Quantitative Measures of Time to Loss of 15% Vital Capacity and Survival Extension in Slowly Progressive Amyotrophic Lateral Sclerosis (ALS) Patients Treated with the Immune Regulator NP001 Suggests an Immunopathogenic Subset of ALS
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Trials and Data
2.2. Definition of Analytical Clinical Factors
2.2.1. Analysis of Predicted Vital Capacity (PVC) Change from Baseline
2.2.2. Definition of ALS Disease Progression Rate (DPR) and Slow vs. Rapid ALS Groups
2.3. Time-to-Event (TTE) Analyses of 15% PVC Loss
2.4. Overall Survival (OS) Analyses
2.5. Statistical Analyses
3. Results
3.1. Analysis of NP001 Activity Defined by Time-to-Event (TTE) Loss of 15% PVC
3.2. Relationship Between 15% PVC Loss in Slowly vs. Rapidly Progressive ALS
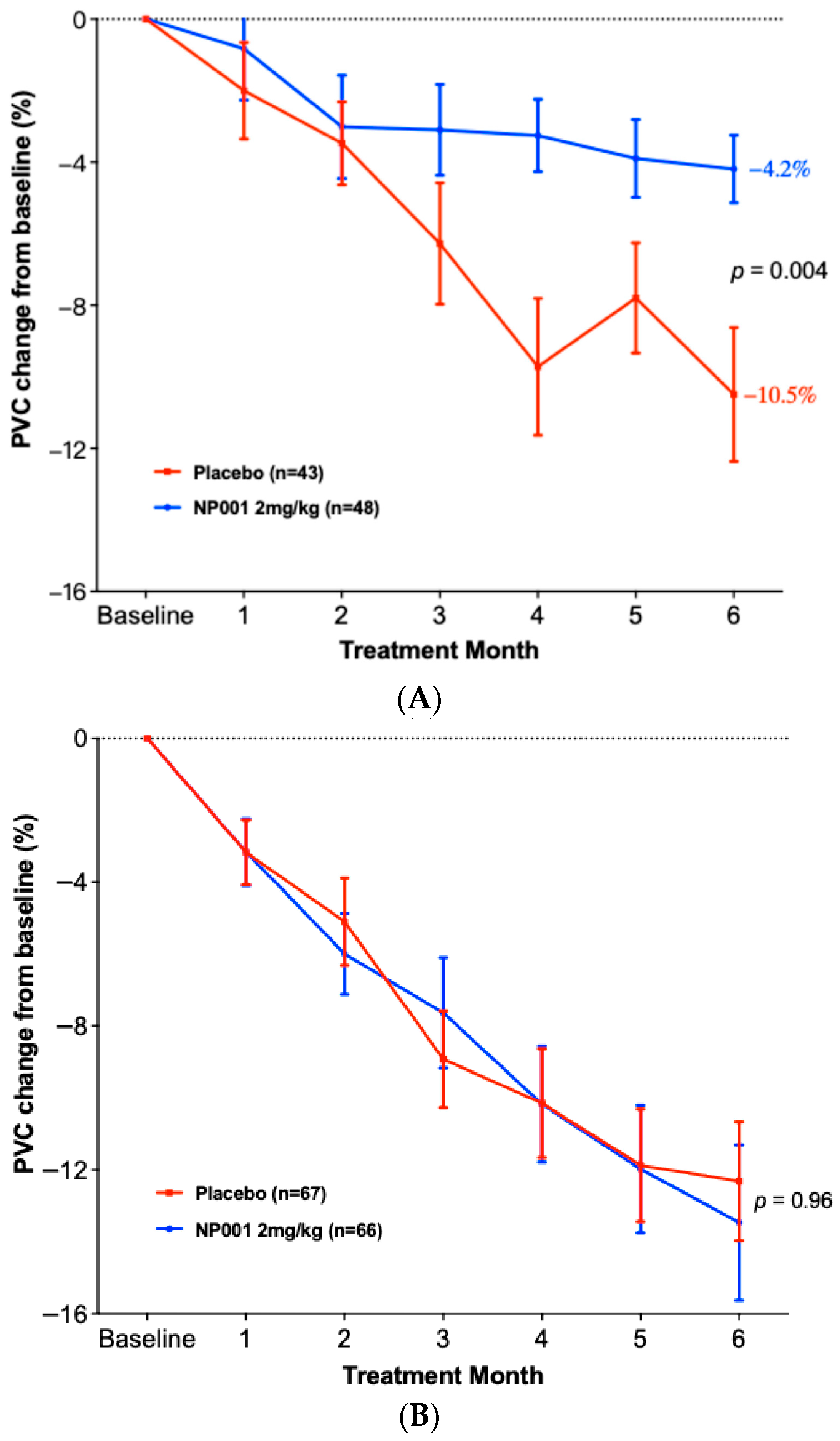
3.3. Change in PVC from Baseline in ALS Patients Treated with NP001 vs. Placebo in Slow vs. Rapid Disease
3.4. Overall Survival Analysis in ALS Patients Treated with NP001 vs. Placebo in Slow vs. Rapid Disease
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALS | Amyotrophic Lateral Sclerosis |
| ALSFRS-R | Revised ALS Functional Rating Scale Score |
| DPR | Disease Progression Rate |
| EOS | End of Study |
| FDA | Food and Drug Administration |
| FVC | Forced Vital Capacity |
| HR | Hazard Ratio |
| IPF | Idiopathic Pulmonary Fibrosis |
| ITT | Intention-to-Treat |
| NF-kB | Nuclear Factor-kappa B |
| OS | Overall Survival |
| PVC | Predicted Vital Capacity |
| SEM | Standard Error of the Mean |
| SD | Standard Deviation |
| SOD1 | Superoxide Dismutase-1 |
| SVC | Slow Vital Capacity |
| TauCl | Taurine Chloramine |
| TNF-α | Tumor Necrosis Factor alpha |
| TTE | Time to Event |
| VC | Vital Capacity |
References
- Zhang, R.; Gascon, R.; Miller, R.G.; Gelinas, D.F.; Mass, J.; Hadlock, K.; Jin, X.; Reis, J.; Narvaez, A.; McGrath, M.S. Evidence for systemic immune system alterations in sporadic amyotrophic lateral sclerosis (sALS). J. Neuroimmunol. 2005, 159, 215–224. [Google Scholar] [CrossRef]
- Zhang, R.; Bracci, P.M.; Azhir, A.; Forrest, B.D.; McGrath, M.S. Macrophage-Targeted Sodium Chlorite (NP001) Slows Progression of Amyotrophic Lateral Sclerosis (ALS) through Regulation of Microbial Translocation. Biomedicines 2022, 10, 2907. [Google Scholar] [CrossRef] [PubMed]
- Giese, T.; McGrath, M.S.; Stumm, S.; Schempp, H.; Elstner, E.; Meuer, S.C. Differential effects on innate versus adaptive immune responses by WF10. Cell. Immunol. 2004, 229, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Barua, M.; Liu, Y.; Quinn, M.R. Taurine chloramine inhibits inducible nitric oxide synthase and TNF-alpha gene expression in activated alveolar macrophages: Decreased NF-κB activation and IκB kinase activity. J. Immunol. 2001, 167, 2275–2281. [Google Scholar] [CrossRef]
- Joo, K.; Lee, Y.; Choi, D.; Han, J.; Hong, S.; Kim, Y.M.; Jung, Y. An anti-inflammatory mechanism of taurine conjugated 5-aminosalicylic acid against experimental colitis: Taurine chloramine potentiates inhibitory effect of 5-aminosalicylic acid on IL-1beta-mediated NFkappaB activation. Eur. J. Pharmacol. 2009, 618, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Seol, S.-I.; Kang, I.S.; Lee, J.S.; Lee, J.-K.; Kim, C. Taurine Chloramine-Mediated Nrf2 Activation and HO-1 Induction Confer Protective Effects in Astrocytes. Antioxidants 2024, 13, 169. [Google Scholar] [CrossRef]
- Miller, R.G.; Zhang, R.; Block, G.; Katz, J.; Barohn, R.; Kasarskis, E.; Forshew, D.; Gopalakrishnan, V.; McGrath, M.S. NP001 regulation of macrophage activation markers in ALS: A phase I clinical and biomarker study. Amyotroph. Lateral Scler. Front. Degener. 2014, 15, 601–609. [Google Scholar] [CrossRef]
- Miller, R.G.; Block, G.; Katz, J.S.; Barohn, R.J.; Gopalakrishnan, V.; Cudkowicz, M.; Zhang, J.R.; McGrath, M.S.; Ludington, E.; Appel, S.H. Randomized phase 2 trial of NP001-a novel immune regulator: Safety and early efficacy in ALS. Neurol. Neuroimmunol. Neuroinflamm 2015, 2, e100. [Google Scholar] [CrossRef]
- Miller, R.G.; Zhang, R.; Bracci, P.M.; Azhir, A.; Barohn, R.; Bedlack, R.; Benatar, M.; Berry, J.D.; Cudkowicz, M.; Kasarskis, E.J.; et al. Phase 2B randomized controlled trial of NP001 in amyotrophic lateral sclerosis: Pre-specified and post hoc analyses. Muscle Nerve 2022, 66, 39–49. [Google Scholar] [CrossRef]
- McGrath, M.S.; Zhang, R.; Bracci, P.M.; Azhir, A.; Forrest, B.D. Regulation of the Innate Immune System as a Therapeutic Approach to Supporting Respiratory Function in ALS. Cells 2023, 12, 1031. [Google Scholar] [CrossRef]
- Zhang, R.; Azhir, A.; McGrath, M.S. Respiratory Function Improvement and Lifespan Extension Following Immunotherapy with NP001 Support the Concept That Amyotrophic Lateral Sclerosis (ALS) Is an Immuno-Neurologic Disease. Int. J. Mol. Sci. 2025, 26, 4349. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.A.; Meng, L.; Kulke, S.F.; Rudnicki, S.A.; Wolff, A.A.; Bozik, M.E.; Malik, F.I.; Shefner, J.M. Association Between Decline in Slow Vital Capacity and Respiratory Insufficiency, Use of Assisted Ventilation, Tracheostomy, or Death in Patients With Amyotrophic Lateral Sclerosis. JAMA Neurol. 2018, 75, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Forrest, B.D.; Goyal, N.A.; Fleming, T.R.; Bracci, P.M.; Brett, N.R.; Khan, Z.; Robinson, M.; Azhir, A.; McGrath, M. The Effectiveness of NP001 on the Long-Term Survival of Patients with Amyotrophic Lateral Sclerosis. Biomedicines 2024, 12, 2367. [Google Scholar] [CrossRef] [PubMed]
- Pinto, S.; de Carvalho, M. SVC Is a Marker of Respiratory Decline Function, Similar to FVC, in Patients With ALS. Front. Neurol. 2019, 10, 109. [Google Scholar] [CrossRef]
- Richeldi, L.; Du Bois, R.M.; Raghu, G.; Azuma, A.; Brown, K.K.; Costabel, U.; Cottin, V.; Flaherty, K.R.; Hansell, D.M.; Inoue, Y.; et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014, 370, 2071–2082. [Google Scholar] [CrossRef]
- Karimi-Shah, B.A.; Chowdhury, B.A. Chowdhury, Forced vital capacity in idiopathic pulmonary fibrosis—FDA review of pirfenidone and nintedanib. N. Engl. J. Med. 2015, 372, 1189–1191. [Google Scholar] [CrossRef]
- King, T.E., Jr.; Bradford, W.Z.; Castro-Bernardini, S.; Fagan, E.A.; Glaspole, I.; Glassberg, M.K.; Gorina, E.; Hopkins, P.M.; Kardatzke, D.; Lancaster, L.; et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014, 370, 2083–2092. [Google Scholar] [CrossRef]
- Amyotrophic Lateral Sclerosis: Developing Drugs for Treatment Guidance for Industry (US Food and Drug Administration, September 2019). Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/amyotrophic-lateral-sclerosis-developing-drugs-treatment-guidance-industry (accessed on 1 July 2024).
- Cao, W.; Fan, D. Neutrophils: A subgroup of neglected immune cells in ALS. Front. Immunol. 2023, 14, 1246768. [Google Scholar] [CrossRef]
- Cui, C.; Sun, J.; Pawitan, Y.; Piehl, F.; Chen, H.; Ingre, C.; Wirdefeldt, K.; Evans, M.; Andersson, J.; Carrero, J.-J.; et al. Creatinine and C-reactive protein in amyotrophic lateral sclerosis, multiple sclerosis and Parkinson’s disease. Brain Commun. 2020, 2, fcaa152. [Google Scholar] [CrossRef]
- Hardiman, O.; Al-Chalabi, A.; Brayne, C.; Beghi, E.; van den Berg, L.H.; Chio, A.; Martin, S.; Logroscino, G.; Rooney, J. The changing picture of amyotrophic lateral sclerosis: Lessons from European registers. J. Neurol. Neurosurg. Psychiatry 2017, 88, 557–563. [Google Scholar] [CrossRef]
- Sugimoto, K.; Han, Y.; Song, Y.; Gao, Y. Correlational Analysis of ALS Progression and Serum NfL Measured by Simoa Assay in Chinese Patients. Front. Neurol. 2020, 11, 579094. [Google Scholar] [CrossRef]
- Therneau, T.M. A Package for Survival Analysis in R. R Package Version 3.8-3. 2024. Available online: https://cran.r-project.org/web/packages/survival/ (accessed on 15 May 2025).
- Kassambara, A.; Kassambara, M.; Biecek, P. Survminer: Drawing Survival Curves Using ‘ggplot2’. R Package Version 0.5.0. 2021. Available online: https://CRAN.R-project.org/package=survminer (accessed on 15 May 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.r-project.org/ (accessed on 15 May 2025).
- Schempp, H.; Reim, M.; Dornisch, K.; Elstner, E.F. Chlorite-hemoprotein interaction as key role for the pharmacological activity of the chlorite-based drug WF10. Arzneimittelforschung 2001, 51, 554–562. [Google Scholar] [CrossRef]
- McGrath, M.S.; OKahn, J.; Herndier, B.G. Development of WF10, a novel macrophage-regulating agent. Curr. Opin. Investig. Drugs 2002, 3, 365–373. [Google Scholar]
- Veerasarn, V.; Boonnuch, W.; Kakanaporn, C. A phase II study to evaluate WF10 in patients with late hemorrhagic radiation cystitis and proctitis. Gynecol. Oncol. 2006, 100, 179–184. [Google Scholar] [CrossRef]
- Veerasarn, V.; Khorprasert, C.; Lorvidhaya, V.; Sangruchi, S.; Tantivatana, T.; Narkwong, L.; Kongthanarat, Y.; Chitapanarux, I.; Tesavibul, C.; Panichevaluk, A.; et al. Reduced recurrence of late hemorrhagic radiation cystitis by WF10 therapy in cervical cancer patients: A multicenter, randomized, two-arm, open-label trial. Radiother. Oncol. 2004, 73, 179–185. [Google Scholar]
- Flemmig, J.; Schlorke, D.; Kühne, F.-W.; Arnhold, J. Inhibition of the heme-induced hemolysis of red blood cells by thechlorite-based drug WF10. Free Radic. Res. 2016, 50, 1386–1395. [Google Scholar] [CrossRef]
- Lu, C.H.; Macdonald-Wallis, C.; Gray, E.; Pearce, N.; Petzold, A.; Norgren, N.; Giovannoni, G.; Fratta, P.; Sidle, K.; Fish, M.; et al. Neurofilament light chain: A prognostic biomarker in amyotrophic lateral sclerosis. Neurology 2015, 84, 2247–2257. [Google Scholar] [CrossRef] [PubMed]




| DPR < 0.50 | DPR ≥ 0.50 | ||
|---|---|---|---|
| Characteristics | (n = 91) | (n = 133) | p Value |
| Sex, n (%) | 1.0 | ||
| Female | 29 (31.9%) | 43 (32.3%) | |
| Male | 62 (68.1%) | 90 (67.7%) | |
| Age at baseline (years), mean ± SD | 56.1 ± 10.1 | 55.7 ± 10.9 | 0.87 |
| Site of ALS onset, n (%) | 0.70 | ||
| Bulbar | 14 (15.4%) | 18 (13.5%) | |
| Limb | 77 (84.6%) | 115 (86.5%) | |
| El Escorial criteria for ALS, n (%) | NS | ||
| Definite | 39 (42.9%) | 57 (42.9%) | |
| Possible | 9 (9.9%) | 8 (6.0%) | |
| Probable | 38 (41.8%) | 59 (44.4%) | |
| Probable Laboratory Supported | 5 (5.5%) | 9 (6.8%) | |
| Concurrent riluzole use, n (%) | 0.15 | ||
| Yes | 56 (61.5%) | 95 (71.4%) | |
| No | 35 (38.5%) | 38 (28.6%) | |
| ALSFRS-R score at baseline, mean ± SD | 40.6 ± 3.6 | 35.4 ± 5.3 | <0.0001 |
| PVC 3 at baseline (%), mean ± SD | 89.7 ± 15.9 | 85.3 ± 15.5 | 0.08 |
| Duration of ALS symptom (months), mean ± SD | 22.79 ± 7.68 | 14.91 ± 7.15 | <0.0001 |
| DPR 4 at baseline (ALSFRS-R score lost/month) | 0.33 ± 0.12 | 1.03 ± 0.89 | <0.0001 |
| CRP at baseline (mg/L), mean ± SD | 3.62 ± 3.59 | 3.48 ± 4.86 | 0.65 |
| NP001 2 mg/kg | Placebo | ||
|---|---|---|---|
| Characteristics | (n = 48) | (n = 43) | p Value |
| Sex, n (%) | 0.26 | ||
| Female | 18 (37.5%) | 11 (25.6%) | |
| Male | 30 (62.5%) | 32 (74.4%) | |
| Age at baseline (years), mean ± SD | 56.1 ± 9.4 | 56.0 ± 11.0 | 0.73 |
| Site of ALS onset, n (%) | 0.56 | ||
| Bulbar | 6 (12.5%) | 8 (18.6%) | |
| Limb | 42 (87.5%) | 35 (81.4%) | |
| El Escorial criteria for ALS, n (%) | NS | ||
| Definite | 18 (37.5%) | 21 (48.8%) | |
| Possible | 6 (12.5%) | 3 (7.0%) | |
| Probable | 21 (43.8%) | 17 (39.5%) | |
| Probable Laboratory Supported | 3 (6.3%) | 2 (4.7%) | |
| Concurrent riluzole use, n (%) | 1.0 | ||
| Yes | 30 (62.5%) | 26 (60.5%) | |
| No | 18 (37.5%) | 17 (39.5%) | |
| ALSFRS-R score at baseline, mean ± SD | 40.2 ± 4.1 | 41.0 ± 3.0 | 0.54 |
| PVC 3 at baseline (%), mean ± SD | 90.5 ± 16.8 | 88.7 ± 15.1 | 0.49 |
| Duration of ALS symptom (months), mean ± SD | 23.67 ± 7.84 | 21.79 ± 7.47 | 0.28 |
| DPR 4 at baseline (ALSFRS-R score lost/month) | 0.33 ± 0.12 | 0.33 ± 0.11 | 1.0 |
| CRP at baseline (mg/L), mean ± SD | 4.05 ± 4.20 | 3.17 ± 2.80 | 0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goyal, N.A.; Andrews, J.A.; Oskarsson, B.E.; Wiedau, M.H.; Kasarskis, E.J.; Forrest, B.D.; Zhang, R.; Bracci, P.M.; Davis, M.W.; Azhir, A.; et al. Quantitative Measures of Time to Loss of 15% Vital Capacity and Survival Extension in Slowly Progressive Amyotrophic Lateral Sclerosis (ALS) Patients Treated with the Immune Regulator NP001 Suggests an Immunopathogenic Subset of ALS. Biomedicines 2025, 13, 3060. https://doi.org/10.3390/biomedicines13123060
Goyal NA, Andrews JA, Oskarsson BE, Wiedau MH, Kasarskis EJ, Forrest BD, Zhang R, Bracci PM, Davis MW, Azhir A, et al. Quantitative Measures of Time to Loss of 15% Vital Capacity and Survival Extension in Slowly Progressive Amyotrophic Lateral Sclerosis (ALS) Patients Treated with the Immune Regulator NP001 Suggests an Immunopathogenic Subset of ALS. Biomedicines. 2025; 13(12):3060. https://doi.org/10.3390/biomedicines13123060
Chicago/Turabian StyleGoyal, Namita A., Jinsy A. Andrews, Björn E. Oskarsson, Martina H. Wiedau, Edward J. Kasarskis, Bruce D. Forrest, Rongzhen Zhang, Paige M. Bracci, Matthew W. Davis, Ari Azhir, and et al. 2025. "Quantitative Measures of Time to Loss of 15% Vital Capacity and Survival Extension in Slowly Progressive Amyotrophic Lateral Sclerosis (ALS) Patients Treated with the Immune Regulator NP001 Suggests an Immunopathogenic Subset of ALS" Biomedicines 13, no. 12: 3060. https://doi.org/10.3390/biomedicines13123060
APA StyleGoyal, N. A., Andrews, J. A., Oskarsson, B. E., Wiedau, M. H., Kasarskis, E. J., Forrest, B. D., Zhang, R., Bracci, P. M., Davis, M. W., Azhir, A., & McGrath, M. S. (2025). Quantitative Measures of Time to Loss of 15% Vital Capacity and Survival Extension in Slowly Progressive Amyotrophic Lateral Sclerosis (ALS) Patients Treated with the Immune Regulator NP001 Suggests an Immunopathogenic Subset of ALS. Biomedicines, 13(12), 3060. https://doi.org/10.3390/biomedicines13123060





