All-Trans Retinoic Acid Impacts Early Palatal Shelves Development via the Wnt and TGF-β Signaling Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and All-Trans Retinoic Acid Exposure
2.2. Tissue Dissociation and Single-Cell Sequencing
2.3. Data Quality Control and Clustering
2.4. Differentially Expressed Genes and Pathway Enrichment Analysis
2.5. Estimation of Pathway Activity
2.6. Analysis of Intercellular Communication
2.7. Cell Cycle Analysis
2.8. hdWGCNA Analysis
2.9. Simulation of Hub Gene Knockouts
2.10. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.11. Western Blot
2.12. Statistical Analysis
3. Results
3.1. All-Trans Retinoic Acid Significantly Impacts the Mesenchymal and Epithelial Cells in the Initial Phase of Mouse Palatal Shelves Development
3.2. All-Trans Retinoic Acid Exposure May Influence Mesenchymal and Epithelial Cells by Modulating Wnt and TGF-β Pathways
3.3. All-Trans Retinoic Acid Inhibits the Proliferation and Migration of Mesenchymal and Epithelial Cells
3.4. All-Trans Retinoic Acid Intricately Regulates the Wnt and Bmp Pathways Between Mesenchymal and Epithelial Cells
3.5. hdWGCNA Analysis Revealed Key Gene Modules and Core Transcription Factors Related to the All-Trans Retinoic Acid-Exposed Group
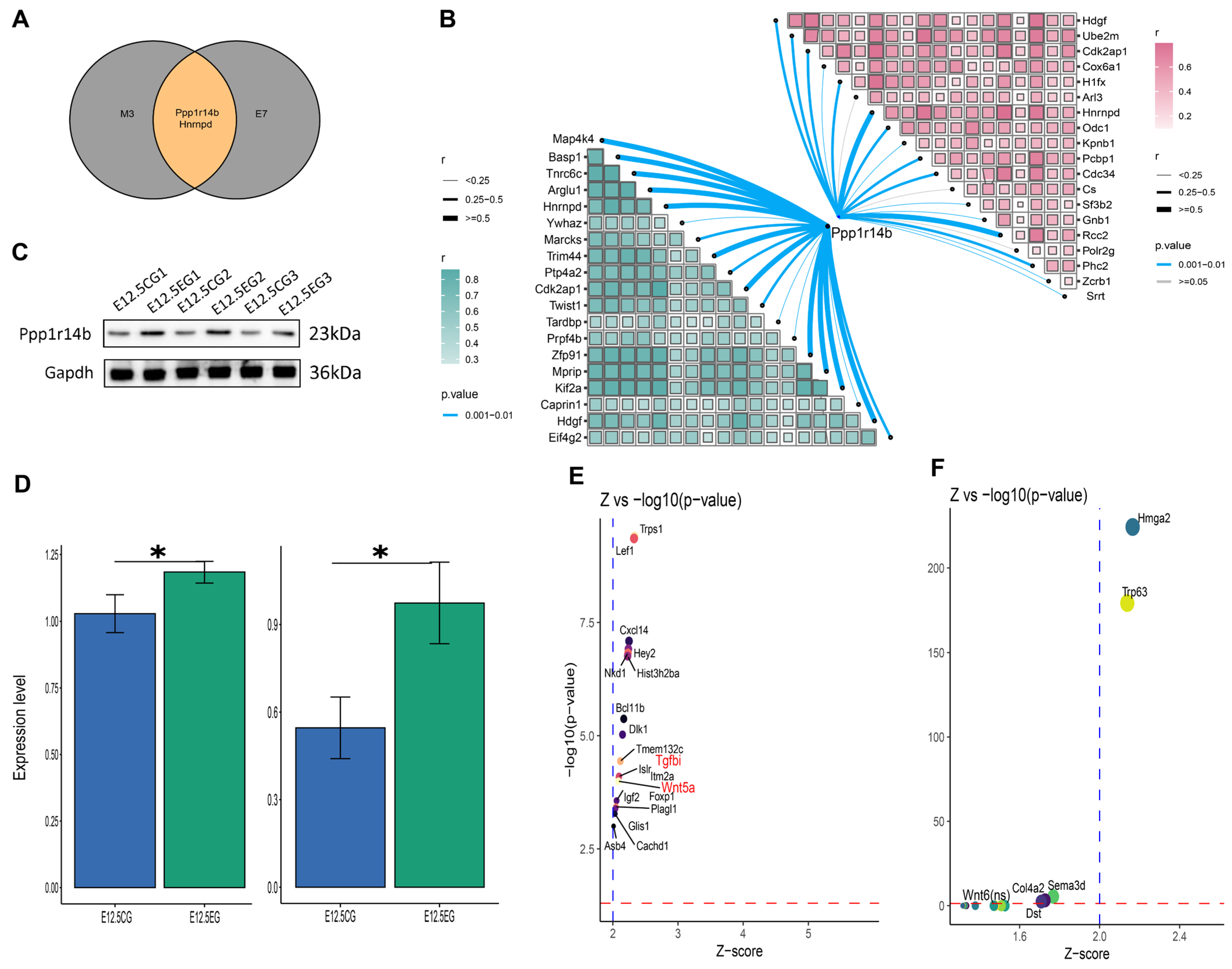
3.6. Ppp1r114b Is an Important Gene Mediating All-Trans Retinoic Acid’s Effect on Palatal Shelves Development
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| atRA | All-trans retinoic acid |
| E12.5 | embryonic day 12.5 |
| E12.5–E16.5 | embryonic days 12.5–16.5 |
| UMI | unique molecular identifier |
| DEGs | Differentially expressed genes |
| GOBP | Gene Ontology biological process |
| GOMF | Gene Ontology molecular function |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| qRT-PCR | Quantitative Real-Time Polymerase Chain Reaction |
| SDS-PAGE | Sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| PVDF | Polyvinylidene Fluoride |
| PCP | planar cell polarity |
| JNK | c-Jun N-terminal kinase |
References
- Kam, R.K.; Deng, Y.; Chen, Y.; Zhao, H. Retinoic acid synthesis and functions in early embryonic development. Cell Biosci. 2012, 2, 11. [Google Scholar] [CrossRef]
- Bush, J.O.; Jiang, R. Palatogenesis: Morphogenetic and molecular mechanisms of secondary palate development. Development 2012, 139, 231–243. [Google Scholar] [CrossRef]
- Donofrio, M.T.; Moon-Grady, A.J.; Hornberger, L.K.; Copel, J.A.; Sklansky, M.S.; Abuhamad, A.; Cuneo, B.F.; Huhta, J.C.; Jonas, R.A.; Krishnan, A.; et al. Diagnosis and treatment of fetal cardiac disease: A scientific statement from the American Heart Association. Circulation 2014, 129, 2183–2242. [Google Scholar] [CrossRef] [PubMed]
- Lammer, E.J.; Chen, D.T.; Hoar, R.M.; Agnish, N.D.; Benke, P.J.; Braun, J.T.; Curry, C.J.; Fernhoff, P.M.; Grix, A.W., Jr.; Lott, I.T.; et al. Retinoic acid embryopathy. N. Engl. J. Med. 1985, 313, 837–841. [Google Scholar] [CrossRef]
- Wu, C.C.; Huang, F.; Hsieh, C.H.; Fu, C.P.; Tsai, Y.L.; Lai, J.P. Velar Closing Ratio as a Predictor for the Verlopharyngeal Function After Double Opposing Z-Plasty for Postpalatoplasty Velopharyngeal Insufficiency in Patients with Cleft Palate. Cleft Palate Craniofac. J. 2021, 58, 407–413. [Google Scholar] [CrossRef]
- Ize-Iyamu, I.N.; Saheeb, B.D. Feeding intervention in cleft lip and palate babies: A practical approach to feeding efficiency and weight gain. Int. J. Oral Maxillofac. Surg. 2011, 40, 916–919. [Google Scholar] [CrossRef]
- Phippen, G. Articulating the issues: Speech assessment and intervention in cleft lip and palate. Br. Dent. J. 2023, 234, 912–917. [Google Scholar] [CrossRef]
- Mossey, P.A.; Little, J.; Munger, R.G.; Dixon, M.J.; Shaw, W.C. Cleft lip and palate. Lancet 2009, 374, 1773–1785. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, X.H.; Su, C.N.; Qiao, W.W.; Gao, Q.; Sun, X.F.; Meng, L.Y. RNA-seq analysis of palatal transcriptome changes in all-trans retinoic acid-induced cleft palate of mice. Environ. Toxicol. Pharmacol. 2020, 80, 103438. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, Z.; Zhao, J.; Ma, Y.; Wang, Y.; Yin, N.; Song, T. Spatiotemporal Evolution of Developing Palate in Mice. J. Dent. Res. 2024, 103, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Stuart, T.; Kowalski, M.H.; Choudhary, S.; Hoffman, P.; Hartman, A.; Srivastava, A.; Molla, G.; Madad, S.; Fernandez-Granda, C.; et al. Dictionary learning for integrative, multimodal and scalable single-cell analysis. Nat. Biotechnol. 2024, 42, 293–304. [Google Scholar] [CrossRef]
- Kerseviciute, I.; Gordevicius, J. aPEAR: An R package for autonomous visualization of pathway enrichment networks. Bioinformatics 2023, 39, btad672. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Badia, I.M.P.; Vélez Santiago, J.; Braunger, J.; Geiss, C.; Dimitrov, D.; Müller-Dott, S.; Taus, P.; Dugourd, A.; Holland, C.H.; Ramirez Flores, R.O.; et al. decoupleR: Ensemble of computational methods to infer biological activities from omics data. Bioinform. Adv. 2022, 2, vbac016. [Google Scholar] [CrossRef]
- Jin, S.; Plikus, M.V.; Nie, Q. CellChat for systematic analysis of cell-cell communication from single-cell transcriptomics. Nat. Protoc. 2025, 20, 180–219. [Google Scholar] [CrossRef] [PubMed]
- Browaeys, R.; Saelens, W.; Saeys, Y. NicheNet: Modeling intercellular communication by linking ligands to target genes. Nat. Methods 2020, 17, 159–162. [Google Scholar] [CrossRef]
- Zheng, S.C.; Stein-O’Brien, G.; Augustin, J.J.; Slosberg, J.; Carosso, G.A.; Winer, B.; Shin, G.; Bjornsson, H.T.; Goff, L.A.; Hansen, K.D. Universal prediction of cell-cycle position using transfer learning. Genome Biol. 2022, 23, 41. [Google Scholar] [CrossRef]
- Morabito, S.; Reese, F.; Rahimzadeh, N.; Miyoshi, E.; Swarup, V. hdWGCNA identifies co-expression networks in high-dimensional transcriptomics data. Cell Rep. Methods 2023, 3, 100498. [Google Scholar] [CrossRef] [PubMed]
- Osorio, D.; Zhong, Y.; Li, G.; Xu, Q.; Yang, Y.; Tian, Y.; Chapkin, R.S.; Huang, J.Z.; Cai, J.J. scTenifoldKnk: An efficient virtual knockout tool for gene function predictions via single-cell gene regulatory network perturbation. Patterns 2022, 3, 100434. [Google Scholar] [CrossRef]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinform. 2016, 54, 1.30.31–1.30.33. [Google Scholar] [CrossRef] [PubMed]
- Hyakusoku, H.; Sano, D.; Takahashi, H.; Hatano, T.; Isono, Y.; Shimada, S.; Ito, Y.; Myers, J.N.; Oridate, N. JunB promotes cell invasion, migration and distant metastasis of head and neck squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2016, 35, 6. [Google Scholar] [CrossRef]
- Fritzmann, J.; Morkel, M.; Besser, D.; Budczies, J.; Kosel, F.; Brembeck, F.H.; Stein, U.; Fichtner, I.; Schlag, P.M.; Birchmeier, W. A colorectal cancer expression profile that includes transforming growth factor beta inhibitor BAMBI predicts metastatic potential. Gastroenterology 2009, 137, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Yang, K.M.; Park, Y.; Hong, E.; Hong, C.P.; Park, J.; Pang, K.; Lee, J.; Park, B.; Lee, S.; et al. Identification of Epithelial-Mesenchymal Transition-related Target Genes Induced by the Mutation of Smad3 Linker Phosphorylation. J. Cancer Prev. 2018, 23, 1–9. [Google Scholar] [CrossRef]
- Kim, N.; Ryu, H.; Kim, S.; Joo, M.; Jeon, H.J.; Lee, M.W.; Song, I.C.; Kim, M.N.; Kim, J.M.; Lee, H.J. CXCR7 promotes migration and invasion in head and neck squamous cell carcinoma by upregulating TGF-β1/Smad2/3 signaling. Sci. Rep. 2019, 9, 18100. [Google Scholar] [CrossRef]
- Qiu, Z.; Liang, N.; Huang, Q.; Sun, T.; Xue, H.; Xie, T.; Wang, X.; Wang, Q. Downregulation of DUSP9 Promotes Tumor Progression and Contributes to Poor Prognosis in Human Colorectal Cancer. Front. Oncol. 2020, 10, 547011. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, J.; Hong, L.; Zhou, Y. EGFLAM correlates with cell proliferation, migration, invasion and poor prognosis in glioblastoma. Cancer Biomark. 2019, 24, 343–350. [Google Scholar] [CrossRef]
- Tanaka, M.; Koyama, T.; Sakurai, T.; Kamiyoshi, A.; Ichikawa-Shindo, Y.; Kawate, H.; Liu, T.; Xian, X.; Imai, A.; Zhai, L.; et al. The endothelial adrenomedullin-RAMP2 system regulates vascular integrity and suppresses tumour metastasis. Cardiovasc. Res. 2016, 111, 398–409. [Google Scholar] [CrossRef]
- Huang, T.; Zhang, Y.; Wu, Y.; Han, X.; Li, L.; Guo, Z.; Li, K.; Xin, Y.; Wang, W. CEBPB dampens the cuproptosis sensitivity of colorectal cancer cells by facilitating the PI3K/AKT/mTOR signaling pathway. Saudi J. Gastroenterol. 2024, 30, 381–388. [Google Scholar] [CrossRef]
- Loubeau, G.; Boudra, R.; Maquaire, S.; Lours-Calet, C.; Beaudoin, C.; Verrelle, P.; Morel, L. NPM1 silencing reduces tumour growth and MAPK signalling in prostate cancer cells. PLoS ONE 2014, 9, e96293. [Google Scholar] [CrossRef]
- Li, L.; Li, F.; Xia, Y.; Yang, X.; Lv, Q.; Fang, F.; Wang, Q.; Bu, W.; Wang, Y.; Zhang, K.; et al. UVB induces cutaneous squamous cell carcinoma progression by de novo ID4 methylation via methylation regulating enzymes. EBioMedicine 2020, 57, 102835. [Google Scholar] [CrossRef] [PubMed]
- Okano, J.; Suzuki, S.; Shiota, K. Involvement of apoptotic cell death and cell cycle perturbation in retinoic acid-induced cleft palate in mice. Toxicol. Appl. Pharmacol. 2007, 221, 42–56. [Google Scholar] [CrossRef]
- Wang, M.; Huang, H.; Chen, Y. Smad2/3 is involved in growth inhibition of mouse embryonic palate mesenchymal cells induced by all-trans retinoic acid. Birth Defects Res. A Clin. Mol. Teratol. 2009, 85, 780–790. [Google Scholar] [CrossRef]
- Yu, Z.; Lin, J.; Xiao, Y.; Han, J.; Zhang, X.; Jia, H.; Tang, Y.; Li, Y. Induction of cell-cycle arrest by all-trans retinoic acid in mouse embryonic palatal mesenchymal (MEPM) cells. Toxicol. Sci. 2005, 83, 349–354. [Google Scholar] [CrossRef]
- Suwa, F.; Jin, Y.; Lu, H.; Li, X.; Tipoe, G.L.; Lau, T.Y.; Tamada, Y.; Kuroki, K.; Fang, Y.R. Alteration of apoptosis in cleft palate formation and ectomesenchymal stem cells influenced by retinoic acid. Okajimas Folia Anat. Jpn. 2001, 78, 179–186. [Google Scholar] [CrossRef]
- Qin, K.; Yu, M.; Fan, J.; Wang, H.; Zhao, P.; Zhao, G.; Zeng, W.; Chen, C.; Wang, Y.; Wang, A.; et al. Canonical and noncanonical Wnt signaling: Multilayered mediators, signaling mechanisms and major signaling crosstalk. Genes Dis. 2024, 11, 103–134. [Google Scholar] [CrossRef]
- Ohkawara, B.; Glinka, A.; Niehrs, C. Rspo3 binds syndecan 4 and induces Wnt/PCP signaling via clathrin-mediated endocytosis to promote morphogenesis. Dev. Cell 2011, 20, 303–314. [Google Scholar] [CrossRef]
- He, F.; Xiong, W.; Yu, X.; Espinoza-Lewis, R.; Liu, C.; Gu, S.; Nishita, M.; Suzuki, K.; Yamada, G.; Minami, Y.; et al. Wnt5a regulates directional cell migration and cell proliferation via Ror2-mediated noncanonical pathway in mammalian palate development. Development 2008, 135, 3871–3879. [Google Scholar] [CrossRef]
- Jiang, Z.; Pan, L.; Chen, X.; Chen, Z.; Xu, D. Wnt6 influences the viability of mouse embryonic palatal mesenchymal cells via the β-catenin pathway. Exp. Ther. Med. 2017, 14, 5339–5344. [Google Scholar] [CrossRef]
- Yuan, G.; Zhan, Y.; Gou, X.; Chen, Y.; Yang, G. TGF-β signaling inhibits canonical BMP signaling pathway during palate development. Cell Tissue Res. 2018, 371, 283–291. [Google Scholar] [CrossRef]
- Liu, W.; Sun, X.; Braut, A.; Mishina, Y.; Behringer, R.R.; Mina, M.; Martin, J.F. Distinct functions for Bmp signaling in lip and palate fusion in mice. Development 2005, 132, 1453–1461. [Google Scholar] [CrossRef]
- Hilliard, S.A.; Yu, L.; Gu, S.; Zhang, Z.; Chen, Y.P. Regional regulation of palatal growth and patterning along the anterior-posterior axis in mice. J. Anat. 2005, 207, 655–667. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, Y.; Zhao, X.; Zhang, X.; Fermin, C.; Chen, Y. Rescue of cleft palate in Msx1-deficient mice by transgenic Bmp4 reveals a network of BMP and Shh signaling in the regulation of mammalian palatogenesis. Development 2002, 129, 4135–4146. [Google Scholar] [CrossRef]
- He, F.; Chen, Y. Wnt signaling in lip and palate development. Front. Oral Biol. 2012, 16, 81–90. [Google Scholar] [CrossRef]
- Mikels, A.J.; Nusse, R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006, 4, e115. [Google Scholar] [CrossRef]
- Schmierer, B.; Hill, C.S. TGFbeta-SMAD signal transduction: Molecular specificity and functional flexibility. Nat. Rev. Mol. Cell Biol. 2007, 8, 970–982. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, H.; Gao, L.; Yin, Y.; Pan, X.; Li, Z.; Li, N.; Li, H.; Yu, Z. Negative interplay of retinoic acid and TGF-β signaling mediated by TG-interacting factor to modulate mouse embryonic palate mesenchymal-cell proliferation. Birth Defects Res. B Dev. Reprod. Toxicol. 2014, 101, 403–409. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Shen, L.; He, Z.; Chen, Y.; Li, N.; Zhang, X.; Zhang, T.; Gao, S.; Yue, H.; et al. LncRNA Meg3-mediated regulation of the Smad pathway in atRA-induced cleft palate. Toxicol. Lett. 2021, 341, 51–58. [Google Scholar] [CrossRef]
- He, F.; Xiong, W.; Wang, Y.; Matsui, M.; Yu, X.; Chai, Y.; Klingensmith, J.; Chen, Y. Modulation of BMP signaling by Noggin is required for the maintenance of palatal epithelial integrity during palatogenesis. Dev. Biol. 2010, 347, 109–121. [Google Scholar] [CrossRef]
- Shi, Y. Serine/threonine phosphatases: Mechanism through structure. Cell 2009, 139, 468–484. [Google Scholar] [CrossRef]
- Ceulemans, H.; Stalmans, W.; Bollen, M. Regulator-driven functional diversification of protein phosphatase-1 in eukaryotic evolution. Bioessays 2002, 24, 371–381. [Google Scholar] [CrossRef]
- Choy, M.S.; Page, R.; Peti, W. Regulation of protein phosphatase 1 by intrinsically disordered proteins. Biochem. Soc. Trans. 2012, 40, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Weidenauer, K.; Schmidt, C.; Rohde, C.; Pauli, C.; Blank, M.F.; Heid, D.; Waclawiczek, A.; Corbacioglu, A.; Göllner, S.; Lotze, M.; et al. The ribosomal protein S6 kinase alpha-1 (RPS6KA1) induces resistance to venetoclax/azacitidine in acute myeloid leukemia. Leukemia 2023, 37, 1611–1625. [Google Scholar] [CrossRef]
- Zhao, M.; Shao, Y.; Xu, J.; Zhang, B.; Li, C.; Gong, J. LINC00466 Impacts Cell Proliferation, Metastasis and Sensitivity to Temozolomide of Glioma by Sponging miR-137 to Regulate PPP1R14B Expression. OncoTargets Ther. 2021, 14, 1147–1159. [Google Scholar] [CrossRef]
- He, K.; Wang, T.; Huang, X.; Yang, Z.; Wang, Z.; Zhang, S.; Sui, X.; Jiang, J.; Zhao, L. PPP1R14B is a diagnostic prognostic marker in patients with uterine corpus endometrial carcinoma. J. Cell. Mol. Med. 2023, 27, 846–863. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Guo, C.; Du, J.; Xu, Q.; Li, J.; Huang, D.; Zheng, X.; Tu, L. PPP1R14B-mediated phosphorylation enhances protein stability of RPS6KA1 to promote hepatocellular carcinoma tumorigenesis. Biochim. Biophys. Acta Mol. Cell Res. 2024, 1871, 119840. [Google Scholar] [CrossRef] [PubMed]
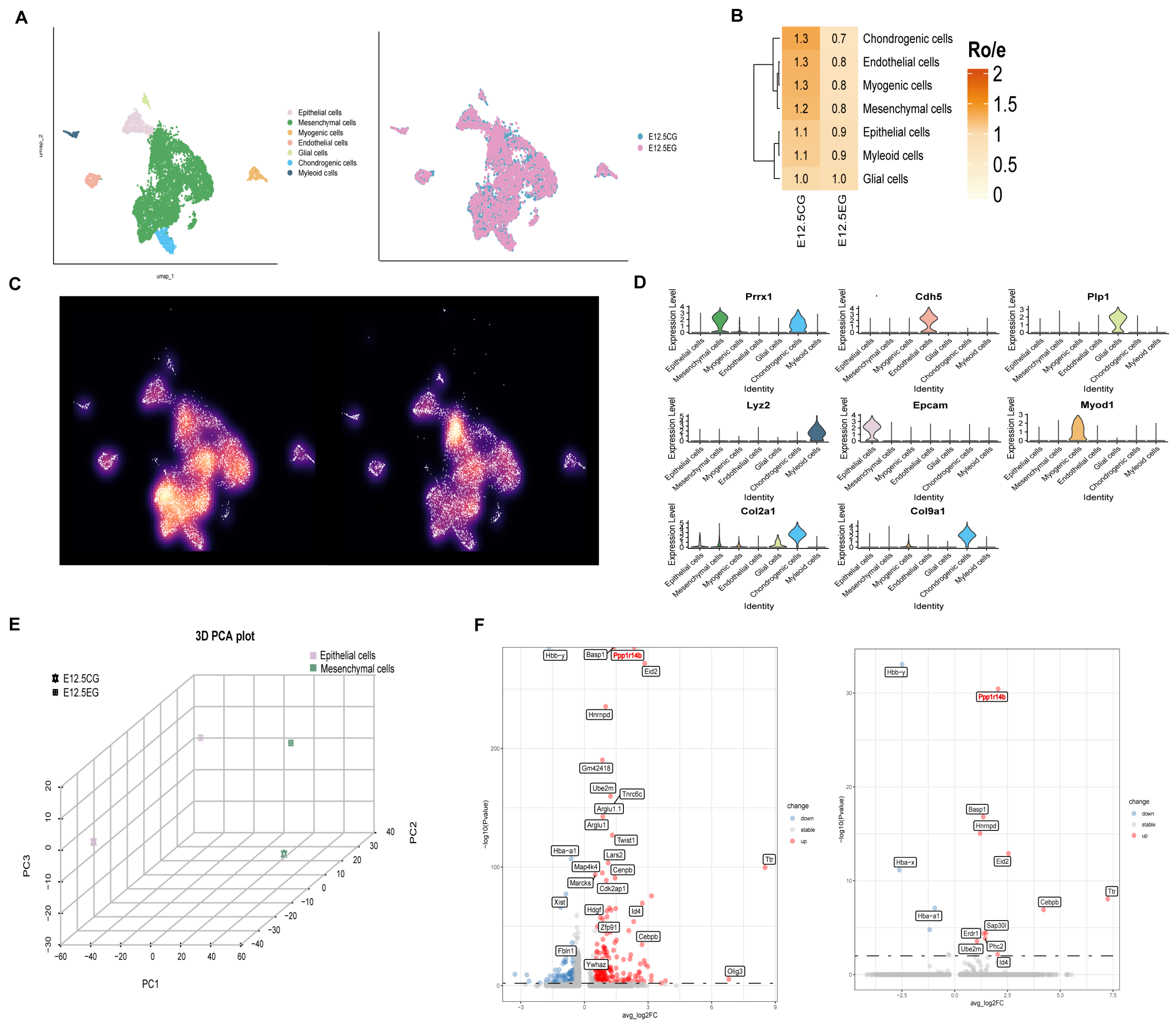
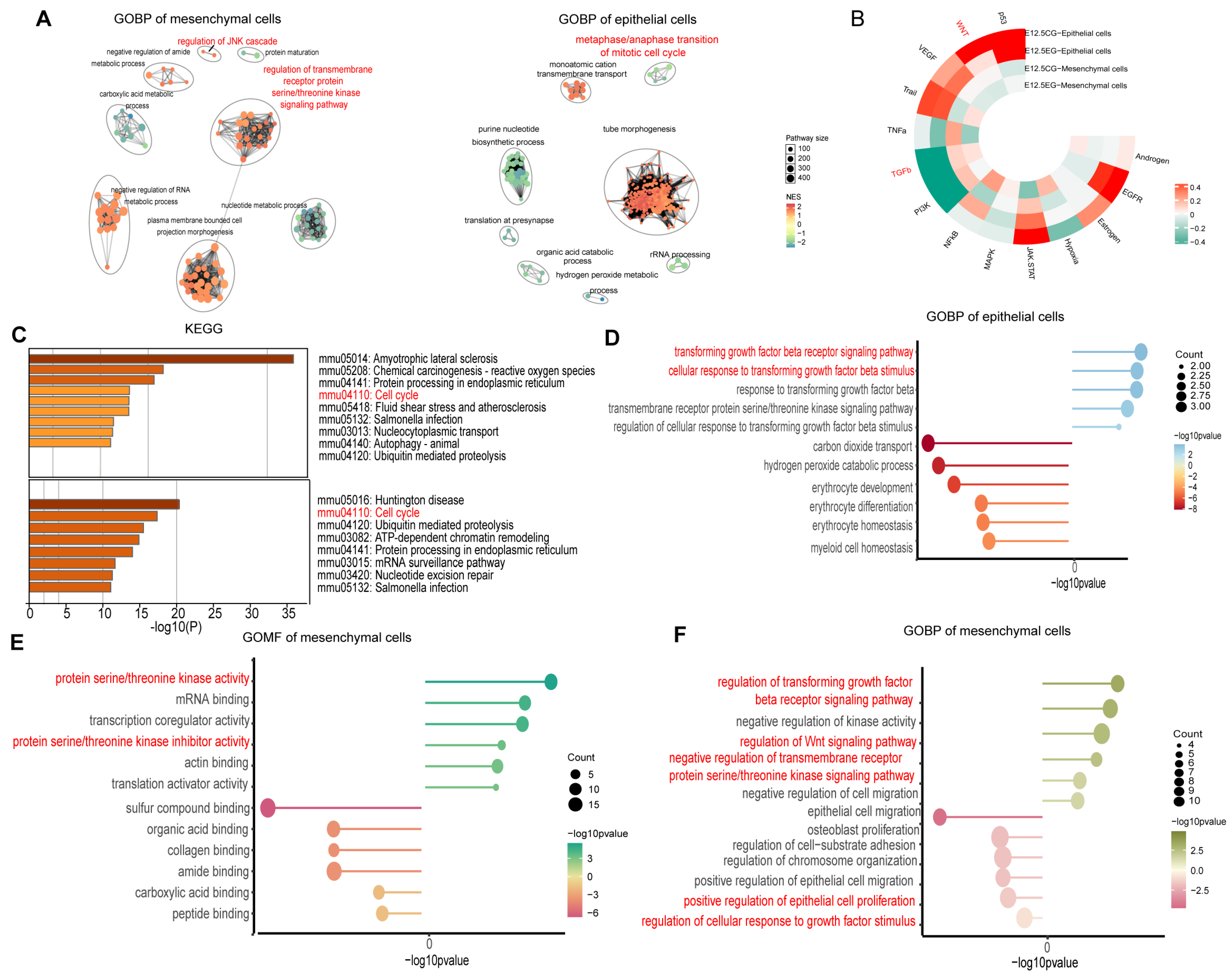
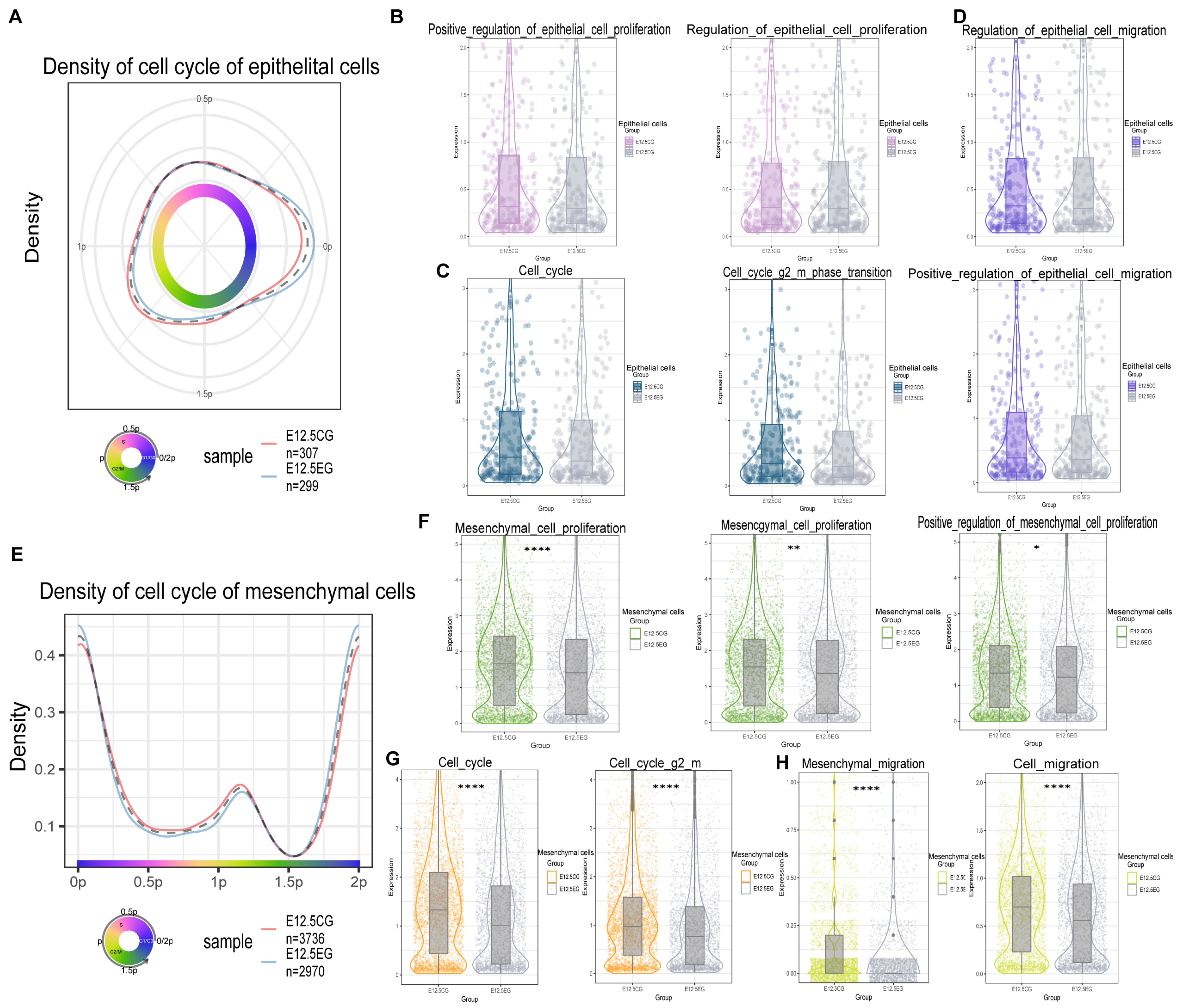
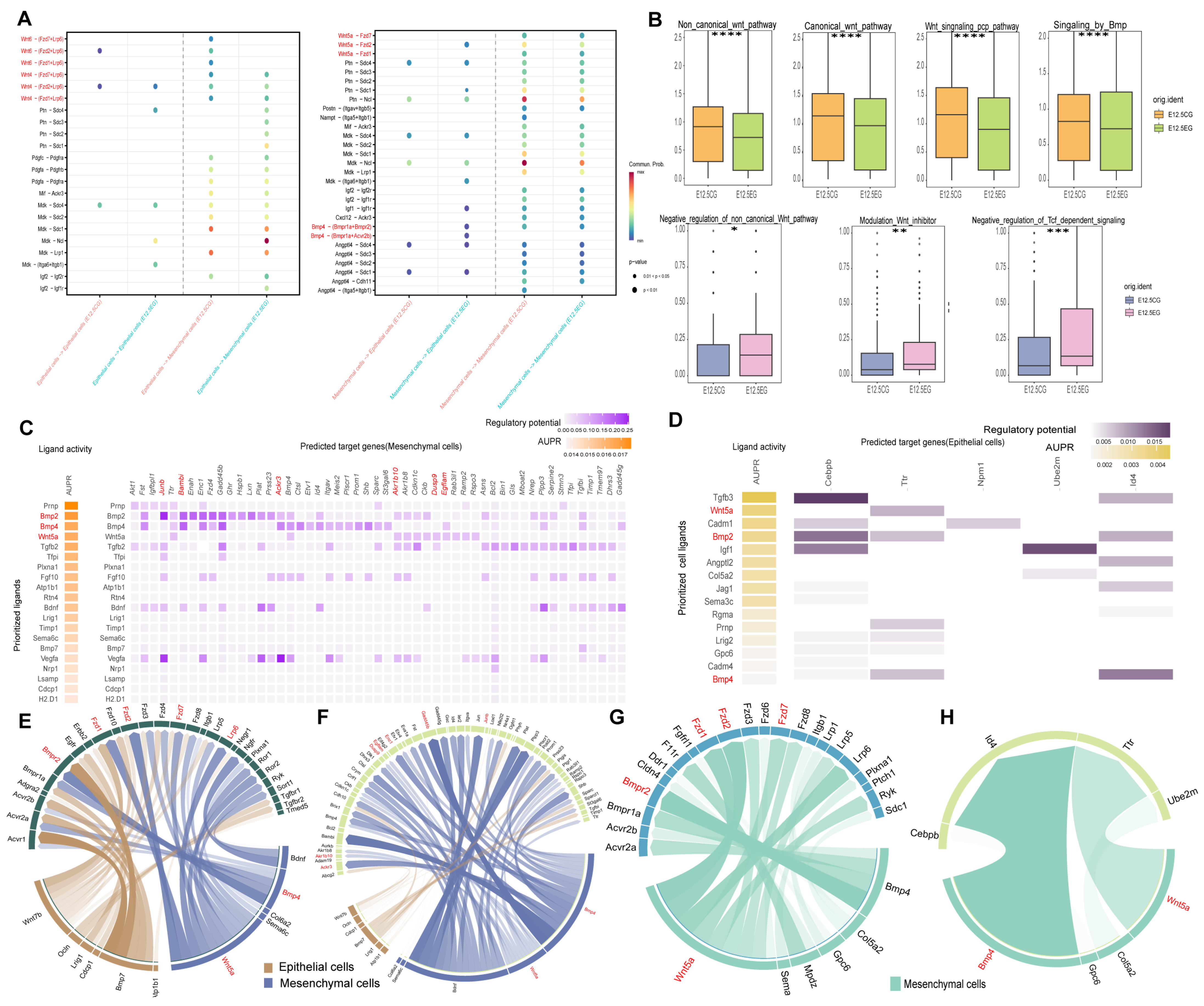


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Wang, B.; Gao, S.; Song, T. All-Trans Retinoic Acid Impacts Early Palatal Shelves Development via the Wnt and TGF-β Signaling Pathways. Biomedicines 2025, 13, 2836. https://doi.org/10.3390/biomedicines13112836
Ma Y, Wang B, Gao S, Song T. All-Trans Retinoic Acid Impacts Early Palatal Shelves Development via the Wnt and TGF-β Signaling Pathways. Biomedicines. 2025; 13(11):2836. https://doi.org/10.3390/biomedicines13112836
Chicago/Turabian StyleMa, Yaping, Binqing Wang, Shikang Gao, and Tao Song. 2025. "All-Trans Retinoic Acid Impacts Early Palatal Shelves Development via the Wnt and TGF-β Signaling Pathways" Biomedicines 13, no. 11: 2836. https://doi.org/10.3390/biomedicines13112836
APA StyleMa, Y., Wang, B., Gao, S., & Song, T. (2025). All-Trans Retinoic Acid Impacts Early Palatal Shelves Development via the Wnt and TGF-β Signaling Pathways. Biomedicines, 13(11), 2836. https://doi.org/10.3390/biomedicines13112836




