Energy-Metabolic Imbalance of Oligodendrocytes in Multiple Sclerosis: Mechanisms, Network Coupling, and Advances in Metabolism-Targeted Therapies
Abstract
1. Introduction
2. Energy Metabolism of OLs/OPCs and Their Networked Cooperation
3. Association Between MS and Dysregulated Energy Metabolism in OLs
3.1. Myelin Loss in MS Is Accompanied by Dysregulated Energy Metabolism in OLs
3.2. Demyelination Is Accompanied by Mitochondrial Metabolic Dysfunction and Oxidative Stress in OLs
4. Therapeutic Strategies Based on Metabolic Regulation
4.1. Cell-Permeable Metabolic Inhibitors
4.2. AMPK Agonists
4.3. Agents Targeting Lipid Metabolism
4.4. Regulatory Roles of Metabolic Intermediates
4.5. Clinical Trials Involving Metabolic Interventions
4.6. Other Interventions That Promote Remyelination
5. Challenges and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cunnane, S.C.; Trushina, E.; Morland, C.; Prigione, A.; Casadesus, G.; Andrews, Z.B.; Beal, M.F.; Bergersen, L.H.; Brinton, R.D.; de la Monte, S.; et al. Brain energy rescue: An emerging therapeutic concept for neurodegenerative disorders of ageing. Nat. Rev. Drug Discov. 2020, 19, 609–633. [Google Scholar] [CrossRef]
- López-Muguruza, E.; Matute, C. Alterations of Oligodendrocyte and Myelin Energy Metabolism in Multiple Sclerosis. Int. J. Mol. Sci. 2023, 24, 12912. [Google Scholar] [CrossRef] [PubMed]
- Oppong, A.E.; Coelewij, L.; Robertson, G.; Martin-Gutierrez, L.; Waddington, K.E.; Dönnes, P.; Nytrova, P.; Farrell, R.; Pineda-Torra, I.; Jury, E.C. Blood metabolomic and transcriptomic signatures stratify patient subgroups in multiple sclerosis according to disease severity. iScience 2024, 27, 109225. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.J.; Ffrench-Constant, C. Remyelination in the CNS: From biology to therapy. Nat. Rev. Neurosci. 2008, 9, 839–855. [Google Scholar] [CrossRef]
- McKenzie, I.A.; Ohayon, D.; Li, H.; de Faria, J.P.; Emery, B.; Tohyama, K.; Richardson, W.D. Motor skill learning requires active central myelination. Science 2014, 346, 318–322. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.; Groh, A.M.R.; Yaqubi, M.; Cui, Q.L.; Stratton, J.A.; Moore, G.R.W.; Antel, J.P. Heterogeneity of mature oligodendrocytes in the central nervous system. Neural Regen. Res. 2025, 20, 1336–1349. [Google Scholar] [CrossRef]
- Campbell, G.R.; Ziabreva, I.; Reeve, A.K.; Krishnan, K.J.; Reynolds, R.; Howell, O.; Lassmann, H.; Turnbull, D.M.; Mahad, D.J. Mitochondrial DNA deletions and neurodegeneration in multiple sclerosis. Ann. Neurol. 2011, 69, 481–492. [Google Scholar] [CrossRef]
- Falcão, A.M.; van Bruggen, D.; Marques, S.; Meijer, M.; Jäkel, S.; Agirre, E.; Samudyata; Floriddia, E.M.; Vanichkina, D.P.; Ffrench-Constant, C.; et al. Disease-specific oligodendrocyte lineage cells arise in multiple sclerosis. Nat. Med. 2018, 24, 1837–1844. [Google Scholar] [CrossRef]
- Cui, Q.L.; Kuhlmann, T.; Miron, V.E.; Leong, S.Y.; Fang, J.; Gris, P.; Kennedy, T.E.; Almazan, G.; Antel, J. Oligodendrocyte progenitor cell susceptibility to injury in multiple sclerosis. Am. J. Pathol. 2013, 183, 516–525. [Google Scholar] [CrossRef]
- Rao, V.T.S.; Khan, D.; Cui, Q.L.; Fuh, S.C.; Hossain, S.; Almazan, G.; Multhaup, G.; Healy, L.M.; Kennedy, T.E.; Antel, J.P. Distinct age and differentiation-state dependent metabolic profiles of oligodendrocytes under optimal and stress conditions. PLoS ONE 2017, 12, e0182372. [Google Scholar] [CrossRef]
- Fünfschilling, U.; Supplie, L.M.; Mahad, D.; Boretius, S.; Saab, A.S.; Edgar, J.; Brinkmann, B.G.; Kassmann, C.M.; Tzvetanova, I.D.; Möbius, W.; et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 2012, 485, 517–521. [Google Scholar] [CrossRef]
- Lee, Y.; Morrison, B.M.; Li, Y.; Lengacher, S.; Farah, M.H.; Hoffman, P.N.; Liu, Y.; Tsingalia, A.; Jin, L.; Zhang, P.W.; et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 2012, 487, 443–448. [Google Scholar] [CrossRef]
- Rinholm, J.E.; Hamilton, N.B.; Kessaris, N.; Richardson, W.D.; Bergersen, L.H.; Attwell, D. Regulation of oligodendrocyte development and myelination by glucose and lactate. J. Neurosci. 2011, 31, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Philips, T.; Rothstein, J.D. Oligodendroglia: Metabolic supporters of neurons. J. Clin. Investig. 2017, 127, 3271–3280. [Google Scholar] [CrossRef] [PubMed]
- Späte, E.; Zhou, B.; Sun, T.; Kusch, K.; Asadollahi, E.; Siems, S.B.; Depp, C.; Werner, H.B.; Saher, G.; Hirrlinger, J.; et al. Downregulated expression of lactate dehydrogenase in adult oligodendrocytes and its implication for the transfer of glycolysis products to axons. Glia 2024, 72, 1374–1391. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, E.Y.; Sohn, H.J.; Kim, S.W.; Kim, C.H.; Ahn, H.Y.; Kim, D.W.; Cho, S.S.; Seo, J.H. Differential expression of αB-crystallin causes maturation-dependent susceptibility of oligodendrocytes to oxidative stress. BMB Rep. 2013, 46, 501–506. [Google Scholar] [CrossRef]
- Sánchez-Abarca, L.I.; Tabernero, A.; Medina, J.M. Oligodendrocytes use lactate as a source of energy and as a precursor of lipids. Glia 2001, 36, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Camargo, N.; Goudriaan, A.; van Deijk, A.F.; Otte, W.M.; Brouwers, J.F.; Lodder, H.; Gutmann, D.H.; Nave, A.; Dijkhuizen, R.M.; Mansvelder, H.D.; et al. Oligodendroglial myelination requires astrocyte-derived lipids. PLoS Biol. 2017, 15, e1002605. [Google Scholar] [CrossRef]
- Gallegos, C.E.; Bartos, M.; Gumilar, F.; Minetti, A.; Baier, C.J. Behavioral and neurochemical impairments after intranasal administration of chlorpyrifos formulation in mice. Pestic. Biochem. Physiol. 2023, 189, 105315. [Google Scholar] [CrossRef]
- Tepavčević, V. Oligodendroglial Energy Metabolism and (re)Myelination. Life 2021, 11, 238. [Google Scholar] [CrossRef]
- Barnes-Vélez, J.A.; Aksoy Yasar, F.B.; Hu, J. Myelin lipid metabolism and its role in myelination and myelin maintenance. Innovation 2023, 4, 100360. [Google Scholar] [CrossRef]
- Asadollahi, E.; Trevisiol, A.; Saab, A.S.; Looser, Z.J.; Dibaj, P.; Ebrahimi, R.; Kusch, K.; Ruhwedel, T.; Möbius, W.; Jahn, O.; et al. Oligodendroglial fatty acid metabolism as a central nervous system energy reserve. Nat. Neurosci. 2024, 27, 1934–1944. [Google Scholar] [CrossRef]
- Khandker, L.; Jeffries, M.A.; Chang, Y.J.; Mather, M.L.; Evangelou, A.V.; Bourne, J.N.; Tafreshi, A.K.; Ornelas, I.M.; Bozdagi-Gunal, O.; Macklin, W.B.; et al. Cholesterol biosynthesis defines oligodendrocyte precursor heterogeneity between brain and spinal cord. Cell Rep. 2022, 38, 110423. [Google Scholar] [CrossRef]
- Jeffries, M.A.; McLane, L.E.; Khandker, L.; Mather, M.L.; Evangelou, A.V.; Kantak, D.; Bourne, J.N.; Macklin, W.B.; Wood, T.L. mTOR Signaling Regulates Metabolic Function in Oligodendrocyte Precursor Cells and Promotes Efficient Brain Remyelination in the Cuprizone Model. J. Neurosci. 2021, 41, 8321–8337. [Google Scholar] [CrossRef]
- Suhail, H.; Nematullah, M.; Rashid, F.; Sajad, M.; Fatma, M.; Singh, J.; Zahoor, I.; Cheung, W.L.; Tiwari, N.; Ayasolla, K.; et al. An early glycolysis burst in microglia regulates mitochondrial dysfunction in oligodendrocytes under neuroinflammation. iScience 2023, 26, 107921. [Google Scholar] [CrossRef] [PubMed]
- Chandler, H.L.; Stickland, R.C.; Patitucci, E.; Germuska, M.; Chiarelli, A.M.; Foster, C.; Bhome-Dhaliwal, S.; Lancaster, T.M.; Saxena, N.; Khot, S.; et al. Reduced brain oxygen metabolism in patients with multiple sclerosis: Evidence from dual-calibrated functional MRI. J. Cereb. Blood Flow. Metab. 2023, 43, 115–128. [Google Scholar] [CrossRef]
- Wang, P.F.; Jiang, F.; Zeng, Q.M.; Yin, W.F.; Hu, Y.Z.; Li, Q.; Hu, Z.L. Mitochondrial and metabolic dysfunction of peripheral immune cells in multiple sclerosis. J. Neuroinflamm. 2024, 21, 28. [Google Scholar] [CrossRef] [PubMed]
- Mahad, D.H.; Trapp, B.D.; Lassmann, H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015, 14, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Steudler, J.; Ecott, T.; Ivan, D.C.; Bouillet, E.; Walthert, S.; Berve, K.; Dick, T.P.; Engelhardt, B.; Locatelli, G. Autoimmune neuroinflammation triggers mitochondrial oxidation in oligodendrocytes. Glia 2022, 70, 2045–2061. [Google Scholar] [CrossRef]
- Signorile, A.; Ferretta, A.; Ruggieri, M.; Paolicelli, D.; Lattanzio, P.; Trojano, M.; De Rasmo, D. Mitochondria, Oxidative Stress, cAMP Signalling and Apoptosis: A Crossroads in Lymphocytes of Multiple Sclerosis, a Possible Role of Nutraceutics. Antioxidants 2020, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Sun, Y.; Li, L. Mitochondrial dysfunction in chronic neuroinflammatory diseases (Review). Int. J. Mol. Med. 2024, 53, 47. [Google Scholar] [CrossRef]
- An, H.; He, L. Current understanding of metformin effect on the control of hyperglycemia in diabetes. J. Endocrinol. 2016, 228, R97–R106. [Google Scholar] [CrossRef]
- Umapathy, S.; Pan, I. Glucose reduced nano-Se mitigates Cu-induced ROS by upregulating antioxidant genes in zebrafish larvae. Nanoscale Adv. 2025, 7, 2502–2517. [Google Scholar] [CrossRef] [PubMed]
- Rajan, A.; Fame, R.M. Brain development and bioenergetic changes. Neurobiol. Dis. 2024, 199, 106550. [Google Scholar] [CrossRef]
- Luo, S.; Wu, F.; Fang, Q.; Hu, Y.; Zhang, H.; Yuan, S.; Yang, C.; Shi, Y.; Luo, Y. Antidepressant effect of teriflunomide via oligodendrocyte protection in a mouse model. Heliyon 2024, 10, e29481. [Google Scholar] [CrossRef]
- Spaas, J.; van Veggel, L.; Schepers, M.; Tiane, A.; van Horssen, J.; Wilson, D.M., 3rd; Moya, P.R.; Piccart, E.; Hellings, N.; Eijnde, B.O.; et al. Oxidative stress and impaired oligodendrocyte precursor cell differentiation in neurological disorders. Cell Mol. Life Sci. 2021, 78, 4615–4637. [Google Scholar] [CrossRef] [PubMed]
- French, H.M.; Reid, M.; Mamontov, P.; Simmons, R.A.; Grinspan, J.B. Oxidative stress disrupts oligodendrocyte maturation. J. Neurosci. Res. 2009, 87, 3076–3087. [Google Scholar] [CrossRef]
- Huang, H.; Taraboletti, A.; Shriver, L.P. Dimethyl fumarate modulates antioxidant and lipid metabolism in oligodendrocytes. Redox Biol. 2015, 5, 169–175. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Kaplanis, S.I.; Kaffe, D.; Ktena, N.; Lygeraki, A.; Kolliniati, O.; Savvaki, M.; Karagogeos, D. Nicotinamide enhances myelin production after demyelination through reduction of astrogliosis and microgliosis. Front. Cell Neurosci. 2023, 17, 1201317. [Google Scholar] [CrossRef]
- Neumann, B.; Baror, R.; Zhao, C.; Segel, M.; Dietmann, S.; Rawji, K.S.; Foerster, S.; McClain, C.R.; Chalut, K.; van Wijngaarden, P.; et al. Metformin Restores CNS Remyelination Capacity by Rejuvenating Aged Stem Cells. Cell Stem Cell 2019, 25, 473–485.e478. [Google Scholar] [CrossRef]
- Narine, M.; Azmi, M.A.; Umali, M.; Volz, A.; Colognato, H. The AMPK activator metformin improves recovery from demyelination by shifting oligodendrocyte bioenergetics and accelerating OPC differentiation. Front. Cell Neurosci. 2023, 17, 1254303. [Google Scholar] [CrossRef]
- Lee, H.; Zandkarimi, F.; Zhang, Y.; Meena, J.K.; Kim, J.; Zhuang, L.; Tyagi, S.; Ma, L.; Westbrook, T.F.; Steinberg, G.R.; et al. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat. Cell Biol. 2020, 22, 225–234. [Google Scholar] [CrossRef]
- Li, C.; Dong, X.; Du, W.; Shi, X.; Chen, K.; Zhang, W.; Gao, M. LKB1-AMPK axis negatively regulates ferroptosis by inhibiting fatty acid synthesis. Signal Transduct. Target. Ther. 2020, 5, 187. [Google Scholar] [CrossRef]
- De Keersmaecker, A.V.; Van Doninck, E.; Popescu, V.; Willem, L.; Cambron, M.; Laureys, G.; D’ Haeseleer, M.; Bjerke, M.; Roelant, E.; Lemmerling, M.; et al. A metformin add-on clinical study in multiple sclerosis to evaluate brain remyelination and neurodegeneration (MACSiMiSE-BRAIN): Study protocol for a multi-center randomized placebo controlled clinical trial. Front. Immunol. 2024, 15, 1362629. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Shen, J.; Wang, C.; Huang, Y.; Wang, L.; Yang, Y.; Hu, W.; Li, P.; Wu, H. Mechanism of aconitine mediated neuronal apoptosis induced by mitochondrial calcium overload caused by MCU. Toxicol. Lett. 2023, 384, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Roth, A.D.; Leisewitz, A.V.; Jung, J.E.; Cassina, P.; Barbeito, L.; Inestrosa, N.C.; Bronfman, M. PPAR gamma activators induce growth arrest and process extension in B12 oligodendrocyte-like cells and terminal differentiation of cultured oligodendrocytes. J. Neurosci. Res. 2003, 72, 425–435. [Google Scholar] [CrossRef]
- Bernardo, A.; Giammarco, M.L.; De Nuccio, C.; Ajmone-Cat, M.A.; Visentin, S.; De Simone, R.; Minghetti, L. Docosahexaenoic acid promotes oligodendrocyte differentiation via PPAR-γ signalling and prevents tumor necrosis factor-α-dependent maturational arrest. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Dimas, P.; Montani, L.; Pereira, J.A.; Moreno, D.; Trötzmüller, M.; Gerber, J.; Semenkovich, C.F.; Köfeler, H.C.; Suter, U. CNS myelination and remyelination depend on fatty acid synthesis by oligodendrocytes. Elife 2019, 8, e44702. [Google Scholar] [CrossRef]
- Levy, M.J.F.; Garcia-Diaz, B.; Sedel, F.; Baron-Van Evercooren, A.; Mozafari, S. High Dose Pharmaceutical Grade Biotin (MD1003) Accelerates Differentiation of Murine and Grafted Human Oligodendrocyte Progenitor Cells In Vivo. Int. J. Mol. Sci. 2022, 23, 15733. [Google Scholar] [CrossRef] [PubMed]
- Peyro Saint Paul, L.; Debruyne, D.; Bernard, D.; Mock, D.M.; Defer, G.L. Pharmacokinetics and pharmacodynamics of MD1003 (high-dose biotin) in the treatment of progressive multiple sclerosis. Expert. Opin. Drug Metab. Toxicol. 2016, 12, 327–344. [Google Scholar] [CrossRef] [PubMed]
- Cree, B.A.C.; Cutter, G.; Wolinsky, J.S.; Freedman, M.S.; Comi, G.; Giovannoni, G.; Hartung, H.P.; Arnold, D.; Kuhle, J.; Block, V.; et al. Safety and efficacy of MD1003 (high-dose biotin) in patients with progressive multiple sclerosis (SPI2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 2020, 19, 988–997. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xin, D.E.; Zhong, X.; Zhao, C.; Li, Z.; Zhang, L.; Dourson, A.J.; Lee, L.; Mishra, S.; Bayat, A.E.; et al. Small-molecule-induced epigenetic rejuvenation promotes SREBP condensation and overcomes barriers to CNS myelin regeneration. Cell 2024, 187, 2465–2484.E22. [Google Scholar] [CrossRef]
- Yeung, M.S.Y.; Djelloul, M.; Steiner, E.; Bernard, S.; Salehpour, M.; Possnert, G.; Brundin, L.; Frisén, J. Dynamics of oligodendrocyte generation in multiple sclerosis. Nature 2019, 566, 538–542. [Google Scholar] [CrossRef]
- Huang, G.; Li, Z.; Liu, X.; Guan, M.; Zhou, S.; Zhong, X.; Zheng, T.; Xin, D.; Gu, X.; Mu, D.; et al. DOR activation in mature oligodendrocytes regulates α-ketoglutarate metabolism leading to enhanced remyelination in aged mice. Nat. Neurosci. 2024, 27, 2073–2085. [Google Scholar] [CrossRef]
- Yang, Y.N.; Zhang, M.Q.; Yu, F.L.; Bing, H.; Bao, M.Y.; Yan, H.; Li, X.; Zhang, Y. Peroxisome Proliferator-Activated Receptor-γ Coactivator-1α in the Spotlight with Multiple Sclerosis. Neurosci. Bull. 2024, 40, 268–272. [Google Scholar] [CrossRef]
- Choi, I.Y.; Piccio, L.; Childress, P.; Bollman, B.; Ghosh, A.; Brandhorst, S.; Suarez, J.; Michalsen, A.; Cross, A.H.; Morgan, T.E.; et al. A Diet Mimicking Fasting Promotes Regeneration and Reduces Autoimmunity and Multiple Sclerosis Symptoms. Cell Rep. 2016, 15, 2136–2146. [Google Scholar] [CrossRef]
- Brenton, J.N.; Lehner-Gulotta, D.; Woolbright, E.; Banwell, B.; Bergqvist, A.G.C.; Chen, S.; Coleman, R.; Conaway, M.; Goldman, M.D. Phase II study of ketogenic diets in relapsing multiple sclerosis: Safety, tolerability and potential clinical benefits. J. Neurol. Neurosurg. Psychiatry 2022, 93, 637–644. [Google Scholar] [CrossRef]
- Ghezzi, L.; Tosti, V.; Shi, L.; Cantoni, C.; Mikesell, R.; Lancia, S.; Zhou, Y.; Obert, K.; Dula, C.; Sen, M.K.; et al. Randomised controlled trial of intermittent calorie restriction in people with multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2025, 96, 158–169. [Google Scholar] [CrossRef]
- Kocot, J.; Kosa, P.; Ashida, S.; Pirjanian, N.A.; Goldbach-Mansky, R.; Peterson, K.; Fossati, V.; Holland, S.M.; Bielekova, B. Clemastine fumarate accelerates accumulation of disability in progressive multiple sclerosis by enhancing pyroptosis. J. Clin. Investig. 2025, 135, e183941. [Google Scholar] [CrossRef]
- Kaiser, C.C.; Shukla, D.K.; Stebbins, G.T.; Skias, D.D.; Jeffery, D.R.; Stefoski, D.; Katsamakis, G.; Feinstein, D.L. A pilot test of pioglitazone as an add-on in patients with relapsing remitting multiple sclerosis. J. Neuroimmunol. 2009, 211, 124–130. [Google Scholar] [CrossRef]
- Green, A.J.; Gelfand, J.M.; Cree, B.A.; Bevan, C.; Boscardin, W.J.; Mei, F.; Inman, J.; Arnow, S.; Devereux, M.; Abounasr, A.; et al. Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): A randomised, controlled, double-blind, crossover trial. Lancet 2017, 390, 2481–2489. [Google Scholar] [CrossRef]
- Ren, J.; Dewey, R.B., 3rd; Rynders, A.; Evan, J.; Evan, J.; Ligozio, S.; Ho, K.S.; Sguigna, P.V.; Glanzman, R.; Hotchkin, M.T.; et al. Evidence of brain target engagement in Parkinson’s disease and multiple sclerosis by the investigational nanomedicine, CNM-Au8, in the REPAIR phase 2 clinical trials. J. Nanobiotechnol. 2023, 21, 478. [Google Scholar] [CrossRef]
- Ma, X.R.; Zhu, X.; Xiao, Y.; Gu, H.M.; Zheng, S.S.; Li, L.; Wang, F.; Dong, Z.J.; Wang, D.X.; Wu, Y.; et al. Restoring nuclear entry of Sirtuin 2 in oligodendrocyte progenitor cells promotes remyelination during ageing. Nat. Commun. 2022, 13, 1225. [Google Scholar] [CrossRef]
- Zhang, Q.; Song, Q.; Yu, R.; Wang, A.; Jiang, G.; Huang, Y.; Chen, J.; Xu, J.; Wang, D.; Chen, H.; et al. Nano-Brake Halts Mitochondrial Dysfunction Cascade to Alleviate Neuropathology and Rescue Alzheimer’s Cognitive Deficits. Adv. Sci. 2023, 10, e2204596. [Google Scholar] [CrossRef]
- Williams, M.J.; Okai, A.F.; Cross, A.H.; Monson, N.L.; Vartanian, T.; Thrower, B.W.; Reder, A.T.; English, J.B.; Wu, G.F.; Bernitsas, E.; et al. Demographics and baseline disease characteristics of Black and Hispanic patients with multiple sclerosis in the open-label, single-arm, multicenter, phase IV CHIMES trial. Mult. Scler. Relat. Disord. 2023, 76, 104794. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Isobe, N.; Niino, M.; Nakashima, I.; Matsushita, T.; Sakai, Y.; Nakahara, J.; Kawachi, I.; Ochi, H.; Nakatsuji, Y.; et al. Prevalence of, and Disability Due to, Multiple Sclerosis and Neuromyelitis Optica Spectrum Disorder in Japan by the Fifth Nationwide Survey. Neurology 2024, 103, e209992. [Google Scholar] [CrossRef] [PubMed]
- Langer-Gould, A.M.; Gonzales, E.G.; Smith, J.B.; Li, B.H.; Nelson, L.M. Racial and Ethnic Disparities in Multiple Sclerosis Prevalence. Neurology 2022, 98, e1818–e1827. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Paez, H.G.; Pitzer, C.R.; Alway, S.E. The Therapeutic Potential of Mitochondria Transplantation Therapy in Neurodegenerative and Neurovascular Disorders. Curr. Neuropharmacol. 2023, 21, 1100–1116. [Google Scholar] [CrossRef]
- Krzak, G. Microglial Succinate Receptor 1 (SUCNR1) Sustains Chronic CNS Inflammation. Ph.D. Thesis, University of Cambridge, Cambridge, UK, 2023. [Google Scholar] [CrossRef]
- Heidker, R.M.; Emerson, M.R.; LeVine, S.M. Metabolic pathways as possible therapeutic targets for progressive multiple sclerosis. Neural Regen. Res. 2017, 12, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Wetmore, E.; Lehner-Gulotta, D.; Florenzo, B.; Banwell, B.; Bergqvist, A.G.C.; Coleman, R.; Conaway, M.; Goldman, M.D.; Brenton, J.N. Ketogenic diet in relapsing multiple sclerosis: Patient perceptions, post-trial diet adherence & outcomes. Clin. Nutr. 2023, 42, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
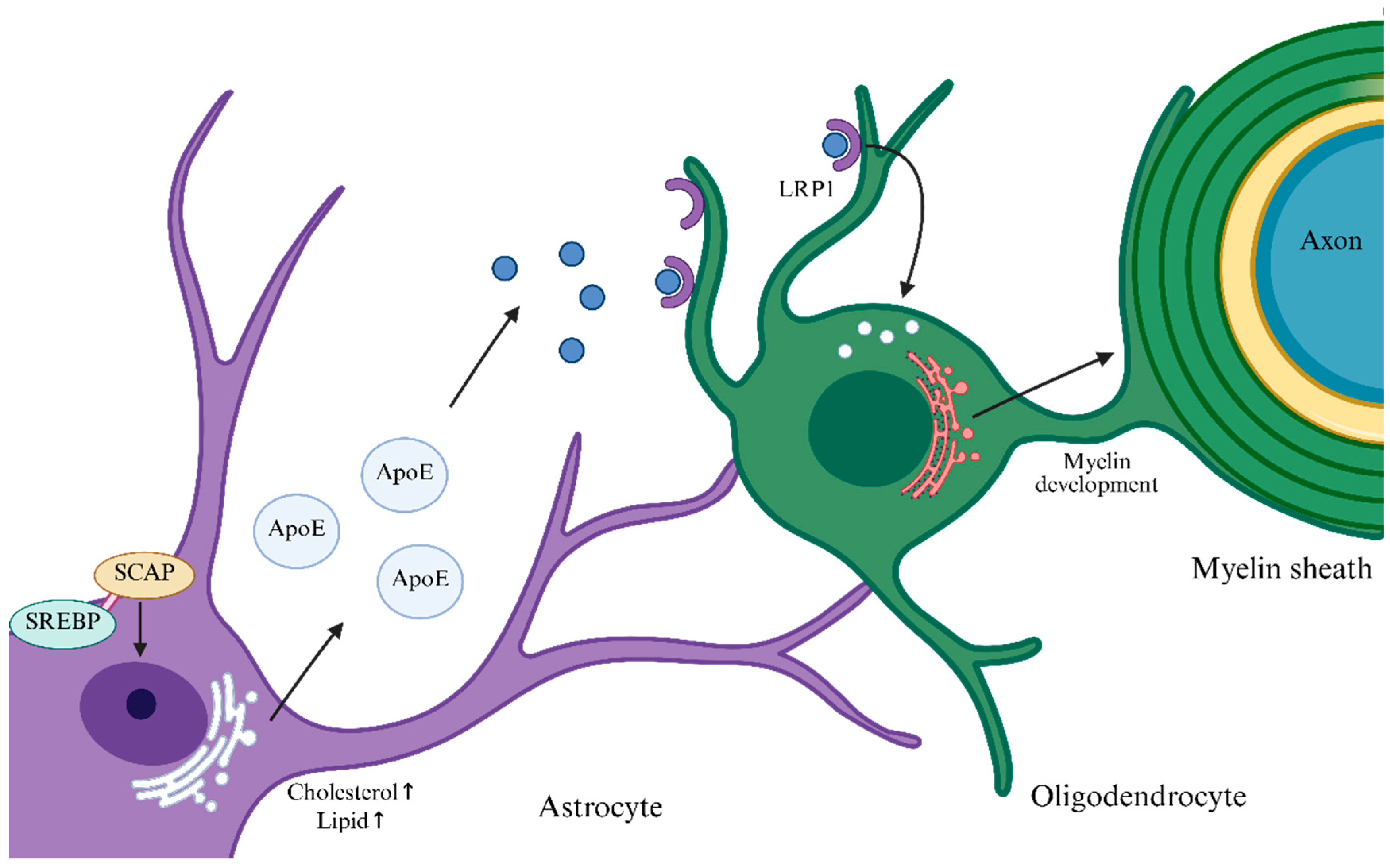
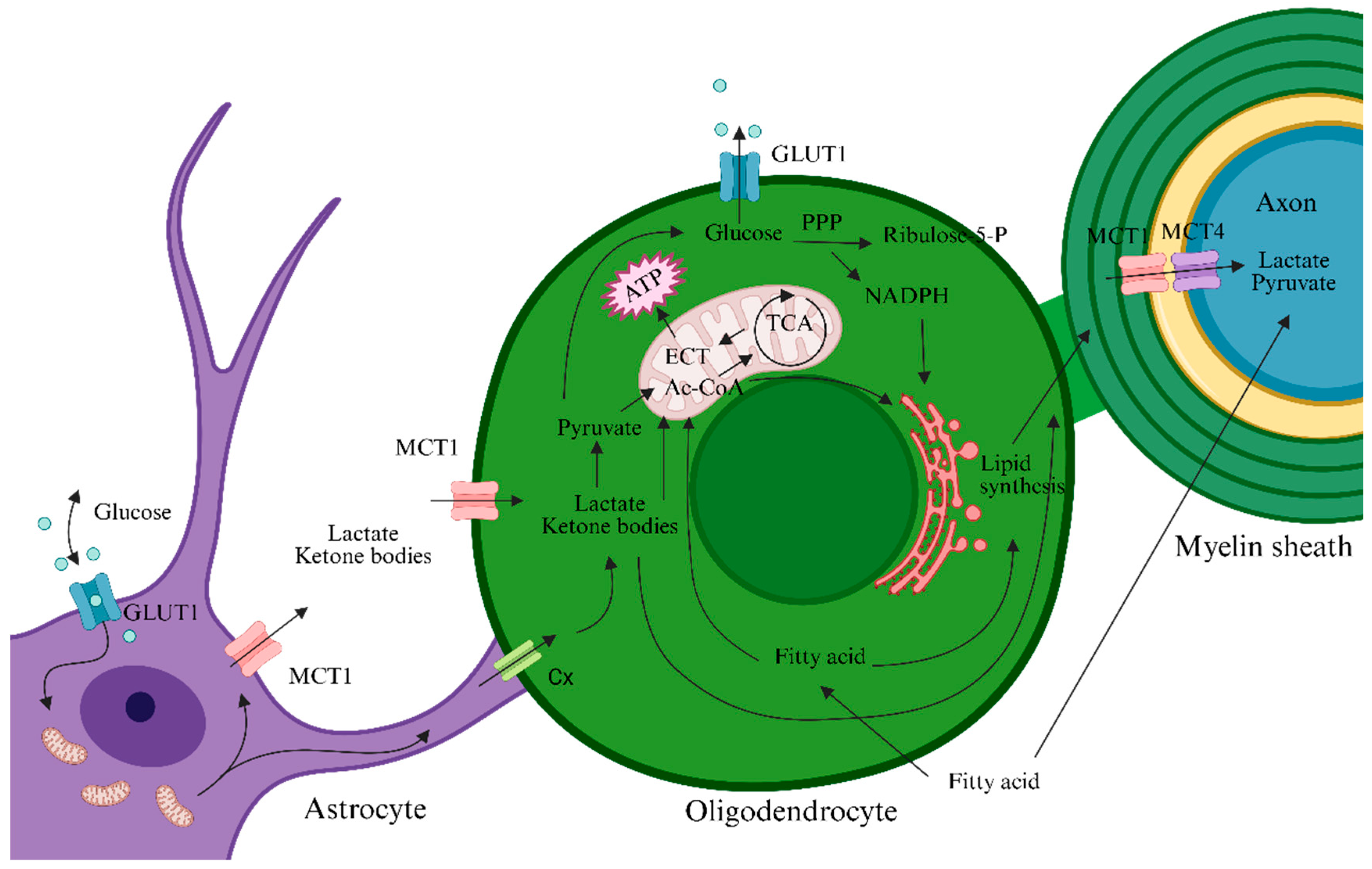


| Metabolic Substrates | Acute Relapse Phase | Remission Phase | Chronic Progressive Phase |
|---|---|---|---|
| Glucose | ↓ | ≈ | ↓ |
| Lactate | ↑ | ≈ | ↑ |
| Ketone Bodies | ≈ | ≈ | ↑ |
| Fatty Acids | ↑ | ≈ | ↑ |
| Registration No. | Intervention | Primary Endpoint | Observation Period | Participants | Results | Start Year |
|---|---|---|---|---|---|---|
| NCT01538355 [58] | FMD, KD | Change in health-related quality of life (HRQOL) score | 6 months | 60 RRMS | HRQOL improvement significantly better than control (p < 0.05), magnitude ≥5 points | 2012 |
| NCT03508414 [58] | KD, FD | Number of new T2-weighted MRI lesions | 18 months | 111 RRMS | Reduction in MRI lesions reached statistical significance in the KD group; no significant difference in the FD group | 2017 |
| NCT03718247 [59] | modified Atkins KD (KDMAD) | Tolerability and safety | 6 months | 65 RMS | KDMAD is safe and tolerable and can improve symptoms in RMS patients | 2018 |
| NCT03539094 [60] | ICR | Serum leptin level | 12 weeks | 42 RRMS | iCR improved the inflammatory microenvironment by lowering leptin and increasing lipid mediators (e.g., LPE, PI) | 2018 |
| NCT06715436 | KD group; Mediterranean-diet group | KD group; Mediterranean-diet group | 9 months | 108 RRMS | Not completed | 2023 |
| NCT06454162 | KD plus exercise | Neurocognitive tests and neuroelectrophysiology; MSQoL-54 scale | 3 months | 60 diagnosed MS | Not completed | 2024 |
| NCT00242177 [62] | Pioglitazone 45 mg qd | Safety, adverse events | 1 year | 24 RRMS | Significantly slowed gray-matter atrophy (46% reduction in gray-matter volume loss); safety supports long-term use | 2006 |
| NCT02040298 [63] | Clemastine fumarate 5.3 mg bid | Visual evoked potential (VEP) latency | 150 days | 50 RMS | Clemastine can reverse chronic demyelinating injury | 2017 |
| NCT03109288 [61] | 1.34 mg CLM qd | Time to disability progression (cCDP) | 12 weeks | 16 PMS | CLM accelerated disability progression in PMS | 2020 |
| NCT04057868 [64] | CNM-Au8, 15 mg/30 mg qd | Change in NAD+/NADH ratio | 12 weeks | 24 participants; RRMS, SPMS | Demonstrated the potential of CNM-Au8 to target brain energy metabolism | 2019 |
| NCT05131828 | Baseline: Clemastine 1.34 mg; Add-on: Metformin 500 mg | mf-VEP P100 latency | 26 weeks | 70 RRMS | Not completed | 2021 |
| NCT05740722 | NR 1000 mg qd | Worsening of any of EDSS, T25FW, or 9HPT as a disability-progression marker | 30 months | PMS, SPMS | Not completed | 2023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Kang, J.; Guo, X.; Jiang, T.; Li, X. Energy-Metabolic Imbalance of Oligodendrocytes in Multiple Sclerosis: Mechanisms, Network Coupling, and Advances in Metabolism-Targeted Therapies. Biomedicines 2025, 13, 2880. https://doi.org/10.3390/biomedicines13122880
Zhang Z, Kang J, Guo X, Jiang T, Li X. Energy-Metabolic Imbalance of Oligodendrocytes in Multiple Sclerosis: Mechanisms, Network Coupling, and Advances in Metabolism-Targeted Therapies. Biomedicines. 2025; 13(12):2880. https://doi.org/10.3390/biomedicines13122880
Chicago/Turabian StyleZhang, Zhimian, Jihe Kang, Xudong Guo, Taotao Jiang, and Xiaoling Li. 2025. "Energy-Metabolic Imbalance of Oligodendrocytes in Multiple Sclerosis: Mechanisms, Network Coupling, and Advances in Metabolism-Targeted Therapies" Biomedicines 13, no. 12: 2880. https://doi.org/10.3390/biomedicines13122880
APA StyleZhang, Z., Kang, J., Guo, X., Jiang, T., & Li, X. (2025). Energy-Metabolic Imbalance of Oligodendrocytes in Multiple Sclerosis: Mechanisms, Network Coupling, and Advances in Metabolism-Targeted Therapies. Biomedicines, 13(12), 2880. https://doi.org/10.3390/biomedicines13122880




