The Effect of Anti-Viral Treatment of HCV Infection on Outcomes of Renal Transplant Patients with Chronic HCV Infection: A Real-World Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Population
2.3. Main Outcome and Covariates
- (1)
- Death.
- (2)
- Overall liver: Liver cell carcinoma (ICD-10: C22.0), Liver (ICDO3: C22.0), Fibrosis and cirrhosis of liver (ICD-10: K74), Hepatic failure, not elsewhere classified (ICD-10: K72).
- (3)
- Hepatoma: Liver cell carcinoma (ICD-10: C22.0), Liver (ICDO3: C22.0).
- (4)
- Cirrhosis (ICD-10: K74).
- (5)
- Hepatic failure (ICD-10: K72).
- (6)
- Graft failure (ICD-10: N18.6).
- (7)
- Serum creatinine level greater than 6 mg/dL (TNX: 9024) as severe renal dysfunction.
2.4. Statistical Analyses
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sawinski, D.; Bloom, R.D. Novel Hepatitis C Treatment and the Impact on Kidney Transplantation. Transplantation 2015, 99, 2458–2466. [Google Scholar] [CrossRef]
- Sawinski, D.; Forde, K.A.; Lo Re, V., 3rd; Goldberg, D.S.; Cohen, J.B.; Locke, J.E.; Bloom, R.D.; Brensinger, C.; Weldon, J.; Shults, J.; et al. Mortality and Kidney Transplantation Outcomes Among Hepatitis C Virus-Seropositive Maintenance Dialysis Patients: A Retrospective Cohort Study. Am. J. Kidney Dis. 2019, 73, 815–826. [Google Scholar] [CrossRef]
- Roth, D.; Gaynor, J.J.; Reddy, K.R.; Ciancio, G.; Sageshima, J.; Kupin, W.; Guerra, G.; Chen, L.; Burke, G.W., 3rd. Effect of kidney transplantation on outcomes among patients with hepatitis C. J. Am. Soc. Nephrol. 2011, 22, 1152–1160. [Google Scholar] [CrossRef]
- Tonelli, M.; Wiebe, N.; Knoll, G.; Bello, A.; Browne, S.; Jadhav, D.; Klarenbach, S.; Gill, J. Systematic review: Kidney transplantation compared with dialysis in clinically relevant outcomes. Am. J. Transplant. 2011, 11, 2093–2109. [Google Scholar] [CrossRef] [PubMed]
- Esforzado, N.; Morales, J.M. Hepatitis C and kidney transplant: The eradication time of the virus has arrived. Nefrologia 2019, 39, 458–472. [Google Scholar] [CrossRef] [PubMed]
- Larsen, F.S.; Vainer, B.; Eefsen, M.; Bjerring, P.N.; Adel Hansen, B. Low-dose tacrolimus ameliorates liver inflammation and fibrosis in steroid refractory autoimmune hepatitis. World J. Gastroenterol. 2007, 13, 3232–3236. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Youssef, M.M.; Grace, J.A.; Sinclair, M. Relative carcinogenicity of tacrolimus vs mycophenolate after solid organ transplantation and its implications for liver transplant care. World J. Hepatol. 2024, 16, 650–660. [Google Scholar] [CrossRef]
- Rodríguez-Perálvarez, M.; Colmenero, J.; González, A.; Gastaca, M.; Curell, A.; Caballero-Marcos, A.; Sánchez-Martínez, A.; Di Maira, T.; Herrero, J.I.; Almohalla, C.; et al. Chronic immunosuppression, cancer Spanish consortium. Cumulative exposure to tacrolimus and incidence of cancer after liver transplantation. Am. J. Transplant. 2022, 22, 1671–1682. [Google Scholar] [CrossRef]
- Kaye, A.D.; Shah, S.S.; Johnson, C.D.; De Witt, A.S.; Thomassen, A.S.; Daniel, C.P.; Ahmadzadeh, S.; Tirumala, S.; Bembenick, K.N.; Kaye, A.M.; et al. Tacrolimus- and Mycophenolate-Mediated Toxicity: Clinical Considerations and Options in Management of Post-Transplant Patients. Curr. Issues Mol. Biol. 2024, 47, 2. [Google Scholar] [CrossRef]
- Mauss, S.; Valenti, W.; DePamphilis, J.; Duff, F.; Cupelli, L.; Passe, S.; Solsky, J.; Torriani, F.J.; Dieterich, D.; Larrey, D. Risk factors for hepatic decompensation in patients with HIV/HCV coinfection and liver cirrhosis during interferon-based therapy. AIDS 2004, 18, F21–F25. [Google Scholar] [CrossRef]
- Vo, T.D.; Bui, V.T.T.; Lam, H.T.; Bui, Q.T.H. High efficacy and safety of direct-acting antivirals for the treatment of chronic hepatitis C: A cohort study conducted in Vietnam. Pharmacol. Res. Perspect. 2024, 12, e70007. [Google Scholar] [CrossRef]
- Ji, F.; Wei, B.; Yeo, Y.H.; Ogawa, E.; Zou, B.; Stave, C.D.; Li, Z.; Dang, S.; Furusyo, N.; Cheung, R.C.; et al. Systematic review with meta-analysis: Effectiveness and tolerability of interferon-free direct-acting antiviral regimens for chronic hepatitis C genotype 1 in routine clinical practice in Asia. Aliment. Pharmacol. Ther. 2018, 47, 550–562. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Ghaffar, T.Y.; Sira, M.M.; El Naghi, S. Hepatitis C genotype 4: The past, present, and future. World J. Hepatol. 2015, 7, 2792–2810. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, E.; Chien, N.; Kam, L.; Yeo, Y.H.; Ji, F.; Huang, D.Q.; Cheung, R.; Nguyen, M.H. Association of Direct-Acting Antiviral Therapy With Liver and Nonliver Complications and Long-term Mortality in Patients With Chronic Hepatitis C. JAMA Intern. Med. 2023, 183, 97–105. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B.; Duong, H.; Luster, M.; Kanwal, F.; Hill, D.D.; Burroughs, M.; Hernandez, C.; Haber, B.A.; Larsen, L.M.; Marcinak, J.F.; et al. Risk of Hepatocellular Cancer in U.S. Patients With Compensated Cirrhosis Treated with Direct-Acting Antivirals Versus Interferon. Aliment. Pharmacol. Ther. 2025, 61, 1226–1237, Erratum in Aliment. Pharmacol. Ther. 2025, Epub ahead of print. [Google Scholar] [CrossRef]
- Jongraksak, T.; Chuncharunee, A.; Intaraprasong, P.; Tansawet, A.; Thakkinstian, A.; Sobhonslidsuk, A. Outcomes of direct-acting antivirals in patients with HCV decompensated cirrhosis: A systematic review and meta-analysis. Front. Med. 2023, 10, 1295857. [Google Scholar] [CrossRef]
- Janjua, N.Z.; Wong, S.; Abdia, Y.; Jeong, D.; Buller-Taylor, T.; Adu, P.A.; Samji, H.; Wilton, J.; Pearce, M.; Butt, Z.A.; et al. Impact of direct-acting antivirals for HCV on mortality in a large population-based cohort study. J. Hepatol. 2021, 75, 1049–1057. [Google Scholar] [CrossRef]
- Kalidindi, Y.; Jung, J.; Feldman, R.; Riley, T., 3rd. Association of Direct-Acting Antiviral Treatment With Mortality Among Medicare Beneficiaries with Hepatitis C. JAMA Netw. Open 2020, 3, e2011055. [Google Scholar] [CrossRef]
- Zhu, X.; Jia, L.; Yue, M.; Zhang, A.; Xia, X.; Yu, R.; Chen, H.; Huang, P. DAA treatment for HCV reduce risk of hepatocellular carcinoma: A 10-years follow-up study based on Chinese patients with hepatitis C. Sci. Rep. 2024, 14, 23760. [Google Scholar] [CrossRef]
- Goetsch, M.R.; Tamhane, A.; Overton, E.T.; Towns, G.C.; Franco, R.A. Direct Acting Antivirals in Hepatitis C-Infected Kidney Transplant Recipients: Associations with Long-term Graft Failure and Patient Mortality. Pathog. Immun. 2020, 5, 275–290. [Google Scholar] [CrossRef]
- Pacheco, L.S.; Ventura, P.E.; Kist, R.; Garcia, V.D.; Meinerz, G.; Tovo, C.V.; Cantisani, G.P.C.; Zanotelli, M.L.; Mucenic, M.; Keitel, E. Real-world effectiveness and safety of direct-acting antivirals for the treatment of hepatitis C virus in kidney and liver transplant recipients: Experience of a large transplant center in Brazil. Rev. Inst. Med. Trop. Sao Paulo 2023, 65, e59. [Google Scholar] [CrossRef] [PubMed]
- Molnar, M.Z.; Azhar, A.; Tsujita, M.; Talwar, M.; Balaraman, V.; Bhalla, A.; Podila, P.S.B.; Kothadia, J.; Agbim, U.A.; Maliakkal, B.; et al. Transplantation of Kidneys From Hepatitis C Virus-Infected Donors to Hepatitis C Virus-Negative Recipients: One-Year Kidney Allograft Outcomes. Am. J. Kidney Dis. 2021, 77, 739–747.e1. [Google Scholar] [CrossRef] [PubMed]
- Kashani, M.; Karimi, M.; Sharifi Rayeni, A.; Azizi Nadian, M.A.; Mortezazadeh, M.; Parsaei, A.; Abolghasemi, N.; Shirsalimi, N.; Mofidi, A.; Seyyed Mahmoudi, S.T. Efficacy of Direct Acting Antivirals (DAA) therapy in patients with recurrent hepatitis C after liver and kidney transplantation: A cross-sectional study. Front. Med. 2024, 11, 1460372. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.T.; Chuang, Y.W.; Chiu, C.W.; Sun, K.T.; Chang, S.S.; Wang, I.K.; Lee, B.K.; Kung, S.C.; Li, C.Y.; Yu, T.M. High risk of hepatic complications in kidney transplantation with chronic hepatitis C virus infection. Sci. Rep. 2025, 15, 29275. [Google Scholar] [CrossRef]

| Before Propensity Score Matching | After Propensity Score Matching | |||||||
|---|---|---|---|---|---|---|---|---|
| HCV + Anti-Viral Treatment | HCV + Non-Treatment | HCV + Anti-Viral Treatment | HCV + Non-Treatment | |||||
| Demographics | N (%) | N (%) | p-Value | Std Diff. | N (%) | N (%) | p-Value | Std Diff. |
| Age | ||||||||
| Mean ± SD | 60.2 ± 8.8 | 57.8 ± 10.0 | <0.001 | 0.248 | 60.1 ± 8.8 | 60.7 ± 8.8 | 0.143 | 0.066 |
| Sex | ||||||||
| Male | 710 (71.60%) | 3542 (69.30%) | 0.150 | 0.050 | 702 (71.50%) | 710 (72.30%) | 0.688 | 0.018 |
| Female | 264 (26.60%) | 1479 (28.90%) | 0.140 | 0.052 | 262 (26.70%) | 253 (25.80%) | 0.644 | 0.021 |
| Race | ||||||||
| Hispanic or Latino | 119 (12.00%) | 677 (13.20%) | 0.287 | 0.037 | 118 (12.00%) | 128 (13.00%) | 0.495 | 0.031 |
| Not Hispanic or Latino | 636 (64.10%) | 3234 (63.30%) | 0.606 | 0.018 | 628 (64.00%) | 630 (64.20%) | 0.925 | 0.004 |
| White | 573 (57.80%) | 1317 (41.70%) | <0.001 | 0.210 | 564 (57.40%) | 556 (56.60%) | 0.715 | 0.016 |
| Black or African American | 251 (25.30%) | 1619 (31.70%) | <0.001 | 0.141 | 250 (25.50%) | 255 (26.00%) | 0.796 | 0.012 |
| Asian | 30 (3.00%) | 266 (5.20%) | 0.003 | 0.110 | 30 (3.10%) | 28 (2.90%) | 0.790 | 0.012 |
| Comorbidities | ||||||||
| Hypertensive diseases | 880 (88.70%) | 4228 (82.70%) | <0.001 | 0.173 | 870 (88.60%) | 879 (89.50%) | 0.515 | 0.029 |
| Type 2 diabetes mellitus | 570 (57.50%) | 2609 (51.00%) | <0.001 | 0.129 | 562 (57.20%) | 560 (57.00%) | 0.927 | 0.004 |
| Overweight and obesity | 340 (34.30%) | 1203 (23.50%) | <0.001 | 0.239 | 333 (33.90%) | 317 (32.30%) | 0.443 | 0.035 |
| Heart failure | 268 (27.00%) | 120 (23.50%) | 0.019 | 0.080 | 264 (26.90%) | 248 (25.30%) | 0.411 | 0.037 |
| Glomerular diseases | 164 (16.50%) | 801 (15.70%) | 0.494 | 0.024 | 161 (16.40%) | 158 (16.10%) | 0.854 | 0.008 |
| Medications | ||||||||
| Glucocorticoids | 443 (44.70%) | 1306 (25.50%) | <0.001 | 0.409 | 433 (44.10%) | 444 (45.20%) | 0.618 | 0.023 |
| Tacrolimus | 597 (60.20%) | 1903 (37.20%) | <0.001 | 0.472 | 587 (59.80%) | 570 (58.00%) | 0.436 | 0.035 |
| Mycophenolate mofetil | 510 (51.40%) | 1673 (32.70%) | <0.001 | 0.386 | 501 (51.00%) | 484 (49.30%) | 0.443 | 0.035 |
| Mycophenolic acid | 243 (24.50%) | 690 (13.50%) | <0.001 | 0.283 | 235 (23.90%) | 230 (23.40%) | 0.791 | 0.012 |
| Basiliximab | 184 (18.50%) | 421 (8.20%) | <0.001 | 0.306 | 174 (17.70%) | 169 (17.20%) | 0.766 | 0.013 |
| Before PSM | After PSM | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HCV + Anti-Viral Treatment | HCV + Non-Treatment | HCV + Anti-Viral Treatment | HCV + Non-Treatment | |||||||
| Number | Events | Number | Events | Hazard Ratio (95% CI) | Number | Events | Number | Events | Hazard Ratio (95% CI) | |
| Death | 992 | 157 | 5113 | 1083 | 0.936 (0.791, 1.107) | 982 | 156 | 982 | 195 | 0.901 (0.728, 1.114) |
| Overall liver complication | 31 | 10 | 590 | 172 | 0.474 (0.151, 1.485) | 31 | 10 | 77 | 15 | 0.469 (0.136, 1.621) |
| Hepatoma | 639 | 14 | 4064 | 108 | 1.029 (0.588, 1.800) | 636 | 14 | 706 | 16 | 1.137 (0.551, 2.347) |
| Cirrhosis | 36 | 10 | 667 | 188 | 0.532 (0.197, 1.433) | 36 | 10 | 87 | 16 | 0.550 (0.184, 1.647) |
| Hepatic failure | 445 | 20 | 3274 | 184 | 1.025 (0.645, 1.628) | 442 | 20 | 514 | 33 | 0.775 (0.443, 1.353) |
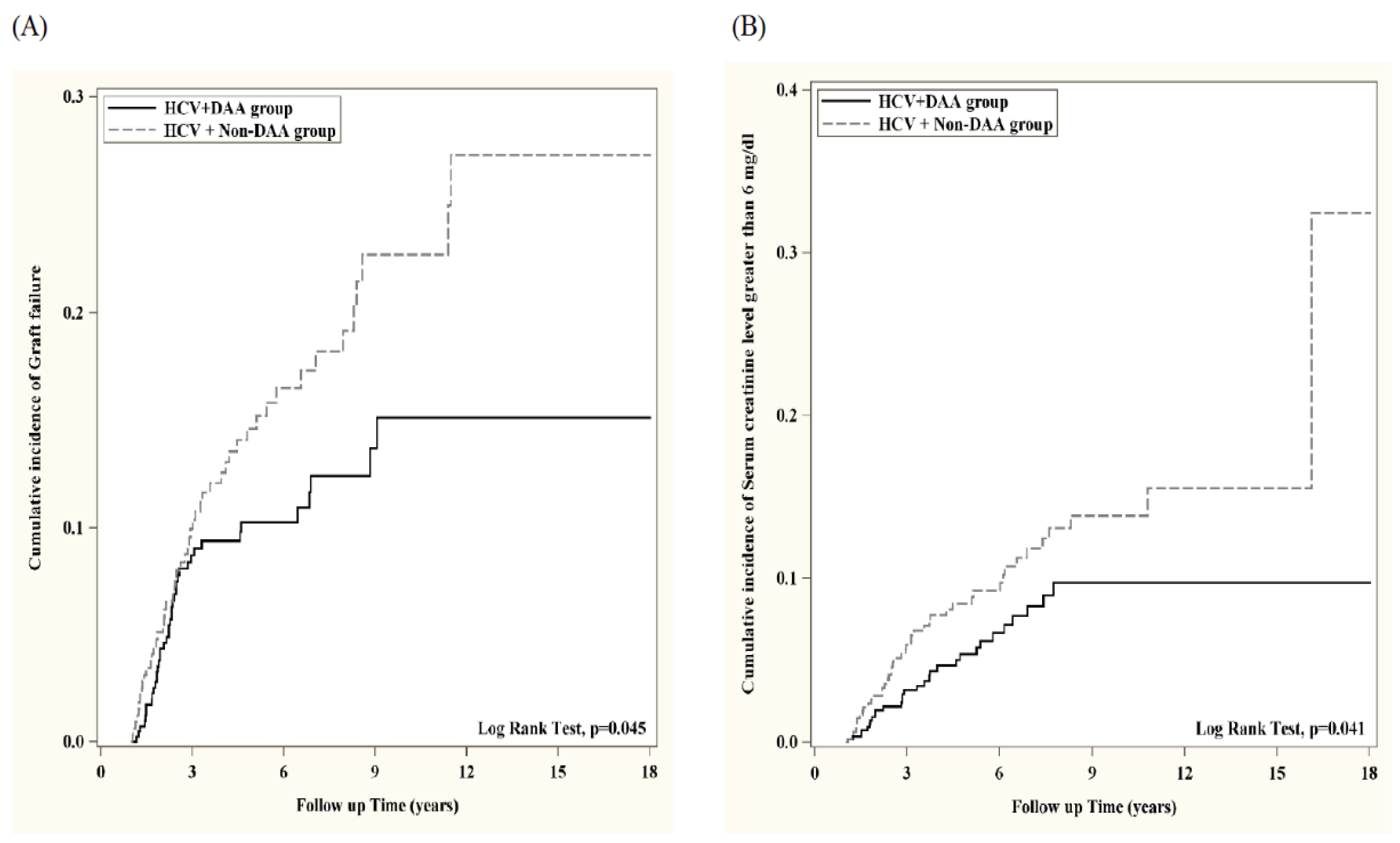
| Graft failure | 495 | 41 | 1863 | 284 | 0.638 (0.459, 0.886) ** | 491 | 41 | 383 | 50 | 0.656 (0.434, 0.993) * |
| Serum creatinine level greater than 6 mg/dL | 657 | 30 | 2829 | 238 | 0.616 (0.421, 0.901) * | 652 | 30 | 587 | 45 | 0.619 (0.390, 0.984) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, C.-W.; Sun, K.-T.; Huang, S.-T.; Wang, I.-K.; Li, C.-Y.; Yu, T.-M. The Effect of Anti-Viral Treatment of HCV Infection on Outcomes of Renal Transplant Patients with Chronic HCV Infection: A Real-World Cohort Study. Biomedicines 2025, 13, 2842. https://doi.org/10.3390/biomedicines13112842
Chiu C-W, Sun K-T, Huang S-T, Wang I-K, Li C-Y, Yu T-M. The Effect of Anti-Viral Treatment of HCV Infection on Outcomes of Renal Transplant Patients with Chronic HCV Infection: A Real-World Cohort Study. Biomedicines. 2025; 13(11):2842. https://doi.org/10.3390/biomedicines13112842
Chicago/Turabian StyleChiu, Chih-Wei, Kuo-Ting Sun, Shih-Ting Huang, I-Kuan Wang, Chi-Yuan Li, and Tung-Min Yu. 2025. "The Effect of Anti-Viral Treatment of HCV Infection on Outcomes of Renal Transplant Patients with Chronic HCV Infection: A Real-World Cohort Study" Biomedicines 13, no. 11: 2842. https://doi.org/10.3390/biomedicines13112842
APA StyleChiu, C.-W., Sun, K.-T., Huang, S.-T., Wang, I.-K., Li, C.-Y., & Yu, T.-M. (2025). The Effect of Anti-Viral Treatment of HCV Infection on Outcomes of Renal Transplant Patients with Chronic HCV Infection: A Real-World Cohort Study. Biomedicines, 13(11), 2842. https://doi.org/10.3390/biomedicines13112842






