The Bidirectional Relationship Between Myocardial Infarction and Depression: Risk Factors, Mechanisms, and Interventions
Abstract
1. Introduction
2. Risk Factors
2.1. Sex and Age
2.2. Lifestyle
2.3. Social Background
2.4. Complications
2.5. Genetics
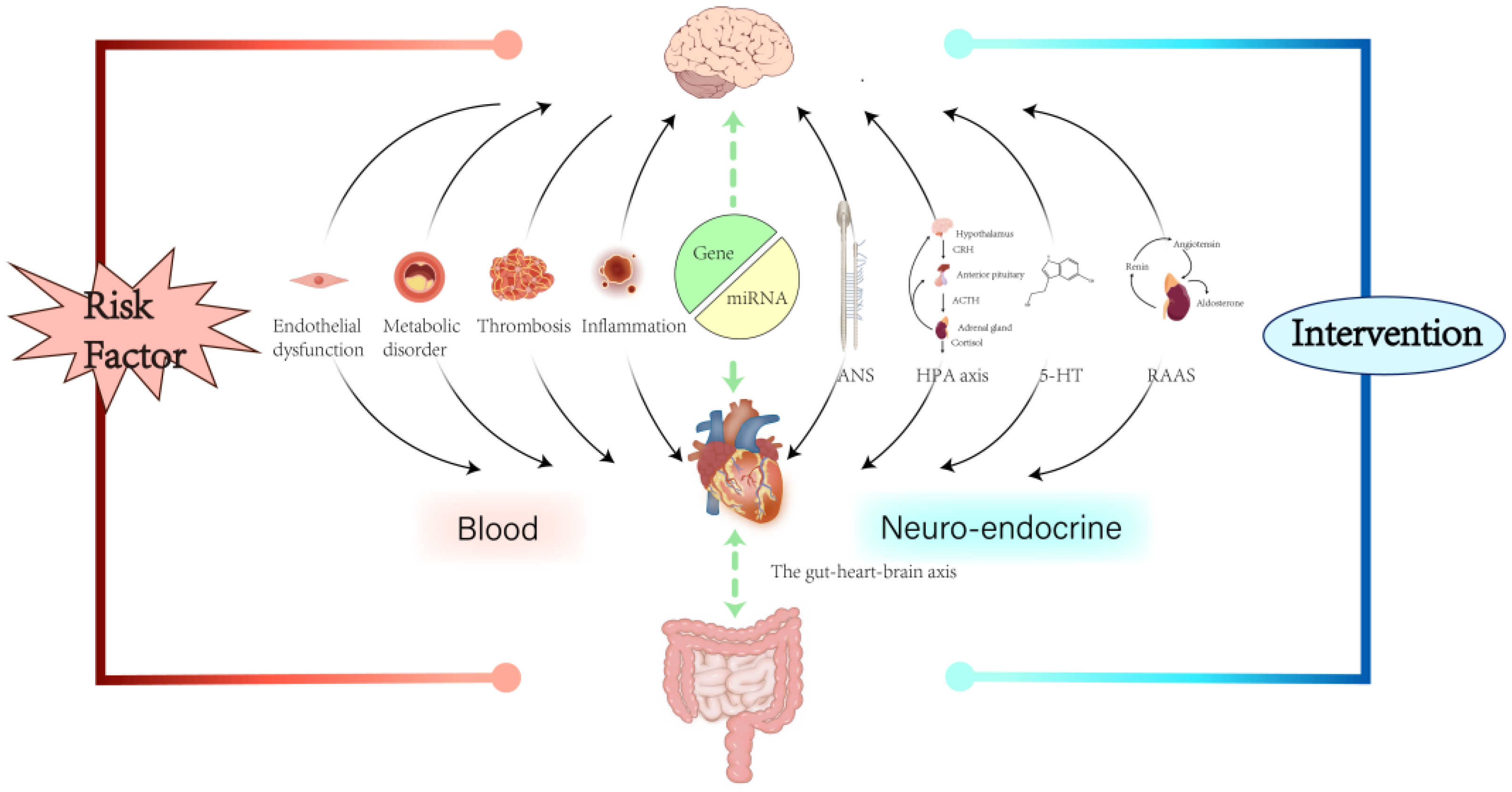
3. Mechanisms
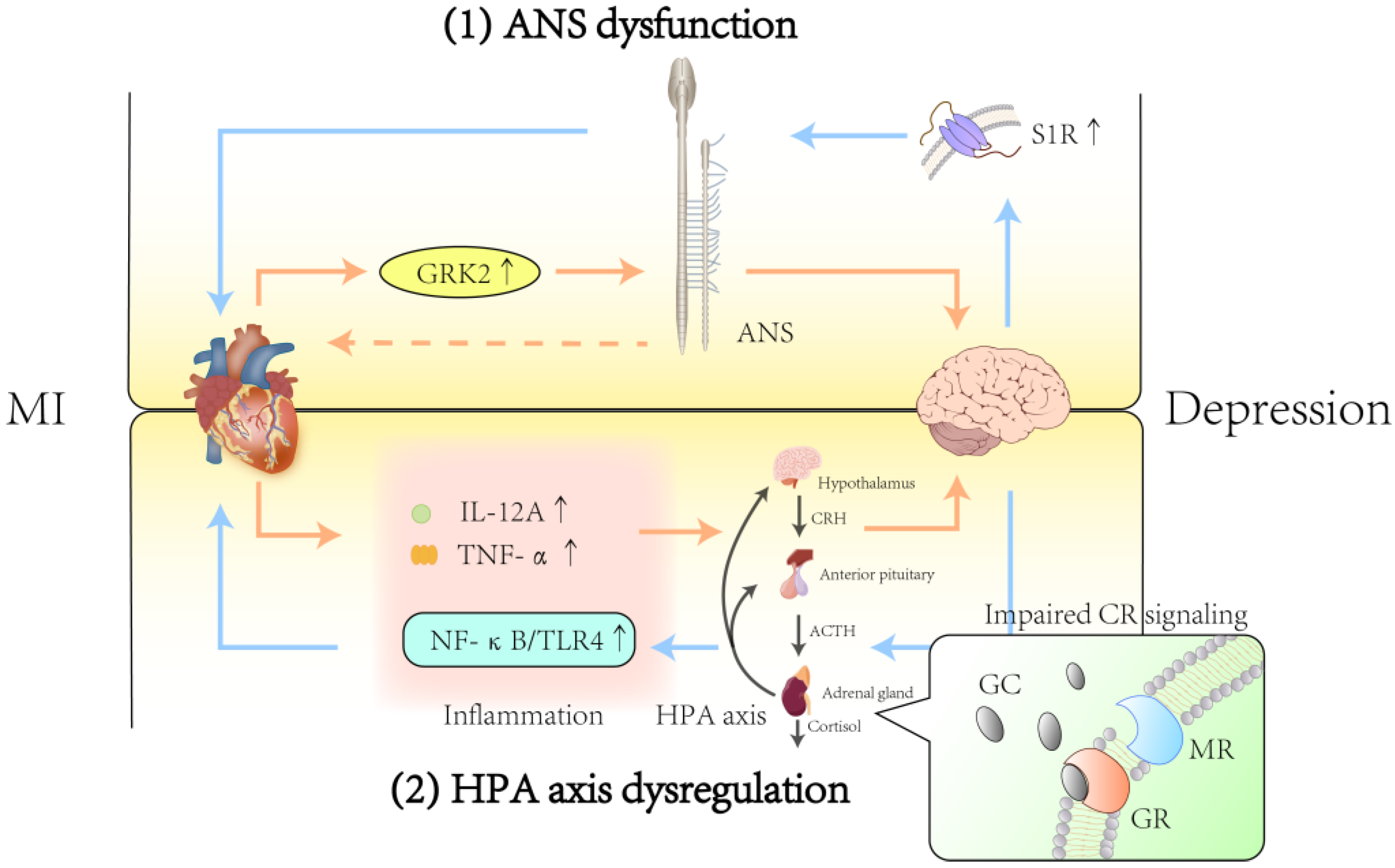
3.1. Autonomic Nervous System
3.1.1. Effect of ANS Dysfunction After MI on Depression
3.1.2. Effect of ANS Dysfunction After Depression on MI
3.2. Hypothalamic–Pituitary–Adrenal Axis
3.2.1. Effect of HPA Axis Dysfunction After MI on Depression
3.2.2. Effects of HPA Axis Dysfunction After Depression on MI
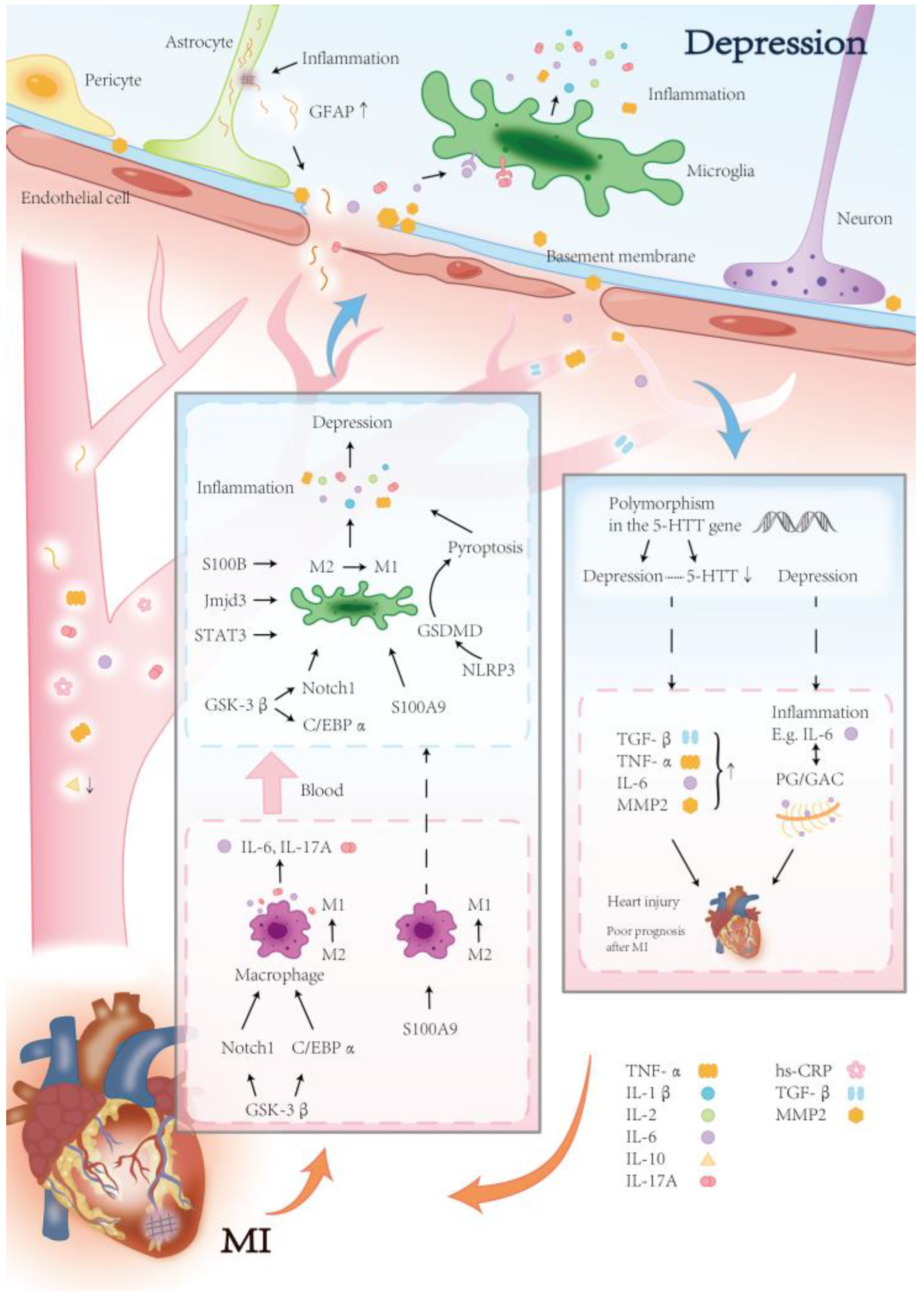
3.3. Inflammation
3.3.1. Effect of Inflammation After MI on Depression
3.3.2. Effects of Inflammation After Depression on MI
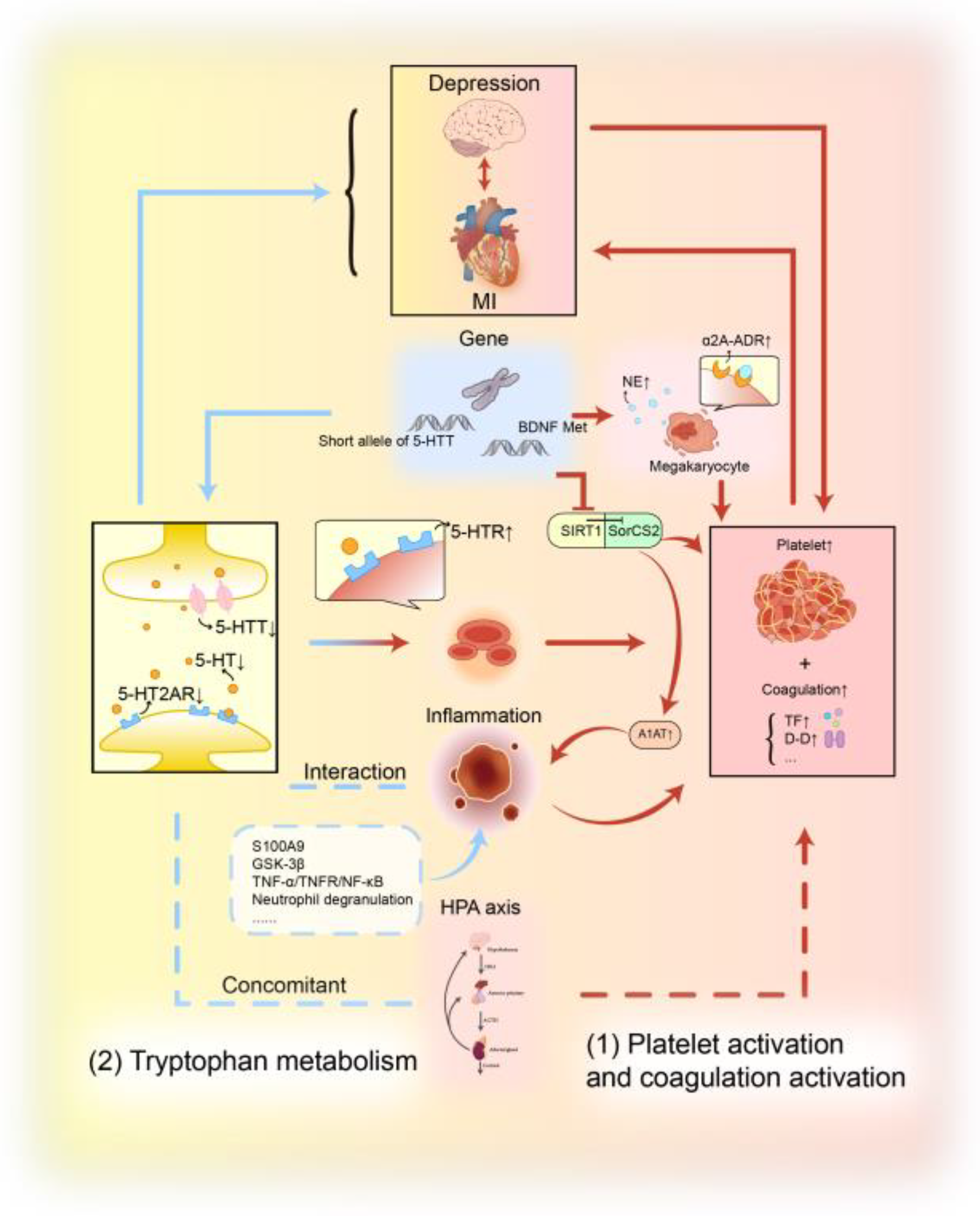
3.4. Platelet Activation and Coagulation Activation
3.5. Tryptophan Metabolism
3.6. Other Mechanisms
3.6.1. Renin–Angiotensin–Aldosterone System
3.6.2. Endothelial Dysfunction
3.6.3. MicroRNAs
3.6.4. Gut Microbiome
4. Interventions and Treatments
4.1. Interventions
4.1.1. Psychotherapy
4.1.2. Exercise Therapy
4.2. Medication
4.2.1. Chemical Drugs
4.2.2. Natural Compounds
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MI | Myocardial Infarction |
| ANS | Autonomic Nervous System |
| HPA | Hypothalamic-pituitary-pdrenal |
| RAAS | Renin–angiotensin–aldosterone System |
| MDD | Major Depressive Disorder |
| CVD | Cardiovascular Disease |
| MACE | Major Adverse Cardiovascular Events |
| CAD | Coronary Artery Disease |
| CHD | Coronary Heart Disease |
| ACS | Acute Coronary Syndrome |
| IHD | Ischaemic Heart Disease |
| LDL | Low-density Lipoprotein |
| PCSK9 | Pro-protein Convertase Subtilisin/kexin Type 9 |
| HRV | Heart Rate Variability |
| FKBP5 | FK506 binding Protein 51 |
| APLNR | Apelin receptor |
| SNS | Sympathetic Nervous System |
| PNS | Parasympathetic Nervous System |
| HR | Heart Rate |
| NE | Norepinephrine |
| ACh | Acetylcholine |
| S1R | Sigma-1 Receptor |
| GRK2 | G Protein-coupled Receptor Kinase-2 |
| LVEF | Left Ventricular Ejection Fractions |
| BP | Blood Pressure |
| SSRI | Selective Serotonin Reuptake Inhibitor |
| LVEDP | Left Ventricular End-diastolic Pressure |
| I/R | Ischemia/Reperfusion |
| CRH | Corticotropin-releasing Hormone |
| ACTH | Adrenocorticotropic Hormone |
| GC | Glucocorticoid |
| CRs | Cortisol Receptors |
| MRs | Mineralocorticoid Receptors |
| GRs | glucocorticoid receptors |
| DCS | Diurnal Cortisol Slope |
| GFAP | Glial Fibrillary Acidic Protein |
| IL | Interleukin |
| TNF | Tumour Necrosis Factor |
| NR3C1 | Nuclear Receptor Subfamily 3 Group C Member 1 |
| HDL-C | High-density Lipoprotein Cholesterol |
| TLR4 | Toll-like Receptor 4 |
| NF-κB | Nuclear Factor Kappa B |
| CR | Corticosteroid Receptor |
| CAR | Cortisol Awakening Response |
| BBB | Blood–Brain Barrier |
| MMPs | Matrix Metalloproteinases |
| CNS | Central Nervous System |
| hs-CRP | High-sensitivity CRP |
| PCI | Percutaneous Coronary Intervention |
| NLRP3 | NOD-like Receptor Thermal Protein Domain Associated Protein 3 |
| STEMI | ST-segment Elevation Myocardial Infarction |
| NLR | Neutrophil To Lymphocyte Ratio |
| GSK-3β | Glycogen Synthase Kinase 3 beta |
| S100A9 | S100 Calcium Binding Protein A9 |
| S100B | S100 Calcium Binding Protein B |
| JMJD3 | Jumonji Domain-containing Protein 3 |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| Notch1 | Notch Receptor 1 |
| C/EBPα | CCAAT/Enhancer-Binding Protein Alpha |
| GSDMD | Gasdermin-D |
| SII | Systemic Immune Inflammation Index |
| MIR | Recurrent Myocardial Infarction |
| MIF | Migration Inhibitory Factor |
| AMPK | Adenosine 5′-monophosphate-activated Protein Kinase |
| 5-HTT/SERT | 5-HT Transporter |
| TGF-β | Transforming Growth Factor-β |
| PG | Proteoglycan |
| GAG | Glycosaminoglycan |
| HS | Heparan Sulphate |
| CS | Chondroitin Sulphate |
| MPO | Myeloperoxidase |
| TF | Tissue Factor |
| 5-HT | 5-Hydroxytryptamine |
| BDNF | Brain-Derived Neurotrophic Factor |
| SIRT1 | Sirtuin 1 |
| SorCS2 | Sortilin Related VPS10 Domain Containing Receptor 2 |
| A1AT | α1-Antitrypsin |
| α2A-ADR | α2A-Adrenergic Receptor |
| L-Trp | L-tryptophan |
| KYNU | kynureninas |
| KYN | kynurenine |
| TNFR | TNF-α Receptor |
| ACE2 | Angiotensin-Converting Enzyme 2 |
| Ang II | Angiotensin II |
| ACEI | Angiotensin-Converting Enzyme Inhibitor |
| EPC | Endothelial Progenitor Cell |
| ET-1 | Endothelin-1 |
| MiRNAs | MicroRNAs |
| NOS1 | Nitric Oxide Synthase 1 |
| FMT | Faecal Microbiota Transplantation |
| CBT | Cognitive Behavioural Therapy |
| QoL | Quality of life |
| NW | Nordic Walking |
| RCT | Randomised Controlled Trial |
| SNRIs | Serotonin-norepinephrine Reuptake Inhibitors |
| TCA | Tricyclic Antidepressants |
| ES | Escitalopram |
References
- GBD 2023 Disease and Injury and Risk Factor Collaborators. Burden of 375 Diseases and Injuries, Risk-Attributable Burden of 88 Risk Factors, and Healthy Life Expectancy in 204 Countries and Territories, Including 660 Subnational Locations, 1990–2023: A Systematic Analysis for the Global Burden of Disease Study 2023. Lancet 2025, 406, 1873–1922. [Google Scholar] [CrossRef]
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef]
- Chan, J.K.N.; Solmi, M.; Lo, H.K.Y.; Chan, M.W.Y.; Choo, L.L.T.; Lai, E.T.H.; Wong, C.S.M.; Correll, C.U.; Chang, W.C. All-Cause and Cause-Specific Mortality in People with Depression: A Large-Scale Systematic Review and Meta-Analysis of Relative Risk and Aggravating or Attenuating Factors, Including Antidepressant Treatment. World Psychiatry 2025, 24, 404–421. [Google Scholar] [CrossRef]
- Zeng, J.; Qiu, Y.; Yang, C.; Fan, X.; Zhou, X.; Zhang, C.; Zhu, S.; Long, Y.; Hashimoto, K.; Chang, L.; et al. Cardiovascular Diseases and Depression: A Meta-Analysis and Mendelian Randomization Analysis. Mol. Psychiatry 2025, 30, 4234–4246, Erratum in Mol. Psychiatry 2025, 30, 4444. [Google Scholar] [CrossRef] [PubMed]
- Levine, G.N.; Carney, R.M.; Cohen, B.E.; Dunn, S.L.; Gaffey, A.E.; Kronish, I.M.; Olsson, E.M.G.; Huffman, J.C.; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; et al. Post-Myocardial Infarction Psychological Distress: A Scientific Statement From the American Heart Association. Circulation 2025, 152, e298–e310. [Google Scholar] [CrossRef] [PubMed]
- Narendrula, A.; Ajani, K.; Lang, J.; Brinza, E.; Longenecker, C.T. Psychological Distress and Health Perception in Patients with a Previous Myocardial Infarction or Stroke: A National Cross-Sectional Study. BMC Cardiovasc. Disord. 2023, 23, 430. [Google Scholar] [CrossRef]
- Feng, L.; Li, L.; Liu, W.; Yang, J.; Wang, Q.; Shi, L.; Luo, M. Prevalence of Depression in Myocardial Infarction: A PRISMA-Compliant Meta-Analysis. Medicine 2019, 98, e14596. [Google Scholar] [CrossRef]
- Lin, Y.; Bai, W.; Liu, H.-H.; Li, Z.-Z.; Gao, Z.-Z.; Han, T.; Ren, H.-H.; Ng, C.H.; Xiang, Y.-T. Prevalence, Correlates, and Network Analysis of Depression and Its Association with Quality of Life in Survivors with Myocardial Infarction during the COVID-19 Pandemic. J. Affect. Disord. 2023, 336, 106–111. [Google Scholar] [CrossRef]
- Meijer, A.; Conradi, H.J.; Bos, E.H.; Anselmino, M.; Carney, R.M.; Denollet, J.; Doyle, F.; Freedland, K.E.; Grace, S.L.; Hosseini, S.H.; et al. Adjusted Prognostic Association of Depression Following Myocardial Infarction with Mortality and Cardiovascular Events: Individual Patient Data Meta-Analysis. Br. J. Psychiatry 2013, 203, 90–102. [Google Scholar] [CrossRef]
- Sreenivasan, J.; Kaul, R.; Khan, M.S.; Malik, A.; Usman, M.S.; Michos, E.D. Mental Health Disorders and Readmissions Following Acute Myocardial Infarction in the United States. Sci. Rep. 2022, 12, 3327. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.O.; Rashid, M.; Farooq, S.; Siddiqui, N.; Parwani, P.; Shiers, D.; Thamman, R.; Gulati, M.; Shoaib, A.; Chew-Graham, C.; et al. Acute Myocardial Infarction in Severe Mental Illness: Prevalence, Clinical Outcomes, and Process of Care in U.S. Hospitalizations. Can. J. Cardiol. 2019, 35, 821–830. [Google Scholar] [CrossRef]
- Lee, S.N.; Yun, J.-S.; Ko, S.-H.; Ahn, Y.-B.; Yoo, K.-D.; Her, S.-H.; Moon, D.; Jung, S.-H.; Won, H.-H.; Kim, D. Impacts of Gender and Lifestyle on the Association between Depressive Symptoms and Cardiovascular Disease Risk in the UK Biobank. Sci. Rep. 2023, 13, 10758. [Google Scholar] [CrossRef]
- Lichtman, J.H.; Froelicher, E.S.; Blumenthal, J.A.; Carney, R.M.; Doering, L.V.; Frasure-Smith, N.; Freedland, K.E.; Jaffe, A.S.; Leifheit-Limson, E.C.; Sheps, D.S.; et al. Depression as a Risk Factor for Poor Prognosis among Patients with Acute Coronary Syndrome: Systematic Review and Recommendations: A Scientific Statement from the American Heart Association. Circulation 2014, 129, 1350–1369. [Google Scholar] [CrossRef]
- Wu, S.; Yang, X.; Chen, Y.; Wang, Y.; Liang, H.; Xu, W.; Wang, J.; Shao, X.; Zhang, H.; Zhong, Z.; et al. Changes in Depressive Symptoms as Predictors of Incident Cardiovascular Disease: Insights from Four Prospective Cohorts. Eur. J. Prev. Cardiol. 2025, zwaf586. [Google Scholar] [CrossRef] [PubMed]
- Walli-Attaei, M.; Rosengren, A.; Rangarajan, S.; Breet, Y.; Abdul-Razak, S.; Sharief, W.A.; Alhabib, K.F.; Avezum, A.; Chifamba, J.; Diaz, R.; et al. Metabolic, Behavioural, and Psychosocial Risk Factors and Cardiovascular Disease in Women Compared with Men in 21 High-Income, Middle-Income, and Low-Income Countries: An Analysis of the PURE Study. Lancet 2022, 400, 811–821. [Google Scholar] [CrossRef]
- Kovess-Masfety, V.; Boyd, A.; van de Velde, S.; de Graaf, R.; Vilagut, G.; Haro, J.M.; Florescu, S.; O’Neill, S.; Weinberg, L.; Alonso, J.; et al. Are There Gender Differences in Service Use for Mental Disorders across Countries in the European Union? Results from the EU-World Mental Health Survey. J. Epidemiol. Community Health 2014, 68, 649–656. [Google Scholar] [CrossRef]
- Al-Zaru, I.M.; Alhalaiqa, F.; Dalky, H.F.; Arramadan, K.A.; Batiha, A.-M. Depression in Nonhospitalized Jordanian Patients With Coronary Artery Disease. J. Nurs. Res. 2020, 28, e66. [Google Scholar] [CrossRef] [PubMed]
- Dikić, A.; Radmilo, L.; Živanović, Ž.; Keković, G.; Sekulić, S.; Kovačić, Z.; Radmilo, R. Cognitive Impairment and Depression after Acute Myocardial Infarction: Associations with Ejection Fraction and Demographic Characteristics. Acta Neurol. Belg. 2021, 121, 1615–1622. [Google Scholar] [CrossRef]
- Zhu, C.; Tran, P.; Dreyer, R.; Lichtman, J. The Association of Depression With Cardiac Rehabilitation Attendance by Age and Sex Among Patients With Myocardial Infarction: Results From the Behavioral Risk Factor Surveillance System. Circulation 2021, 144, A12931. [Google Scholar] [CrossRef]
- Nyström, A.; Strömberg, S.; Jansson, K.; Faresjö, Å.O.; Faresjö, T. Cardiovascular Risks before Myocardial Infarction Differences between Men and Women. BMC Cardiovasc. Disord. 2022, 22, 110. [Google Scholar] [CrossRef]
- Lu, Y.; Li, S.-X.; Liu, Y.; Rodriguez, F.; Watson, K.E.; Dreyer, R.P.; Khera, R.; Murugiah, K.; D’Onofrio, G.; Spatz, E.S.; et al. Sex-Specific Risk Factors Associated With First Acute Myocardial Infarction in Young Adults. JAMA Netw. Open 2022, 5, e229953. [Google Scholar] [CrossRef]
- Moreno, G.; Vicent, L.; Rosillo, N.; Delgado, J.; Cerro, E.P.D.; Bueno, H. Do Sex and Gender Aspects Influence Non-Adherence to Secondary Prevention Measures after Myocardial Infarction? Am. J. Prev. Cardiol. 2024, 19, 100713. [Google Scholar] [CrossRef]
- Theofilis, P.; Oikonomou, E.; Lazaros, G.; Vogiatzi, G.; Niarchou, P.; Goliopoulou, A.; Anastasiou, M.; Mistakidi, V.C.; Tsalamandris, S.; Fountoulakis, P.; et al. The Association of Depression With QT Duration: A Comparison Between Individuals Younger or Older Than 65 Years. Psychosom. Med. 2023, 85, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Nygren, A.; Reutfors, J.; Karlsson, P.; Tiger, M.; Faxén, J.; Brenner, P. Risk of Major Adverse Cardiovascular Events in Treatment-Resistant or Severe Depression. J. Affect. Disord. 2025, 386, 119419. [Google Scholar] [CrossRef]
- Erdman, J.; Kornspun, A.; Stein, L.; Rossi, K.; Tuhrim, S.; Dhamoon, M. Readmission for Depression and Suicide Attempt Following Stroke and Myocardial Infarction. Stroke 2020, 51, A43. [Google Scholar] [CrossRef]
- Fleetwood, K.; Wild, S.H.; Smith, D.J.; Mercer, S.W.; Licence, K.; Sudlow, C.L.M.; Jackson, C.A. Severe Mental Illness and Mortality and Coronary Revascularisation Following a Myocardial Infarction: A Retrospective Cohort Study. BMC Med. 2021, 19, 67. [Google Scholar] [CrossRef]
- Lang, X.; Liu, Z.; Islam, S.; Han, G.; Rangarajan, S.; Tse, L.A.; Mushtaha, M.; Wang, J.; Hu, L.; Qiang, D.; et al. Interaction of Depression and Unhealthy Diets on the Risk of Cardiovascular Diseases and All-Cause Mortality in the Chinese Population: A PURE Cohort Substudy. Nutrients 2022, 14, 5172. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, F.; Ma, H.; Yin, H.; Wang, P.; Bai, B.; Guo, L.; Geng, Q. Associations between Depression, Nutrition, and Outcomes among Individuals with Coronary Artery Disease. Nutrition 2021, 86, 111157. [Google Scholar] [CrossRef]
- Pogosova, N.; Boytsov, S.; De Bacquer, D.; Sokolova, O.; Ausheva, A.; Kursakov, A.; Saner, H. Factors Associated with Anxiety and Depressive Symptoms in 2775 Patients with Arterial Hypertension and Coronary Heart Disease: Results from the COMETA Multicenter Study. Glob. Heart 2021, 16, 73. [Google Scholar] [CrossRef]
- Kim, M.; Kim, H.; Han, K.; Yoo, J.; Yang, K.; Jeon, H.J. Changes in Alcohol Consumption and the Risk of Cardiovascular Diseases in Patients with Depression Who Had Not Consumed Alcohol: A Nationwide Cohort Study. J. Psychiatr. Res. 2022, 155, 458–464. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Z.; Georgakis, M.K.; Lin, H.; Zheng, L. Genetic Liability to Depression and Risk of Coronary Artery Disease, Myocardial Infarction, and Other Cardiovascular Outcomes. J. Am. Heart Assoc. 2021, 10, e017986. [Google Scholar] [CrossRef]
- Li, G.H.-Y.; Cheung, C.-L.; Chung, A.K.-K.; Cheung, B.M.-Y.; Wong, I.C.-K.; Fok, M.L.Y.; Au, P.C.-M.; Sham, P.-C. Evaluation of Bi-Directional Causal Association between Depression and Cardiovascular Diseases: A Mendelian Randomization Study. Psychol. Med. 2022, 52, 1765–1776. [Google Scholar] [CrossRef]
- Zhang, W.-Y.; Nan, N.; He, Y.; Zuo, H.-J.; Song, X.-T.; Zhang, M.; Zhou, Y. Prevalence of Depression and Anxiety Symptoms and Their Associations with Cardiovascular Risk Factors in Coronary Patients. Psychol. Health Med. 2023, 28, 1275–1287. [Google Scholar] [CrossRef]
- Furlong-Millones, M.R.; Mostacero-Becerra, K.; Aguirre-Milachay, E.; Alvarez-Risco, A.; Del-Aguila-Arcentales, S.; Garcia Guerra, A.; Davies, N.M.; Yañez, J.A.; Valladares-Garrido, M.J. Quality of Life, Anxiety, and Depression in Peruvian Patients with Acute Coronary Syndrome. Sustainability 2022, 14, 14970. [Google Scholar] [CrossRef]
- Murphy, B.; Le Grande, M.; Alvarenga, M.; Worcester, M.; Jackson, A. Anxiety and Depression After a Cardiac Event: Prevalence and Predictors. Front. Psychol. 2019, 10, 3010. [Google Scholar] [CrossRef]
- Krasieva, K.; Clair, C.; Gencer, B.; Carballo, D.; Klingenberg, R.; Raber, L.; Windecker, S.; Rodondi, N.; Matter, C.M.; Luscher, T.F.; et al. Impact of Smoking Cessation on Depression after Acute Coronary Syndrome. Eur. Heart J. 2021, 42, ehab724.2598. [Google Scholar] [CrossRef]
- Krasieva, K.; Clair, C.; Gencer, B.; Carballo, D.; Klingenberg, R.; Räber, L.; Windecker, S.; Rodondi, N.; Matter, C.M.; Lüscher, T.F.; et al. Smoking Cessation and Depression after Acute Coronary Syndrome. Prev. Med. 2022, 163, 107177. [Google Scholar] [CrossRef]
- Deschênes, S.S.; Burns, R.J.; Graham, E.; Schmitz, N. Depressive Symptoms and Sleep Problems as Risk Factors for Heart Disease: A Prospective Community Study. Epidemiol. Psychiatr. Sci. 2019, 29, e50. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Ouyang, F.; Ma, T.; He, L.; Gong, L.; Yin, J.; Zhang, G.; Bai, Y. The Individual and Joint Associations of Depression and Sleep Duration with Cardiometabolic Diseases and Mortality: A Prospective Cohort Study. Atherosclerosis 2022, 361, 10–17. [Google Scholar] [CrossRef]
- Feng, Z.; Tong, W.K.; Tang, Z. Longitudinal Trends in the Prevalence and Treatment of Depression among Adults with Cardiovascular Disease: An Analysis of National Health and Nutrition Examination Survey 2009–2020. Front. Psychiatry 2022, 13, 943165. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Lee, S.C.; Shin, Y.S.; Park, S.; Won, K.B.; Ann, S.H.; Ko, E.J. Severity, Progress, and Related Factors of Mood Disorders in Patients with Coronary Artery Disease: A Retrospective Study. Healthcare 2020, 8, 568. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Lee, M.G.; Jo, M.; Kim, G.; Park, S. Joint Effect of Depression and Health Behaviors or Conditions on Incident Cardiovascular Diseases: A Korean Population-Based Cohort Study. J. Affect. Disord. 2020, 276, 616–622. [Google Scholar] [CrossRef]
- Rajan, S.; McKee, M.; Rangarajan, S.; Bangdiwala, S.; Rosengren, A.; Gupta, R.; Kutty, V.R.; Wielgosz, A.; Lear, S.; AlHabib, K.F.; et al. Association of Symptoms of Depression With Cardiovascular Disease and Mortality in Low-, Middle-, and High-Income Countries. JAMA Psychiatry 2020, 77, 1052–1063. [Google Scholar] [CrossRef]
- Silventoinen, K.; Korhonen, K.; Lahtinen, H.; Jelenkovic, A.; Havulinna, A.S.; Ripatti, S.; Salomaa, V.; Davey Smith, G.; Martikainen, P. Joint Associations of Depression, Genetic Susceptibility and the Area of Residence for Coronary Heart Disease Incidence. J. Epidemiol. Community Health 2022, 76, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Power, N.; Deschênes, S.S.; Ferri, F.; Schmitz, N. The Association between Job Strain, Depressive Symptoms, and Cardiovascular Disease Risk: Results from a Cross-Sectional Population-Based Study in Québec, Canada. Int. Arch. Occup. Environ. Health 2020, 93, 1013–1021. [Google Scholar] [CrossRef]
- Jones, D.P.; Wootton, R.E.; Gill, D.; Carter, A.R.; Gunnell, D.; Munafò, M.R.; Sallis, H.M. Mental Health as a Mediator of the Association Between Educational Inequality and Cardiovascular Disease: A Mendelian Randomization Study. J. Am. Heart Assoc. 2021, 10, e019340. [Google Scholar] [CrossRef] [PubMed]
- Figura, A.; Kuhlmann, S.L.; Rose, M.; Slagman, A.; Schenk, L.; Möckel, M. Mental Health Conditions in Older Multimorbid Patients Presenting to the Emergency Department for Acute Cardiac Symptoms: Cross-Sectional Findings from the EMASPOT Study. Acad. Emerg. Med. 2021, 28, 1262–1276. [Google Scholar] [CrossRef]
- Niedhammer, I.; Sultan-Taïeb, H.; Parent-Thirion, A.; Chastang, J.-F. Update of the Fractions of Cardiovascular Diseases and Mental Disorders Attributable to Psychosocial Work Factors in Europe. Int. Arch. Occup. Environ. Health 2022, 95, 233–247, Erratum in Int. Arch. Occup. Environ. Health 2023, 96, 639–640. [Google Scholar] [CrossRef]
- Munyombwe, T.; Dondo, T.B.; Aktaa, S.; Wilkinson, C.; Hall, M.; Hurdus, B.; Oliver, G.; West, R.M.; Hall, A.S.; Gale, C.P. Association of Multimorbidity and Changes in Health-Related Quality of Life Following Myocardial Infarction: A UK Multicentre Longitudinal Patient-Reported Outcomes Study. BMC Med. 2021, 19, 227. [Google Scholar] [CrossRef]
- Jia, Z.; Li, S. Risk of Cardiovascular Disease Mortality in Relation to Depression and 14 Common Risk Factors. Int. J. Gen. Med. 2021, 14, 441–449. [Google Scholar] [CrossRef]
- Inoue, K.; Mayeda, E.R.; Nianogo, R.; Paul, K.; Yu, Y.; Haan, M.; Ritz, B. Estimating the Joint Effect of Diabetes and Subsequent Depressive Symptoms on Mortality among Older Latinos. Ann. Epidemiol. 2021, 64, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Jung, I.; Kwon, H.; Park, S.E.; Han, K.-D.; Park, Y.-G.; Kim, Y.-H.; Rhee, E.-J.; Lee, W.-Y. Increased Risk of Cardiovascular Disease and Mortality in Patients with Diabetes and Coexisting Depression: A Nationwide Population-Based Cohort Study. Diabetes Metab. J. 2021, 45, 379–389. [Google Scholar] [CrossRef]
- Peter, R.S.; Jaensch, A.; Mons, U.; Schöttker, B.; Schmucker, R.; Koenig, W.; Brenner, H.; Rothenbacher, D. Prognostic Value of Long-Term Trajectories of Depression for Incident Diabetes Mellitus in Patients with Stable Coronary Heart Disease. Cardiovasc. Diabetol. 2021, 20, 108. [Google Scholar] [CrossRef]
- Zareini, B.; Sørensen, K.K.; Blanche, P.; Falkentoft, A.C.; Fosbøl, E.; Køber, L.; Torp-Pedersen, C. Incidence of Depression in Patients with Cardiovascular Disease and Type 2 Diabetes: A Nationwide Cohort Study. Clin. Res. Cardiol. 2024, 113, 1523–1533. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yin, H.; Liu, Q.; Chen, Y.; Liang, Y.; Zhou, H.; Ma, H.; Geng, Q. Associations Among Depression, Hemoglobin A1c Level, and Prognosis in Patients With Coronary Artery Disease: A Prospective Study. Front. Psychiatry 2022, 13, 815196. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Z.; Yang, Y.; Chen, G.; Wan, Q.; Qin, G.; Yan, L.; Wang, G.; Qin, Y.; Luo, Z.; et al. Depression Status, Lifestyle, and Metabolic Factors With Subsequent Risk for Major Cardiovascular Events: The China Cardiometabolic Disease and Cancer Cohort (4C) Study. Front. Cardiovasc. Med. 2022, 9, 865063. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Yan, L.; Li, Z.; Zhao, S.; Feng, Y.; Zeng, J.; Chen, L.; Huang, A.; Chen, Y.; Lei, S.; et al. Potential Shared Gene Signatures and Molecular Mechanisms between Atherosclerosis and Depression: Evidence from Transcriptome Data. Comput. Biol. Med. 2023, 152, 106450. [Google Scholar] [CrossRef]
- Lin, J.; Yang, R.; Zhang, Y.; Hou, Y.; Yang, H.; Zhou, X.; Liu, T.; Yang, Q.; Wang, Y. The Mediation Effects of Metabolic and Immune-Inflammation Factors on the Depression-Premature Coronary Heart Disease Association. J. Affect. Disord. 2023, 331, 434–441. [Google Scholar] [CrossRef]
- Katzmann, J.L.; Mahfoud, F.; Böhm, M.; Schulz, M.; Laufs, U. Association of Medication Adherence and Depression with the Control of Low-Density Lipoprotein Cholesterol and Blood Pressure in Patients at High Cardiovascular Risk. Patient Prefer. Adherence 2019, 13, 9–19. [Google Scholar] [CrossRef]
- Macchi, C.; Favero, C.; Ceresa, A.; Vigna, L.; Conti, D.M.; Pesatori, A.C.; Racagni, G.; Corsini, A.; Ferri, N.; Sirtori, C.R.; et al. Depression and Cardiovascular Risk-Association among Beck Depression Inventory, PCSK9 Levels and Insulin Resistance. Cardiovasc. Diabetol. 2020, 19, 187. [Google Scholar] [CrossRef]
- Akyol, O.; Chowdhury, I.; Akyol, H.R.; Tessier, K.; Vural, H.; Akyol, S. Why Are Cardiovascular Diseases More Common among Patients with Severe Mental Illness? The Potential Involvement of Electronegative Low-Density Lipoprotein (LDL) L5. Med. Hypotheses 2020, 142, 109821. [Google Scholar] [CrossRef]
- Gutlapalli, S.D.; Farhat, H.; Irfan, H.; Muthiah, K.; Pallipamu, N.; Taheri, S.; Thiagaraj, S.S.; Shukla, T.S.; Giva, S.; Penumetcha, S.S. The Anti-Depressant Effects of Statins in Patients With Major Depression Post-Myocardial Infarction: An Updated Review 2022. Cureus 2022, 14, e32323. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, G.M.; Zuber, V.; Rees, J.M.B.; Carvalho, L.; Mason, A.M.; Foley, C.N.; Gkatzionis, A.; Jones, P.B.; Burgess, S. Correction: Shared Mechanisms between Coronary Heart Disease and Depression: Findings from a Large UK General Population-Based Cohort. Mol. Psychiatry 2021, 26, 3659–3661. [Google Scholar] [CrossRef]
- Shell, A.L.; Gonzenbach, V.; Sawhney, M.; Crawford, C.A.; Stewart, J.C. Associations between Affective Factors and High-Frequency Heart Rate Variability in Primary Care Patients with Depression. J. Psychosom. Res. 2022, 161, 110992. [Google Scholar] [CrossRef]
- Yıldırım, D.; Kocatepe, V. Evaluating Death Anxiety and Death Depression Levels among Patients with Acute Myocardial Infarction. Omega 2023, 86, 1402–1414. [Google Scholar] [CrossRef]
- Bobo, W.V.; Grossardt, B.R.; Virani, S.; St Sauver, J.L.; Boyd, C.M.; Rocca, W.A. Association of Depression and Anxiety With the Accumulation of Chronic Conditions. JAMA Netw. Open 2022, 5, e229817. [Google Scholar] [CrossRef]
- Wang, Q.; Shelton, R.C.; Dwivedi, Y. Interaction between Early-Life Stress and FKBP5 Gene Variants in Major Depressive Disorder and Post-Traumatic Stress Disorder: A Systematic Review and Meta-Analysis. J. Affect. Disord. 2018, 225, 422–428. [Google Scholar] [CrossRef]
- Brandt, J.; Warnke, K.; Jörgens, S.; Arolt, V.; Beer, K.; Domschke, K.; Haverkamp, W.; Kuhlmann, S.L.; Müller-Nordhorn, J.; Rieckmann, N.; et al. Association of FKBP5 Genotype with Depressive Symptoms in Patients with Coronary Heart Disease: A Prospective Study. J. Neural Transm. 2020, 127, 1651–1662. [Google Scholar] [CrossRef]
- Wang, P.; Xu, C.; Wang, C.; Wu, Y.; Wang, D.; Chen, S.; Zhao, Y.; Wang, X.; Li, S.; Yang, Q.; et al. Association of SNP Rs9943582 in APLNR with Left Ventricle Systolic Dysfunction in Patients with Coronary Artery Disease in a Chinese Han GeneID Population. PLoS ONE 2015, 10, e0125926. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wu, B.; Lin, R. Association of Apelin and Apelin Receptor with the Risk of Coronary Artery Disease: A Meta-Analysis of Observational Studies. Oncotarget 2017, 8, 57345–57355. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, W.; Xiao, Y.; Yuan, H.; Wang, F.; Jiang, P.; Luo, Z. Association of Apelin and Apelin Receptor Polymorphisms With the Risk of Comorbid Depression and Anxiety in Coronary Heart Disease Patients. Front. Genet. 2020, 11, 893. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, H.; Wang, B.; Chen, C.; Chen, Y.; Chen, Y.; Xia, F.; Tan, X.; Zhang, J.; Li, Q.; et al. Joint Exposure to Positive Affect, Life Satisfaction, Broad Depression, and Neuroticism and Risk of Cardiovascular Diseases: A Prospective Cohort Study. Atherosclerosis 2022, 359, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Aires, R.; Pimentel, E.B.; Forechi, L.; Dantas, E.M.; Mill, J.G. Time Course of Changes in Heart Rate and Blood Pressure Variability in Rats with Myocardial Infarction. Braz. J. Med. Biol. Res. 2017, 50, e5511. [Google Scholar] [CrossRef]
- Mulkey, S.B.; du Plessis, A.J. Autonomic Nervous System Development and Its Impact on Neuropsychiatric Outcome. Pediatr. Res. 2019, 85, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Pyhälä, R.; Wolford, E.; Kautiainen, H.; Andersson, S.; Bartmann, P.; Baumann, N.; Brubakk, A.-M.; Evensen, K.A.I.; Hovi, P.; Kajantie, E.; et al. Self-Reported Mental Health Problems Among Adults Born Preterm: A Meta-Analysis. Pediatrics 2017, 139, e20162690. [Google Scholar] [CrossRef]
- Porges, S.W.; Furman, S.A. The Early Development of the Autonomic Nervous System Provides a Neural Platform for Social Behavior: A Polyvagal Perspective. Infant Child Dev. 2011, 20, 106–118. [Google Scholar] [CrossRef]
- Fyfe, K.L.; Yiallourou, S.R.; Wong, F.Y.; Odoi, A.; Walker, A.M.; Horne, R.S.C. The Effect of Gestational Age at Birth on Post-Term Maturation of Heart Rate Variability. Sleep 2015, 38, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- Yiallourou, S.R.; Witcombe, N.B.; Sands, S.A.; Walker, A.M.; Horne, R.S.C. The Development of Autonomic Cardiovascular Control Is Altered by Preterm Birth. Early Hum. Dev. 2013, 89, 145–152. [Google Scholar] [CrossRef]
- Wilkowska, A.; Rynkiewicz, A.; Wdowczyk, J.; Landowski, J.; Cubała, W.J. Heart Rate Variability and Incidence of Depression during the First Six Months Following First Myocardial Infarction. Neuropsychiatr. Dis. Treat. 2019, 15, 1951–1956. [Google Scholar] [CrossRef]
- Yu, L.-C.; Lin, I.-M.; Fan, S.-Y.; Chien, C.-L.; Lin, T.-H. One-Year Cardiovascular Prognosis of the Randomized, Controlled, Short-Term Heart Rate Variability Biofeedback Among Patients with Coronary Artery Disease. Int. J. Behav. Med. 2018, 25, 271–282. [Google Scholar] [CrossRef]
- Limmer, A.; Laser, M.; Schütz, A. Mobile Heart Rate Variability Biofeedback as a Complementary Intervention After Myocardial Infarction: A Randomized Controlled Study. Int. J. Behav. Med. 2022, 29, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Qiao, J.; Wang, Y.; Liu, Z.; Liu, Z.; Tan, W.; Wang, C.; Peng, C.; Cheng, S.; Han, X.; et al. PENG-Based Self-Powered Transcutaneous Auricular Vagus Nerve Stimulation Attenuated Myocardial Infarction-Induced Heart-Brain Remodeling via Ameliorating the Neuroinflammatory Response in Central Amygdala. Int. Immunopharmacol. 2025, 162, 115085. [Google Scholar] [CrossRef]
- Ciccarelli, M.; Sorriento, D.; Fiordelisi, A.; Gambardella, J.; Franco, A.; Del Giudice, C.; Sala, M.; Monti, M.G.; Bertamino, A.; Campiglia, P.; et al. Pharmacological Inhibition of GRK2 Improves Cardiac Metabolism and Function in Experimental Heart Failure. ESC Heart Fail. 2020, 7, 1571–1584. [Google Scholar] [CrossRef]
- Machhada, A.; Hosford, P.S.; Dyson, A.; Ackland, G.L.; Mastitskaya, S.; Gourine, A.V. Optogenetic Stimulation of Vagal Efferent Activity Preserves Left Ventricular Function in Experimental Heart Failure. JACC Basic Transl. Sci. 2020, 5, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Machhada, A.; Trapp, S.; Marina, N.; Stephens, R.C.M.; Whittle, J.; Lythgoe, M.F.; Kasparov, S.; Ackland, G.L.; Gourine, A.V. Vagal Determinants of Exercise Capacity. Nat. Commun. 2017, 8, 15097. [Google Scholar] [CrossRef]
- Tian, X.; Wang, Q.; Guo, R.; Xu, L.; Chen, Q.M.; Hou, Y. Effects of Paroxetine-Mediated Inhibition of GRK2 Expression on Depression and Cardiovascular Function in Patients with Myocardial Infarction. Neuropsychiatr. Dis. Treat. 2016, 12, 2333–2341. [Google Scholar] [CrossRef]
- Ngampramuan, S.; Tungtong, P.; Mukda, S.; Jariyavilas, A.; Sakulisariyaporn, C. Evaluation of Autonomic Nervous System, Saliva Cortisol Levels, and Cognitive Function in Major Depressive Disorder Patients. Depress. Res. Treat. 2018, 2018, 7343592. [Google Scholar] [CrossRef]
- Morais-Silva, G.; Costa-Ferreira, W.; Gomes-de-Souza, L.; Pavan, J.C.; Crestani, C.C.; Marin, M.T. Cardiovascular Outcomes Related to Social Defeat Stress: New Insights from Resilient and Susceptible Rats. Neurobiol. Stress. 2019, 11, 100181. [Google Scholar] [CrossRef] [PubMed]
- Euteneuer, F.; Neuert, M.; Salzmann, S.; Fischer, S.; Ehlert, U.; Rief, W. Does Psychological Treatment of Major Depression Reduce Cardiac Risk Biomarkers? An Exploratory Randomized Controlled Trial. Psychol. Med. 2023, 53, 3735–3749. [Google Scholar] [CrossRef]
- Helman, T.J.; Headrick, J.P.; Peart, J.N.; Stapelberg, N.J.C. Central and Cardiac Stress Resilience Consistently Linked to Integrated Immuno-Neuroendocrine Responses across Stress Models in Male Mice. Eur. J. Neurosci. 2022, 56, 4333–4362. [Google Scholar] [CrossRef]
- Liu, X.; Qu, C.; Yang, H.; Shi, S.; Zhang, C.; Zhang, Y.; Liang, J.; Yang, B. Chronic Stimulation of the Sigma-1 Receptor Ameliorates Autonomic Nerve Dysfunction and Atrial Fibrillation Susceptibility in a Rat Model of Depression. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1521–H1531. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.X.; Milaneschi, Y.; Lamers, F.; Nolte, I.M.; Snieder, H.; Dolan, C.V.; Penninx, B.W.J.H.; de Geus, E.J.C. The Association of Depression and Anxiety with Cardiac Autonomic Activity: The Role of Confounding Effects of Antidepressants. Depress. Anxiety 2019, 36, 1163–1172. [Google Scholar] [CrossRef]
- Devarajan, A.; Wang, K.; Lokhandwala, Z.A.; Emamimeybodi, M.; Shannon, K.; Tompkins, J.D.; Hevener, A.L.; Lusis, A.J.; Abel, E.D.; Vaseghi, M. Myocardial Infarction Causes Sex-Dependent Dysfunction in Vagal Sensory Glutamatergic Neurotransmission That Is Mitigated by 17β-Estradiol. JCI Insight 2024, 9, e181042. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Pan, X.; Wang, F.; Ma, J.; Su, G.; Dong, Y.; Yang, J.; Wu, C. Baicalin Promotes Hippocampal Neurogenesis via SGK1- and FKBP5-Mediated Glucocorticoid Receptor Phosphorylation in a Neuroendocrine Mouse Model of Anxiety/Depression. Sci. Rep. 2016, 6, 30951. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Li, X.; Tang, C.; Chen, J.; Fan, B.; Liang, J.; Zhen, X.; Tao, R.; Zhang, S.; Cong, Z.; et al. Mechanisms of Xiong-Pi-Fang in Treating Coronary Heart Disease Associated with Depression: A Systematic Pharmacology Strategy and in Vivo Pharmacological Validation. J. Ethnopharmacol. 2022, 298, 115631. [Google Scholar] [CrossRef]
- Verma, H.; Bhattacharjee, A.; Shivavedi, N.; Nayak, P.K. Evaluation of Rosmarinic Acid against Myocardial Infarction in Maternally Separated Rats. Naunyn Schmiedeberg’s Arch. Pharmacol. 2022, 395, 1189–1207. [Google Scholar] [CrossRef]
- Park, J.-Y.; Kim, J.-W.; Kang, H.-J.; Choi, W.; Lee, J.-Y.; Kim, S.-W.; Shin, I.-S.; Ahn, Y.; Jeong, M.H.; Kim, J.-M. Effect Modification of Cortisol on the Associations Between Obsessive-Compulsive Symptoms on Suicidality in Patients With Acute Coronary Syndrome. Psychiatry Investig. 2023, 20, 707–713. [Google Scholar] [CrossRef]
- Wilkowska, A.; Rynkiewicz, A.; Wdowczyk, J.; Landowski, J. Morning and Afternoon Serum Cortisol Level in Patients with Post-Myocardial Infarction Depression. Cardiol. J. 2019, 26, 550–554. [Google Scholar] [CrossRef]
- Poole, L.; Kidd, T.; Ronaldson, A.; Leigh, E.; Jahangiri, M.; Steptoe, A. Depression 12-Months after Coronary Artery Bypass Graft Is Predicted by Cortisol Slope over the Day. Psychoneuroendocrinology 2016, 71, 155–158. [Google Scholar] [CrossRef]
- Bruns, B.; Daub, R.; Schmitz, T.; Hamze-Sinno, M.; Spaich, S.; Dewenter, M.; Schwale, C.; Gass, P.; Vogt, M.; Katus, H.; et al. Forebrain Corticosteroid Receptors Promote Post-Myocardial Infarction Depression and Mortality. Basic Res. Cardiol. 2022, 117, 44. [Google Scholar] [CrossRef]
- Kun, W.; Jie, Z.; Shuai, C.; Xin, W.U.; Guoqi, Z.; Shengbing, W.U.; Meiqi, Z. Electroacupuncture Ameliorates Cardiac Dysfunction in Myocardial Ischemia Model Rats: A Potential Role of the Hypothalamic-Pituitary-Adrenal Axis. J. Tradit. Chin. Med. 2023, 43, 944–954. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, X.; Villalba, N.; Chatterjee, V.; Reynolds, A.; Spence, S.; Wu, M.H.; Yuan, S.Y. Circulating Lymphocyte Trafficking to the Bone Marrow Contributes to Lymphopenia in Myocardial Infarction. Am. J. Physiol. Heart Circ. Physiol. 2022, 322, H622–H635. [Google Scholar] [CrossRef]
- Kang, H.-J.; Stewart, R.; Kim, J.-W.; Kim, S.-W.; Shin, I.-S.; Kim, M.-C.; Hong, Y.J.; Ahn, Y.; Shin, M.-G.; Jeong, M.H.; et al. Synergistic Effects of Depression and NR3C1 Methylation on Prognosis of Acute Coronary Syndrome. Sci. Rep. 2020, 10, 5519. [Google Scholar] [CrossRef]
- Nikkheslat, N.; McLaughlin, A.P.; Hastings, C.; Zajkowska, Z.; Nettis, M.A.; Mariani, N.; Enache, D.; Lombardo, G.; Pointon, L.; Cowen, P.J.; et al. Childhood Trauma, HPA Axis Activity and Antidepressant Response in Patients with Depression. Brain Behav. Immun. 2020, 87, 229–237. [Google Scholar] [CrossRef]
- Gan, L.; Li, N.; Heizati, M.; Lin, M.; Zhu, Q.; Hong, J.; Wu, T.; Tong, L.; Xiamili, Z.; Lin, Y. Diurnal Cortisol Features with Cardiovascular Disease in Hypertensive Patients: A Cohort Study. Eur. J. Endocrinol. 2022, 187, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Labad, J.; Soria, V.; Salvat-Pujol, N.; Segalàs, C.; Real, E.; Urretavizcaya, M.; de Arriba-Arnau, A.; Ferrer, A.; Crespo, J.M.; Jiménez-Murcia, S.; et al. Hypothalamic-Pituitary-Adrenal Axis Activity in the Comorbidity between Obsessive-Compulsive Disorder and Major Depression. Psychoneuroendocrinology 2018, 93, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Nowacki, J.; Wingenfeld, K.; Kaczmarczyk, M.; Chae, W.R.; Salchow, P.; Abu-Tir, I.; Piber, D.; Hellmann-Regen, J.; Otte, C. Steroid Hormone Secretion after Stimulation of Mineralocorticoid and NMDA Receptors and Cardiovascular Risk in Patients with Depression. Transl. Psychiatry 2020, 10, 109. [Google Scholar] [CrossRef]
- Haj-Mirzaian, A.; Ramezanzadeh, K.; Shariatzadeh, S.; Tajik, M.; Khalafi, F.; Tafazolimoghadam, A.; Radmard, M.; Rahbar, A.; Pirri, F.; Kazemi, K.; et al. Role of Hypothalamic-Pituitary Adrenal-Axis, Toll-like Receptors, and Macrophage Polarization in Pre-Atherosclerotic Changes Induced by Social Isolation Stress in Mice. Sci. Rep. 2021, 11, 19091. [Google Scholar] [CrossRef] [PubMed]
- Scarborough, J.; Mueller, F.S.; Weber-Stadlbauer, U.; Mattei, D.; Opitz, L.; Cattaneo, A.; Richetto, J. A Novel Murine Model to Study the Impact of Maternal Depression and Antidepressant Treatment on Biobehavioral Functions in the Offspring. Mol. Psychiatry 2021, 26, 6756–6772. [Google Scholar] [CrossRef]
- Holsboer, F. The Corticosteroid Receptor Hypothesis of Depression. Neuropsychopharmacology 2000, 23, 477–501. [Google Scholar] [CrossRef]
- Bunevicius, A.; Gintauskiene, V.; Podlipskyte, A.; Zaliunas, R.; Brozaitiene, J.; Prange, A.J.; Bunevicius, R. Fatigue in Patients with Coronary Artery Disease: Association with Thyroid Axis Hormones and Cortisol. Psychosom. Med. 2012, 74, 848–853. [Google Scholar] [CrossRef]
- Nikkheslat, N.; Zunszain, P.A.; Horowitz, M.A.; Barbosa, I.G.; Parker, J.A.; Myint, A.-M.; Schwarz, M.J.; Tylee, A.T.; Carvalho, L.A.; Pariante, C.M. Insufficient Glucocorticoid Signaling and Elevated Inflammation in Coronary Heart Disease Patients with Comorbid Depression. Brain Behav. Immun. 2015, 48, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Malan, L.; Schutte, C.E.; Alkerwi, A.; Stranges, S.; Malan, N.T. Hypothalamic-Pituitary-Adrenal-Axis Dysregulation and Double Product Increases Potentiate Ischemic Heart Disease Risk in a Black Male Cohort: The SABPA Study. Hypertens. Res. 2017, 40, 590–597. [Google Scholar] [CrossRef]
- von Känel, R.; Schmid, J.-P.; Abbas, C.C.; Gander, M.-L.; Saner, H.; Begré, S. Stress Hormones in Patients with Posttraumatic Stress Disorder Caused by Myocardial Infarction and Role of Comorbid Depression. J. Affect. Disord. 2010, 121, 73–79. [Google Scholar] [CrossRef]
- Messerli-Bürgy, N.; Molloy, G.J.; Wikman, A.; Perkins-Porras, L.; Randall, G.; Steptoe, A. Cortisol Levels and History of Depression in Acute Coronary Syndrome Patients. Psychol. Med. 2012, 42, 1815–1823. [Google Scholar] [CrossRef]
- Kwok, M.K.; Kawachi, I.; Rehkopf, D.; Schooling, C.M. The Role of Cortisol in Ischemic Heart Disease, Ischemic Stroke, Type 2 Diabetes, and Cardiovascular Disease Risk Factors: A Bi-Directional Mendelian Randomization Study. BMC Med. 2020, 18, 363. [Google Scholar] [CrossRef]
- Fang, L.; Moore, X.-L.; Dart, A.M.; Wang, L.-M. Systemic Inflammatory Response Following Acute Myocardial Infarction. J. Geriatr. Cardiol. 2015, 12, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. The Inflammatory Response in Myocardial Injury, Repair, and Remodelling. Nat. Rev. Cardiol. 2014, 11, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Raison, C.L. The Role of Inflammation in Depression: From Evolutionary Imperative to Modern Treatment Target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef]
- Galea, I. The Blood-Brain Barrier in Systemic Infection and Inflammation. Cell. Mol. Immunol. 2021, 18, 2489–2501. [Google Scholar] [CrossRef]
- Huppert, J.; Closhen, D.; Croxford, A.; White, R.; Kulig, P.; Pietrowski, E.; Bechmann, I.; Becher, B.; Luhmann, H.J.; Waisman, A.; et al. Cellular Mechanisms of IL-17-Induced Blood-Brain Barrier Disruption. FASEB J. 2010, 24, 1023–1034. [Google Scholar] [CrossRef]
- Traub, J.; Grondey, K.; Gassenmaier, T.; Schmitt, D.; Fette, G.; Frantz, S.; Boivin-Jahns, V.; Jahns, R.; Störk, S.; Stoll, G.; et al. Sustained Increase in Serum Glial Fibrillary Acidic Protein after First ST-Elevation Myocardial Infarction. Int. J. Mol. Sci. 2022, 23, 10304. [Google Scholar] [CrossRef]
- Baysak, E.; Yildirim, C.; Sayar, N.; Sayar, M.K.; Halaris, A.; Aricioglu, F. The Possible Role of NLRP3 Inflammasome in Depression and Myocardial Infarction Comorbidity. J. Pers. Med. 2023, 13, 1295. [Google Scholar] [CrossRef]
- Lu, H.; Yang, Q.; Zhang, Y. The Relation of Common Inflammatory Cytokines with Anxiety and Depression and Their Values in Estimating Cardiovascular Outcomes in Coronary Heart Disease Patients. J. Clin. Lab. Anal. 2022, 36, e24404. [Google Scholar] [CrossRef]
- Moludi, J.; Alizadeh, M.; Mohammadzad, M.H.S.; Davari, M. The Effect of Probiotic Supplementation on Depressive Symptoms and Quality of Life in Patients After Myocardial Infarction: Results of a Preliminary Double-Blind Clinical Trial. Psychosom. Med. 2019, 81, 770–777. [Google Scholar] [CrossRef]
- Li, C.; Wan, S.; Li, W.; Wang, Y.; Li, B.; Chen, Y.; Sun, P.; Lyu, J. Higher Neutrophil to Lymphocyte Ratio at Admission Is Association with Post-PCI Depressive Symptoms in Patients with ACS. Neuropsychiatr. Dis. Treat. 2022, 18, 2981–2990. [Google Scholar] [CrossRef] [PubMed]
- Najjar, F.; Ahmad, M.; Lagace, D.; Leenen, F.H.H. Role of Myocardial Infarction-Induced Neuroinflammation for Depression-Like Behavior and Heart Failure in Ovariectomized Female Rats. Neuroscience 2019, 415, 201–214. [Google Scholar] [CrossRef]
- Wang, H.-W.; Ahmad, M.; Jadayel, R.; Najjar, F.; Lagace, D.; Leenen, F.H.H. Inhibition of Inflammation by Minocycline Improves Heart Failure and Depression-like Behaviour in Rats after Myocardial Infarction. PLoS ONE 2019, 14, e0217437. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Huang, T.; Zhang, H.; Li, X.; Shi, S.; Tian, X.; Huang, Z.; Zhang, R.; Liu, Z.; Cheng, Y. Formononetin Improves Cardiac Function and Depressive Behaviours in Myocardial Infarction with Depression by Targeting GSK-3β to Regulate Macrophage/Microglial Polarization. Phytomedicine 2023, 109, 154602. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Y.; Li, B.; Zhou, Y.; Guan, J.; Huang, F.; Wu, J.; Dong, Y.; Sun, P.; Tian, X.; et al. The Antidepressant Effect of Shexiang Baoxin Pills on Myocardial Infarction Rats with Depression May Be Achieved through the Inhibition of the NLRP3 Inflammasome Pathway. Brain Behav. 2024, 14, e3586. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Z.; Hou, J.; Shi, J.; Tang, Z.; Wang, C.; Zhao, H. Shuangxinfang Prevents S100A9-Induced Macrophage/Microglial Inflammation to Improve Cardiac Function and Depression-Like Behavior in Rats After Acute Myocardial Infarction. Front. Pharmacol. 2022, 13, 832590. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Z.; Wang, C.; Tang, Z.; Zhao, H. Psycho-Cardiology Therapeutic Effects of Shuangxinfang in Rats with Depression-Behavior Post Acute Myocardial Infarction: Focus on Protein S100A9 from Proteomics. Biomed. Pharmacother. 2021, 144, 112303. [Google Scholar] [CrossRef]
- Ge, Y.; Xu, W.; Zhang, L.; Liu, M. Ginkgolide B Attenuates Myocardial Infarction-Induced Depression-like Behaviors via Repressing IL-1β in Central Nervous System. Int. Immunopharmacol. 2020, 85, 106652. [Google Scholar] [CrossRef]
- Su, J.; Wang, J.; Ma, Y.; Li, Q.; Yang, Y.; Huang, L.; Wang, H.; Li, H.; Wang, Z.; Tong, J.; et al. Inflammation Associated with Chronic Heart Failure Leads to Enhanced Susceptibility to Depression. FEBS J. 2019, 286, 2769–2786. [Google Scholar] [CrossRef]
- Tang, X.; Liu, R.; Zhang, Y.; Zhu, L.; Shi, W.; Shan, Y.; Wu, S.; Li, Y.; Liu, G.; Ma, W. Downregulation of Interleukin-1 Beta via Jmjd3 Inhibition Improves Post-Myocardial Infarction Depression. Cardiovasc. Diagn. Ther. 2022, 12, 340–351. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Li, Y.; Liu, R.; Pan, J.; Tang, X.; Sun, S.; Liu, J.; Ma, W. Human Umbilical Cord Mesenchymal Stem Cells Ameliorate Depression by Regulating Jmjd3 and Microglia Polarization in Myocardial Infarction Mice. Psychopharmacology 2021, 238, 2973–2984. [Google Scholar] [CrossRef]
- Sorci, G.; Bianchi, R.; Riuzzi, F.; Tubaro, C.; Arcuri, C.; Giambanco, I.; Donato, R. S100B Protein, A Damage-Associated Molecular Pattern Protein in the Brain and Heart, and Beyond. Cardiovasc. Psychiatry Neurol. 2010, 2010, 656481. [Google Scholar] [CrossRef]
- Abel, W.M.; Scanlan, L.N.; Horne, C.E.; Crane, P.B. Factors Associated with Myocardial Infarction Reoccurrence. J. Cardiovasc. Nurs. 2022, 37, 359–367. [Google Scholar] [CrossRef]
- Mester, A.; Benedek, T.; Ratiu, M.; Morariu, M.; Benedek, A.; Hodas, R.; Opincariu, D.; Rat, N.; Chitu, M.; Benedek, I. 490 Persistently Increased Inflammatory Status in the First Days Post MI Is Associated with Larger Myocardial Scar, Higher Transmurality Extent and Deterioration of Ventricular Function at 1 Month. Eur. Heart J. Cardiovasc. Imaging 2019, 20, jez123.004. [Google Scholar] [CrossRef]
- Petyunina, O.V.; Kopytsya, M.P.; Vyshnevska, I.R. Interactions between Macrophage Inhibitor Factor and Emotional Distress in Patients with St-Segment Elevation Myocardial Infarction. Atherosclerosis 2021, 331, e69. [Google Scholar] [CrossRef]
- Qian, L.; Zhang, S.; Lin, C.; Lin, J.; Li, Z. Depression Exacerbates Myocardial Ischemia-Reperfusion Injury in Mice via CNR2 Gene and MIF-AMPK Signaling Pathway. Int. J. Cardiol. 2024, 416, 132505. [Google Scholar] [CrossRef]
- von Känel, R.; Rosselet, K.; Gessler, K.; Haeussler, A.; Aschmann, J.; Rodriguez, H.; Dzemali, O. Preoperative Depression and Anxiety as Predictors of Postoperative C-Reactive Protein Levels in Patients Undergoing Cardiac Surgery: A Prospective Observational Study. Swiss Med. Wkly. 2022, 152, 40018. [Google Scholar] [CrossRef] [PubMed]
- Min, J.J.; Nam, K.; Kim, T.K.; Kim, H.J.; Seo, J.H.; Hwang, H.Y.; Kim, K.B.; Murkin, J.M.; Hong, D.M.; Jeon, Y. Relationship between Early Postoperative C-Reactive Protein Elevation and Long-Term Postoperative Major Adverse Cardiovascular and Cerebral Events in Patients Undergoing off-Pump Coronary Artery Bypass Graft Surgery: A Retrospective Study. Br. J. Anaesth. 2014, 113, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Hariri, A.R.; Mattay, V.S.; Tessitore, A.; Kolachana, B.; Fera, F.; Goldman, D.; Egan, M.F.; Weinberger, D.R. Serotonin Transporter Genetic Variation and the Response of the Human Amygdala. Science 2002, 297, 400–403. [Google Scholar] [CrossRef]
- Lesch, K.P.; Bengel, D.; Heils, A.; Sabol, S.Z.; Greenberg, B.D.; Petri, S.; Benjamin, J.; Müller, C.R.; Hamer, D.H.; Murphy, D.L. Association of Anxiety-Related Traits with a Polymorphism in the Serotonin Transporter Gene Regulatory Region. Science 1996, 274, 1527–1531. [Google Scholar] [CrossRef]
- Caspi, A.; Sugden, K.; Moffitt, T.E.; Taylor, A.; Craig, I.W.; Harrington, H.; McClay, J.; Mill, J.; Martin, J.; Braithwaite, A.; et al. Influence of Life Stress on Depression: Moderation by a Polymorphism in the 5-HTT Gene. Science 2003, 301, 386–389. [Google Scholar] [CrossRef]
- Popp, S.; Schmitt-Böhrer, A.; Langer, S.; Hofmann, U.; Hommers, L.; Schuh, K.; Frantz, S.; Lesch, K.-P.; Frey, A. 5-HTT Deficiency in Male Mice Affects Healing and Behavior after Myocardial Infarction. J. Clin. Med. 2021, 10, 3104. [Google Scholar] [CrossRef]
- Luong, H.; Singh, S.; Patil, M.; Krishnamurthy, P. Cardiac Glycosaminoglycans and Structural Alterations during Chronic Stress-Induced Depression-like Behavior in Mice. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H2044–H2057. [Google Scholar] [CrossRef]
- Lockhart, M.; Wirrig, E.; Phelps, A.; Wessels, A. Extracellular Matrix and Heart Development. Birth Defects Res. A Clin. Mol. Teratol. 2011, 91, 535–550. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, P.; Zhang, X.; Li, J.-P. Heparan Sulfate Proteoglycans as Relays of Neuroinflammation. J. Histochem. Cytochem. 2018, 66, 305–319. [Google Scholar] [CrossRef]
- Baranyi, A.; Enko, D.; Meinitzer, A.; Von Lewinski, D.; Rothenhäusler, H.-B.; Harpf, L.; Traninger, H.; Obermayer-Pietsch, B.; Harb, B.M.; Schweinzer, M.; et al. Myeloperoxidase as a Potential Biomarker of Acute-Myocardial-Infarction-Induced Depression and Suppression of the Innate Immune System. Antioxidants 2022, 11, 2083. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, G.; Tao, S.; Xia, P.; Chaudhry, N.; Kaura, S.; Stone, S.S.; Liu, M. Ginkgo Biloba Extract Reduces Cardiac and Brain Inflammation in Rats Fed a HFD and Exposed to Chronic Mental Stress through NF-κB Inhibition. Mediat. Inflamm. 2022, 2022, 2408598. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, F.; Zhang, L.; Li, J.; Tang, S.; Li, X.; Peng, M.; Zhao, Q.; Zhu, X. A Bioinformatics Approach to Identifying the Biomarkers and Pathogenesis of Major Depressive Disorder Combined with Acute Myocardial Infarction. Am. J. Transl. Res. 2023, 15, 932–948. [Google Scholar]
- Wang, M.; Cheng, L.; Gao, Z.; Li, J.; Ding, Y.; Shi, R.; Xiang, Q.; Chen, X. Investigation of the Shared Molecular Mechanisms and Hub Genes between Myocardial Infarction and Depression. Front. Cardiovasc. Med. 2023, 10, 1203168. [Google Scholar] [CrossRef]
- Nurillaeva, N.M.; Abdumalikova, F.B. Predictive Importance of Psycho-Emotional Syndrome of Patients with Coronary Heart Disease in the Violation of Platelet Hemostatic System. Atherosclerosis 2021, 331, e204. [Google Scholar] [CrossRef]
- von Känel, R.; Pazhenkottil, A.P.; Meister-Langraf, R.E.; Znoj, H.; Schmid, J.-P.; Zuccarella-Hackl, C.; Barth, J.; Schnyder, U.; Princip, M. Longitudinal Association between Cognitive Depressive Symptoms and D-Dimer Levels in Patients Following Acute Myocardial Infarction. Clin. Cardiol. 2021, 44, 1316–1325. [Google Scholar] [CrossRef] [PubMed]
- Deter, H.-C.; Orth-Gomér, K.; Rauch-Kröhnert, U.; Albus, C.; Ladwig, K.-H.; Söllner, W.; de Zwaan, M.; Grün, A.-S.; Ronel, J.; Hellmich, M.; et al. Depression, Anxiety, and Vital Exhaustion Are Associated with pro-Coagulant Markers in Depressed Patients with Coronary Artery Disease—A Cross Sectional and Prospective Secondary Analysis of the SPIRR-CAD Trial. J. Psychosom. Res. 2021, 151, 110659. [Google Scholar] [CrossRef] [PubMed]
- Rieder, M.; Gauchel, N.; Bode, C.; Duerschmied, D. Serotonin: A Platelet Hormone Modulating Cardiovascular Disease. J. Thromb. Thrombolysis 2021, 52, 42–47. [Google Scholar] [CrossRef]
- Hoirisch-Clapauch, S.; Nardi, A.E.; Gris, J.-C.; Brenner, B. Are the Antiplatelet and Profibrinolytic Properties of Selective Serotonin-Reuptake Inhibitors Relevant to Their Brain Effects? Thromb. Res. 2014, 134, 11–16. [Google Scholar] [CrossRef]
- Serebruany, V.L.; Gurbel, P.A.; O’Connor, C.M. Platelet Inhibition by Sertraline and N-Desmethylsertraline: A Possible Missing Link between Depression, Coronary Events, and Mortality Benefits of Selective Serotonin Reuptake Inhibitors. Pharmacol. Res. 2001, 43, 453–462. [Google Scholar] [CrossRef]
- Youssef, M.M.; Underwood, M.D.; Huang, Y.-Y.; Hsiung, S.-C.; Liu, Y.; Simpson, N.R.; Bakalian, M.J.; Rosoklija, G.B.; Dwork, A.J.; Arango, V.; et al. Association of BDNF Val66Met Polymorphism and Brain BDNF Levels with Major Depression and Suicide. Int. J. Neuropsychopharmacol. 2018, 21, 528–538. [Google Scholar] [CrossRef]
- Amadio, P.; Colombo, G.I.; Tarantino, E.; Gianellini, S.; Ieraci, A.; Brioschi, M.; Banfi, C.; Werba, J.P.; Parolari, A.; Lee, F.S.; et al. BDNFVal66met Polymorphism: A Potential Bridge between Depression and Thrombosis. Eur. Heart J. 2017, 38, 1426–1435. [Google Scholar] [CrossRef]
- Petyunina, O.V.; Kopytsya, M.P.; Berezin, A.E. Brain-Derived Neurotrophic Factor Gene Polymorphism in Post-ST-Elevation Myocardial Infarction Patients Undergoing Primary Percutaneous Intervention. Biomed. Res. Ther. 2020, 7, 3921–3932. [Google Scholar] [CrossRef]
- Sandrini, L.; Castiglioni, L.; Amadio, P.; Werba, J.P.; Eligini, S.; Fiorelli, S.; Zarà, M.; Castiglioni, S.; Bellosta, S.; Lee, F.S.; et al. Impact of BDNF Val66Met Polymorphism on Myocardial Infarction: Exploring the Macrophage Phenotype. Cells 2020, 9, 1084. [Google Scholar] [CrossRef] [PubMed]
- Sandrini, L.; Amadio, P.; Ieraci, A.; Malara, A.; Werba, J.P.; Soprano, P.M.; Balduini, A.; Zarà, M.; Bonomi, A.; Veglia, F.; et al. The A2-Adrenergic Receptor Pathway Modulating Depression Influences the Risk of Arterial Thrombosis Associated with BDNFVal66Met Polymorphism. Biomed. Pharmacother. 2022, 146, 112557. [Google Scholar] [CrossRef] [PubMed]
- Verhagen, M.; van der Meij, A.; van Deurzen, P.A.M.; Janzing, J.G.E.; Arias-Vásquez, A.; Buitelaar, J.K.; Franke, B. Meta-Analysis of the BDNF Val66Met Polymorphism in Major Depressive Disorder: Effects of Gender and Ethnicity. Mol. Psychiatry 2010, 15, 260–271. [Google Scholar] [CrossRef]
- Wang, Y.; Li, O.; Li, N.; Sha, Z.; Zhao, Z.; Xu, J. Association between the BDNF Val66Met Polymorphism and Major Depressive Disorder: A Systematic Review and Meta-Analysis. Front. Psychiatry 2023, 14, 1143833. [Google Scholar] [CrossRef]
- Geiser, F.; Urbach, A.S.; Harbrecht, U.; Conrad, R.; Pötzsch, B.; Amann, N.; Kiesewetter, K.; Sieke, A.; Wolffs, K.; Skowasch, D. Anxiety and Depression in Patients Three Months after Myocardial Infarction: Association with Markers of Coagulation and the Relevance of Age. J. Psychosom. Res. 2017, 99, 162–168. [Google Scholar] [CrossRef]
- Empana, J.P.; Sykes, D.H.; Luc, G.; Juhan-Vague, I.; Arveiler, D.; Ferrieres, J.; Amouyel, P.; Bingham, A.; Montaye, M.; Ruidavets, J.B.; et al. Contributions of Depressive Mood and Circulating Inflammatory Markers to Coronary Heart Disease in Healthy European Men: The Prospective Epidemiological Study of Myocardial Infarction (PRIME). Circulation 2005, 111, 2299–2305. [Google Scholar] [CrossRef]
- Piantella, S.; Dragano, N.; Marques, M.; McDonald, S.J.; Wright, B.J. Prospective Increases in Depression Symptoms and Markers of Inflammation Increase Coronary Heart Disease Risk—The Whitehall II Cohort Study. J. Psychosom. Res. 2021, 151, 110657. [Google Scholar] [CrossRef] [PubMed]
- Sama, J.; Vaidya, D.; Mukherjee, M.; Williams, M. Effects of Clinical Depression on Left Ventricular Dysfunction in Patients with Acute Coronary Syndrome. J. Thromb. Thrombolysis 2021, 51, 693–700. [Google Scholar] [CrossRef]
- Shimokhina, N.Y.; Savchenko, A.A.; Petrova, M.M. Peculiarities of Platelet Metabolism in Patients with Acute Coronary Syndrome with Anxiety-Depressive Disorders and Informativity of Enzymes in the Forecast of Development of Cardiovascular Complications. Pharmaceuticals 2020, 13, 169. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Wu, R.-M.; Li, H.-D.; Li, K.; Li, H.; Dang, W.-Z.; Feng, G.-Z.; Bao, W.-L.; Ye, G.; Shen, X.-Y. Yixin Ningshen Tablet Alleviates Comorbidity of Myocardial Infarction and Depression by Enhancing Myocardial Energy Metabolism and Increasing Availability of Monoamine Neurotransmitter. Chin. J. Integr. Med. 2022, 28, 586–593. [Google Scholar] [CrossRef]
- Hamdan, D.I.; Hafez, S.S.; Hassan, W.H.B.; Morsi, M.M.; Khalil, H.M.A.; Ahmed, Y.H.; Ahmed-Farid, O.A.; El-Shiekh, R.A. Chemical Profiles with Cardioprotective and Anti-Depressive Effects of Morus Macroura Miq. Leaves and Stem Branches Dichloromethane Fractions on Isoprenaline Induced Post-MI Depression. RSC Adv. 2022, 12, 3476–3493. [Google Scholar] [CrossRef]
- Liu, M.-Y.; Ren, Y.-P.; Zhang, L.-J.; Ding, J.Y. Pretreatment with Ginseng Fruit Saponins Affects Serotonin Expression in an Experimental Comorbidity Model of Myocardial Infarction and Depression. Aging Dis. 2016, 7, 680–686. [Google Scholar] [CrossRef] [PubMed]
- He, D.-F.; Ren, Y.-P.; Liu, M.-Y. Effects of Ginseng Fruit Saponins on Serotonin System in Sprague-Dawley Rats with Myocardial Infarction, Depression, and Myocardial Infarction Complicated with Depression. Chin. Med. J. 2016, 129, 2913–2919. [Google Scholar] [CrossRef] [PubMed]
- Manjarrez-Gutiérrez, G.; Ramírez-Campillo, R.; Borrayo-Sánchez, G.; Hernández-Rodríguez, J. Disturbance of Serotonergic Neurotransmission in Patients with Postmyocardial Infarction and Depression. Metab. Brain Dis. 2013, 28, 15–20. [Google Scholar] [CrossRef]
- Shaw, D.M.; Macsweeney, D.A.; Hewland, R.; Johnson, A.L. Tricyclic Antidepressants and Tryptophan in Unipolar Depression. Psychol. Med. 1975, 5, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Fraer, M.; Kilic, F. Serotonin: A Different Player in Hypertension-Associated Thrombosis. Hypertension 2015, 65, 942–948. [Google Scholar] [CrossRef]
- Brattelid, T.; Qvigstad, E.; Moltzau, L.R.; Bekkevold, S.V.S.; Sandnes, D.L.; Birkeland, J.A.K.; Skomedal, T.; Osnes, J.-B.; Sjaastad, I.; Levy, F.O. The Cardiac Ventricular 5-HT4 Receptor Is Functional in Late Foetal Development and Is Reactivated in Heart Failure. PLoS ONE 2012, 7, e45489. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, Y.S.; Kim, M.G.; Song, Y.-K.; Kim, Y.; Jang, H.; Kim, J.H.; Han, N.; Ji, E.; Kim, I.-W.; et al. The Effect of Selective Serotonin Reuptake Inhibitors on Major Adverse Cardiovascular Events: A Meta-Analysis of Randomized-Controlled Studies in Depression. Int. Clin. Psychopharmacol. 2019, 34, 9–17. [Google Scholar] [CrossRef]
- Rami, M.; Guillamat-Prats, R.; Rinne, P.; Salvermoser, M.; Ring, L.; Bianchini, M.; Blanchet, X.; Megens, R.T.A.; Döring, Y.; Walzog, B.; et al. Chronic Intake of the Selective Serotonin Reuptake Inhibitor Fluoxetine Enhances Atherosclerosis. Arter. Thromb. Vasc. Biol. 2018, 38, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-Y.; Ren, Y.-P.; Wei, W.-L.; Tian, G.-X.; Li, G. Changes of Serotonin (5-HT), 5-HT2A Receptor, and 5-HT Transporter in the Sprague-Dawley Rats of Depression, Myocardial Infarction and Myocardial Infarction Co-Exist with Depression. Chin. Med. J. 2015, 128, 1905–1909. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cai, Q.; Su, Z.; Chen, Z.; Cao, J.; Xu, F. Could Peripheral 5-HT Level Be Used as a Biomarker for Depression Diagnosis and Treatment? A Narrative Minireview. Front. Pharmacol. 2023, 14, 1149511, Erratum in Front. Pharmacol. 2023, 14, 1318433. [Google Scholar] [CrossRef] [PubMed]
- Mauler, M.; Herr, N.; Schoenichen, C.; Witsch, T.; Marchini, T.; Härdtner, C.; Koentges, C.; Kienle, K.; Ollivier, V.; Schell, M.; et al. Platelet Serotonin Aggravates Myocardial Ischemia/Reperfusion Injury via Neutrophil Degranulation. Circulation 2019, 139, 918–931. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, M.; Chen, H.; Li, Y.; Rao, P. Antidepressant in Treating Myocardial Infarction Complicated with Depression via 5-HT/Inflammation from Heart to Brain. J. Affect. Disord. 2025, 391, 120048. [Google Scholar] [CrossRef]
- Zhang, L.-J.; Zeng, X.-T.; Zhao, M.-J.; He, D.-F.; Liu, J.-Y.; Liu, M.-Y. The Important Effect of 5-HTTLPR Polymorphism on the Risk of Depression in Patients with Coronary Heart Disease: A Meta-Analysis. BMC Cardiovasc. Disord. 2020, 20, 141. [Google Scholar] [CrossRef]
- Doggrell, S.A. The Role of 5-HT on the Cardiovascular and Renal Systems and the Clinical Potential of 5-HT Modulation. Expert. Opin. Investig. Drugs 2003, 12, 805–823. [Google Scholar] [CrossRef]
- Fernandes, N.; Prada, L.; Rosa, M.M.; Ferreira, J.J.; Costa, J.; Pinto, F.J.; Caldeira, D. The Impact of SSRIs on Mortality and Cardiovascular Events in Patients with Coronary Artery Disease and Depression: Systematic Review and Meta-Analysis. Clin. Res. Cardiol. 2021, 110, 183–193. [Google Scholar] [CrossRef]
- Karlsen, H.R.; Løchen, M.-L.; Langvik, E. Antidepressant Use and Risk of Myocardial Infarction: A Longitudinal Investigation of Sex-Specific Associations in the HUNT Study. Psychosom. Med. 2023, 85, 26–33. [Google Scholar] [CrossRef]
- Li, M.; Kwok, M.K.; Fong, S.S.M.; Schooling, C.M. Effects of Tryptophan, Serotonin, and Kynurenine on Ischemic Heart Diseases and Its Risk Factors: A Mendelian Randomization Study. Eur. J. Clin. Nutr. 2020, 74, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Bahr, F.S.; Ricke-Hoch, M.; Ponimaskin, E.; Müller, F.E. Serotonin Receptors in Myocardial Infarction: Friend or Foe? ACS Chem. Neurosci. 2024, 15, 1619–1634. [Google Scholar] [CrossRef]
- Han, W.; Wei, Z.; Dang, R.; Guo, Y.; Zhang, H.; Geng, C.; Wang, C.; Feng, Q.; Jiang, P. Angiotensin-Ⅱ and Angiotensin-(1–7) Imbalance Affects Comorbidity of Depression and Coronary Heart Disease. Peptides 2020, 131, 170353. [Google Scholar] [CrossRef]
- Mathews, R.; Wang, T.Y.; Honeycutt, E.; Henry, T.D.; Zettler, M.; Chang, M.; Fonarow, G.C.; Peterson, E.D.; TRANSLATE-ACS Study Investigators. Persistence with Secondary Prevention Medications after Acute Myocardial Infarction: Insights from the TRANSLATE-ACS Study. Am. Heart J. 2015, 170, 62–69. [Google Scholar] [CrossRef]
- Lima, B.B.; Hammadah, M.; Kim, J.H.; Uphoff, I.; Shah, A.; Levantsevych, O.; Almuwaqqat, Z.; Moazzami, K.; Sullivan, S.; Ward, L.; et al. Association of Transient Endothelial Dysfunction Induced by Mental Stress With Major Adverse Cardiovascular Events in Men and Women with Coronary Artery Disease. JAMA Cardiol. 2019, 4, 988–996. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, R.; Felice, F.; Pini, S.; Mazzotta, G.; Bovenzi, F.M.; Bertoli, D.; Abelli, M.; Borelli, L.; Cardini, A.; Lari, L.; et al. Impact of Depression on Circulating Endothelial Progenitor Cells in Patients with Acute Coronary Syndromes: A Pilot Study. J. Cardiovasc. Med. 2014, 15, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Madva, E.N.; Celano, C.M.; Smith, D.M.; Januzzi, J.L.; Huffman, J.C. Recurrent versus New-Onset Depressive Symptoms: Relationships with Biomarkers of Cardiovascular Health Following Acute Coronary Syndrome. J. Psychosom. Res. 2021, 140, 110291. [Google Scholar] [CrossRef] [PubMed]
- Yammine, L.; Frazier, L.; Padhye, N.S.; Sanner, J.E.; Burg, M.M. Two-Year Prognosis after Acute Coronary Syndrome in Younger Patients: Association with Feeling Depressed in the Prior Year, and BDI-II Score and Endothelin-1. J. Psychosom. Res. 2017, 99, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Yammine, L.; Frazier, L.; Padhye, N.S.; Burg, M.M.; Meininger, J.C. Severe Depressive Symptoms Are Associated with Elevated Endothelin-1 in Younger Patients with Acute Coronary Syndrome. J. Psychosom. Res. 2014, 77, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Jazdzewski, K.; Murray, E.L.; Franssila, K.; Jarzab, B.; Schoenberg, D.R.; de la Chapelle, A. Common SNP in Pre-miR-146a Decreases Mature miR Expression and Predisposes to Papillary Thyroid Carcinoma. Proc. Natl. Acad. Sci. USA 2008, 105, 7269–7274. [Google Scholar] [CrossRef]
- Zhang, X.; Huo, Q.; Sun, W.; Zhang, C.; Wu, Z.; Xing, B.; Li, Q. Rs2910164 in microRNA-146a Confers an Elevated Risk of Depression in Patients with Coronary Artery Disease by Modulating the Expression of NOS1. Mol. Med. Rep. 2018, 18, 603–609. [Google Scholar] [CrossRef]
- Gao, S.-F.; Lu, Y.-R.; Shi, L.-G.; Wu, X.-Y.; Sun, B.; Fu, X.-Y.; Luo, J.-H.; Bao, A.-M. Nitric Oxide Synthase and Nitric Oxide Alterations in Chronically Stressed Rats: A Model for Nitric Oxide in Major Depressive Disorder. Psychoneuroendocrinology 2014, 47, 136–140. [Google Scholar] [CrossRef]
- Steinert, J.R.; Chernova, T.; Forsythe, I.D. Nitric Oxide Signaling in Brain Function, Dysfunction, and Dementia. Neuroscientist 2010, 16, 435–452. [Google Scholar] [CrossRef]
- Zhou, Q.-G.; Zhu, L.-J.; Chen, C.; Wu, H.-Y.; Luo, C.-X.; Chang, L.; Zhu, D.-Y. Hippocampal Neuronal Nitric Oxide Synthase Mediates the Stress-Related Depressive Behaviors of Glucocorticoids by Downregulating Glucocorticoid Receptor. J. Neurosci. 2011, 31, 7579–7590. [Google Scholar] [CrossRef]
- Pögün, S.; Kuhar, M.J. Regulation of Neurotransmitter Reuptake by Nitric Oxide. Ann. N. Y. Acad. Sci. 1994, 738, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, L.; Li, Y.; Meng, X.; Chi, Y.; Liu, M. Gut Microbiota and Its Metabolites: The Emerging Bridge Between Coronary Artery Disease and Anxiety and Depression? Aging Dis. 2024, 16, 1265–1284. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, X.; Lv, Y.; Yang, C.; Zhou, C.; Wang, L. Changes in Rats’ Gut Microbiota Composition Caused by Induced Chronic Myocardial Infarction Lead to Depression-Like Behavior. Front. Microbiol. 2021, 12, 641084. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Zhang, L.; Yang, C.; Wang, T.; Feng, L.; Peng, C.; Long, Y.; Dai, G.; Chang, L.; Wei, Y.; et al. Sotagliflozin Attenuates Cardiac Dysfunction and Depression-like Behaviors in Mice with Myocardial Infarction through the Gut-Heart-Brain Axis. Neurobiol. Dis. 2024, 199, 106598. [Google Scholar] [CrossRef]
- Sun, B.; Ma, T.; Li, Y.; Yang, N.; Li, B.; Zhou, X.; Guo, S.; Zhang, S.; Kwok, L.-Y.; Sun, Z.; et al. Bifidobacterium Lactis Probio-M8 Adjuvant Treatment Confers Added Benefits to Patients with Coronary Artery Disease via Target Modulation of the Gut-Heart/-Brain Axes. mSystems 2022, 7, e0010022. [Google Scholar] [CrossRef]
- Moludi, J.; Khedmatgozar, H.; Nachvak, S.M.; Abdollahzad, H.; Moradinazar, M.; Sadeghpour Tabaei, A. The Effects of Co-Administration of Probiotics and Prebiotics on Chronic Inflammation, and Depression Symptoms in Patients with Coronary Artery Diseases: A Randomized Clinical Trial. Nutr. Neurosci. 2022, 25, 1659–1668. [Google Scholar] [CrossRef]
- Gagnon, E.; Mitchell, P.L.; Manikpurage, H.D.; Abner, E.; Taba, N.; Esko, T.; Ghodsian, N.; Thériault, S.; Mathieu, P.; Arsenault, B.J. Impact of the Gut Microbiota and Associated Metabolites on Cardiometabolic Traits, Chronic Diseases and Human Longevity: A Mendelian Randomization Study. J. Transl. Med. 2023, 21, 60. [Google Scholar] [CrossRef]
- Verma, H.; Shivavedi, N.; Tej, G.N.V.C.; Kumar, M.; Nayak, P.K. Prophylactic Administration of Rosmarinic Acid Ameliorates Depression-Associated Cardiac Abnormalities in Wistar Rats: Evidence of Serotonergic, Oxidative, and Inflammatory Pathways. J. Biochem. Mol. Toxicol. 2022, 36, e23160. [Google Scholar] [CrossRef]
- Khalil, H.M.A.; Mahmoud, D.B.; El-Shiekh, R.A.; Bakr, A.F.; Boseila, A.A.; Mehanna, S.; Naggar, R.A.; Eliwa, H.A. Antidepressant and Cardioprotective Effects of Self-Nanoemulsifying Self-Nanosuspension Loaded with Hypericum Perforatum on Post-Myocardial Infarction Depression in Rats. AAPS PharmSciTech 2022, 23, 243. [Google Scholar] [CrossRef] [PubMed]
- Sylvia, L.G.; Gold, A.K.; Rakhilin, M.; Amado, S.; Modrow, M.F.; Albury, E.A.; George, N.; Peters, A.T.; Selvaggi, C.A.; Horick, N.; et al. Healthy Hearts Healthy Minds: A Randomized Trial of Online Interventions to Improve Physical Activity. J. Psychosom. Res. 2023, 164, 111110. [Google Scholar] [CrossRef]
- Chen, X.; Zeng, M.; Chen, C.; Zhu, D.; Chen, L.; Jiang, Z. Efficacy of Psycho-Cardiology Therapy in Patients with Acute Myocardial Infarction Complicated with Mild Anxiety and Depression. Front. Cardiovasc. Med. 2022, 9, 1031255. [Google Scholar] [CrossRef]
- Shi, W.; Ghisi, G.L.M.; Zhang, L.; Hyun, K.; Pakosh, M.; Gallagher, R. A Systematic Review, Meta-Analysis, and Meta-Regression of Patient Education for Secondary Prevention in Patients with Coronary Heart Disease: Impact on Psychological Outcomes. Eur. J. Cardiovasc. Nurs. 2022, 21, 643–654. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, H. Effects of Comprehensive Nursing Intervention Based on Self-Disclosure on Improving Alexithymia in Elder Patients with Coronary Heart Disease. BMC Nurs. 2022, 21, 216. [Google Scholar] [CrossRef] [PubMed]
- Westas, M.; Lundgren, J.; Andersson, G.; Mourad, G.; Johansson, P. Effects of Internet-Delivered Cognitive Behavioural Therapy Adapted for Patients with Cardiovascular Disease and Depression: A Long-Term Follow-up of a Randomized Controlled Trial at 6 and 12 Months Posttreatment. Eur. J. Cardiovasc. Nurs. 2022, 21, 559–567. [Google Scholar] [CrossRef]
- Chen, B.; Wen, J.; You, D.; Zhang, Y. Implication of Cognitive-Behavioral Stress Management on Anxiety, Depression, and Quality of Life in Acute Myocardial Infarction Patients after Percutaneous Coronary Intervention: A Multicenter, Randomized, Controlled Study. Ir. J. Med. Sci. 2024, 193, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Chew, T.R.; Yeo, T.M.; Teo, J.Y.C.; Seah, C.W.A.; Soh, C.S.Q.; Meng, J.; Wang, W. Effectiveness of Psychological Interventions in Reducing Post-Traumatic Stress among Post-Myocardial Infarction Patients: A Systematic Review and Meta-Analysis. Eur. J. Cardiovasc. Nurs. 2025, 24, 375–386. [Google Scholar] [CrossRef]
- Carli, V.; Petros, N.G.; Hadlaczky, G.; Vitcheva, T.; Berchialla, P.; Bianchi, S.; Carletto, S.; Christinaki, E.; Citi, L.; Dinis, S.; et al. The NEVERMIND E-Health System in the Treatment of Depressive Symptoms among Patients with Severe Somatic Conditions: A Multicentre, Pragmatic Randomised Controlled Trial. EClinicalMedicine 2022, 48, 101423. [Google Scholar] [CrossRef]
- Ni, R.; Liu, M.; Huang, S.; Yang, J. Effects of eHealth Interventions on Quality of Life and Psychological Outcomes in Cardiac Surgery Patients: Systematic Review and Meta-Analysis. J. Med. Internet Res. 2022, 24, e40090. [Google Scholar] [CrossRef]
- Boszko, M.; Krzowski, B.; Peller, M.; Hoffman, P.; Żurawska, N.; Skoczylas, K.; Osak, G.; Kołtowski, Ł.; Grabowski, M.; Opolski, G.; et al. Impact of AfterAMI Mobile App on Quality of Life, Depression, Stress and Anxiety in Patients with Coronary Artery Disease: Open Label, Randomized Trial. Life 2023, 13, 2015. [Google Scholar] [CrossRef]
- Deng, L.; Wu, Q.; Ding, F.; Liu, Y.; Shen, J.; Lin, Y.; Shi, K.; Zeng, B.; Wu, L.; Tong, H. The Effect of Telemedicine on Secondary Prevention of Atherosclerotic Cardiovascular Disease: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 1020744. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Yoo, J.; Han, K.; Jeon, H.J. Physical Activity and Cardiovascular Health in Depression: Links between Changes in Physical Activity and Cardiovascular Risk. Gen. Hosp. Psychiatry 2022, 78, 35–41. [Google Scholar] [CrossRef]
- Terada, T.; Cotie, L.M.; Tulloch, H.; Mistura, M.; Vidal-Almela, S.; O’Neill, C.D.; Reid, R.D.; Pipe, A.; Reed, J.L. Sustained Effects of Different Exercise Modalities on Physical and Mental Health in Patients with Coronary Artery Disease: A Randomized Clinical Trial. Can. J. Cardiol. 2022, 38, 1235–1243. [Google Scholar] [CrossRef]
- Reed, J.L.; Terada, T.; Cotie, L.M.; Tulloch, H.E.; Leenen, F.H.; Mistura, M.; Hans, H.; Wang, H.-W.; Vidal-Almela, S.; Reid, R.D.; et al. The Effects of High-Intensity Interval Training, Nordic Walking and Moderate-to-Vigorous Intensity Continuous Training on Functional Capacity, Depression and Quality of Life in Patients with Coronary Artery Disease Enrolled in Cardiac Rehabilitation: A Randomized Controlled Trial (CRX Study). Prog. Cardiovasc. Dis. 2022, 70, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Dong, Y.; Yu, H.; Liu, X.; Gu, Y.; Song, J.; Ouyang, P.; Hong, Z. The Effect of Sitting Baduanjin in Patients with ST-Segment Elevation Acute Myocardial Infarction after Percutaneous Coronary Intervention: A Quasi-Experimental Study. Heart Lung 2024, 66, 78–85. [Google Scholar] [CrossRef]
- Lyu, S.; Wang, H.; Wei, Q.; Cui, M.; Li, Y.; Chen, Z.; Zhang, J.; Peng, F. Effects of Tai Chi Cardiac Rehabilitation Program on Anxiety and Depression in Patients with Coronary Heart Disease: A Randomized Controlled Clinical Trial. Eur. J. Integr. Med. 2022, 53, 102147. [Google Scholar] [CrossRef]
- Kalra, S.; Miraj, M.; Ajmera, P.; Shaik, R.A.; Seyam, M.K.; Shawky, G.M.; Alasiry, S.M.; Mohamed, E.H.; Alasiri, H.M.; Alzhrani, M.; et al. Effects of Yogic Interventions on Patients Diagnosed With Cardiac Diseases. A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 942740. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A. Yogic Exercise—Its Effect on Blood Pressure and Depression Score in Myocardial Infarction. J. Hypertens. 2023, 41, e300. [Google Scholar] [CrossRef]
- Zhang, L.; Bao, Y.; Tao, S.; Zhao, Y.; Liu, M. The Association between Cardiovascular Drugs and Depression/Anxiety in Patients with Cardiovascular Disease: A Meta-Analysis. Pharmacol. Res. 2022, 175, 106024. [Google Scholar] [CrossRef]
- Molero, Y.; Kaddoura, S.; Kuja-Halkola, R.; Larsson, H.; Lichtenstein, P.; D’Onofrio, B.M.; Fazel, S. Associations between β-Blockers and Psychiatric and Behavioural Outcomes: A Population-Based Cohort Study of 1.4 Million Individuals in Sweden. PLoS Med. 2023, 20, e1004164. [Google Scholar] [CrossRef]
- Leissner, P.; Mars, K.; Humphries, S.; Karlström, P.; Yndigegn, T.; Jernberg, T.; Hofmann, R.; Held, C.; Olsson, E.M.G. Short- and Long-Term Effects of Beta-Blockers on Symptoms of Anxiety and Depression in Patients with Myocardial Infarction and Preserved Left Ventricular Function: A Pre-Specified Quality of Life Sub-Study from the REDUCE-AMI Trial. Eur. Heart J. Acute Cardiovasc. Care 2024, 13, 789–797. [Google Scholar] [CrossRef]
- Kim, J.H.; Song, Y.-K.; Jang, H.Y.; Shin, J.-Y.; Lee, H.-Y.; Ahn, Y.M.; Oh, J.M.; Kim, I.-W. Major Adverse Cardiovascular Events in Antidepressant Users Within Patients With Ischemic Heart Diseases: A Nationwide Cohort Study. J. Clin. Psychopharmacol. 2020, 40, 475–481. [Google Scholar] [CrossRef]
- Biffi, A.; Rea, F.; Scotti, L.; Lucenteforte, E.; Vannacci, A.; Lombardi, N.; Chinellato, A.; Onder, G.; Vitale, C.; Cascini, S.; et al. Antidepressants and the Risk of Cardiovascular Events in Elderly Affected by Cardiovascular Disease: A Real-Life Investigation From Italy. J. Clin. Psychopharmacol. 2020, 40, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lu, N.; Liu, M. Selective Serotonin Reuptake Inhibitors Regulate the Interrelation between 5-HT and Inflammation after Myocardial Infarction. BMC Cardiovasc. Disord. 2023, 23, 342. [Google Scholar] [CrossRef] [PubMed]
- Gutlapalli, S.D.; Prakash, K.; Swarnakari, K.M.; Bai, M.; Manoharan, M.P.; Raja, R.; Jamil, A.; Csendes, D.; Desai, A.; Desai, D.M.; et al. The Risk of Fatal Arrhythmias Associated With Sertraline in Patients With Post-Myocardial Infarction Depression. Cureus 2022, 14, e28946. [Google Scholar] [CrossRef]
- Desai, R.; Park, H.; Brown, J.D.; Mohandas, R.; Smith, S.M. Norepinephrine Reuptake Inhibitors and Risk of Antihypertensive Treatment Intensification and Major Adverse Cardiovascular Events in Patients with Stable Hypertension and Depression. Pharmacotherapy 2022, 42, 472–482. [Google Scholar] [CrossRef]
- Gutlapalli, S.D.; Lavu, V.K.; Mohamed, R.A.; Huang, R.; Potla, S.; Bhalla, S.; Al Qabandi, Y.; Nandula, S.A.; Boddepalli, C.S.; Hamid, P. The Risk of Fatal Arrhythmias in Post-Myocardial Infarction Depression in Association With Venlafaxine. Cureus 2022, 14, e29107. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, Y.-B.; Lee, J.; Kang, D.; Kim, G.; Jin, S.-M.; Kim, J.H.; Hur, K.Y.; Jeon, H.J. Association between Depression, Antidepressant Use, and the Incidence of Atherosclerotic Cardiovascular Diseases. J. Affect. Disord. 2024, 352, 214–221. [Google Scholar] [CrossRef]
- Gagné, M.-A.; Frégeau, G.; Godbout, R.; Rousseau, G. The Role of Probiotics in Modulating Myocardial Infarction and Depression-like Symptoms: A Study on Sex-Specific Responses. Biomedicines 2024, 12, 2511. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Teng, Y.; Li, Y.; Lai, S.; Wu, Y.; Chen, S.; Li, T.; Han, X.; Zhou, H.; Wang, Y.; et al. Evidence and Characteristics of Traditional Chinese Medicine for Coronary Heart Disease Patients With Anxiety or Depression: A Meta-Analysis and Systematic Review. Front. Pharmacol. 2022, 13, 854292. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, F.; Vafa, M.; Ebrahimkhani, A.; Găman, M.-A.; Sezavar Seyedi Jandaghi, S.H. Effects of Quercetin Supplementation on Endothelial Dysfunction Biomarkers and Depression in Post-Myocardial Infarction Patients: A Double-Blind, Placebo-Controlled, Randomized Clinical Trial. Clin. Nutr. ESPEN 2023, 56, 73–80. [Google Scholar] [CrossRef] [PubMed]
| Effect of MI on Depression | Effects of Depression on MI | |||||
|---|---|---|---|---|---|---|
| Phenotypes | Mechanisms | Interventions | Phenotypes | Mechanisms | Interventions | |
| ANS | HRV ↓ [79] | GRK2 ↑→ HRV ↓, depression and MI [83,84,85,86] | Paroxetine [86] | HRV ↓, plasma epinephrine and NE levels ↑ [87,88,89,90] | S1R↓ (depression-related) → ANS dysfunction → Cardiac function and structure ↓ [91] | |
| HPA axis | GC ↑, adrenal hypertrophy and flattened DCS [97,98,99] | MI→ Inflammation (mRNA expression of IL-12A and TNF-α in the hypothalamus ↑) → HPA axis dysregulation → Depression [102]. MI → Trigger HPA axis dysfunction after MR/GR imbalance → Depression [100] Impaired CR signalling [100,103,108,110] | GC ↑ and flattened DCS [104,105,106,107] | Depression → Abnormal activation of the HPA axis → GR-mediated NF-κB/TLR4 signalling pathway ↑ → CVD [108,109] Impaired CR signalling [100,103,108,110] | Rosmarinic acid [212] | |
| Inflammation | Hs-CRP, NLRP3, TNF-α, IL-17A, IL-1β, IL-2, IL-6, NLR and microglia ↑ [123,124,125,126,127,128] | GSK-3β/Notch1 and GSK-3β/C/EBPα signalling pathways induce macrophage/microglial polarisation [129] NLRP3-mediated GSDMD-induced microglial pyroptosis [130] S100A9-mediated macrophage/microglial inflammation [131,132] S100B, JMJD3 or STAT3-mediated microglial polarization [133,134,135,136,137] | Minocycline [128] Formononetin [129] HP.SNESNS [213] GBE [152] Ginkgolide B [133] | Occurrence: SII and hs-CRP [58,138] Prognosis: MIF and CRP [140,141,142,143] | Depression → 5-HTT ↓ →inflammation (TGF-β ↑, TNF-α ↑, IL-6 ↑ and MMP-2 ↑) → The early healing in MI ↓ [144,145,146,147] Depression → PG/GAG structural and functional changes → Heart disease [148,149,150] | Formononetin [129] Rosmarinic acid [212] |
| Platelet activation and coagulation activation | Platelet activation: the average PLT, PDW and MPV ↑ [155]. Coagulation activation: TF and D-dimer ↑ [156,157] | Depression → 5-HT ↓ → Platelet activation → CVD ↑ [158,159,160] The BDNF Val66Met gene polymorphism: BDNF Met → SIRT1 ↓/SorCS2 ↑ pathway → regulates coagulation (TF ↑ and gelsolin ↑) and inflammation (A1AT ↑), promotes thrombosis and increases CVD risk [162] BDNF Met→ NE/α2A-ADR pathway ↑ → Thrombosis → CVD risk ↑ [165] | Sertraline and N-nitrosodimethylamine [160] Desipramine [165] Rauwolscine [165] | |||
| RAAS | Ang-(1-7) ↓ Ang-Ⅱ/Ang-(1-7) ↑ [193] | ACE2 genetic polymorphisms: ACE2 genetic polymorphisms in patients with CHD → ACE2 ↓ → Ang II/Ang-(1-7) imbalance → Depression [193] | Depression reduces adherence to ACEIs in patients with MI [194]. | |||
| Endothelial dysfunction | Vasodilatory capacity ↓, EPC ↓ and ET-1 ↑ [195,196,197]. | |||||
| MiRNA | MiR-146a rs2910164 C allele → MiR-146a ↓ (patients with CAD) → NOS1 ↑ → Depression ↓ [200,201,202,203,204,205] | |||||
| Phenotypes | Mechanisms | Interventions | ||||
| Tryptophan metabolism | 5-HT levels in serum and brain tissue ↓ [131,173,174,175] 5-HT2A receptors expression in brain tissue ↓ [175,176] Altered plasma-free L-Trp scores alongside abnormal IDAEP [177] The mRNA expression of tryptophan metabolism-related enzymes such as IDO1, KMO and KYNU in the hippocampus↑ [173] Serum KYN levels ↑ [134] KYN-to-tryptophan ratio ↑ [134] | HPA axis [134,173] Inflammation (may be mediated by S100A9, GSK-3β, TNF-α/TNFR/NF-κB signalling pathway or neutrophil degranulation) → The inflammation in the infarcted cardiac tissue ↑ and depression ↑ [129,131,134,173,185,186] Regulation of 5-HTT gene polymorphisms → Depression and 5-HTT ↓ → Inflammation ↑ → Cardiac outcomes ↓ [144,145,147,187] 5-HT ↓ → Thrombosis ↑ → MI risk ↑ [158,159,160,188,189,190] | SSRIs [189,190] Dichloromethane fractions of Morus macroura [174] Rosmarinic acid [212] | |||
| Gut microbiome | Staphylococcus, Escherichia coli, Helicobacter pylori, Shigella and Streptococcus ↑ [206] Prevotella, Lactobacillus, E. pumilus, Collinsella, Bifidobacterium and Actinobacteriaceae ↓ [206] AUC values for Streptococcaceae and Aspergillus ↑ [207] | The gut–heart–brain axis | Sotagliflozin, [208] probiotic and prebiotic inulin [209,210] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Z.; Yang, Q.; Yang, F.; Yang, Y.; Yang, X.; Yu, Y.; Cai, Y.; Fan, X.; Bai, R. The Bidirectional Relationship Between Myocardial Infarction and Depression: Risk Factors, Mechanisms, and Interventions. Biomedicines 2025, 13, 2838. https://doi.org/10.3390/biomedicines13112838
Cui Z, Yang Q, Yang F, Yang Y, Yang X, Yu Y, Cai Y, Fan X, Bai R. The Bidirectional Relationship Between Myocardial Infarction and Depression: Risk Factors, Mechanisms, and Interventions. Biomedicines. 2025; 13(11):2838. https://doi.org/10.3390/biomedicines13112838
Chicago/Turabian StyleCui, Zhuorui, Qiaoning Yang, Furong Yang, Yankai Yang, Xuexin Yang, Yanqiao Yu, Yajie Cai, Xiaodi Fan, and Ruina Bai. 2025. "The Bidirectional Relationship Between Myocardial Infarction and Depression: Risk Factors, Mechanisms, and Interventions" Biomedicines 13, no. 11: 2838. https://doi.org/10.3390/biomedicines13112838
APA StyleCui, Z., Yang, Q., Yang, F., Yang, Y., Yang, X., Yu, Y., Cai, Y., Fan, X., & Bai, R. (2025). The Bidirectional Relationship Between Myocardial Infarction and Depression: Risk Factors, Mechanisms, and Interventions. Biomedicines, 13(11), 2838. https://doi.org/10.3390/biomedicines13112838







