, inflammation, and apoptosis in the pathogenesis of heatstroke a study in baboon model. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.S.; Tang, Y.Q.; Chen, Y.; Qiu, J.M.; Wen, Q.; Su, L. Early elevated HMGB1 level predicting the outcome in exertional heatstroke. J. Trauma Inj. Infect. Crit. Care 2011, 71, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Segev, G.; Daminet, S.; Meyer, E.; De Loor, J.; Cohen, A.; Aroch, I.; Bruchim, Y. Characterization of kidney damage using several renal biomarkers in dogs with naturally occurring heatstroke. Vet. J. 2015, 206, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Bruchim, Y.; Avital, Y.; Horowitz, M.; Mazaki-Tovi, M.; Aroch, I.; Segev, G. Urinary heat shock protein 72 as a biomarker of acute kidney injury in dogs. Vet. J. 2017, 225, 32–34. [Google Scholar] [CrossRef] [PubMed]
- Capacchione, J.F.; Muldoon, S.M. The relationship between exertional heat illness, exertional rhabdomyolysis, and malignant hyperthermia. Anesth. Analg. 2009, 109, 1065–1069. [Google Scholar] [CrossRef] [PubMed]
- Grogan, H.; Hopkins, P.M. Heat stroke: Implications for critical care and anaesthesia. Br. J. Anaesth. 2002, 88, 700–707. [Google Scholar] [CrossRef]
- Poussel, M.; Guerci, P.; Kaminsky, P.; Heymonet, M.; Roux-Buisson, N.; Faure, J.; Fronzaroli, E.; Chenuel, B. Exertional heat stroke and susceptibility to malignant hyperthermia in an athlete: Evidence for a link? J. Athl. Train. 2015, 50, 1212–1214. [Google Scholar] [CrossRef]
- Bruchim, Y.; Ginsburg, I.; Segev, G.; Mreisat, A.; Avital, Y.; Aroch, I.; Horowitz, M. Serum histones as biomarkers of the severity of heatstroke in dogs. Cell Stress Chaperones 2017, 22, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Gu, Z.; Li, H.; Su, L.; Liu, Z. Cryptdin-2 predicts intestinal injury during heatstroke in mice. Int. J. Mol. Med. 2018, 41, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Stanger, D.; Mihajlovic, V.; Singer, J.; Desai, S.; El-Sayegh, R.; Wong, G.C. Editor’s Choice-Effects of targeted temperature management on mortality and neurological outcome: A systematic review and meta-analysis. Eur. Hear. J. Acute Cardiovasc. Care 2018, 7, 467–477. [Google Scholar] [CrossRef]
- Liu, S.Y.; Song, J.C.; Mao, H.D.; Zhao, J.B.; Song, Q.; Liu, D.W.; Yu, K.J.; Li, J.G.; Lin, H.Y.; Lin, S.; et al. Expert consensus on the diagnosis and treatment of heat stroke in China. Mil. Med. Res. 2020, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Bouchama, A.; Dehbi, M.; Chaves-Carballo, E. Cooling and hemodynamic management in heatstroke: Practical recommendations. Crit. Care 2007, 11, R54. [Google Scholar] [CrossRef]
- Armstrong, L.E.; Crago, A.E.; Adams, R.; Roberts, W.O.; Maresh, C.M. Whole-body cooling of hyperthermic runners: Comparison of two field therapies. Am. J. Emerg. Med. 1996, 14, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Douma, M.J.; Aves, T.; Allan, K.S.; Bendall, J.C.; Berry, D.C.; Chang, W.T.; Epstein, J.; Hood, N.; Singletary, E.M.; Zideman, D.; et al. First aid cooling techniques for heat stroke and exertional hyperthermia: A systematic review and meta-analysis. Resuscitation 2020, 148, 173–190. [Google Scholar] [CrossRef]
- Hsu, S.F.; Niu, K.C.; Lin, C.L.; Lin, M.T. Brain cooling causes attenuation of cerebral oxidative stress, systemic inflammation, activated coagulation, and tissue ischemia/injury during heatstroke. Shock 2006, 26, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.S.; Huang, M.S.; Lin, M.T.; Lee, C.H. Hypothermic retrograde jugular vein flush in heatstroke rats provides brain protection by maintaining cerebral blood flow but not by hemodilution. Crit. Care Med. 2004, 32, 1391–1395. [Google Scholar] [CrossRef]
- Li, Y.N.; Tao, H.; Hong, J.H.; Xiong, Y.L.; Pan, X.C.; Liu, Y.; Yang, X.S.; Zhang, H.G. The Chinese Herbal Formula Huoxiang Zhengqi Dropping Pills Prevents Acute Intestinal Injury Induced by Heatstroke by Increasing the Expression of Claudin-3 in Rats. Evid.-Based Complement. Altern. Med. 2022, 2022, 9230341. [Google Scholar] [CrossRef]
- Bai, X.; Zheng, E.; Tong, L.; Liu, Y.; Li, X.; Yang, H.; Jiang, J.; Chang, Z.; Yang, H. Angong Niuhuang Wan inhibit ferroptosis on ischemic and hemorrhagic stroke by activating PPARγ/AKT/GPX4 pathway. J. Ethnopharmacol. 2024, 321, 117438. [Google Scholar] [CrossRef] [PubMed]
- Walter, E.; Gibson, O.R. The efficacy of steroids in reducing morbidity and mortality from extreme hyperthermia and heatstroke—A systematic review. Pharmacol. Res. Perspect. 2020, 8, e00626. [Google Scholar] [CrossRef]
- Tang, J.; Deng, P.; Jiang, Y.; Tang, Y.; Chen, B.; Su, L.; Liu, Z. Role of HMGB1 in propofol protection of rat intestinal epithelial cells injured by heat shock. Cell Biol. Int. 2013, 37, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.K.; Sheikh, L.H.; Iwaniec, J.D.; Robinson, G.P.; Berlet, R.A.; Mattingly, A.J.; Murray, K.O.; Laitano, O.; Clanton, T.L. Effects of Ibuprofen during Exertional Heat Stroke in Mice. Med. Sci. Sports Exerc. 2020, 52, 1870–1878. [Google Scholar] [CrossRef]
- Umemura, Y.; Ogura, H.; Matsuura, H.; Ebihara, T.; Shimizu, K.; Shimazu, T. Bone marrow-derived mononuclear cell therapy can attenuate systemic inflammation in rat heatstroke. Scand. J. Trauma. Resusc. Emerg. Med. 2018, 26, 97. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Chang, F.M.; Chang, H.K.; Chen, W.C.; Huang, K.F.; Lin, M.T. Human umbilical cord blood-derived CD34+ cells cause attenuation of multiorgan dysfunction during experimental heatstroke. Shock 2007, 27, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Deng, Z.; Zhao, Y.; Yuan, R.; Yang, M.; Zhang, Y.; Li, Y.; Liu, Y.; Zhou, F.; Kang, H. Mesenchymal stem cells regulate activation of microglia cells to improve hippocampal injury of heat stroke rats. J. Therm. Biol. 2021, 101, 103081. [Google Scholar] [CrossRef]
- Chang, C.P.; Huang, W.T.; Cheng, B.C.; Hsu, C.C.; Lin, M.T. The flavonoid baicalin protects against cerebrovascular dysfunction and brain inflammation in experimental heatstroke. Neuropharmacology 2007, 52, 1024–1033. [Google Scholar] [CrossRef]
- Li, P.; Shen, T.; Luo, X.; Yang, J.; Luo, Z.; Tan, Y.; He, G.; Wang, Z.; Yu, X.; Wang, Y.; et al. Modulation of microglial phenotypes by dexmedetomidine through TREM2 reduces neuroinflammation in heatstroke. Sci. Rep. 2021, 11, 13345. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Yuan, F.; Geng, Y.; Qiu, X.; Liu, Z.; Lu, J.; Tang, L.; Zhang, Y.; Su, L. Eicosapentaenoic acid enhances heatstroke-impaired intestinal epithelial barrier function in rats. Shock 2015, 44, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.Y.; Hsu, Y.J.; Lu, C.Y.; Tsai, M.C.; Hung, W.C.; Chen, P.C.; Wang, J.C.; Hsu, L.A.; Yeh, Y.H.; Chu, P.; et al. Pharmacological Activation Of Aldehyde Dehydrogenase 2 Protects Against Heatstroke-Induced Acute Lung Injury by Modulating Oxidative Stress and Endothelial Dysfunction. Front. Immunol. 2021, 12, 740562. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.C.; Lam, K.K.; Peng, Y.J.; Lee, Y.M.; Yang, C.Y.; Tsai, Y.J.; Yen, M.H.; Cheng, P.Y. Heat shock protein 70 and AMP-activated protein kinase contribute to 17-DMAG-dependent protection against heat stroke. J. Cell. Mol. Med. 2016, 20, 1889–1897. [Google Scholar] [CrossRef]
- Hii, H.P.; Lo, W.Z.; Fu, Y.H.; Chen, M.H.; Shih, C.C.; Tsao, C.M.; Ka, S.M.; Chiu, Y.L.; Wu, C.C.; Shih, C.C. Improvement in heat stress-induced multiple organ dysfunction and intestinal damage through protection of intestinal goblet cells from prostaglandin E1 analogue misoprostol. Life Sci. 2022, 310, 121039. [Google Scholar] [CrossRef] [PubMed]
- Varasteh, S.; Braber, S.; Kraneveld, A.D.; Garssen, J.; Fink-Gremmels, J. L-Arginine supplementation prevents intestinal epithelial barrier breakdown under heat stress conditions by promoting nitric oxide synthesis. Nutr. Res. 2018, 57, 45–55. [Google Scholar] [CrossRef]
- Lin, X.; Zhao, T.; Lin, C.H.; Zuo, D.; Ye, Z.; Lin, S.; Wen, S.; Liu, L.; Lin, M.T.; Chang, C.P.; et al. Melatonin provides protection against heat stroke-induced myocardial injury in male rats. J. Pharm. Pharmacol. 2018, 70, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.J.; Mei, G.P.; Liu, J.; Li, Y.L.; Zuo, D.; Liu, S.J.; Zhao, T.B.; Lin, M.T. Therapeutic effects of melatonin on heatstroke-induced multiple organ dysfunction syndrome in rats. J. Pineal Res. 2011, 50, 436–444. [Google Scholar] [CrossRef]
- Wu, W.S.; Chou, M.T.; Chao, C.M.; Chang, C.K.; Lin, M.T.; Chang, C.P. Melatonin reduces acute lung inflammation, edema, and hemorrhage in heatstroke rats. Acta Pharmacol. Sin. 2012, 33, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.D.; Fan, H.; Zhu, J.H. Salidroside pretreatment protects against myocardial injury induced by heat stroke in mice. J. Int. Med. Res. 2019, 47, 5229–5238. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Lin, C.H.; Zhao, T.; Zuo, D.; Ye, Z.; Liu, L.; Lin, M.T. Quercetin protects against heat stroke-induced myocardial injury in male rats: Antioxidative and antiinflammatory mechanisms. Chem. Biol. Interact. 2017, 265, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-Q.; Gao, J.-T.; Liu, S.-H.; Wu, Y.; Lin, M.-T.; Fan, M. Geranylgeranylacetone preconditioning may attenuate heat-induced inflammation and multiorgan dysfunction in rats. J. Pharm. Pharmacol. 2010, 62, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.M.; Buettner, G.R.; Oberley, L.W.; Xu, L.; Matthes, R.D.; Gisolfi, C.V. Mechanisms of circulatory and intestinal barrier dysfunction during whole body hyperthermia. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H509–H521. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Li, H.; Zhu, G.; Chen, X.; Tang, Z. Sodium tanshinone IIA sulfonate improves inflammation, aortic endothelial cell apoptosis, disseminated intravascular coagulation and multiple organ damage in a rat heat stroke model. Mol. Med. Rep. 2017, 16, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.K.; Chang, C.P.; Liu, S.Y.; Lin, M.T. Oxidative stress and ischemic injuries in heat stroke. Prog. Brain Res. 2007, 162, 525–546. [Google Scholar] [PubMed]
- Mota, R.A.; Hernández-Espinosa, D.; Galbis-Martinez, L.; Ordoñez, A.; Miñano, A.; Parrilla, P.; Vicente, V.; Corral, J.; Yélamos, J. Poly (ADP-ribose) polymerase-1 inhibition increases expression of heat shock proteins and attenuates heat stroke-induced liver injury. Crit. Care Med. 2008, 36, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Kao, T.Y.; Jin, Y.T.; Chen, C.F. Interleukin-1 receptor antagonist attenuates the heat stroke-induced neuronal damage by reducing the cerebral ischemia in rats. Brain Res. Bull. 1995, 37, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.S.; Tang, Y.Q.; Chen, Y.; Yuan, F.F.; Liu, Z.F.; Peng, N.; Tang, L.Q.; Su, L. HMGB1 activity inhibition alleviating liver injury in heatstroke. J. Trauma Acute Care Surg. 2013, 74, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Ohbe, H.; Isogai, S.; Jo, T.; Matsui, H.; Fushimi, K.; Yasunaga, H. Treatment with Antithrombin or Thrombomodulin and Mortality from Heatstroke-Induced Disseminated Intravascular Coagulation: A Nationwide Observational Study. Semin. Thromb. Hemost. 2019, 45, 760–766. [Google Scholar] [CrossRef]
- Li, L.; Tan, H.; Zou, Z.; Gong, J.; Zhou, J.; Peng, N.; Su, L.; Maegele, M.; Cai, D.; Gu, Z. Preventing necroptosis by scavenging ROS production alleviates heat stress-induced intestinal injury. Int. J. Hyperth. 2020, 37, 517–530. [Google Scholar] [CrossRef]
- Moon, M.; Huh, E.; Lee, W.; Song, E.J.; Hwang, D.S.; Lee, T.H.; Oh, M.S. Coptidis Rhizoma prevents heat stress-induced brain damage and cognitive impairment in mice. Nutrients 2017, 9, 1057. [Google Scholar] [CrossRef]
- Li, L.; Wang, M.; Chen, J.; Xu, Z.; Wang, S.; Xia, X.; Liu, D.; Wang, S.; Xie, C.; Wu, J.; et al. Preventive Effects of Bacillus licheniformis on Heat Stroke in Rats by Sustaining Intestinal Barrier Function and Modulating Gut Microbiota. Front. Microbiol. 2021, 12, 630841. [Google Scholar] [CrossRef]
- Ren, M.Q.; Kazman, J.B.; Abraham, P.A.; Atias-Varon, D.; Heled, Y.; Deuster, P.A. Gene expression profiling of humans under exertional heat stress: Comparisons between persons with and without exertional heat stroke. J. Therm. Biol. 2019, 85, 102423. [Google Scholar] [CrossRef] [PubMed]
- Durham, W.J.; Aracena-Parks, P.; Long, C.; Rossi, A.E.; Goonasekera, S.A.; Boncompagni, S.; Galvan, D.L.; Gilman, C.P.; Baker, M.R.; Shirokova, N.; et al. RyR1 S-Nitrosylation Underlies Environmental Heat Stroke and Sudden Death in Y522S RyR1 Knockin Mice. Cell 2008, 133, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Yamazawa, T.; Kobayashi, T.; Kurebayashi, N.; Konishi, M.; Noguchi, S.; Inoue, T.; Inoue, Y.U.; Nishino, I.; Mori, S.; Iinuma, H.; et al. A novel RyR1-selective inhibitor prevents and rescues sudden death in mouse models of malignant hyperthermia and heat stroke. Nat. Commun. 2021, 12, 4293. [Google Scholar] [CrossRef] [PubMed]
- Protasi, F.; Paolini, C.; Dainese, M. Calsequestrin-1: A new candidate gene for malignant hyperthermia and exertional/environmental heat stroke. Proc. J. Physiol. 2009, 587, 3095–3100. [Google Scholar] [CrossRef] [PubMed]
| Treatment/Therapy | Mechanism of Action | References |
|---|---|---|
| Targeted temperature management | Maintains body temperature. | [43,44] |
| Total cold water immersion | Maintains body temperature. | [46,47] |
| Hypothermic retrograde jugular vein flush | Reduces systemic inflammation, oxidative stress, ischemic injury, and coagulation. | [48,49] |
| Bone marrow-derived mononuclear cell (BMMNC) transplantation therapy | Reduces acute systemic inflammation and vascular endothelial injury by reducing proinflammatory cytokines. | [55] |
| Human umbilical cord blood-derived CD34+ cell therapy | Protects against multiorgan dysfunction by reducing systemic inflammation. | [56] |
| Mesenchymal stem cell therapy | Reduces mortality by protecting against neurological defects and hippocampal damage, by restricting the overactivation of hippocampal microglia. | [28,57] |
| Regular physical exercise for at least 3 weeks | Reduction in heatstroke-induced ROS and lipid peroxidation. | [58] |
| Drugs/Strategies | Source | Action Against Heatstroke Pathologies | Study Model | References |
|---|---|---|---|---|
| Dexmedetomidine | Synthetic | Reduces neuroinflammation by activating PI3K/Akt via TREM2 in microglia. | Mice | [59] |
| Eicosapentaenoic acid | Cold water fish | Maintaining intestinal barrier permeability by protecting tight junctions and reducing plasma endotoxin levels. | Rats | [60] |
| Alad-1 | Synthetic | An agonist of aldehyde dehydrogenase 2, alleviates oxidative stress by reducing ROS. | Mice | [61] |
| 17-dimethylaminoethylamino-17-demethoxy-geldanamycin (17-DMAG) | Semisynthetic derivative of geldanamycin | Decreases hypotension and organ dysfunction via upregulation of Hsp70 and phosphorylation of AMPK. | Rats | [62] |
| Misoprostol | Synthetic | Prostaglandin protects against multiorgan dysfunction and intestinal damage. | Rats | [63] |
| l-Arginine | Natural amino acid | Protects intestinal epithelial integrity by replenishing E-calmodulin downregulation. | Human colorectal adenocarcinoma (Caco-2) cells | [64] |
| Melatonin | Natural hormone | Diminishes hyperthermia and hypotension, and protects against myocardial injury by attenuating oxidative stress and inflammation. Reduces neutrophil infiltration and gene expression of pro-inflammatory factors. | Rats | [65,66,67] |
| Salidroside | Roots of Rhodiola rosea | Protects against myocardial injury by reducing inflammation and oxidative stress. | Mice | [68] |
| Quercetin | Plant pigment (flavonoid) | Protects against myocardial injury by reducing inflammation and oxidative stress. | Rats | [69] |
| Geranylgeranylacetone | Synthetic | Protects against hepatic and renal dysfunction by inducing HSP70, and anti-inflammatory cytokines. | Rats | [70] |
| Allopurinol | Synthetic | Reduces portal vein endotoxins and protects the integrity of the circulatory and intestinal barrier. | Rats | [71] |
| Sodium tanshinone IIA | Derivative of tanshinone IIA (extracted from Salvia miltiorrhiza) | Protects against multiorgan failure by reducing inflammatory response, intravascular coagulation, and endothelial cell apoptosis. | Rats | [72] |
| Magnolol | Bark of Magnolia tree | Reduces oxidative damage. | Rats | [73] |
| Baicalin | Roots of Scutellaria baicalensis | Protects against cerebrovascular dysfunction and reduces brain inflammation. | Rats | [58] |
| PARP inhibitor | Synthetic | Reduces heatstroke injury via inducing the expression of HSPs. | Mice | [74] |
| IL-1 receptor antagonist | Naturally produced by the body | Protects neuronal damage by restricting cerebral ischemia. | Rats | [75] |
| HMGB1 monoclonal antibody | Synthetic | Alleviates inflammatory response and reduces liver injury. | Rats | [76] |
| Recombinant human thrombomodulin | Synthetic | Protects against disseminated intravascular coagulation. | Humans | [77] |
| Mannitol | Natural sugar alcohol | Reduces intestinal damage via inhibition of RIPK1/RIPK3-dependent necroptosis. | Mice | [78] |
| α-tocopherol | Natural | Reduces intestinal damage via inhibition of RIPK1/RIPK3-dependent necroptosis. | Mice | [78] |
| N-acetyl-l-cysteine | Synthetic | Reduces intestinal damage via inhibition of RIPK1/RIPK3-dependent necroptosis. | Mice | [78] |
| Huanglian | Extracted from the rhizome of Coptis chinensis | Protects against brain injury via attenuating hypothermia and neuroinflammation and upregulation of HSPs and c-Fos. | Mice | [79] |
| Bacillus licheniformis | Bacteria | Reduces intestinal damage by maintaining the integrity of the intestinal barrier and improving the health of gut microbiota. | Rats | [80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baindara, P.; Jana, A.; Dinata, R.; Mandal, S.M. Heatstroke-Induced Inflammatory Response and Therapeutic Biomarkers. Biomedicines 2025, 13, 261. https://doi.org/10.3390/biomedicines13020261
Baindara P, Jana A, Dinata R, Mandal SM. Heatstroke-Induced Inflammatory Response and Therapeutic Biomarkers. Biomedicines. 2025; 13(2):261. https://doi.org/10.3390/biomedicines13020261
Chicago/Turabian StyleBaindara, Piyush, Aritra Jana, Roy Dinata, and Santi M. Mandal. 2025. "Heatstroke-Induced Inflammatory Response and Therapeutic Biomarkers" Biomedicines 13, no. 2: 261. https://doi.org/10.3390/biomedicines13020261
APA StyleBaindara, P., Jana, A., Dinata, R., & Mandal, S. M. (2025). Heatstroke-Induced Inflammatory Response and Therapeutic Biomarkers. Biomedicines, 13(2), 261. https://doi.org/10.3390/biomedicines13020261








