Recent Advances in Periodontal Regenerative Medicine: A Focus on the Role of Mechanical Stimulation
Abstract
1. Introduction
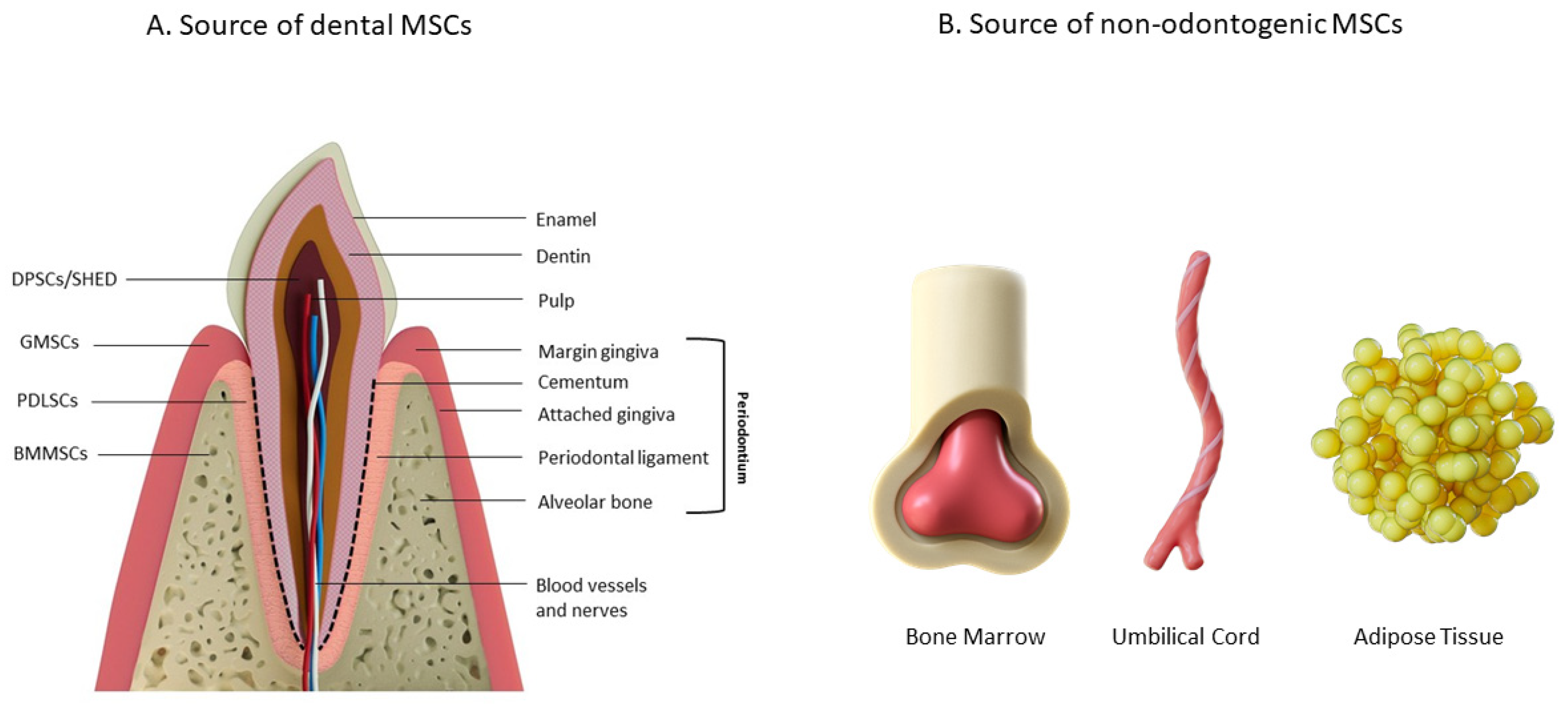
2. Periodontal Ligaments
3. Cellular Considerations
3.1. Odontogenic Cells
3.2. Mesenchymal Stem Cells (MSCs)
4. Biomaterial for Periodontal Regeneration (A Brief Overview of Different Types of Biomaterials)
4.1. Polymers for the Matrix
4.2. Additives for Periodontal Regeneration Constructs
4.2.1. Antibacterial/Antioxidants
4.2.2. Growth Factors
4.2.3. Nanoparticles
4.2.4. Inorganic Additives
4.3. Structural Considerations for Periodontal Regeneration
4.3.1. Porosity and Permeability
4.3.2. Mechanical Properties
4.3.3. Biodegradation
4.3.4. Structural Alignment
5. Biofabrication Strategies for Periodontal Regeneration
5.1. Conventional Methods of Biofabrication
5.2. Decellularized Structures
5.3. Bioprinting and 3D Printing
5.4. Electrospinning
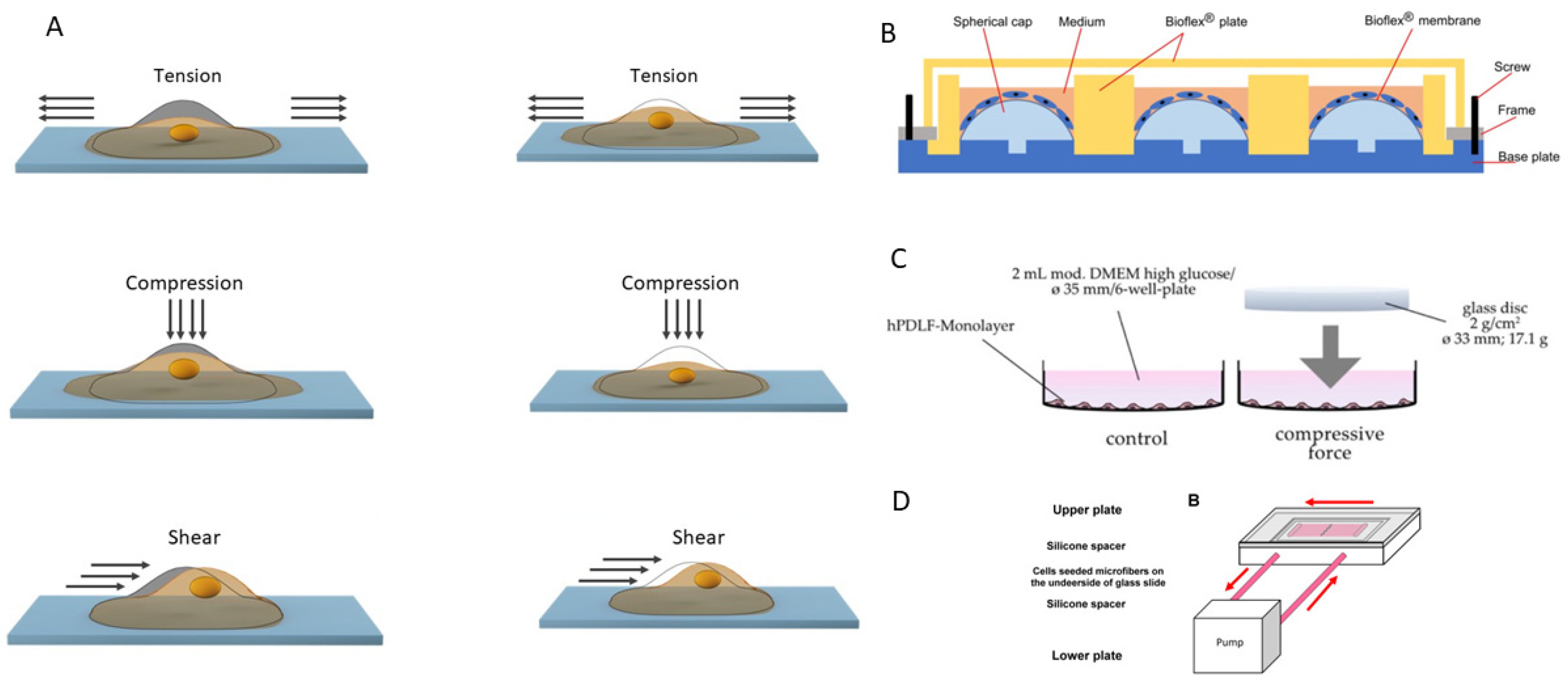
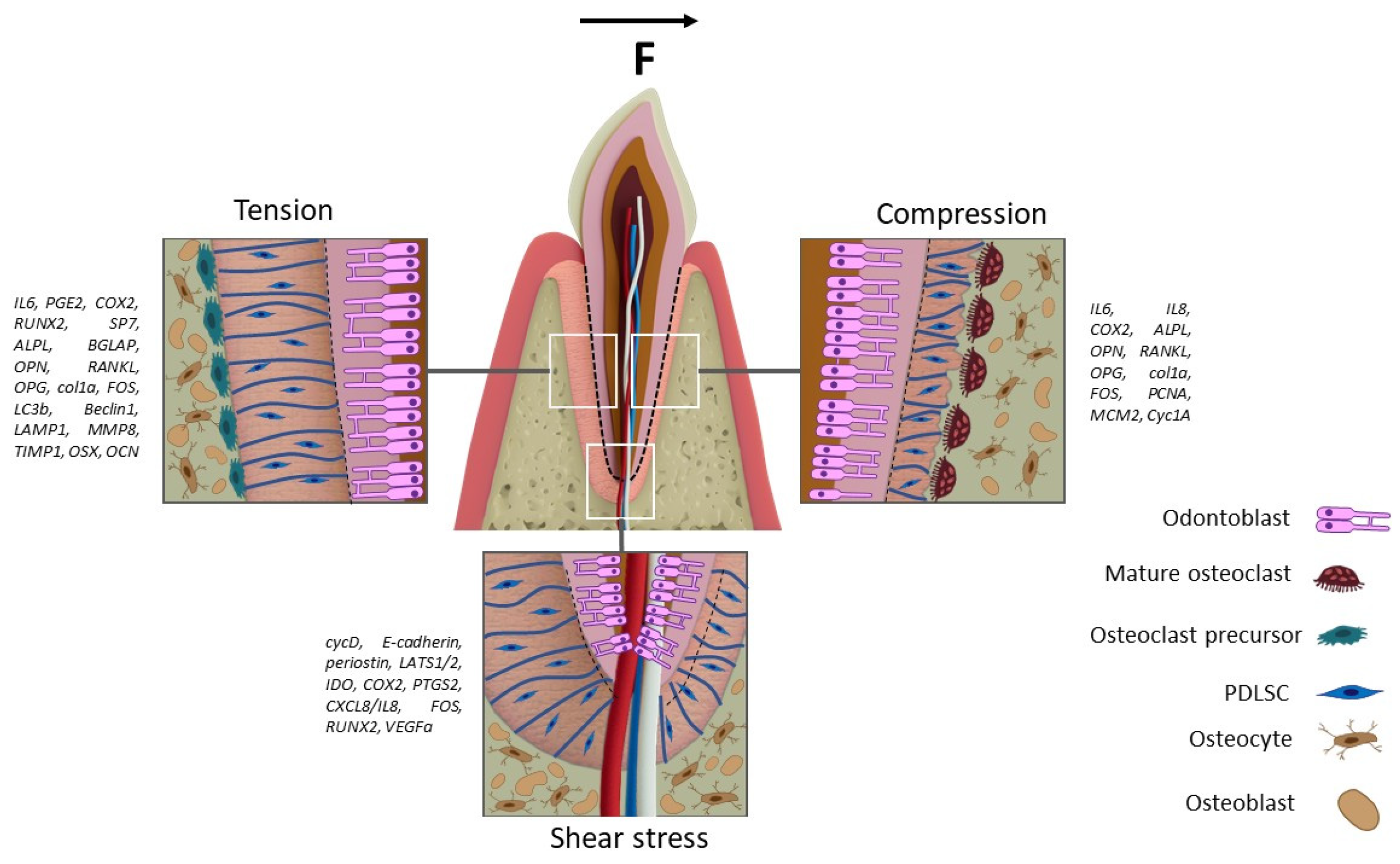
6. Mechanical Stimuli in Periodontal Ligaments Formation
| ECM Component | Stretching | Compression | References |
|---|---|---|---|
| Collagen I | ↑ gene expression, protein ↓ fibers on immunohistochemistry | ↓ gene expression, protein ↓ fibers on immunohistochemistry | [59,247,248,249] |
| Collagen III | ↑ fibers on immunohistochemistry | ↓ fibers on immunohistochemistry | [249] |
| Fibronectin (FN1) | ↑ gene expression, protein | ↓ gene expression | [59,250] |
| Osteopontin | ↑ gene expression | NA | [250] |
| Osteonectin | ↑ gene expression | NA | [250] |
| Stimuli | Type of Cells | Description of the Bioreactor Design | Changes in Gene Expression | Changes in Protein Expression | Changes in Cytokine Production | Changes in microRNA Levels | Magnitude | Frequency | Duration | Medium | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Stretching | PDLSC | 6-well plate BioFlex® (Flexcell International Corporation, Burlington, VT, USA) with elastic membrane at the bottom; stretching achieved by a pushing mechanism from beneath the membrane. | RUNX2 (↑1d → ↓3d; ↑3% → ↓20%); ALPL (↑1d → ↓3d; ↑3% → ↓20%); Osterix (↑1d → ↓3d; ↑3% → ↑↑6% → ↓20%); OCN (↓1d → ↑3d; ↓3% → ↑↑10% → ↑↑15% → ↑20%); FOS (↓1d →-↑3d; no dependence on the percentage of stretching.); IL6 (↑1d → ↓3d; ↑3% → ↓10% → ↑20%); PTGS2 (↑1d → ↓3d; ↓3% → ↑20%) | ___ | ↑ IL1β ↑ TNFα ↑ IL6 ↑ IL8 ↑ PGE2 | ___ | 3%, 6%, 10%, 15%, 20% | NA | 1, 2, 3 d | Low-glucose DMEM supplemented with 10% FBS, 2% MEM vitamins and 1% of antibiotic/antimycotic | [16] |
| PDLSC (modification related to YAP) | Tension Plus System: 6-well plate with an elastic membrane at the bottom. Stretching was generated by pulling the membrane inward using a vacuum pump. | After 3 days of stretching in osteogenic differentiation medium: ↑↑ OPN ↑ RUNX2 ↑ Col1 ↑ ALPL ↑ Osterix ↑ OCN | ↑ OPN ↑ OCN ↑ ALP | ___ | ___ | 10% | 0.1 Hz (5 s stress and 5 s rest) | 24 h (mRNA extraction); 72 h (ALP staining, Western blots analyses and immunofluorescence detections) | Osteogenic differentiation media (α-MEM containing 10 mM β-glycerophosphate, 0.5 μM dexamethasone, 50 mg/mL ascorbic acid, and 10% FBS) | [217] | |
| PDLSC | 6-well plate BioFlex combined with the Flexercell FX4000 Strain Unit. Stretching was generated by pulling the membrane inward using a vacuum pump. | ↑ OCN ↑ ALPL | ___ | ___ | ↓ miR-434-5p ↓ miR-1297 ↓ miR-3607-5p ↓ miR-145-5p ↓ miR-4328 ↓ miR-224-5p ↓ miR-195-5p | 12% | 6 cycles/min (5 s on and 5 s off) | 6, 12, 24, 48, 72 h | αMEM supplemented with 10% FBS, 100 U ml−1 penicillin, and 100 mg ml−1 streptomycin | [228] | |
| PDLSC | Elastic membrane with a circularly fixed membrane, which is subjected to hydrostatic pressure and/or using a plate with Teflon rings. | ↑ Col1A1 | ↑ Total Protein ↑ Col1 (10%, 24 h) | ___ | ___ | 10% | 30 cycles/min | 24 h | Serum-free growth media containing 50 μg/mL of both ascorbic acid and β-aminopropionitrile | [59] | |
| PDLSC | Flexercell Strain Unit (Flexcell Corp). | ___ | ___ | ↑ IL1β (3, 5 days) | ___ | 9%, 18% | 6 cycles/min (5 s elongation and 5 s relaxation) | 1, 3, 5 d | αMEM supplemented with 100 μg/mL penicillin-G, 50 μg/mL gentamycin sulfate, 0,3 μg/mL amphotericin B, 2% fetal calf serum (FCS) | [58] | |
| PDLSC | BioFlex c гибким днoм (Flexcell), Flexcell® FX-6000 TM Tension Unit (Flexcell International Corporation, Burlington, VT, USA). | ↑ OPN (12, 48 h); ↑↑ OPN (24 h); ↑ RUNX2 (12, 24 h); ↑ OCN (12, 24, 48 h); ↑ corresponding LC3B-II/LC3B-I; ↑ Beclin1; ↑ LAMP1. | ↑ OPN (12, 24, 48 h); ↑ RUNX2 (12, 24 h); ↓ RUNX2 (48 h); ↑ OCN (12, 24, 48 h); ↑ corresponding LC3B-II/LC3B-I; ↑ Beclin1; ↑ LAMP1. | ___ | ___ | 12% | 0.1 Hz 6 cycles/min (5 s on and 5 s off) | 12, 24, 48 h | α-MEM, 100 U/mL penicillin/streptomycin and 10% FBS | [17] | |
| PDLSC | Collagen-coated 6-well BioFlex® (Flexcell International Corporation, Burlington, VT, USA)culture plates + Flexcell® tension system (Flexcell International Corporation, Burlington, VT, USA) | ↓ FGFR2 (72 h); ↓ NOG (72 h); ↓ SULF1 (72 h); ↓ SFRP1 (72 h) | ___ | ___ | ___ | Starting with a maximum tension intensity of 10% for the first 6 h and then gradually decreasing to 3% | NA | 72 h | αMEM supplemented with 10% fetal calf serum (FCS), 2 mM L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin and 2.5 μg/mL amphotericin | [229] | |
| PDLSC | 6-well BioFlex® plates with flexible membranes; (Flexcell International Corporation, Burlington, VT, USA)coated with fibronectin + Flexercell Strain Unit (Model FX 3000) (Flexcell International Corporation, Burlington, VT, USA). | ↑ IL-6 (10%, 12 h) ↓ IL-6 (1, 5%, 12 h) ↑ COX-2 (10%, 12 h) | ↑ PGE2 10%, 12 h) ↑ IL-6 (10%, 12 h) ↑ MMP-8 (10%, 12 h) ↑ TIMP-1 (5, 10%, 12 h) ↑ TIMP-1/MMP-8 (5%, 12 h) | ___ | ___ | 1%, 5%, 10% | NA | 12 h | DMEM containing 1% l-glutamine, and 1% penicillin/streptomycin/neomycin | [19] | |
| PDLSC | 6-well BioFlex® plate (Flexcell International Corporation, Burlington, VT, USA) combined with the Flexercell® 5000 unit. | ↑ RUNX2 (12 h); ↑ Col1A1 (12 h); ↑ CYTOR (12 h); ↑ MIR22HG (12 h); ↑ SNHG3 (12 h); ↑ EGFR (12 h); ↑ FGF5 (12 h); ↑ HIF1A (12 h); ↑ VEGFA (12 h); ↓ FOXO1(12 h) | ↑ RUNX2 (3 d.); ↑ Col1A1 (3 d.) | ___ | ___ | 12% | NA | 12 h, 3 d | αMEM supplemented with 100 U/mL penicillin, and 0.1 mg/mL streptomycin | [221] | |
| Compression | PDLSC | Elastic membrane with a circularly fixed membrane, which is subjected to force using a plate with Teflon rings. | ↓ Col1A1 | ↓ Col1 ↓ FN | ___ | ___ | 10% | 30 cycles/min | 24 h | Serum-free growth media containing 50 μg/mL of both ascorbic acid and β-aminopropionitrile | [59] |
| PDLSC | 6-well plate + application of force using round glass cylinders. | ↓ PCNA (24, 48, 72 h); ↓ MCM2 (24, 48, 72 h); ↓ Cyclin A1 (24, 48, 72 h); | ↓ PCNA (24, 48, 72 h); ↓ MCM2 (24, 48, 72 h); ↓ Cyclin A1 (24, 48, 72 h); | ↑ IL6 ↑ IL8 | ___ | 2 g/cm2 (0.02 N/cm2, respectively) | NA | 24, 48, 72 h | DMEM containing 100 units/mL of penicillin, 100 µg/mL of streptomycin, 10% FCS and 50 mg/L-ascorbic acid | [215] | |
| PDLSC | Three-dimensional cell culture on PLGA scaffolds placed in a 6-well plate, with a cover glass and a granule bottle positioned on the scaffold with cells to generate a compression of 25 g/cm2. | ↑ RANKL (6 h); ↑ NFATC2 (6, 12 h); ↓ OPG (3 h); ↑ OPG (12 h); ↓ OPG/RANKL (3, 6 h); ↑ OPG/RANKL (12 h) | ↑ RANKL (12 h); ↑ OPG (12 h) | ___ | ___ | 25 g/cm2 | NA | 3, 6, 12 h | NA | [60] | |
| PDLSC | 6-well plate + application of force using round glass cylinders (2 g/cm2). | ↑ VEGFA (24 h); | ↑ IL6 (24 h); ↑ TLR4 (3 h); ↓ TLR4 (24 h); ↓ pAKT (3, 24 h); ↑ pERK (3 h); ↑ p-p38 (3, 24 h); | ↑ IL6 (24 h); ↑ IL8 (24 h); ↑ COX2 (24 h); | ___ | no information | NA | 3, 24 h | NA | [219] | |
| Shear stress | PDLSC | 24 mm long collagen microfiber with PDLSC. | waveform microfiber: ↑ CycD (1, 4 h); ↑ E-cadherin (1, 4 h); ↑ Periostin (1, 4 h) | ___ | ___ | ___ | 6 dyne/cm2 | 4.2 mL/min | 1, 4 h | NA | [216] |
| PDLSC | Parallel plate flow chamber. | ↑ CTGF (30 min.–4 h); ↑ ANKRD1 (30 min.–4 h); ↓ pLATS1 (5 min.–4 h) ↑ p38 | ↓ Yap (30 min.-4 h); | ___ | ___ | 1, 3, 6, and 9 dyn/cm2 | NA | 5, 10, 30 min, 1, 2, 4 h | High DMEM supplemented with 10% FBS and 0.1 mg/mL penicillin/streptomycin | [235] | |
| PDLSC | Cells into 35 mm culture dishes with a cone-shaped rotating disk. | ↑ IDO (5 dyn/cm2, 3 h); ↑ COX2 (5 dyn/cm2, 3 h); | ↑ TGF-β1 (5 dyn/cm2); ↑ kynurenine (5 dyn/cm2); ↑ IDO (5 и 10 dyn/cm); | ___ | ___ | 0.5, 5 and 10 dyn/cm2 | NA | 24 h | DMEM containing 10% FBS, 1% L-glutamine, 1% antibiotic–antimycotic | [236] | |
| PDLSC | A parallel flow chamber from polydimethylsiloxane (PDMS). | ↑ FOS (6 dyn/cm2, 1 h); ↑ PTGS2 (6 dyn/cm2, 1 h); ↑ CXCL8/IL8 (6 dyn/cm2, 1 h); ↑ RUNX2 (6 dyn/cm2, 1 h); ↑ VEGFA (6 dyn/cm2, 1 h); | ___ | ___ | ___ | 1, 6 dyn/cm2 | NA | 1 h | DMEM/F-12 supplemented with 10% FBS, 1% MEM vitamins, 1% GlutaMAX™, 1% antibiotic/antimycotic, HEPES, odium pyruvate solution | [241] |
7. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AB | alveolar bone |
| ABC | alveolar bone cells |
| ADM | acellular dermal matrix |
| AT-MSCs | Adipose-tissue-derived mesenchymal stem cells |
| ALP | alkaline phosphatase |
| ALPL | gene encoding alkaline phosphatase |
| ASA | acetylsalicylic acid |
| bFGF | basic fibroblast growth factor |
| BGM | bioactive glass microspheres |
| BMP | bone morphogenetic protein |
| BMSCs | bone marrow stem cells |
| b-TCP | b-tricalcium phosphate |
| BV | tissue volume (bone volume) |
| BV/TV | bone volume/total volume |
| CAD | computer-aided design |
| CAL | clinical attachment level |
| CaP | calcium phosphate |
| COL | collagen |
| CT | computed tomography |
| CTGF | connective tissue growth factor |
| DBB | droplet-based bioprinting |
| dECM | decellularized extracellular matrix |
| dPDL | decellularized periodontal ligament |
| dTM | decellularized tooth matrix |
| DLP | digital light processing |
| DPSCs | dental pulp stem cells |
| DSCs | dental stem cells |
| DSP | dentin sialoprotein |
| DSPP | dentin sialophosphoprotein |
| ECM | extracellular matrix |
| EGFR | epidermal growth factor receptor |
| FDM | fused deposition modeling |
| FGF5 | fibroblast growth factor 5 |
| GelMa | gelatin methacryloyl |
| GMCs | gingival margin-derived cells |
| GR | gingival recession |
| GSCs | gingival stem cells |
| GTR | guided tissue regeneration |
| Gum–HA | gum-hydroxyapatite |
| HA | hyaluronic acid |
| HAp | hydroxyapatite |
| HE | hematoxylin and eosin |
| HGF | hepatocyte growth factor |
| HIF-1α | hypoxia-inducible factor 1-alpha |
| IDO | indoleamine 2,3-dioxygenase |
| IFN-γ | interferon-γ |
| IHC | Immunohistochemical |
| IL1β | interleukin 1β |
| IL6 | interleukin 6 |
| IL8 | interleukin 8 |
| MAPK | mitogen-activated protein kinase family |
| MCM2 | minichromosome maintenance complex component 2 |
| MMPs | matrix metalloproteinases |
| MyD88 | myeloid differentiation primary response gene 88 |
| NMMII | non-muscle myosin II (Rho/ROCK) |
| OCN | osteocalcin (bone gamma-carboxyglutamic acid-containing protein) |
| OPG | osteoprotegerin (TNFRSF11B) |
| OPN | osteopontin (SPP1) |
| PCL | polycaprolactone |
| PCNA | proliferating cell nuclear antigen |
| PD | periodontal desease |
| PDL | periodontal ligament |
| PDLSCs | periodontal ligament-derived stem cells |
| PGE2 | prostaglandin E2 |
| PLGA | poly(lactic-co-glycolic acid) |
| PTGS2 | prostaglandin–endoperoxide synthase 2 |
| RANK | kappa-B nuclear factor activator receptor |
| RANKL | receptor activator of nuclear factor kappa ligand |
| SA | sodium alginate |
| SCAP | stem cells from the apical papilla |
| SHED | stem cells from human exfoliated deciduous teeth |
| SLA | stereolithography |
| SOD | superoxide dismutase |
| TGF-β | transforming growth factor beta |
| TIMP-1 | tissue inhibitor of metalloproteinases-1 |
| TLR4 | toll-like receptor 4 |
| TLS | triple-layered structure |
| TNFα | tumor necrosis factor alpha |
| TRAF6 | TNF receptor-associated Factor 6 |
| TT | trabecular thickness of bone |
| UC-MSCs | umbilical cord-derived mesenchymal stem cells |
| VEGF | vascular endothelial growth factor |
| YAP | Yes-associated protein |
References
- Chen, M.X.; Zhong, Y.J.; Dong, Q.Q.; Wong, H.M.; Wen, Y.F. Global, regional, and national burden of severe periodontitis, 1990–2019: An analysis of the Global Burden of Disease Study 2019. J. Clin. Periodontol. 2021, 48, 1165–1188. [Google Scholar] [CrossRef]
- Usui, M.; Onizuka, S.; Sato, T.; Kokabu, S.; Ariyoshi, W.; Nakashima, K. Mechanism of alveolar bone destruction in periodontitis—Periodontal bacteria and inflammation. Jpn. Dent. Sci. Rev. 2021, 57, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Li, Y.; Feng, M.; Li, L. Understanding the feelings and experiences of patients with periodontal disease: A qualitative meta-synthesis. Health Qual Life Outcomes 2022, 20, 126. [Google Scholar] [CrossRef]
- Paknejad, M.; Eslaminejad, M.B.; Ghaedi, B.; Rokn, A.R.; Khorsand, A.; Etemad-Moghadam, S.; Alaeddini, M.; Dehghan, M.M.; Moslemi, N.; Nowzari, H. Isolation and assessment of mesenchymal stem cells derived from bone marrow: Histologic and histomorphometric study in a canine periodontal defect. J. Oral. Implantol. 2015, 41, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.H.; Chen, J.-Y.; Suo, W.-H.; Shao, W.-R.; Huang, C.-Y.; Li, M.-T.; Li, Y.-Y.; Li, Y.-H.; Liang, E.-L.; Chen, Y.-H.; et al. Unlocking the Future of Periodontal Regeneration: An Interdisciplinary Approach to Tissue Engineering and Advanced Therapeutics. Biomedicines 2024, 12, 1090. [Google Scholar] [CrossRef] [PubMed]
- Kadkhoda, Z.; Motie, P.; Rad, M.R.; Mohaghegh, S.; Kouhestani, F.; Motamedian, S.R. Comparison of Periodontal Ligament Stem Cells with Mesenchymal Stem Cells from Other Sources: A Scoping Systematic Review of In vitro and In vivo Studies. Curr. Stem Cell Res. Ther. 2024, 19, 497–522. [Google Scholar] [CrossRef]
- Nguyen-Thi, T.D.; Nguyen-Huynh, B.H.; Vo-Hoang, T.T.; Nguyen-Thanh, T. Stem cell therapies for perio-dontal tissue regeneration: A meta-analysis of clinical trials. J. Oral Biol. Craniofac. Res. 2023, 13, 589–597. [Google Scholar] [CrossRef]
- Cai, C.; Li, C.; Wei, B.; Ye, Z.; Mao, Q.; Ye, W.; Rong, M.; Zeng, J. Current Perspectives on Mesenchymal Stem Cells as a Potential Treatment for Periodontal Diseases and Conditions. Genesis 2025, 63, e70024. [Google Scholar] [CrossRef]
- Mohammed, E.; Khalil, E.; Sabry, D. Effect of adipose-derived stem cells and their exo as adjunctive therapy to nonsurgical periodontal treatment: A histologic and histomorphometric study in rats. Biomolecules 2018, 8, 167. [Google Scholar] [CrossRef]
- Galal, G.; Elba, G.; Kawana, K.; Fahmy, R.; Mehanna, R. Biological Evaluation of Adipose Tissue-Derived Stem Cells on Alveolar Bone Healing in rats with Ligature Induced Periodontitis. Alex. Dent. J. 2022, 47, 54–62. [Google Scholar] [CrossRef]
- Galli, M.; Yao, Y.; Giannobile, W.V.; Wang, H.L. Current and future trends in periodontal tissue engineering and bone regeneration. Plast Aesthet Res 2021, 8, 3. [Google Scholar] [CrossRef]
- Angjelova, A.; Jovanova, E.; Polizzi, A.; Annunziata, M.; Laganà, L.; Santonocito, S.; Isola, G. Insights and Advancements in Periodontal Tissue Engineering and Bone Regeneration. Medicina 2024, 60, 773. [Google Scholar] [CrossRef]
- Swanson, W.B.; Yao, Y.; Mishina, Y. Novel approaches for periodontal tissue engineering. Genesis 2022, 60, e23499. [Google Scholar] [CrossRef]
- Bakhshandeh, B.; Ranjbar, N.; Abbasi, A.; Amiri, E.; Abedi, A.; Mehrabi, M.; Dehghani, Z.; Pennisi, C.P. Recent progress in the manipulation of biochemical and biophysical cues for engineering functional tissues. Bioeng. Transl. Med. 2023, 8, e10383. [Google Scholar] [CrossRef]
- Jin, S.S.; He, D.; Wang, Y.; Zhang, T.; Yu, H.; Li, Z.; Zhu, L.; Zhou, Y.; Liu, Y. Mechanical force modulates periodontal ligament stem cell characteristics during bone remodelling via TRPV4. Cell Prolif. 2020, 53, e12912. [Google Scholar] [CrossRef]
- Sun, C.; Rankovic, M.J.; Folwaczny, M.; Stocker, T.; Otto, S.; Wichelhaus, A.; Baumert, U. Effect of Different Parameters of In Vitro Static Tensile Strain on Human Periodontal Ligament Cells Simulating the Tension Side of Orthodontic Tooth Movement. Int. J. Mol. Sci. 2022, 23, 1525. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Hu, Z.; Su, H.; Wang, Y.; Lin, Y. Effects of tension on mitochondrial autophagy and osteogenic differentiation of periodontal ligament stem cells. Cell Prolif. 2023, 57, e13561. [Google Scholar] [CrossRef]
- Wada, S.; Kanzaki, H.; Narimiya, T.; Nakamura, Y. Novel device for application of continuous mechanical tensile strain to mammalian cells. Biol. Open 2017, 6, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, C.; Walter, C.; Ziebart, T.; Grimm, S.; Meila, D.; Krieger, E.; Wehrbein, H. Induction of IL-6 and MMP-8 in human periodontal fibroblasts by static tensile strain. Clin. Oral. Investig. 2014, 18, 901–908. [Google Scholar] [CrossRef]
- Bae, H.J.; Shin, S.-J.; Bin Jo, S.; Li, C.J.; Lee, D.-J.; Lee, H.-H.; Kim, H.-W.; Lee, J.-H. Cyclic stretch-induced epigenetic activation of periodontal ligament cells. Mater. Today Bio 2024, 26, 101050, Erratum in Mater. Today Bio 2024, 29, 101277. https://doi.org/10.1016/J.MTBIO.2024.101277. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.P.; Yu, B.; Yun, W.; Marshall, G.W.; Ryder, M.I.; Marshall, S.J. Structure, chemical composition and mechanical properties of human and rat cementum and its interface with root dentin. Acta Biomater. 2009, 5, 707–718. [Google Scholar] [CrossRef]
- Yoshida, N.; Koga, Y.; Peng, C.L.; Tanaka, E.; Kobayashi, K. In vivo measurement of the elastic modulus of the human periodontal ligament. Med. Eng. Phys. 2001, 23, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, R.; Jeannin, C.; Attik, N.; Trunfio-Sfarghiu, A.M.; Gritsch, K.; Grosgogeat, B. Tissue Engineering for Periodontal Ligament Regeneration: Biomechanical Specifications. J. Biomech. Eng. 2021, 143, 030801. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Pei, F.; Jin, Y.; Zhao, Z. Exploring the mechanical and biological interplay in the periodontal ligament. Int. J. Oral Sci. 2025, 17, 23. [Google Scholar] [CrossRef]
- Ovy, E.G.; Romanyk, D.L.; Mir, C.F.; Westover, L. Modelling and evaluating periodontal ligament mechanical behaviour and properties: A scoping review of current approaches and limitations. Orthod. Craniofac. Res. 2022, 25, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Nanci, A.; Bosshardt, D.D. Structure of periodontal tissues in health and disease. Periodontol. 2000 2006, 40, 11–28. [Google Scholar] [CrossRef]
- Buduneli, N. Anatomy of Periodontal Tissues. In Biomarkers in Periodontal Health and Disease; Springer: Cham, Switzerland, 2020; pp. 1–7. [Google Scholar] [CrossRef]
- Cope, G.; Cope, A. The periodontium: An anatomical guide. Dent. Nurs. 2011, 7, 376–378. [Google Scholar] [CrossRef]
- Zhang, Z.; Deng, M.; Hao, M.; Tang, J. Periodontal ligament stem cells in the periodontitis niche: Inseparable interactions and mechanisms. J. Leukoc. Biol. 2021, 110, 565–576. [Google Scholar] [CrossRef]
- de Jong, T.; Bakker, A.D.; Everts, V.; Smit, T.H. The intricate anatomy of the periodontal ligament and its development: Lessons for periodontal regeneration. J. Periodontal Res. 2017, 52, 965–974. [Google Scholar] [CrossRef]
- Liu, M.; Wu, B.; Yang, F.; Jiang, D.; Izadikhah, I.; Chen, Y.; Li, N.; Yan, B. Understanding the hierarchical structure of collagen fibers of the human periodontal ligament: Implications for biomechanical characteristics. Acta Biomater. 2024, 188, 253–265. [Google Scholar] [CrossRef]
- Huang, Y.; Tang, Y.; Zhang, R.; Wu, X.; Yan, L.; Chen, X.; Wu, Q.; Chen, Y.; Lv, Y.; Su, Y. Role of periodontal ligament fibroblasts in periodontitis: Pathological mechanisms and therapeutic potential. J. Transl. Med. 2024, 22, 1136. [Google Scholar] [CrossRef]
- Villar, C.C.; Cochran, D.L. Regeneration of Periodontal Tissues: Guided Tissue Regeneration. Dent. Clin. N. Am. 2010, 54, 73–92. [Google Scholar] [CrossRef]
- Ivanovski, S.; Han, P.; Peters, O.A.; Sanz, M.; Bartold, P.M. The Therapeutic Use of Dental Mesenchymal Stem Cells in Human Clinical Trials. J. Dent. Res. 2024, 103, 1173–1184. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, J.; Bronckaers, A.; Dillen, Y.; Gervois, P.; Vangansewinkel, T.; Driesen, R.B.; Wolfs, E.; Lambrichts, I.; Hilkens, P. The Neurovascular Properties of Dental Stem Cells and Their Importance in Dental Tissue Engineering. Stem Cells Int. 2016, 2016, 9762871. [Google Scholar] [CrossRef]
- Shi, S.; Miura, M.; Seo, B.M.; Robey, P.G.; Bartold, P.M.; Gronthos, S. The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthod. Craniofac Res. 2005, 8, 191–199. [Google Scholar] [CrossRef]
- Qiao, X.; Tang, J.; Dou, L.; Yang, S.; Sun, Y.; Mao, H.; Yang, D. Dental Pulp Stem Cell-Derived Exosomes Regulate Anti-Inflammatory and Osteogenesis in Periodontal Ligament Stem Cells and Promote the Repair of Experimental Periodontitis in Rats. Int. J. Nanomed. 2023, 18, 4683–4703. [Google Scholar] [CrossRef]
- Aimetti, M.; Ferrarotti, F.; Mariani, G.M.; Cricenti, L.; Romano, F. Use of Dental Pulp Stem Cells/Collagen Sponge Biocomplex in the Treatment of Non-Contained Intrabony Defects: A Case Series. Clin. Adv. Periodontics 2015, 5, 104–109. [Google Scholar] [CrossRef] [PubMed]
- D’Aquino, R.; De Rosa, A.; Lanza, V.; Tirino, V.; Laino, L.; Graziano, A.; Desiderio, V.; Laino, G.; Papaccio, G. Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur. Cells Mater. 2009, 18, 75–83. [Google Scholar] [CrossRef]
- Ferrarotti, F.; Romano, F.; Gamba, M.N.; Quirico, A.; Giraudi, M.; Audagna, M.; Aimetti, M. Human intrabony defect regeneration with micrografts containing dental pulp stem cells: A randomized controlled clinical trial. J. Clin. Periodontol. 2018, 45, 841–850. [Google Scholar] [CrossRef]
- Bartold, M.; Ivanovski, S. Stem Cell Applications in Periodontal Regeneration. Dent. Clin. N. Am. 2022, 66, 53–74. [Google Scholar] [CrossRef]
- Hu, J.; Cao, Y.; Xie, Y.; Wang, H.; Fan, Z.; Wang, J.; Zhang, C.; Wu, C.; Wang, S. Periodontal regeneration in swine after cell injection and cell sheet transplantation of human dental pulp stem cells following good manufacturing practice. Stem Cell Res. Ther. 2016, 7, 130. [Google Scholar] [CrossRef]
- Khorsand, A.; Eslaminejad, M.B.; Arabsolghar, M.; Paknejad, M.; Ghaedi, B.; Rokn, A.R.; Moslemi, N.; Nazarian, H.; Jahangir, S. Autologous dental pulp stem cells in regeneration of defect created in canine periodontal tissue. J. Oral. Implantol. 2013, 39, 433–443. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, Z.; Xie, Y.; Hu, J.; Wang, H.; Fan, Z.; Zhang, C.; Wang, J.; Wu, C.-T.; Wang, S. Adenovirus-mediated transfer of hepatocyte growth factor gene to human dental pulp stem cells under good manufacturing practice improves their potential for periodontal regeneration in swine. Stem Cell Res. Ther. 2015, 6, 249. [Google Scholar] [CrossRef]
- Hernández-Monjaraz, B.; Santiago-Osorio, E.; Ledesma-Martínez, E.; Aguiñiga-Sánchez, I.; Sosa-Hernández, N.A.; Mendoza-Núñez, V.M. Dental Pulp Mesenchymal Stem Cells as a Treatment for Periodontal Disease in Older Adults. Stem Cells Int. 2020, 2020, 8890873. [Google Scholar] [CrossRef]
- Iwayama, T.; Sakashita, H.; Takedachi, M.; Murakami, S. Periodontal tissue stem cells and mesenchymal stem cells in the periodontal ligament. Jpn. Dent. Sci. Rev. 2022, 58, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Menicanin, D.; Mrozik, K.M.; Wada, N.; Marino, V.; Shi, S.; Bartold, P.M.; Gronthos, S. Periodontal-Ligament-Derived Stem Cells Exhibit the Capacity for Long-Term Survival, Self-Renewal, and Regeneration of Multiple Tissue Types in Vivo. Stem Cells Dev. 2013, 23, 1001–1011. [Google Scholar] [CrossRef]
- Iwasaki, K.; Komaki, M.; Yokoyama, N.; Tanaka, Y.; Taki, A.; Kimura, Y.; Takeda, M.; Oda, S.; Izumi, Y.; Morita, I. Periodontal Ligament Stem Cells Possess the Characteristics of Pericytes. J. Periodontol. 2013, 84, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Behm, C.; Miłek, O.; Schwarz, K.; Kovar, A.; Derdak, S.; Rausch-Fan, X.; Moritz, A.; Andrukhov, O. Heterogeneity in Dental Tissue-Derived MSCs Revealed by Single-Cell RNA-seq. J. Dent. Res. 2024, 103, 1141–1152. [Google Scholar] [CrossRef]
- Ng, T.K.; Chen, C.-B.; Xu, C.; Xu, Y.; Yao, X.; Huang, L.; Liang, J.-J.; Cheung, H.S.; Pang, C.P.; Huang, Y. Attenuated regenerative properties in human periodontal ligament–derived stem cells of older donor ages with shorter telomere length and lower SSEA4 expression. Cell Tissue Res. 2020, 381, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, B.; Wang, H.; Zhao, X.; Zhang, Z.; Ding, G.; Wei, F. The effect of aging on the biological and immunological characteristics of periodontal ligament stem cells. Stem Cell Res. Ther. 2020, 11, 326. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Gauthier, P.; Tran, Q.T.; El-Ayachi, I.; Bhatti, F.U.R.; Bahabri, R.; Al-Habib, M.; Huang, G.T. Differential Properties of Human ALP+ Periodontal Ligament Stem Cells vs Their ALP- Counterparts. J. Stem Cell Res. Ther. 2015, 5, 292. [Google Scholar] [CrossRef]
- Seo, B.-M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Zhang, Q.; Qian, L.; Shi, Z.; Wang, X.; Jia, L.; Xia, Y. Antibacterial periodontal ligament stem cells enhance periodontal regeneration and regulate the oral microbiome. Stem Cell Res. Ther. 2024, 15, 334. [Google Scholar] [CrossRef]
- Iwasaki, K.; Akazawa, K.; Nagata, M.; Komaki, M.; Honda, I.; Morioka, C.; Yokoyama, N.; Ayame, H.; Yamaki, K.; Tanaka, Y.; et al. The fate of transplanted periodontal ligament stem cells in surgically created periodontal defects in rats. Int. J. Mol. Sci. 2019, 20, 192. [Google Scholar] [CrossRef]
- Raju, R.; Oshima, M.; Inoue, M.; Morita, T.; Huijiao, Y.; Waskitho, A.; Baba, O.; Inoue, M.; Matsuka, Y. Three-dimensional periodontal tissue regeneration using a bone-ligament complex cell sheet. Sci. Rep. 2020, 10, 1656. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, N.; Yamaguchi, M.; Goseki, T.; Ozawa, Y.; Saito, K.; Takiguchi, H.; Iwasawa, T.; Abiko, Y. Cyclic-tension force stimulates interleukin-1 beta production by human periodontal ligament cells. J. Periodontal Res. 1994, 29, 328–333. [Google Scholar] [CrossRef]
- He, Y.; Macarak, E.J.; Korostoff, J.M.; Howard, P.S. Compression and tension: Differential effects on matrix accumulation by periodontal ligament fibroblasts in vitro. Connect. Tissue Res. 2004, 45, 28–39. [Google Scholar] [CrossRef]
- Jianru, Y.; MeiLe, L.; Yang, Y.; Zheng, W.; Yu, L.; Zhao, Z. Static compression regulates OPG expression in periodontal ligament cells via the CAMK II pathway. J. Appl. Oral. Sci. 2015, 23, 549–554. [Google Scholar] [CrossRef]
- Shetty, S.S.; Sowmya, S.; Pradeep, A.; Jayakumar, R. Gingival Mesenchymal Stem Cells: A Periodontal Regenerative Substitute. Tissue Eng. Regen. Med. 2025, 22, 1–21. [Google Scholar] [CrossRef]
- Peng, Y.; Jaar, J.; Tran, S.D. Gingival mesenchymal stem cells: Biological properties and therapeutic applications. J. Oral. Biol. Craniofac Res. 2024, 14, 547–569. [Google Scholar] [CrossRef]
- Balaban, Y.E.; Akbaba, S.; Bozkurt, S.B.; Buyuksungur, A.; Akgun, E.E.; Gonen, Z.B.; Salkin, H.; Tezcaner, A.; Hakki, S.S. Local application of gingiva-derived mesenchymal stem cells on experimental periodontitis in rats. J. Periodontol. 2024, 95, 456–468. [Google Scholar] [CrossRef]
- El-Sayed, K.M.F.; Mekhemar, M.K.; Beck-Broichsitter, B.E.; Bähr, T.; Hegab, M.; Receveur, J.; Heneweer, C.; Becker, S.T.; Wiltfang, J.; Dörfer, C.E. Periodontal regeneration employing gingival margin-derived stem/progenitor cells in conjunction with IL-1ra-hydrogel synthetic extracellular matrix. J. Clin. Periodontol. 2015, 42, 448–457. [Google Scholar] [CrossRef]
- Yu, X.; Ge, S.; Chen, S.; Xu, Q.; Zhang, J.; Guo, H.; Yang, P. Human gingiva-derived mesenchymal stromal cells contribute to periodontal regeneration in beagle dogs. Cells Tissues Organs 2013, 198, 428–437. [Google Scholar] [CrossRef]
- Dan, H.; Vaquette, C.; Fisher, A.G.; Hamlet, S.M.; Xiao, Y.; Hutmacher, D.W.; Ivanovski, S. The influence of cellular source on periodontal regeneration using calcium phosphate coated polycaprolactone scaffold supported cell sheets. Biomaterials 2014, 35, 113–122. [Google Scholar] [CrossRef]
- Vaquette, C.; Saifzadeh, S.; Farag, A.; Hutmacher, D.W.; Ivanovski, S. Periodontal Tissue Engineering with a Multiphasic Construct and Cell Sheets. J. Dent. Res. 2019, 98, 673–681. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, K.M.F.; Paris, S.; Becker, S.T.; Neuschl, M.; De Buhr, W.; Sälzer, S.; Wulff, A.; Elrefai, M.; Darhous, M.S.; El-Masry, M.; et al. Periodontal regeneration employing gingival margin-derived stem/progenitor cells: An animal study. J. Clin. Periodontol. 2012, 39, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Guo, S.; Shi, W.; Liu, Q.; Huo, F.; Wu, Y.; Tian, W. Bone Marrow Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Promote Periodontal Regeneration. Tissue Eng. Part A 2021, 27, 962–976. [Google Scholar] [CrossRef] [PubMed]
- Portron, S.; Soueidan, A.; Marsden, A.-C.; Rakic, M.; Verner, C.; Weiss, P.; Badran, Z.; Struillou, X. Periodontal regenerative medicine using mesenchymal stem cells and biomaterials: A systematic review of pre-clinical studies. Dent. Mater. J. 2019, 38, 867–883. [Google Scholar] [CrossRef]
- Iwasaki, K.; Peng, Y.; Kanda, R.; Umeda, M.; Ishikawa, I. Stem Cell Transplantation and Cell-Free Treatment for Periodontal Regeneration. Int. J. Mol. Sci. 2022, 23, 1011. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.A.; Deliberador, T.M.; Abuna, R.P.F.; Rodrigues, T.L.; de Souza, S.L.S.; Palioto, D.B. Mesenchymal stem cells surpass the capacity of bone marrow aspirate concentrate for periodontal regeneration. J. Appl. Oral. Sci. 2022, 30, e20210359. [Google Scholar] [CrossRef]
- Jung, Y.H.; Park, J.Y.; Kim, H.J.; Lee, S.M.; Kim, S.H.; Yun, J.H. Regenerative Potential of Bone Morphogenetic Protein 7-Engineered Mesenchymal Stem Cells in Ligature-Induced Periodontitis. Tissue Eng. Part. A 2023, 29, 200–210. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, Y.; Chen, X.; Chen, C.; Zhu, Z.; Li, L. Therapeutic effect of bone marrow mesenchymal stem cells pretreated with acetylsalicylic acid on experimental periodontitis in rats. Int. Immunopharmacol. 2018, 54, 320–328. [Google Scholar] [CrossRef]
- Dong, L.; Li, X.; Leng, W.; Guo, Z.; Cai, T.; Ji, X.; Xu, C.; Zhu, Z.; Lin, J. Adipose stem cells in tissue regeneration and repair: From bench to bedside. Regen. Ther. 2023, 24, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Venkataiah, V.S.; Handa, K.; Njuguna, M.M.; Hasegawa, T.; Maruyama, K.; Nemoto, E.; Yamada, S.; Sugawara, S.; Lu, L.; Takedachi, M.; et al. Periodontal Regeneration by Allogeneic Transplantation of Adipose Tissue Derived Multi-Lineage Progenitor Stem Cells in vivo. Sci. Rep. 2019, 9, 921. [Google Scholar] [CrossRef]
- Wang, T.; Zhou, Y.; Zhang, W.; Xue, Y.; Xiao, Z.; Zhou, Y.; Peng, X. Exosomes and exosome composite scaffolds in periodontal tissue engineering. Front. Bioeng. Biotechnol. 2024, 11, 1287714. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, M.; Monsarrat, P.; Blasco-Baque, V.; Loubières, P.; Burcelin, R.; Casteilla, L.; Planat-Bénard, V.; Kémoun, P. Periodontal Tissue Regeneration Using Syngeneic Adipose-Derived Stromal Cells in a Mouse Model. Stem Cells Transl. Med. 2017, 6, 656–665. [Google Scholar] [CrossRef]
- Can, A.; Karahuseyinoglu, S. Concise Review: Human Umbilical Cord Stroma with Regard to the Source of Fetus-Derived Stem Cells. Stem Cells 2007, 25, 2886–2895. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Yang, C.; Xu, S.; Zhao, H. Comparison of osteogenic differentiation capacity in mesenchymal stem cells derived from human amniotic membrane (AM), umbilical cord (UC), chorionic membrane (CM), and decidua (DC). Cell Biosci. 2019, 9, 17. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, Y.; Chen, L.; Ye, L.; Cui, J.; Sun, Q.; Li, K.; Li, Z.; Liu, L. Human Umbilical Cord Mesenchymal Stem Cells: A New Therapeutic Option for Tooth Regeneration. Stem Cells Int. 2015, 2015, 549432. [Google Scholar] [CrossRef]
- Li, Y.; Hou, R.; Wang, Y.; Lu, B.; Zhang, J.; Feng, X.; Liu, Y.; Cao, Q. Fundamental study of application of umbilical cord mesenchymal stem cells to the periodontium to aid healing after autotransplantation of teeth. Br. J. Oral. Maxillofac. Surg. 2014, 52, 501–506. [Google Scholar] [CrossRef]
- Bright, R.; Hynes, K.; Gronthos, S.; Bartold, P.M. Periodontal ligament-derived cells for periodontal regeneration in animal models: A systematic review. J. Periodontal Res. 2015, 50, 160–172. [Google Scholar] [CrossRef]
- Sun, X.-C.; Wang, H.; Li, J.-H.; Zhang, D.; Yin, L.-Q.; Yan, Y.-F.; Ma, X.; Xia, H.-F. Repair of alveolar cleft bone defects by bone collagen particles combined with human umbilical cord mesenchymal stem cells in rabbit. Biomed. Eng. Online 2020, 19, 62. [Google Scholar] [CrossRef]
- Yang, S.; Zhu, B.; Tian, X.Y.; Yu, H.Y.; Qiao, B.; Zhao, L.S.; Zhang, B. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Enhance the Osteoblastic Differentiation of Periodontal Ligament Stem Cells Under High Glucose Conditions Through the PI3K/AKT Signaling Pathway. Biomed. Environ. Sci. 2022, 35, 811–820. [Google Scholar] [CrossRef]
- Chanreiphy, H.; Gupta, V.; Vandana, K.L.; Goswami, V.; Jhawar, B.; Vinitha, V. Evaluation of autologous periodontal stem cells niche in the treatment of periodontal intrabony defects: A split-mouth randomized controlled trial. J. Indian. Soc. Periodontol. 2025, 29, 28–33. [Google Scholar] [CrossRef]
- Du, J.; Shan, Z.; Ma, P.; Wang, S.; Fan, Z. Allogeneic bone marrow mesenchymal stem cell transplantation for periodontal regeneration. J. Dent. Res. 2014, 93, 183–188. [Google Scholar] [CrossRef]
- Putri, I.L.; Fatchiyah; Pramono, C.; Bachtiar, I.; Latief, F.D.E.; Utomo, B.; Rachman, A.; Soesilawati, P.; Hakim, L.; Rantam, F.A.; et al. Alveolar Repair Using Cancellous Bone and Beta Tricalcium Phosphate Seeded with Adipose-Derived Stem Cell. Cleft Palate Craniofacial J. 2024, 61, 555–565. [Google Scholar] [CrossRef]
- Shang, F.; Liu, S.; Ming, L.; Tian, R.; Jin, F.; Ding, Y.; Zhang, Y.; Zhang, H.; Deng, Z.; Jin, Y. Human Umbilical Cord MSCs as New Cell Sources for Promoting Periodontal Regeneration in Inflammatory Periodontal Defect. Theranostics 2017, 7, 4370. [Google Scholar] [CrossRef] [PubMed]
- Yürük, G.; Demir, Y.D.; Vural, Ş.; Kehr, N.S. Polymeric biomaterials for periodontal tissue engineering and periodontitis. RSC Appl. Polym. 2024, 2, 534–556. [Google Scholar] [CrossRef]
- Binlateh, T.; Thammanichanon, P.; Rittipakorn, P.; Thinsathid, N.; Jitprasertwong, P. Collagen-Based Biomaterials in Periodontal Regeneration: Current Applications and Future Perspectives of Plant-Based Collagen. Biomimetics 2022, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Zhang, Z.; Shang, X.; Zhang, J.; Ren, S. Optimization of light-curing ionogels by response surface methodology. RSC Appl. Polym. 2025, 3, 1366–1375. [Google Scholar] [CrossRef]
- Alqahtani, A.M.; Moorehead, R.; Asencio, I.O. Guided Tissue and Bone Regeneration Membranes: A Review of Biomaterials and Techniques for Periodontal Treatments. Polymers 2023, 15, 3355. [Google Scholar] [CrossRef] [PubMed]
- Almeida, N.D.; Carneiro, C.A.; de Marco, A.C.; Porto, V.C.; França, R. 3D Bioprinting Techniques and Bioinks for Periodontal Tissues Regeneration—A Literature Review. Biomimetics 2024, 9, 480. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef]
- Lestari, W.; Irfanita, N.; Haris, M.S.; Lin, G.S.S.; Jaswir, I.; Darnis, D.S.; Ruziantee, N.; Mazlan, N.; Idrus, E.; Amir, L.R.; et al. Advancements and applications of gelatin-based scaffolds in dental engineering: A narrative review. Odontology 2025, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Atia, G.A.N.; Shalaby, H.K.; Zehravi, M.; Ghobashy, M.M.; Attia, H.A.N.; Ahmad, Z.; Khan, F.S.; Dey, A.; Mukerjee, N.; Alexiou, A.; et al. Drug-Loaded Chitosan Scaffolds for Periodontal Tissue Regeneration. Polymers 2022, 14, 3192. [Google Scholar] [CrossRef]
- Hwang, H.S.; Lee, C.-S. Recent Progress in Hyaluronic-Acid-Based Hydrogels for Bone Tissue Engineering. Gels 2023, 9, 588. [Google Scholar] [CrossRef] [PubMed]
- Polizzi, A.; Leanza, Y.; Belmonte, A.; Grippaudo, C.; Leonardi, R.; Isola, G. Impact of Hyaluronic Acid and Other Re-Epithelializing Agents in Periodontal Regeneration: A Molecular Perspective. Int. J. Mol. Sci. 2024, 25, 12347. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.R.; Biswal, T. Alginate and its application to tissue engineering. SN Appl. Sci. 2021, 3, 30. [Google Scholar] [CrossRef]
- Ramamurthy, J.; Bajpai, D. Role of Alginate-based Scaffolds for Periodontal Regeneration of Intrabony Defects: A Systematic Review. World J. Dent. 2024, 15, 181–187. [Google Scholar] [CrossRef]
- Deng, R.; Xie, Y.; Chan, U.; Xu, T.; Huang, Y. Biomaterials and biotechnology for periodontal tissue regeneration: Recent advances and perspectives. J. Dent. Res. Dent. Clin. Dent. Prospect. 2022, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Perinelli, D.R.; Cespi, M.; Bonacucina, G.; Palmieri, G.F. PEGylated polylactide (PLA) and poly (lactic-co-glycolic acid) (PLGA) copolymers for the design of drug delivery systems. J. Pharm. Investig. 2019, 49, 443–458. [Google Scholar] [CrossRef]
- Sun, S.; Cui, Y.; Yuan, B.; Dou, M.; Wang, G.; Xu, H.; Wang, J.; Yin, W.; Wu, D.; Peng, C. Drug delivery systems based on polyethylene glycol hydrogels for enhanced bone regeneration. Front Bioeng Biotechnol 2023, 11, 1117647. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Li, L.; Zhang, D.; Huang, S.; Jing, Z.; Wu, Y.; Zhao, Z.; Zhao, L.; Zhou, S. Incorporation of aligned PCL-PEG nanofibers into porous chitosan scaffolds improved the orientation of collagen fibers in regenerated periodontium. Acta Biomater. 2015, 25, 240–252. [Google Scholar] [CrossRef]
- Chen, E.; Wang, T.; Tu, Y.; Sun, Z.; Ding, Y.; Gu, Z.; Xiao, S. ROS-scavenging biomaterials for periodontitis. J. Mater. Chem. B 2022, 11, 482–499. [Google Scholar] [CrossRef]
- López-Valverde, N.; López-Valverde, A.; Montero, J.; Rodríguez, C.; de Sousa, B.M.; Aragoneses, J.M. Antioxidant, anti-inflammatory and antimicrobial activity of natural products in periodontal disease: A comprehensive review. Front. Bioeng. Biotechnol. 2023, 11, 1226907. [Google Scholar] [CrossRef] [PubMed]
- Gholami, Z.; Hasanpour, S.; Sadigh, S.; Johari, S.; Shahveghar, Z.; Ataei, K.; Javari, E.; Amani, M.; Kia, L.J.; Akbari, Z.D.; et al. Antibacterial agent-releasing scaffolds in dental tissue engineering. J. Adv. Periodontol. Implant. Dent. 2021, 13, 43–47. [Google Scholar] [CrossRef]
- Ding, T.; Kang, W.; Li, J.; Yu, L.; Ge, S. An in situ tissue engineering scaffold with growth factors combining angiogenesis and osteoimmunomodulatory functions for advanced periodontal bone regeneration. J. Nanobiotechnol. 2021, 19, 247. [Google Scholar] [CrossRef]
- Foo, J.B.; Looi, Q.H.; Chong, P.P.; Hassan, N.H.; Yeo, G.E.C.; Ng, C.Y.; Koh, B.; How, C.W.; Lee, S.H.; Law, J.X. Comparing the Therapeutic Potential of Stem Cells and their Secretory Products in Regenerative Medicine. Stem Cells Int. 2021, 2021, 2616807. [Google Scholar] [CrossRef]
- Mijiritsky, E.; Assaf, H.D.; Peleg, O.; Shacham, M.; Cerroni, L.; Mangani, L. Use of PRP, PRF and CGF in Periodontal Regeneration and Facial Rejuvenation—A Narrative Review. Biology 2021, 10, 317. [Google Scholar] [CrossRef]
- Zong, C.; Bronckaers, A.; Willems, G.; He, H.; Cadenas de Llano-Pérula, M. Nanomaterials for Periodontal Tissue Regeneration: Progress, Challenges and Future Perspectives. J. Funct. Biomater. 2023, 14, 290. [Google Scholar] [CrossRef] [PubMed]
- Ul Hassan, S.; Bilal, B.; Nazir, M.S.; Naqvi, S.A.R.; Ali, Z.; Nadeem, S.; Muhammad, N.; Palvasha, B.A.; Mohyuddin, A. Recent progress in materials development and biological properties of GTR membranes for periodontal regeneration. Chem. Biol. Drug Des. 2021, 98, 1007–1024. [Google Scholar] [CrossRef] [PubMed]
- Norahan, M.H.; Amroon, M.; Ghahremanzadeh, R.; Mahmoodi, M.; Baheiraei, N. Electroactive graphene oxide-incorporated collagen assisting vascularization for cardiac tissue engineering. J. Biomed. Mater. Res. A 2019, 107, 204–219. [Google Scholar] [CrossRef]
- Norahan, M.H.; Amroon, M.; Ghahremanzadeh, R.; Rabiee, N.; Baheiraei, N. Reduced graphene oxide: Osteogenic potential for bone tissue engineering. IET Nanobiotechnol. 2019, 13, 720–725. [Google Scholar] [CrossRef] [PubMed]
- An, N.; Yan, X.; Qiu, Q.; Zhang, Z.; Zhang, X.; Zheng, B.; Zhao, Z.; Guo, J.; Liu, Y. Human periodontal ligament stem cell sheets activated by graphene oxide quantum dots repair periodontal bone defects by promoting mitochondrial dynamics dependent osteogenic differentiation. J. Nanobiotechnol. 2024, 22, 133. [Google Scholar] [CrossRef]
- Suo, L.; Wu, H.; Wang, P.; Xue, Z.; Gao, J.; Shen, J. The improvement of periodontal tissue regeneration using a 3D-printed carbon nanotube/chitosan/sodium alginate composite scaffold. J. Biomed. Mater. Res. B Appl. Biomater. 2023, 111, 73–84. [Google Scholar] [CrossRef]
- Li, Y.; Yang, L.; Hou, Y.; Zhang, Z.; Chen, M.; Wang, M.; Liu, J.; Wang, J.; Zhao, Z.; Xie, C.; et al. Polydopamine-mediated graphene oxide and nanohydroxyapatite-incorporated conductive scaffold with an immunomodulatory ability accelerates periodontal bone regeneration in diabetes. Bioact. Mater. 2022, 18, 213–227. [Google Scholar] [CrossRef]
- Shue, L.; Yufeng, Z.; Mony, U. Biomaterials for periodontal regeneration: A review of ceramics and polymers. Biomatter 2012, 2, 271–277. [Google Scholar] [CrossRef]
- AL Jasser, R.; AlSubaie, A.; AlShehri, F. Effectiveness of beta-tricalcium phosphate in comparison with other materials in treating periodontal infra-bony defects around natural teeth: A systematic review and meta-analysis. BMC Oral. Health 2021, 21, 219. [Google Scholar] [CrossRef]
- Tan, N.; Sabalic-Schoener, M.; Nguyen, L.; D’Aiuto, F. β-Tricalcium Phosphate-Loaded Chitosan-Based Thermosensitive Hydrogel for Periodontal Regeneration. Polymers 2023, 15, 4146. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Elsherbini, A.M.; Ibrahim, F.M.; El-Meadawy, S.M.; Youssef, J.M. Biological effect of the nanocrystalline calcium sulfate bone graft in the periodontal regeneration. J. Oral. Biol. Craniofac Res. 2021, 11, 47–52. [Google Scholar] [CrossRef]
- Motta, C.; Cavagnetto, D.; Amoroso, F.; Baldi, I.; Mussano, F. Bioactive glass for periodontal regeneration: A systematic review. BMC Oral. Health 2023, 23, 264. [Google Scholar] [CrossRef]
- Norahan, M.H.; Pedroza-González, S.C.; Sánchez-Salazar, M.G.; Álvarez, M.M.; de Santiago, G.T. Structural and biological engineering of 3D hydrogels for wound healing. Bioact. Mater. 2023, 24, 197–235. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Makkar, H.; Dal-Fabbro, R.; Malda, J.; Sriram, G.; Bottino, M.C. Biofabrication Strategies for Oral Soft Tissue Regeneration. Adv. Health Mater. 2024, 13, 2304537. [Google Scholar] [CrossRef]
- Tran, P.A. Blood clots and tissue regeneration of 3D printed dual scale porous polymeric scaffolds. Mater. Lett. 2021, 285, 129184. [Google Scholar] [CrossRef]
- Shi, H.; Zong, W.; Xu, X.; Chen, J. Improved biphasic calcium phosphate combined with periodontal ligament stem cells may serve as a promising method for periodontal regeneration. Am. J. Transl. Res. 2018, 10, 4030. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC6325501/ (accessed on 25 August 2025).
- Kim, S.; Uroz, M.; Bays, J.L.; Chen, C.S. Harnessing Mechanobiology for Tissue Engineering. Dev. Cell 2021, 56, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.; Callanan, A. Recent Methods for Modifying Mechanical Properties of Tissue-Engineered Scaffolds for Clinical Applications. Biomimetics 2023, 8, 205. [Google Scholar] [CrossRef] [PubMed]
- Osorio, R.; Alfonso-Rodríguez, C.A.; Osorio, E.; Medina-Castillo, A.L.; Alaminos, M.; Toledano-Osorio, M.; Toledano, M. Novel potential scaffold for periodontal tissue engineering. Clin. Oral. Investig. 2017, 21, 2695–2707. [Google Scholar] [CrossRef] [PubMed]
- Yi, B.; Xu, Q.; Liu, W. An overview of substrate stiffness guided cellular response and its applications in tissue regeneration. Bioact. Mater. 2022, 15, 82–102. [Google Scholar] [CrossRef]
- Liu, N.; Zhou, M.; Zhang, Q.; Yong, L.; Zhang, T.; Tian, T.; Ma, Q.; Lin, S.; Zhu, B.; Cai, X. Effect of substrate stiffness on proliferation and differentiation of periodontal ligament stem cells. Cell Prolif. 2018, 51, e12478. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, Y.; Cheng, B.; Zou, R.; Wan, W. Substrate Stiffness Regulates the Osteogenesis of PDLSCs Via ERK-Mediated YAP Nuclear Translocation. Int. Dent. J. 2025, 75, 103852. [Google Scholar] [CrossRef]
- Dallaev, R. Smart and Biodegradable Polymers in Tissue Engineering and Interventional Devices: A Brief Review. Polymers 2025, 17, 1976. [Google Scholar] [CrossRef]
- Kirillova, A.; Yeazel, T.R.; Asheghali, D.; Petersen, S.R.; Dort, S.; Gall, K.; Becker, M.L. Fabrication of Biomedical Scaffolds Using Biodegradable Polymers. Chem. Rev. 2021, 121, 11238–11304. [Google Scholar] [CrossRef]
- Wu, D.T.; Munguia-Lopez, J.G.; Cho, Y.W.; Ma, X.; Song, V.; Zhu, Z.; Tran, S.D. Polymeric Scaffolds for Dental, Oral, and Craniofacial Regenerative Medicine. Molecules 2021, 26, 7043. [Google Scholar] [CrossRef]
- Cheah, C.W.; Al-Namnam, N.M.; Lau, M.N.; Lim, G.S.; Raman, R.; Fairbairn, P.; Ngeow, W.C. Synthetic Material for Bone, Periodontal, and Dental Tissue Regeneration: Where Are We Now, and Where Are We Heading Next? Materials 2021, 14, 6123. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ren, S.; Li, L.; Zhou, Y.; Peng, W.; Xu, Y. Biodegradable engineered fiber scaffolds fabricated by electrospinning for periodontal tissue regeneration. J. Biomater. Appl. 2021, 36, 55–75. [Google Scholar] [CrossRef] [PubMed]
- Ling, K.E.; Roslan, S.M.; Taib, H.; Berahim, Z. Biodegradability of Amniotic Membrane as Potential Scaffold for Periodontal Regeneration. Cureus 2023, 15, e45394. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Dissanayaka, W.L. Current Strategies in Pulp and Periodontal Regeneration Using Biodegradable Biomaterials. Biodegrad. Mater. Their Appl. 2022, 377–427. [Google Scholar] [CrossRef]
- Khlusov, I.A.; Litvinova, L.S.; Yurova, K.A.; Khlusova, M.Y. Precise tissue bioengineering and niches of mesenchymal stem cells: Their size and hierarchy matter. BIOCELL 2022, 46, 1365–1373. [Google Scholar] [CrossRef]
- Wang, W.; Wang, A.; Hu, G.; Bian, M.; Chen, L.; Zhao, Q.; Sun, W.; Wu, Y. Potential of an Aligned Porous Hydrogel Scaffold Combined with Periodontal Ligament Stem Cells or Gingival Mesenchymal Stem Cells to Promote Tissue Regeneration in Rat Periodontal Defects. ACS Biomater. Sci. Eng. 2023, 9, 1961–1975. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, X.; Chen, Y.; Zhang, J.; Gai, K.; Chen, J.; Huo, F.; Guo, Q.; Guo, W.; Gou, M.; et al. Biomimetic Peridontium Patches for Functional Periodontal Regeneration. Adv. Health Mater. 2023, 12, 2202169. [Google Scholar] [CrossRef]
- Kim, J.I.; Kim, J.Y.; Bhattarai, G.; So, H.S.; Kook, S.H.; Lee, J.C. Periodontal Ligament-Mimetic Fibrous Scaffolds Regulate YAP-Associated Fibroblast Behaviors and Promote Regeneration of Periodontal Defect in Relation to the Scaffold Topography. ACS Appl. Mater. Interfaces 2023, 15, 599–616. [Google Scholar] [CrossRef]
- Santos, M.S.; Silva, J.C.; Carvalho, M.S. Hierarchical Biomaterial Scaffolds for Periodontal Tissue Engineering: Recent Progress and Current Challenges. Int. J. Mol. Sci. 2024, 25, 8562. [Google Scholar] [CrossRef]
- Thavornyutikarn, B.; Chantarapanich, N.; Sitthiseripratip, K.; Thouas, G.A.; Chen, Q. Bone tissue engineering scaffolding: Computer-aided scaffolding techniques. Prog. Biomater. 2014, 3, 61–102. [Google Scholar] [CrossRef]
- Mikos, A.G.; Temenoff, J.S. Formation of highly porous biodegradable scaffolds for tissue engineering. Electron. J. Biotechnol. 2000, 3, 23–24. Available online: http://www.scielo.cl/scielo.php?script=sci_arttext&pid=S0717-34582000000200003&lng=es&nrm=iso&tlng=pt (accessed on 7 September 2025). [CrossRef]
- Liang, H.; Wang, Z.; Wu, J.; Li, X.; Semirumi, D.T. Microstructural and micromechanical modeling of gum-gelatin-based soft tissue engineering scaffolds. Int. J. Biol. Macromol. 2023, 241, 124544. [Google Scholar] [CrossRef]
- Huang, S.; Yu, F.; Cheng, Y.; Li, Y.; Chen, Y.; Tang, J.; Bei, Y.; Tang, Q.; Zhao, Y.; Huang, Y.; et al. Transforming Growth Factor-β3/Recombinant Human-like Collagen/Chitosan Freeze-Dried Sponge Primed with Human Periodontal Ligament Stem Cells Promotes Bone Regeneration in Calvarial Defect Rats. Front. Pharmacol. 2021, 12, 678322. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Luo, D.; Qiao, J.; Guo, J.; He, D.; Jin, S.; Tang, L.; Wang, Y.; Shi, X.; Mao, J.; et al. A hierarchical bilayer architecture for complex tissue regeneration. Bioact. Mater. 2022, 10, 93–106. [Google Scholar] [CrossRef]
- Brown, A.; Zaky, S.; Ray, H.; Sfeir, C. Porous magnesium/PLGA composite scaffolds for enhanced bone regeneration following tooth extraction. Acta Biomater. 2015, 11, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Rowley, A.T.; Nagalla, R.R.; Wang, S.; Liu, W.F. Extracellular Matrix-Based Strategies for Immunomodulatory Biomaterials Engineering. Adv. Health Mater. 2019, 8, 1801578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, X.; Hong, H.; Hu, R.; Liu, J.; Liu, C. Decellularized extracellular matrix scaffolds: Recent trends and emerging strategies in tissue engineering. Bioact. Mater. 2022, 10, 15–31. [Google Scholar] [CrossRef]
- Son, H.; Jeon, M.; Choi, H.-J.; Lee, H.-S.; Kim, I.-H.; Kang, C.-M.; Song, J.S. Decellularized human periodontal ligament for periodontium regeneration. PLoS ONE 2019, 14, e0221236. [Google Scholar] [CrossRef]
- Farag, A.; Vaquette, C.; Theodoropoulos, C.; Hamlet, S.M.; Hutmacher, D.W.; Ivanovski, S. Decellularized periodontal ligament cell sheets with recellularization potential. J. Dent. Res. 2014, 93, 1313–1319. [Google Scholar] [CrossRef]
- Farag, A.; Hashimi, S.M.; Vaquette, C.; Bartold, P.M.; Hutmacher, D.W.; Ivanovski, S. The effect of decellularized tissue engineered constructs on periodontal regeneration. J. Clin. Periodontol. 2018, 45, 586–596. [Google Scholar] [CrossRef]
- Farag, A.; Hashimi, S.M.; Vaquette, C.; Volpato, F.Z.; Hutmacher, D.W.; Ivanovski, S. Assessment of static and perfusion methods for decellularization of PCL membrane-supported periodontal ligament cell sheet constructs. Arch. Oral. Biol. 2018, 88, 67–76. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, J.-M.; Huang, J.-P.; Lu, K.-X.; Sun, W.-L.; Tan, J.-Y.; Li, B.-X.; Chen, L.-L.; Wu, Y.-M. Regeneration potential of decellularized periodontal ligament cell sheets combined with 15-deoxy-Δ12,14-prostaglandin J2 nanoparticles in a rat periodontal defect. Biomed. Mater. 2021, 16, 045008. [Google Scholar] [CrossRef]
- Ivanov, A.A.; Danilova, T.I.; Kuznetsova, A.V.; Popova, O.P.; Yanushevich, O.O. Decellularized Matrix Induced Spontaneous Odontogenic and Osteogenic Differentiation in Periodontal Cells. Biomolecules 2023, 13, 122. [Google Scholar] [CrossRef]
- Ivanov, A.A.; Kuznetsova, A.V.; Popova, O.P.; Danilova, T.I.; Latyshev, A.V.; Yanushevich, O.O. Influence of Extracellular Matrix Components on the Differentiation of Periodontal Ligament Stem Cells in Collagen I Hydrogel. Cells 2023, 12, 2335. [Google Scholar] [CrossRef] [PubMed]
- Kaku, M.; Yamauchi, M. Mechano-regulation of collagen biosynthesis in periodontal ligament. J. Prosthodont. Res. 2014, 58, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Zhang, Q.Y.; Huang, L.P.; Huang, K.; Xie, H.Q. Decellularized scaffold and its elicited immune response towards the host: The underlying mechanism and means of immunomodulatory modification. Biomater. Sci. 2021, 9, 4803–4820. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef]
- Gomes, K.T.; Prasad, P.R.; Sandhu, J.S.; Kumar, A.; Kumar, N.A.N.; Shridhar, N.B.; Bisht, B.; Paul, M.K. Decellularization techniques: Unveiling the blueprint for tracheal tissue engineering. Front. Bioeng. Biotechnol. 2025, 13, 1518905. [Google Scholar] [CrossRef] [PubMed]
- Biehl, A.; Martins, A.M.G.; Davis, Z.G.; Sze, D.; Collins, L.; Mora-Navarro, C.; Fisher, M.B.; Freytes, D.O. Towards a standardized multi-tissue decellularization protocol for the derivation of extracellular matrix materials. Biomater. Sci. 2023, 11, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Liao, L.; Tian, W. Advances Focusing on the Application of Decellularized Extracellular Matrix in Periodontal Regeneration. Biomolecules 2023, 13, 673. [Google Scholar] [CrossRef]
- Rahim, T.N.A.T.; Abdullah, A.M.; Akil, H.M. Recent Developments in Fused Deposition Modeling-Based 3D Printing of Polymers and Their Composites. Polym. Rev. 2019, 59, 589–624. [Google Scholar] [CrossRef]
- Jiao, Z.; Luo, B.; Xiang, S.; Ma, H.; Yu, Y.; Yang, W. 3D printing of HA/PCL composite tissue engineering scaffolds. Adv. Ind. Eng. Polym. Res. 2019, 2, 196–202. [Google Scholar] [CrossRef]
- Wang, S.; Ma, Y.; Deng, Z.; Zhang, S.; Cai, J. Effects of fused deposition modeling process parameters on tensile, dynamic mechanical properties of 3D printed polylactic acid materials. Polym. Test. 2020, 86, 106483. [Google Scholar] [CrossRef]
- Li, W.; Wang, M.; Ma, H.; Chapa-Villarreal, F.A.; Lobo, A.O.; Zhang, Y.S. Zhang. Stereolithography apparatus and digital light processing-based 3D bioprinting for tissue fabrication. iScience 2023, 26, 106039. [Google Scholar] [CrossRef]
- O’Halloran, S.; Pandit, A.; Heise, A.; Kellett, A. Two-Photon Polymerization: Fundamentals, Materials, and Chemical Modification Strategies. Adv. Sci. 2023, 10, 2204072. [Google Scholar] [CrossRef]
- Fang, Y.; Guo, Y.; Liu, T.; Xu, R.; Mao, S.; Mo, X.; Zhang, T.; Ouyang, L.; Xiong, Z.; Sun, W. Advances in 3D Bioprinting. Chin. J. Mech. Eng. Addit. Manuf. Front. 2022, 1, 100011. [Google Scholar] [CrossRef]
- Ramesh, S.; Harrysson, O.L.; Rao, P.K.; Tamayol, A.; Cormier, D.R.; Zhang, Y.; Rivero, I.V. Extrusion bioprinting: Recent progress, challenges, and future opportunities. Bioprinting 2021, 21, e00116. [Google Scholar] [CrossRef]
- Gupta, D.; Derman, I.D.; Xu, C.; Huang, Y.; Ozbolat, L.T. Droplet-based bioprinting. Nat. Rev. Methods Primers 2025, 5, 25. [Google Scholar] [CrossRef]
- Miao, G.; Liang, L.; Li, W.; Ma, C.; Pan, Y.; Zhao, H.; Zhang, Q.; Xiao, Y.; Yang, X. 3D Bioprinting of a Bioactive Composite Scaffold for Cell Delivery in Periodontal Tissue Regeneration. Biomolecules 2023, 13, 1062. [Google Scholar] [CrossRef]
- Liu, P.; Li, Q.; Yang, Q.; Zhang, S.; Lin, C.; Zhang, G.; Tang, Z. Three-dimensional cell printing of gingival fibroblast/acellular dermal matrix/gelatin–sodium alginate scaffolds and their biocompatibility evaluation in vitro. RSC Adv. 2020, 10, 15926–15935. [Google Scholar] [CrossRef]
- Liu, P.; Li, Q.; Yang, Q.; Zhang, S.; Yi, K.; Zhang, G.; Tang, Z. Evaluation of the effect of 3D-bioprinted gingival fibroblast-encapsulated ADM scaffolds on keratinized gingival augmentation. J. Periodontal Res. 2023, 58, 564–574. [Google Scholar] [CrossRef]
- Prendergast, M.E.; Davidson, M.D.; Burdick, J.A. A biofabrication method to align cells within bioprinted photocrosslinkable and cell-degradable hydrogel constructs via embedded fibers. Biofabrication 2021, 13, 044108. [Google Scholar] [CrossRef]
- Yang, X.; Ma, Y.; Wang, X.; Yuan, S.; Huo, F.; Yi, G.; Zhang, J.; Yang, B.; Tian, W. A 3D-Bioprinted Functional Module Based on Decellularized Extracellular Matrix Bioink for Periodontal Regeneration. Adv. Sci. 2023, 10, 2205041. [Google Scholar] [CrossRef] [PubMed]
- Pilipchuk, S.P.; Monje, A.; Jiao, Y.; Hao, J.; Kruger, L.; Flanagan, C.L.; Hollister, S.J.; Giannobile, W.V. Integration of 3D Printed and Micropatterned Polycaprolactone Scaffolds for Guidance of Oriented Collagenous Tissue Formation In Vivo. Adv. Health Mater. 2016, 5, 676–687. [Google Scholar] [CrossRef]
- Kim, M.G.; Park, C.H. The Topographical Optimization of 3D Microgroove Pattern Intervals for Ligamentous Cell Orientations: In Vitro. Int. J. Mol. Sci. 2020, 21, 9358. [Google Scholar] [CrossRef] [PubMed]
- Soufivand, A.A.; Abolfathi, N.; Hashemi, S.A.; Lee, S.J. Prediction of mechanical behavior of 3D bioprinted tissue-engineered scaffolds using finite element method (FEM) analysis. Addit. Manuf. 2020, 33, 101181. [Google Scholar] [CrossRef]
- Murenu, N.; Faber, J.; Soufivand, A.A.; Buss, M.; Schaefer, N.; Budday, S. Cell Behavior and Complex Mechanical Properties of 3D Printed Cell-Laden Alginate-Gelatin Macroporous Mesostructures. Macromol. Biosci. 2025, e00204. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, T.D.M.; Do Amaral, G.C.L.S.; Bezerra, G.N.; Nakao, L.Y.S.; Villar, C.C. Three-dimensional-printed scaffolds for periodontal regeneration: A systematic review. J. Indian. Soc. Periodontol. 2023, 27, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Martino, F.; Perestrelo, A.R.; Vinarský, V.; Pagliari, S.; Forte, G. Cellular mechanotransduction: From tension to function. Front. Physiol. 2018, 9, 378185. [Google Scholar] [CrossRef]
- Bakhshandeh, B.; Sorboni, S.G.; Ranjbar, N.; Deyhimfar, R.; Abtahi, M.S.; Izady, M.; Kazemi, N.; Noori, A.; Pennisi, C.P. Mechanotransduction in tissue engineering: Insights into the interaction of stem cells with biomechanical cues. Exp. Cell Res. 2023, 431, 113766. [Google Scholar] [CrossRef]
- Mierke, C.T. Viscoelasticity, Like Forces, Plays a Role in Mechanotransduction. Front. Cell. Dev. Biol. 2022, 10, 789841. [Google Scholar] [CrossRef]
- Brusatin, G.; Panciera, T.; Gandin, A.; Citron, A.; Piccolo, S. Biomaterials and engineered microenvironments to control YAP/TAZ-dependent cell behaviour. Nat. Mater. 2018, 17, 1063–1075. [Google Scholar] [CrossRef]
- Scott, K.E.; Fraley, S.I.; Rangamani, P. A spatial model of YAP/TAZ signaling reveals how stiffness, dimensionality, and shape contribute to emergent outcomes. Proc. Natl. Acad. Sci. USA 2021, 118, e2021571118. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Spill, F.; Zaman, M.H. A Computational Model of YAP/TAZ Mechanosensing. Biophys. J. 2016, 110, 2540–2550. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Mills, D.K.; Urbanska, A.M.; Saeb, M.R.; Venugopal, J.R.; Ramakrishna, S.; Mozafari, M. Electrospinning for tissue engineering applications. Prog. Mater. Sci. 2021, 117, 100721. [Google Scholar] [CrossRef]
- Vaquette, C.; Cooper-White, J.J. Increasing electrospun scaffold pore size with tailored collectors for improved cell penetration. Acta Biomater. 2011, 7, 2544–2557. [Google Scholar] [CrossRef]
- Nitti, P.; Palazzo, B.; Gallo, N.; Scalera, F.; Sannino, A.; Gervaso, F. Smooth-rough asymmetric PLGA structure made of dip coating membrane and electrospun nanofibrous scaffolds meant to be used for guided tissue regeneration of periodontium. Polym. Eng. Sci. 2022, 62, 2061–2069. [Google Scholar] [CrossRef]
- Ivanovski, S.; Vaquette, C.; Gronthos, S.; Hutmacher, D.W.; Bartold, P.M. Multiphasic Scaffolds for Periodontal Tissue Engineering. J. Dent. Res. 2014, 93, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.F.; Vaquette, C.; Zhang, Q.; Reis, R.L.; Ivanovski, S.; Hutmacher, D.W. Advanced tissue engineering scaffold design for regeneration of the complex hierarchical periodontal structure. J. Clin. Periodontol. 2014, 41, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Vaquette, C.; Fan, W.; Xiao, Y.; Hamlet, S.; Hutmacher, D.W.; Ivanovski, S. A biphasic scaffold design combined with cell sheet technology for simultaneous regeneration of alveolar bone/periodontal ligament complex. Biomaterials 2012, 33, 5560–5573. [Google Scholar] [CrossRef]
- Santos, M.S.; Cordeiro, R.; Moura, C.S.; Cabral, J.M.S.; Ferreira, F.C.; Silva, J.C.; Carvalho, M.S. Bioactive Nanofibrous Scaffolds Incorporating Decellularized Cell-Derived Extracellular Matrix for Periodontal Tissue Engineering. ACS Appl. Nano Mater. 2024, 7, 4501–4517. [Google Scholar] [CrossRef]
- Yuan, H.; Zhou, Q.; Zhang, Y. Improving fiber alignment during electrospinning. Electrospun Nanofibers 2017, 125–147. [Google Scholar] [CrossRef]
- Staples, R.; Ivanovski, S.; Vaquette, C. Fibre-guiding biphasic scaffold for perpendicular periodontal ligament attachment. Acta Biomater. 2022, 150, 221–237. [Google Scholar] [CrossRef]
- Liang, C.; Wang, G.; Liang, C.; Li, M.; Sun, Y.; Tian, W.; Liao, L. Hierarchically patterned triple-layered gelatin-based electrospun membrane functionalized by cell-specific extracellular matrix for periodontal regeneration. Dent. Mater. 2024, 40, 90–101. [Google Scholar] [CrossRef]
- Erencia, M.; Cano, F.; Tornero, J.A.; Fernandes, M.M.; Tzanov, T.; Macanás, J.; Carrillo, F. Electrospinning of gelatin fibers using solutions with low acetic acid concentration: Effect of solvent composition on both diameter of electrospun fibers and cytotoxicity. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Khorshidi, S.; Solouk, A.; Mirzadeh, H.; Mazinani, S.; Lagaron, J.M.; Sharifi, S.; Ramakrishna, S. A review of key challenges of electrospun scaffolds for tissue-engineering applications. J. Tissue Eng. Regen. Med. 2016, 10, 715–738. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Shanbhag, S.; Mustafa, K. Scaffolds in Periodontal Regenerative Treatment. Dent. Clin. N. Am. 2022, 66, 111–130. [Google Scholar] [CrossRef]
- Sun, C.; Rankovic, M.J.; Folwaczny, M.; Otto, S.; Wichelhaus, A.; Baumert, U. Effect of Tension on Human Periodontal Ligament Cells: Systematic Review and Network Analysis. Front. Bioeng. Biotechnol. 2021, 9, 695053. [Google Scholar] [CrossRef]
- Hirashima, S.; Ohta, K.; Kanazawa, T.; Togo, A.; Kakuma, T.; Kusukawa, J.; Nakamura, K.-I. Three-dimensional ultrastructural and histomorphological analysis of the periodontal ligament with occlusal hypofunction via focused ion beam/scanning electron microscope tomography. Sci. Rep. 2019, 9, 9520. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, C.; Li, J.; Han, J.; Liu, X.; Yang, H. The physical microenvironment of hematopoietic stem cells and its emerging roles in engineering applications. Stem Cell Res. Ther. 2019, 10, 327. [Google Scholar] [CrossRef]
- Du, Y.; Zheng, J.; Xu, B.; Peng, C.; Yang, K. Piezo1 participates in the tension-driven osteogenic differentiation of periodontal ligament stem cells. BMC Oral Health 2025, 25, 1155. [Google Scholar] [CrossRef]
- Pandya, M.; Gopinathan, G.; Tillberg, C.; Wang, J.; Luan, X.; Diekwisch, T.G.H. The Hippo Pathway Effectors YAP/TAZ Are Essential for Mineralized Tissue Homeostasis in the Alveolar Bone/Periodontal Complex. J. Dev. Biol. 2022, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Dieterle, M.P.; Husari, A.; Steinberg, T.; Wang, X.; Ramminger, I.; Tomakidi, P. From the Matrix to the Nucleus and Back: Mechanobiology in the Light of Health, Pathologies, and Regeneration of Oral Periodontal Tissues. Biomolecules 2021, 11, 824. [Google Scholar] [CrossRef]
- Isaeva, E.V.; Beketov, E.E.; Arguchinskaya, N.V.; Ivanov, S.A.; Shegay, P.V.; Kaprin, A.D. Decellularized Extracellular Matrix for Tissue Engineering (Review). Sovrem. Tehnol. vMed. 2022, 14, 57–69. [Google Scholar] [CrossRef]
- Sauter, J.; Degenhardt, H.; Tuebel, J.; Foehr, P.; Knoeckel, P.; Florian, K.; Charitou, F.; Burgkart, R.; Schmitt, A. Effect of different decellularization protocols on reendothelialization with human cells for a perfused renal bioscaffold of the rat. BMC Biotechnol. 2023, 23, 8. [Google Scholar] [CrossRef] [PubMed]
- Neishabouri, A.; Khaboushan, A.S.; Daghigh, F.; Kajbafzadeh, A.-M.; Zolbin, M.M. Decellularization in Tissue Engineering and Regenerative Medicine: Evaluation, Modification, and Application Methods. Front. Bioeng. Biotechnol. 2022, 10, 805299. [Google Scholar] [CrossRef]
- Garrison, C.M.; Schwarzbauer, J.E. Fibronectin fibril alignment is established upon initiation of extracellular matrix assembly. Mol. Biol. Cell 2021, 32, 739–752. [Google Scholar] [CrossRef]
- Punetha, J.; Kesari, A.; Hoffman, E.P.; Gos, M.; Kamińska, A.; Kostera-Pruszczyk, A.; Hausmanowa-Petrusewicz, I.; Hu, Y.; Zou, Y.; Bönnemann, C.G.; et al. Novel Col12A1 variant expands the clinical picture of congenital myopathies with extracellular matrix defects. Muscle Nerve 2017, 55, 277–281. [Google Scholar] [CrossRef]
- Brockhaus, J.; Craveiro, R.B.; Azraq, I.; Niederau, C.; Schröder, S.K.; Weiskirchen, R.; Jankowski, J.; Wolf, M. In vitro compression model for orthodontic tooth movement modulates human periodontal ligament fibroblast proliferation, apoptosis and cell cycle. Biomolecules 2021, 11, 932. [Google Scholar] [CrossRef]
- Lin, H.-H.; Chao, P.-H.G.; Tai, W.-C.; Chang, P.-C. 3D-Printed Collagen-Based Waveform Microfibrous Scaffold for Periodontal Ligament Reconstruction. Int. J. Mol. Sci. 2021, 22, 7725. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, B.K.; Chang, M.L.; Wan, Z.Q.; Han, G.L. Cyclic Stretch Enhances Osteogenic Differentiation of Human Periodontal Ligament Cells via YAP Activation. BioMed Res. Int. 2018, 2018, 2174824. [Google Scholar] [CrossRef] [PubMed]
- Long, P.; Hu, J.; Piesco, N.; Buckley, M.; Agarwal, S. Low magnitude of tensile strain inhibits IL-1 β-dependent induction of pro-inflammatory cytokines and induces synthesis of IL-10 in human periodontal ligament cells in vitro. J. Dent. Res. 2001, 80, 1416–1420. [Google Scholar] [CrossRef] [PubMed]
- Roth, C.E.; Craveiro, R.B.; Niederau, C.; Malyaran, H.; Neuss, S.; Jankowski, J.; Wolf, M. Mechanical Compression by Simulating Orthodontic Tooth Movement in an In Vitro Model Modulates Phosphorylation of AKT and MAPKs via TLR4 in Human Periodontal Ligament Cells. Int. J. Mol. Sci. 2022, 23, 8062. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, R.; Attik, N.; Chevalier, C.; Salles, V.; Grosgogeat, B.; Gritsch, K.; Trunfio-Sfarghiu, A.-M. 3D Electrospun Polycaprolactone Scaffolds to Assess Human Periodontal Ligament Cells Mechanobiological Behaviour. Biomimetics 2023, 8, 108. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Cheng, T.; Zhu, S.; Gu, M.; Jin, L.; Yang, Y. mRNA and long non-coding RNA expression profiling of human periodontal ligament cells under tension loading. Eur. J. Orthod. 2021, 43, 698–707. [Google Scholar] [CrossRef]
- Han, X.; Wang, L.Y.; Diao, Z.L.; Liu, W.H. Apelin: A novel inhibitor of vascular calcification in chronic kidney disease. Atherosclerosis 2016, 244, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhan, Q.; Bao, M.; Yi, J.; Li, Y. Biomechanical and biological responses of periodontium in orthodontic tooth movement: Up-date in a new decade. Int. J. Oral. Sci. 2021, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Zhang, Y.; Yang, T.; Qi, J.; Zhang, L.; Deng, L. HIF-1α disturbs osteoblasts and osteoclasts coupling in bone remodeling by up-regulating OPG expression. In Vitr. Cell Dev. Biol. Anim. 2015, 51, 808–814. [Google Scholar] [CrossRef]
- Meng, X.; Wielockx, B.; Rauner, M.; Bozec, A. Hypoxia-Inducible Factors Regulate Osteoclasts in Health and Disease. Front. Cell Dev. Biol. 2021, 9, 658893. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, J.; Liu, P.; Wang, Q.; Liu, L.; Zhao, H. The RANK/RANKL/OPG system and tumor bone metastasis: Potential mechanisms and therapeutic strategies. Front. Endocrinol. 2022, 13, 1063815. [Google Scholar] [CrossRef]
- Komori, T. Regulation of Skeletal Development and Maintenance by Runx2 and Sp7. Int. J. Mol. Sci. 2024, 25, 10102. [Google Scholar] [CrossRef]
- Chang, M.; Lin, H.; Luo, M.; Wang, J.; Han, G. Integrated miRNA and mRNA expression profiling of tension force-induced bone formation in periodontal ligament cells. Vitr. Cell Dev. Biol. Anim. 2015, 51, 797–807. [Google Scholar] [CrossRef]
- Janjić, K.; Nemec, M.; Maaser, J.L.; Sagl, B.; Jonke, E.; Andrukhov, O. Differential gene expression and protein-protein interaction networks of human periodontal ligament stromal cells under mechanical tension. Eur. J. Cell Biol. 2023, 102, 151319. [Google Scholar] [CrossRef]
- Terasawa, M.; Shimokawa, R.; Terashima, T.; Ohya, K.; Takagi, Y.; Shimokawa, H. Expression of dentin matrix protein 1 (DMP1) in nonmineralized tissues. J. Bone Min. Metab. 2004, 22, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Phothichailert, S.; Kornsuthisopon, C.; Chansaenroj, A.; Trachoo, V.; Nowwarote, N.; Fournier, B.; Namangkalakul, W.; Osathanon, T. Decellularised matrices from force loaded periodontal ligament stem cells support osteogenic differentiation. Sci. Rep. 2025, 15, 28387. [Google Scholar] [CrossRef] [PubMed]
- Görlitz, S. The Role of Mechanical Forces in Osteogenic Differentiation, BMP Signaling and Early Tissue Formation Processes in the Context of Bone Healing. Doctoral Dissertation, Technische Universitaet Berlin, Berlin, Germany, 2021. [Google Scholar] [CrossRef]
- Svitkina, T. The actin cytoskeleton and actin-based motility. old Spring Harb. Perspect. Biol. 2018, 10, a018267. [Google Scholar] [CrossRef]
- Jiang, Y.; Guan, Y.; Lan, Y.; Chen, S.; Li, T.; Zou, S.; Hu, Z.; Ye, Q. Mechanosensitive Piezo1 in Periodontal Ligament Cells Promotes Alveolar Bone Remodeling During Orthodontic Tooth Movement. Front. Physiol. 2021, 12, 767136. [Google Scholar] [CrossRef]
- Shi, Q.; Zheng, L.; Na, J.; Li, X.; Yang, Z.; Chen, X.; Song, Y.; Li, C.; Zhou, L.; Fan, Y. Fluid shear stress promotes periodontal ligament cells proliferation via p38-AMOT-YAP. Cell. Mol. Life Sci. 2022, 79, 551. [Google Scholar] [CrossRef]
- Suwittayarak, R.; Klincumhom, N.; Ngaokrajang, U.; Namangkalakul, W.; Ferreira, J.N.; Pavasant, P.; Osathanon, T. Shear Stress Enhances the Paracrine-Mediated Immunoregulatory Function of Human Periodontal Ligament Stem Cells via the ERK Signalling Pathway. Int. J. Mol. Sci. 2022, 23, 7119. [Google Scholar] [CrossRef]
- Du, J.; Li, M. Functions of Periostin in dental tissues and its role in periodontal tissues’ regeneration. Cell. Mol. Life Sci. 2017, 74, 4279–4286. [Google Scholar] [CrossRef] [PubMed]
- Norris, R.A.; Damon, B.; Mironov, V.; Kasyanov, V.; Ramamurthi, A.; Moreno-Rodriguez, R.; Trusk, T.; Potts, J.D.; Goodwin, R.L.; Davis, J.; et al. Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J. Cell Biochem. 2007, 101, 695–711. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.C.; Moroishi, T.; Meng, Z.; Jeong, H.-S.; Plouffe, S.W.; Sekido, Y.; Han, J.; Park, H.W.; Guan, K.-L. Regulation of Hippo pathway transcription factor TEAD by p38 MAPK-induced cytoplasmic translocation. Nat. Cell Biol. 2017, 19, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Pallotta, M.T.; Rossini, S.; Suvieri, C.; Coletti, A.; Orabona, C.; Macchiarulo, A.; Volpi, C.; Grohmann, U. Indoleamine 2,3-dioxygenase 1 (IDO1): An up-to-date overview of an eclectic immunoregulatory enzyme. FEBS J. 2022, 289, 6099–6118. [Google Scholar] [CrossRef]
- Nile, M.; Folwaczny, M.; Kessler, A.; Wichelhaus, A.; Rankovic, M.J.; Baumert, U. Development of a Custom Fluid Flow Chamber for Investigating the Effects of Shear Stress on Periodontal Ligament Cells. Cells 2024, 13, 1751. [Google Scholar] [CrossRef]
- McCabe, L.R.; Banerjee, C.; Kundu, R.; Harrison, R.J.; Dobner, P.R.; Stein, J.L.; Lian, J.B.; Stein, G.S. Developmental expression and activities of specific fos and jun proteins are functionally related to osteoblast maturation: Role of Fra-2 and Jun D during differentiation. Endocrinology 1996, 137, 4398–4408. [Google Scholar] [CrossRef]
- Liu, L.; Shao, L.; Li, B.; Zong, C.; Li, J.; Zheng, Q.; Tong, X.; Gao, C.; Wang, J. Extracellular signal-regulated kinase1/2 activated by fluid shear stress promotes osteogenic differentiation of human bone marrow-derived mesenchymal stem cells through novel signaling pathways. Int. J. Biochem. Cell Biol. 2011, 43, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Stańczak, M.; Kacprzak, B.; Gawda, P. Tendon Cell Biology: Effect of Mechanical Loading. Cell. Physiol. Biochem. 2024, 58, 677–701. [Google Scholar] [CrossRef] [PubMed]
- Fitridge, R.; Thompson, M. Mechanisms of vascular disease: A reference book for vascular specialists. In Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists; University of Adelaide Press: Adelaide, Australia, 2011; pp. 1–553. [Google Scholar] [CrossRef]
- Martínez-García, M.; Hernández-Lemus, E. Periodontal Inflammation and Systemic Diseases: An Overview. Front. Physiol. 2021, 12, 709438. [Google Scholar] [CrossRef]
- Chukkapalli, S.S.; Lele, T.P. Periodontal cell mechanotransduction. Open Biol. 2018, 8, 180053. [Google Scholar] [CrossRef]
- Liu, M.; Dai, J.; Lin, Y.; Yang, L.; Dong, H.; Li, Y.; Ding, Y.; Duan, Y. Effect of the cyclic stretch on the expression of osteogenesis genes in human periodontal ligament cells. Gene 2012, 491, 187–193. [Google Scholar] [CrossRef]
- Li, Z.; Yu, M.; Jin, S.; Wang, Y.; Luo, R.; Huo, B.; Liu, D.; He, D.; Zhou, Y.; Liu, Y. Stress Distribution and Collagen Remodeling of Periodontal Ligament During Orthodontic Tooth Movement. Front. Pharmacol. 2019, 10, 1263. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhao, D.; Wu, Y.; Xu, C.; Zhang, F. Cyclic stretch induced gene expression of extracellular matrix and adhesion molecules in human periodontal ligament cells. Arch. Oral. Biol. 2015, 60, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Liu, X.; Chen, C.; Luo, T.; Yan, Z.; Wu, L.; An, Z. Roles for Exosomes from Various Cellular Sources in Spinal Cord Injury. Mol. Neurobiol. 2025, 62, 14660–14682. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, L.; Kikuiri, T.; Akiyama, K.; Chen, C.; Xu, X.; Yang, R.; Chen, W.; Wang, S.; Shi, S. Mesenchymal stem cell-based tissue regeneration is governed by recipient T lymphocytes via IFN-γ and TNF-α. Nat. Med. 2011, 17, 1594–1601. [Google Scholar] [CrossRef]
- Liu, D.; Xu, J.; Liu, O.; Fan, Z.; Liu, Y.; Wang, F.; Ding, G.; Wei, F.; Zhang, C.; Wang, S. Mesenchymal stem cells derived from inflamed periodontal ligaments exhibit impaired immunomodulation. J. Clin. Periodontol. 2012, 39, 1174–1182. [Google Scholar] [CrossRef]
- Zhou, T.; Zheng, K.; Sui, B.; Boccaccini, A.R.; Sun, J. In vitro evaluation of poly (vinyl alcohol)/collagen blended hydrogels for regulating human periodontal ligament fibroblasts and gingival fibroblasts. Int. J. Biol. Macromol. 2020, 163, 1938–1946. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, L.; Ngai, T. Multiphasic Membranes/Scaffolds for Periodontal Guided Tissue Regeneration. Macromol. Mater. Eng. 2023, 308, 2300081. [Google Scholar] [CrossRef]
- Post, J.N.; Loerakker, S.; Merks, R.M.H.; Carlier, A. Implementing Computational Modeling in Tissue Engineering: Where Disciplines Meet. Tissue Eng. Part. A 2022, 28, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Bagherpour, R.; Bagherpour, G.; Mohammadi, P. Application of Artificial Intelligence in Tissue Engineering. Tissue Eng. Part. B Rev. 2025, 31, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.L.; Januszyk, M.; Longaker, M.T. Machine Learning in Tissue Engineering. Tissue Eng. Part. A 2023, 29, 2–19. [Google Scholar] [CrossRef]
- Natali, A.N.; Pavan, P.G.; Scarpa, C. Numerical analysis of tooth mobility: Formulation of a non-linear constitutive law for the periodontal ligament. Dent. Mater. 2004, 20, 623–629. [Google Scholar] [CrossRef]
- Hu, W.W.; Chen, Y.C.; Tsao, C.W.; Chen, S.L.; Tzeng, C.Y. Development of a multifunctional bioreactor to evaluate the promotion effects of cyclic stretching and electrical stimulation on muscle differentiation. Bioeng. Transl. Med. 2023, 9, e10633. [Google Scholar] [CrossRef]
- Zhao, J.; Meng, F.; Qian, J.; Huang, Y.; Fan, Y. In vitro cell stretching devices and their applications: From cardiomyogenic differentiation to tissue engineering. Med. Nov. Technol. Devices 2023, 18, 100220. [Google Scholar] [CrossRef]
- Riehl, B.D.; Park, J.H.; Kwon, I.K.; Lim, J.Y. Mechanical stretching for tissue engineering: Two-dimensional and three-dimensional constructs. Tissue Eng. Part. B Rev. 2012, 18, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.; Leroux, A.C.; Zang, H.; Pollard, D.; Zehe, C.; Akbari, S. Cell-based shear stress sensor for bioprocessing. J. Biotechnol. 2024, 390, 71–79. [Google Scholar] [CrossRef]
- Piwocka, O.; Musielak, M.; Ampuła, K.; Piotrowski, I.; Adamczyk, B.; Fundowicz, M.; Suchorska, W.M.; Malicki, J. Navigating challenges: Optimising methods for primary cell culture isolation. Cancer Cell Int. 2024, 24, 28. [Google Scholar] [CrossRef] [PubMed]
| Cell Type | Delivery Method | Animal | Animal Model | Results | Sample Size and Age | Ref. |
|---|---|---|---|---|---|---|
| BMMSCs | Cell suspension | Rat | Periodontitis caused by binding wire around the bilateral maxillary first molars and subsequently inoculating with Porphyromonas gingivalis. | Periodontal tissue regeneration (histology and X-rays). Inhibition of inflammatory mediators’ interleukin 1β (IL-1β), interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α). | 5 animals per group | [87] |
| hDPSCs | Cell sheets and cell suspension | Miniature pig | Periodontitis caused by surgical AB defect | Twelve weeks after transplantation. Both cell sheets and suspension caused bone repair (histology, CT, formation of cementum-like layer, b-globin expression). hDPSCs sheets demonstrated a greater ability for bone repair than hDPSCs suspension injection. | 6 animals per group 12 months old | [43] |
| hDPSCs | Cells on Bio-Oss® scaffold (Geistlich Pharma, Switzerland) | Dog | Periodontitis model caused by incision from the mandibular canine to the mid-buccal of the first premolar. | After 4 weeks, treatment with cells stimulated new cementum and PDL formation compared to the control group. No significant differences in amount of bone formation. | 6 animals per group | [44] |
| hPDLSCs | Cell suspension | Mice | Periodontitis caused by removal of AB. Defect size 3.5 × 2 × 1.5 mm. | Analysis 2 or 4 weeks after cells injection. Cells increase the values of width of new bone, new bone area fraction and cementum-like tissue was observed. PDLSCs increase the abundance of non-pathogenic bacteria and inhibit growth of pathogenic bacteria. | - | [55] |
| hPDLSCs | hPDLSCs on decellularized amniotic membrane | Rat | Periodontitis model, caused by removing of the buccal bone, PDL, cementum, and dentin from the mesial root of the mandibular first molar to the second molar. Defect size 2 × 3 mm. | hPDLSCs on decellularized amniotic membrane significantly enhances formation of cementum, periodontal ligament, and bone (micro-CT and histological analysis). | 22 7–8 weeks old | [56] |
| Rat PDLSCs and osteoblast-like cells (MC3T3-E1 cells) | Composite cell sheets | Mice | Periodontal tissue injury model caused by removing the PDL fibers and bone near the palatal surface of maxillary first molar tooth. Treatment after 8 weeks. | Complex cell sheet from PDLSCs and osteoblast-like cells promote the regeneration of PDL-like fibers and AB periodontal ligament structure in ectopic and orthotopic transplantation (azan staining for PDL-like fibers, IHC analysis for expression of periostin and OCN, micro-CT) | - 4–5 weeks old | [57] |
| hPDLSCs, human AB stem cells (hABSCs) | A calcium phosphate-coated melt electrospinning polycaprolactone (CaP-PCL) scaffold with cell sheets | Rat | Periodontitis model caused by removing AB and cementum covering the roots of the mandibular first molar, | Cell sheets from hPDLSCs and hABSCs stimulate periodontal attachment formation. At 4 weeks, mineralized tissue (micro-CT), new bone, cementum, and PDL were formed (IHC for bone sialoprotein and OPN, histology). | 3 animals per group 12 weeks old | [66] |
| hABSCs | Rat | |||||
| Human gingival margin-derived cells (GMCs) | Rat | GMCs did not induce periodontal regeneration. | ||||
| PDLSCs | Biphasic electrospinning scaffold with autologous cell sheet | Sheep | Surgically created periodontal defects. Treatment with cell sheet on first day and healing analysis for 5 and 10 weeks | PDLSCs sheet therapy significantly increased BV/TV compared to control scaffold after 10 weeks of therapy. | 5 animals per group | [67] |
| GSCs | Sheep | GC sheet therapy had no positive effect on periodontal tissues and even decreased BV/TV, cementum formation, and ligament fibers attachment compared to scaffold without cells. | ||||
| GSCs | Fibroin/chitosan oligosaccharide lactate hydrogel | Rat | Periodontitis caused by cotton ligatures were bilaterally ligatured at the subgingival portion of the maxillary second molars for 14 days for desired level of periodontal destruction. | Treatment with cells in hydrogel after 14 days of injury. Decreased alveolar bone loss (ABL) revealed by micro-CT after 2 and 8 weeks of treatment. Less inflammation, tissue destruction 2 weeks after cell treatment. Reduction in apical migration of epithelium after 8 weeks revealed by Masson staining. Hydrogel itself also reduced inflammation and destruction, but did not cause reduction in epithelium apical migration. | 10 animals in each group - | [63] |
| STRO-1 positive GSCs | Collagen scaffold and deproteinized bovine cancellous bone | Miniature pig | Periodontal defects in the premolar/molar area. | Cell-loaded scaffolds led to higher CAL, PD, GR and radiographic defect volume between baseline and 12 weeks, and lower junctional epithelium length and connective tissue adhesion after 12 weeks. | 8 animals 4 weeks old | [64] |
| GMCs | IL-1ra+ or IL-1ra- releasing HA-ECM | Miniature pig | Periodontal defects were induced in the premolar/molar area. | Treatment after 4 weeks post-periodontal defects formation with GMCs in HA with or without IL-1re significantly improved PD, CAL, and GR. | [64] | |
| GMSCs | Cell sheets | Dog | Class III furcation defects (5 mm from the furcation fornix to the bottom of the defect) with addition of anaerobic bacteria into the defect. | Treatment enhanced new bone and cementum formation (p < 0.01). In the GMSCs group, newly formed Sharpey’s fibers anchored into the newly regenerated cementum and were arranged perpendicularly to the root surface in contrast to parallel newly formed fibers in the control group. | 4 animals and 4 teeth from each | [65] |
| BM-MSCs | Biphasic electrospinning scaffold with autologous cell sheet | Sheep | Surgically created defect 6 mm × 5 mm size. | BM-MSCs sheet increase BV/TV compared to control scaffold after 10 weeks of therapy, but have no effect on cementum formation and ligament fibers attachment compared to scaffold without cells | 5 animals per time point 4 defects for each animal | [67] |
| BM-MSCs | Cell suspension | Rat | Surgical periodontal fenestration defects (2 × 4 × 1 mm), analysis 15 and 30 days after treatment. | BM-MSCs caused higher bone and cementum formation, and OCN, OPN and OPG synthesis compared to control. | 6 animals per group | [72] |
| Acetylsalicylic acid (ASA) treated BM-MSCs | Cell suspension | Rat | Periodontitis, caused by cotton ligatures with Porphyromonas gingivalis. | ASA-BM-MSCs treatment reduced inflammatory infiltration and AB loss, proved by IHC staining of OPG/RANK-L and micro-CT after 3 weeks. | 6 animals per group 6 weeks old | [74] |
| AT-MSCs | Fibrin gel | Miniature pig | Periodontitis caused by furcation defects in mandibular premolars. | AT-MSCs caused new AB formation (micro-CT), collagen fiber formation (histology + hematoxylin and eosin (HE), Masson’s trichrome (MT) staining) and decreased neutrophil infiltration in periodontal defects. | 8 animals 24–30 months | [76] |
| ADSCs AT-MSCs | b-TCP and a human cancellous freeze-dried graft (HCG) | Rat | Alveolar defect, 5 mm. Dissection of muscles and periosteum followed by maxillary bone and zygoma dissection. | AT-MSCs promoted the regenerative effect of b-TCP and human HCG for AB reconstruction. Eight weeks after surgery application of AT-MSCs with b-TCP and HCG, osteogenic genes expression (RUNX2, Osterix, alkaline phosphatase (ALP), and BMP-2) were stimulated. Micro-CT revealed BV and TT compared to autologous bone graft. | 6 animals per group | [88] |
| ADSCs AT-MSCs | Cell suspension | Rat | Ligature-induced periodontitis. After 2 weeks, the ligature was removed, which caused gingival inflammation, ulceration, and creation of pocket. | The histological and scanning electron microscopy results revealed restoration of the AB level around mandibular first molar. | 12 animals per group 6 months old | [10] |
| ADSCs AT-MSCs | Cell suspension | Mice | Bacteria (Porphyromonas gingivalis, Fusobacterium nucleatum, and Prevotella intermedia)-induced periodontitis in molar regions. | After 12 weeks of cementum, researchers observed regeneration, organization of PDL fibers, an increase in the number of PD vessels, and higher BMP-2 and OPN expression in the treated group. | 6 animals per group 8 weeks old | [78] |
| ADSCs AT-MSCs | Cell suspension | Rat | Ligature-induced periodontitis (14 days) | Four weeks after the AT-MSCs treatment, healthy periodontal tissue was observed and the cells were perpendicular to the cementum and AB; multiple blood vessels; well-oriented periodontal cells and fibers; less inflammatory infiltration compared to control. | 5 animals per group | [9] |
| UC-MSCs | β-tricalcium phosphate bioceramic (β-TCP) | Rat | Periodontitis, caused by removing 2.5 × 1.5 mm of the AB over the mandibular first molar roots and cementum covering the roots of the mandibular first molar. | Better (p < 0.05) formation of new bone tissues, cementum, and PDL fibers compared to β-TCP without cells at 8 weeks after surgery. | 5 animals per group 4–6 weeks old | [89] |
| UC-MSCs | Bone collagen particles | Rabbit | Alveolar clefts obtained by removing the incisors on the left side of the upper jaw. | Bone collagen particles with UC-MSCs stimulated bone repair and regeneration and treated AB alveolar cleft lesions. | 6 animals per group 1 or 2 months old | [84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grinchevskaia, L.; Revokatova, D.; Norahan, M.H.; Senkovenko, A.; David Alencar de Sena Pereira, F.; Kosheleva, N.; Shpichka, A.; Timashev, P. Recent Advances in Periodontal Regenerative Medicine: A Focus on the Role of Mechanical Stimulation. Biomedicines 2025, 13, 2839. https://doi.org/10.3390/biomedicines13112839
Grinchevskaia L, Revokatova D, Norahan MH, Senkovenko A, David Alencar de Sena Pereira F, Kosheleva N, Shpichka A, Timashev P. Recent Advances in Periodontal Regenerative Medicine: A Focus on the Role of Mechanical Stimulation. Biomedicines. 2025; 13(11):2839. https://doi.org/10.3390/biomedicines13112839
Chicago/Turabian StyleGrinchevskaia, Lidiia, Daria Revokatova, Mohammad Hadi Norahan, Alexey Senkovenko, Frederico David Alencar de Sena Pereira, Nastasia Kosheleva, Anastasia Shpichka, and Peter Timashev. 2025. "Recent Advances in Periodontal Regenerative Medicine: A Focus on the Role of Mechanical Stimulation" Biomedicines 13, no. 11: 2839. https://doi.org/10.3390/biomedicines13112839
APA StyleGrinchevskaia, L., Revokatova, D., Norahan, M. H., Senkovenko, A., David Alencar de Sena Pereira, F., Kosheleva, N., Shpichka, A., & Timashev, P. (2025). Recent Advances in Periodontal Regenerative Medicine: A Focus on the Role of Mechanical Stimulation. Biomedicines, 13(11), 2839. https://doi.org/10.3390/biomedicines13112839






