Factors Released by Polarized Neutrophil-like Cells Modulate Cardiac Fibroblast Phenotype and Limit the Inflammatory Response After Myocardial Infarction
Abstract
1. Introduction
2. Materials and Methods
2.1. HL-60 Cell Culture and Polarization into N1 and N2 Neutrophil-like Cells
2.2. Human Cardiac Fibroblasts (Adult)
2.3. Hydrogel Preparation
2.4. The 3D Co-Culture Model
2.5. Generation of N1/N2 Secretome
2.6. Animal Groups and MI Surgical Procedures and SN1/SN2 Treatment
2.7. MI Surgical Procedure and N1/N2 Secretome Injection
2.8. Elastase Activity Detection in Ex Vivo Mouse Hearts
2.9. RNA Extraction and qPCR
2.10. Immunohistochemistry
2.11. Protein Extraction and Western Blotting
2.12. Enzyme-Linked Immunosorbent Assay (ELISA)
2.13. F-Actin Labelling
2.14. Zymography
2.15. DNA Labelling in Plasma
2.16. Proliferation Assay (xCelligence)
2.17. 3-(4,5-Dimethylthiazol-2-Yl)-2,5-diphenyltetrazolium Bromide (MTT) Assay
2.18. Statistical Analysis
3. Results
3.1. Cardiac Fibroblasts Exhibit a Quiescent Phenotype When Cultured on a Hydrogel Derived from Native Cardiac ECM
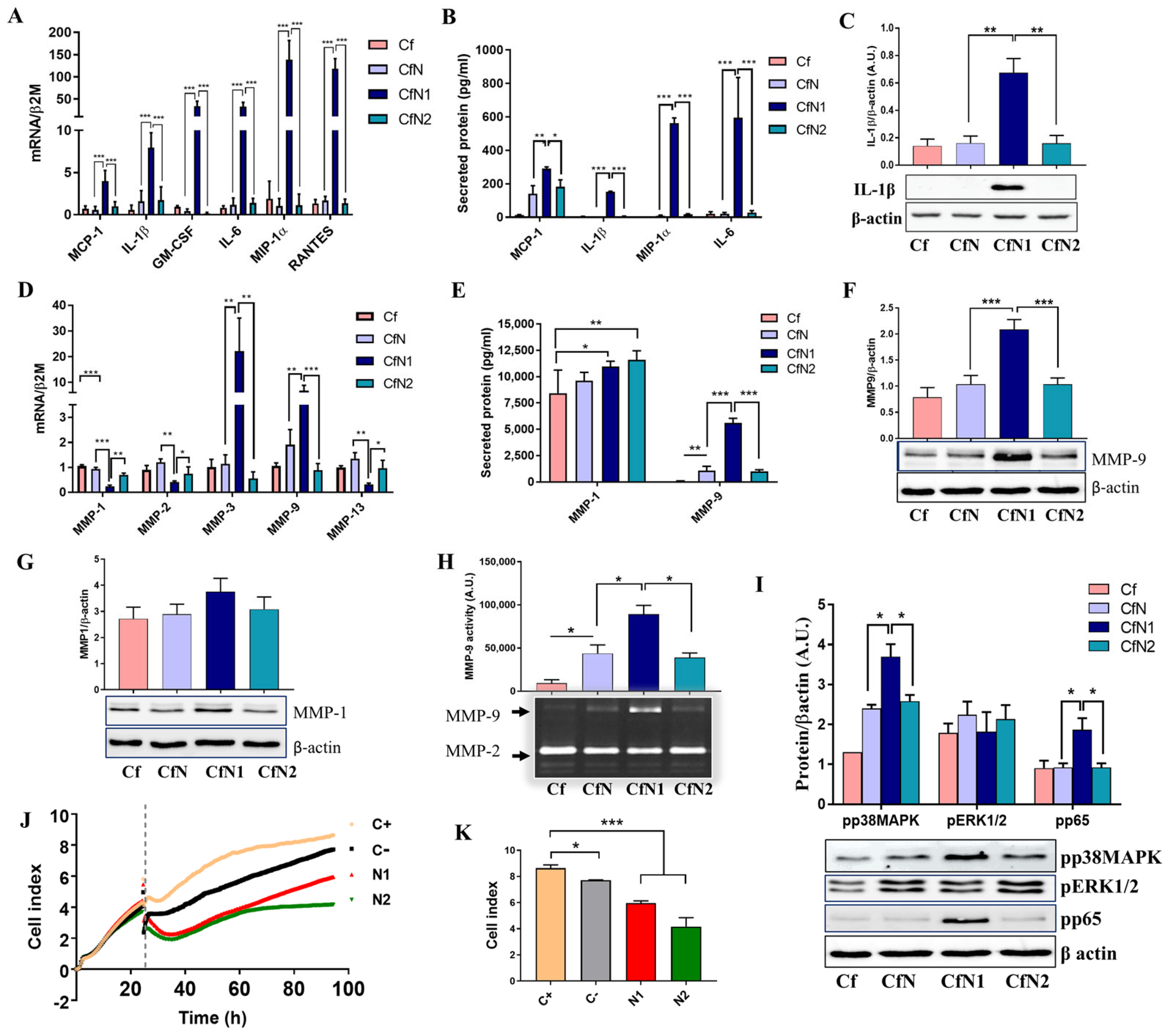
3.2. Cardiac Fibroblasts Acquire a Pro-Inflammatory and Matrix-Remodeling Phenotype, with Reduced Proliferative Capacity upon Exposure to Soluble Mediators Released by Pro-Inflammatory Neutrophil-like Cells
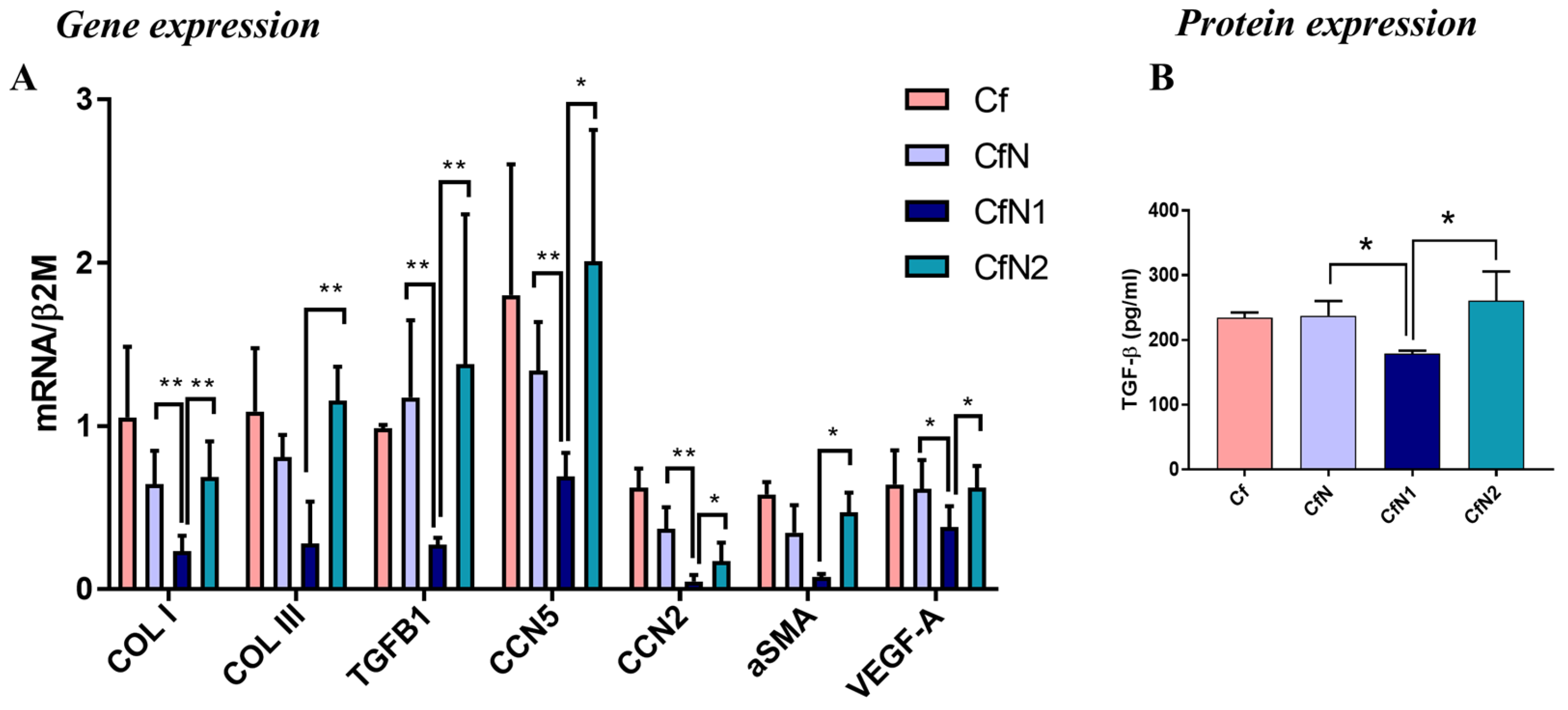
3.3. Pro-Inflammatory Neutrophil-like Cells Impair the Transition of Cardiac Fibroblasts to Myofibroblasts
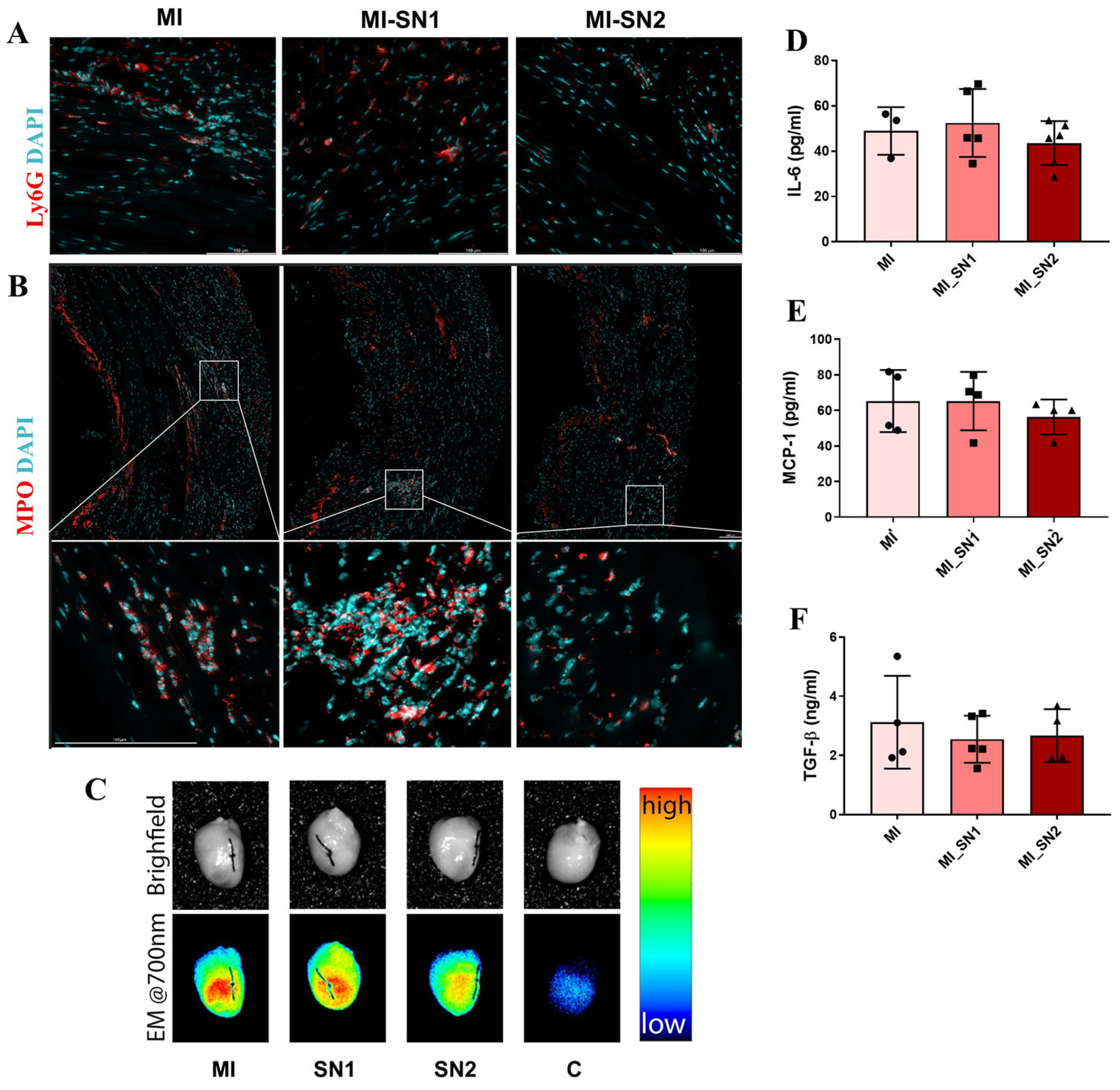
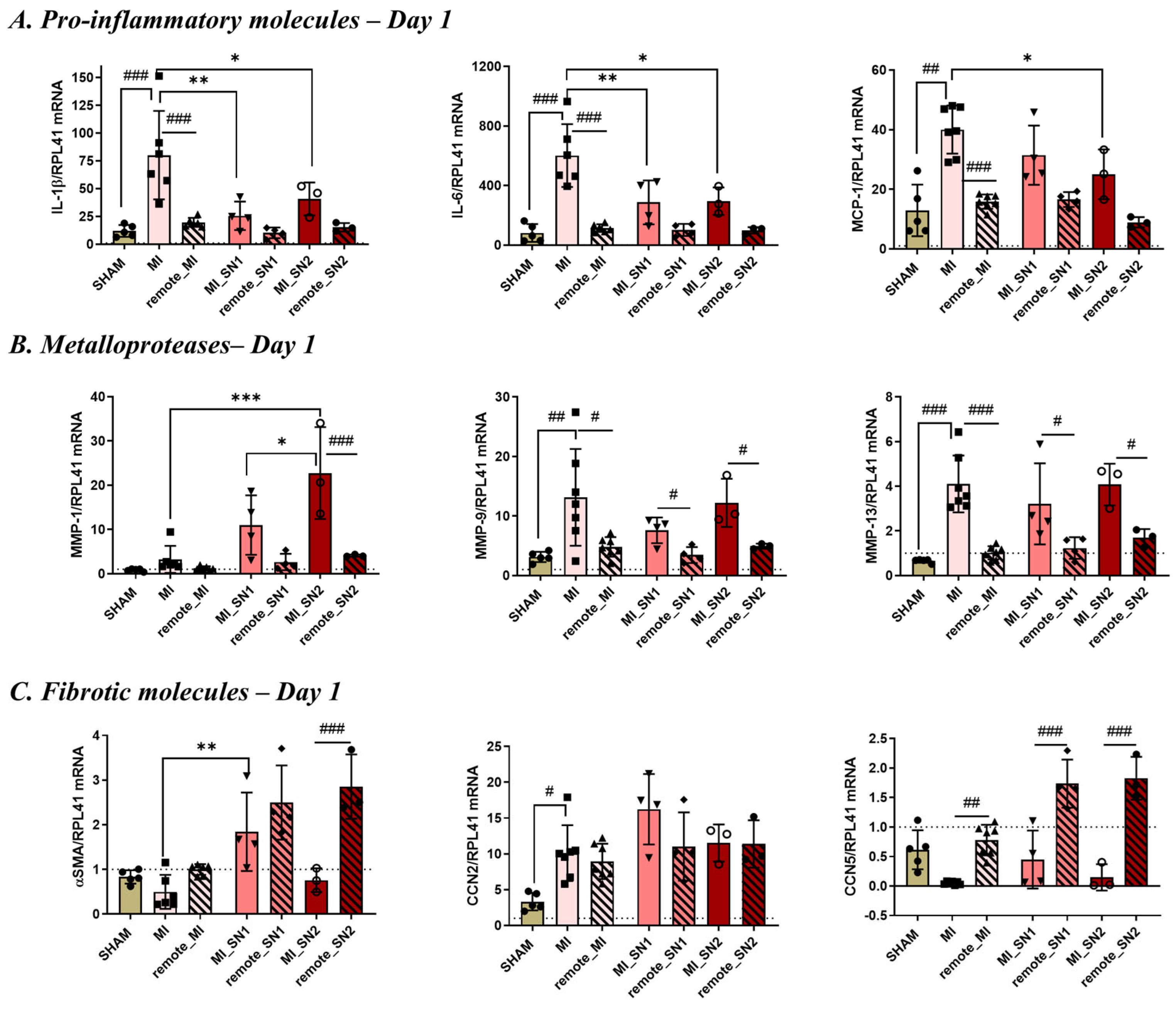
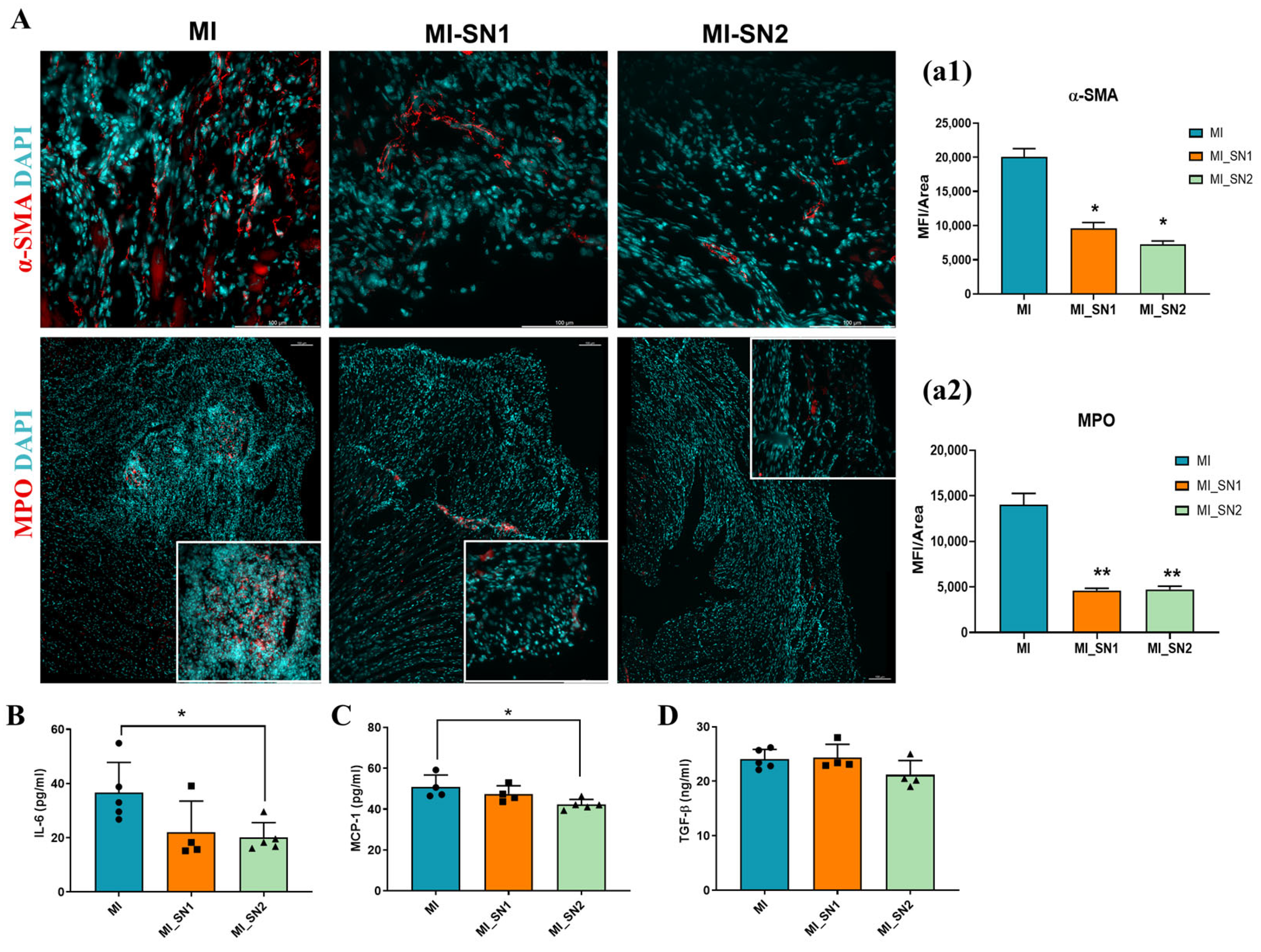
3.4. Administration of N1 and N2 Secretome in the Infarcted Area Diminishes the Inflammatory Response at Day 1 Post-MI
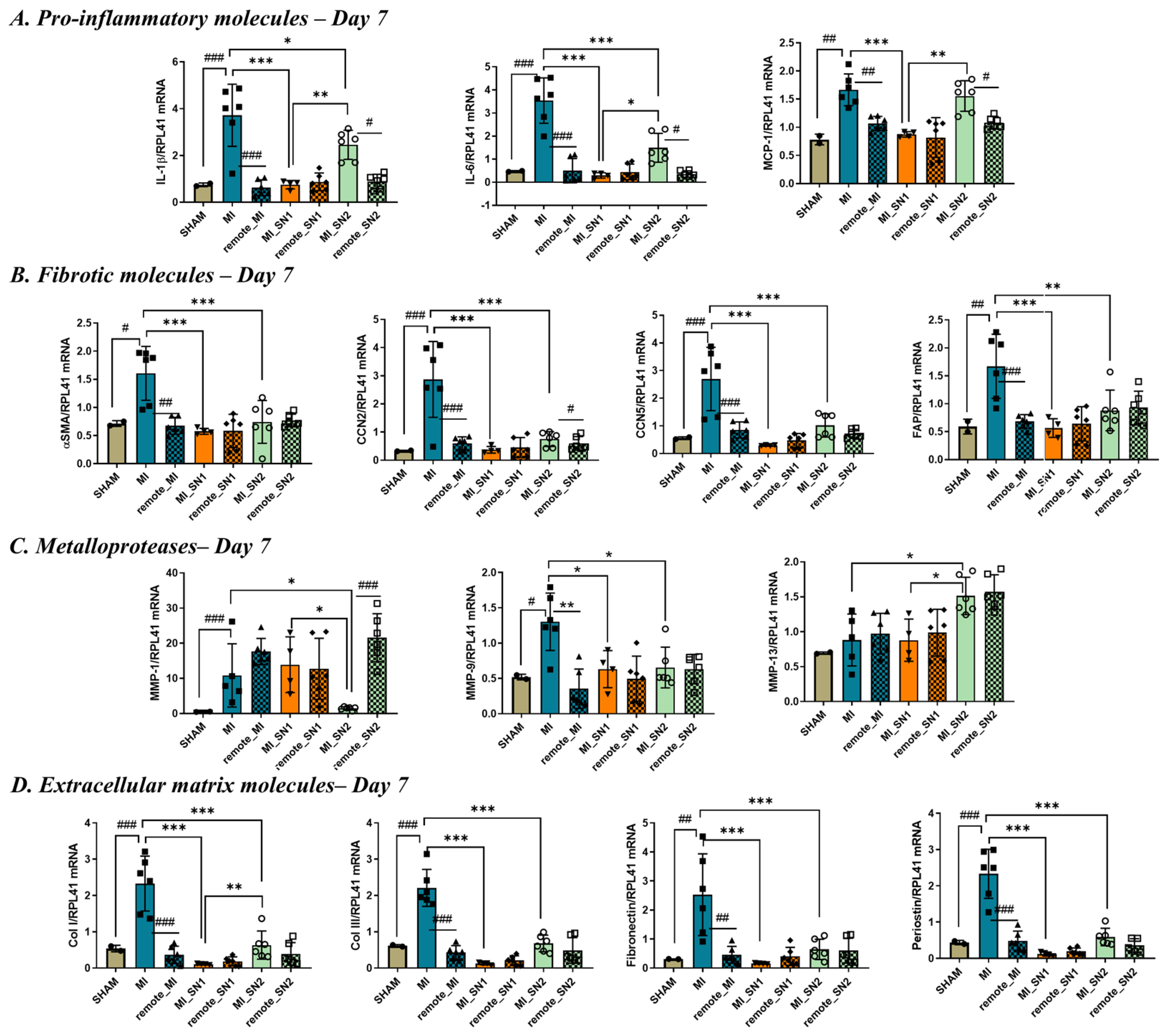
3.5. Factors Released by N1/N2 Neutrophils Reduce FMT and ECM Deposition at 7 Days Post-MI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| α-SMA | Alpha-smooth muscle actin |
| APA | Alternative polyadenylation |
| ARdH | Aortic root derived hydrogel |
| Arg1 | Arginase 1 |
| B2M | Β2-Microglobulin |
| BM | Bone marrow |
| CCN2 | Cellular communication network factor 2 |
| CD | Cluster of differentiation |
| CF | Cardiac fibroblast |
| DAPI | 4′,6-diamidino-2-phenylindole |
| dHL-60 | Differentiated HL-60 cells |
| DMSO | Dimethyl sulfoxide |
| ECM | Extracellular matrix |
| EDTA | Ethylenediaminetetraacetic acid |
| ELISA | Enzyme-linked immunosorbent assay |
| FAP | Fibroblast activation protein |
| FBS | Fetal bovine serum |
| FMT | Fibroblast-to-myofibroblast transition |
| FRET | Fluorescence resonance energy transfer |
| HRP | Horseradish peroxidase |
| IFN-γ | Interferon gamma |
| IHC | Immunohistochemistry |
| IL- | Interleukin- |
| LCA | Left coronary artery |
| LPS | Lipopolysaccharide |
| LV | Left ventricle |
| MAPK | Mitogen-activated protein kinase |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MFI | Mean fluorescence intensity |
| MI | Myocardial infarction |
| MMP | Matrix metalloproteinases |
| MPO | Myeloperoxidase |
| NETs | Neutrophil extracellular traps |
| OCT | Optimal cutting temperature |
| P/S | Penicillin/streptomycin |
| PBS | Phosphate-buffered saline |
| PCR | Polymerase chain reaction |
| PFA | Paraformaldehyde |
| RIPA | Radio-immunoprecipitation Assay |
| ROS | Reactive oxygen species |
| RT | Room temperature |
| SDS-PAGE | Sodium dodecyl sulfate–polyacrylamide gel electrophoresis |
| SN1 | Supernatant derived from N1 polarized BM neutrophils |
| SN2 | Supernatant derived from N2 polarized BM neutrophils |
| TBS | Tris-buffered saline |
| TGF-β | Transforming growth factor beta |
References
- Thulabandu, V.; Chen, D.; Atit, R.P. Dermal fibroblast in cutaneous development and healing. Wiley Interdiscip. Rev. Dev. Biol. 2018, 7, e307. [Google Scholar] [CrossRef]
- Kurose, H. Cardiac Fibrosis and Fibroblasts. Cells 2021, 10, 1716. [Google Scholar] [CrossRef]
- Lynch, M.D.; Watt, F.M. Fibroblast heterogeneity: Implications for human disease. J. Clin. Investig. 2018, 128, 26–35. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Cardiac fibrosis. Cardiovasc. Res. 2021, 117, 1450–1488. [Google Scholar] [CrossRef]
- Salari, N.; Morddarvanjoghi, F.; Abdolmaleki, A.; Rasoulpoor, S.; Khaleghi, A.A.; Hezarkhani, L.A.; Shohaimi, S.; Mohammadi, M. The global prevalence of myocardial infarction: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2023, 23, 206. [Google Scholar] [CrossRef]
- Muhl, L.; Genove, G.; Leptidis, S.; Liu, J.; He, L.; Mocci, G.; Sun, Y.; Gustafsson, S.; Buyandelger, B.; Chivukula, I.V.; et al. Single-cell analysis uncovers fibroblast heterogeneity and criteria for fibroblast and mural cell identification and discrimination. Nat. Commun. 2020, 11, 3953. [Google Scholar] [CrossRef] [PubMed]
- Lendahl, U.; Muhl, L.; Betsholtz, C. Identification, discrimination and heterogeneity of fibroblasts. Nat. Commun. 2022, 13, 3409. [Google Scholar] [CrossRef] [PubMed]
- Kirk, T.; Ahmed, A.; Rognoni, E. Fibroblast Memory in Development, Homeostasis and Disease. Cells 2021, 10, 2840. [Google Scholar] [CrossRef]
- Vadana, M.; Cecoltan, S.; Ciortan, L.; Macarie, R.D.; Tucureanu, M.M.; Mihaila, A.C.; Droc, I.; Butoi, E.; Manduteanu, I. Molecular mechanisms involved in high glucose-induced valve calcification in a 3D valve model with human valvular cells. J. Cell. Mol. Med. 2020, 24, 6350–6361. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.; Law, J.P.; Reyat, J.S.; Cumberland, M.J.; Hang, S.; Vo, N.T.N.; Raniga, K.; Weston, C.J.; O’Shea, C.; Townend, J.N.; et al. Chronic activation of human cardiac fibroblasts in vitro attenuates the reversibility of the myofibroblast phenotype. Sci. Rep. 2023, 13, 12137. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Can Myocardial Fibrosis Be Reversed? J. Am. Coll. Cardiol. 2019, 73, 2283–2285. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yabluchanskiy, A.; Iyer, R.P.; Cannon, P.L.; Flynn, E.R.; Jung, M.; Henry, J.; Cates, C.A.; Deleon-Pennell, K.Y.; Lindsey, M.L. Temporal neutrophil polarization following myocardial infarction. Cardiovasc. Res. 2016, 110, 51–61. [Google Scholar] [CrossRef]
- Shinde, A.V.; Frangogiannis, N.G. Fibroblasts in myocardial infarction: A role in inflammation and repair. J. Mol. Cell. Cardiol. 2014, 70, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N. Transforming growth factor-β in tissue fibrosis. J. Exp. Med. 2020, 217, e20190103. [Google Scholar] [CrossRef]
- Venugopal, H.; Hanna, A.; Humeres, C.; Frangogiannis, N.G. Properties and Functions of Fibroblasts and Myofibroblasts in Myocardial Infarction. Cells 2022, 11, 1386. [Google Scholar] [CrossRef]
- Ma, Y. Role of Neutrophils in Cardiac Injury and Repair Following Myocardial Infarction. Cells 2021, 10, 1676. [Google Scholar] [CrossRef]
- Zhang, N.; Aiyasiding, X.; Li, W.J.; Liao, H.H.; Tang, Q.Z. Neutrophil degranulation and myocardial infarction. Cell Commun. Signal. 2022, 20, 50. [Google Scholar] [CrossRef]
- Mihaila, A.C.; Ciortan, L.; Macarie, R.D.; Vadana, M.; Cecoltan, S.; Preda, M.B.; Hudita, A.; Gan, A.-M.; Jakobsson, G.; Tucureanu, M.M.; et al. Transcriptional Profiling and Functional Analysis of N1/N2 Neutrophils Reveal an Immunomodulatory Effect of S100A9-Blockade on the Pro-Inflammatory N1 Subpopulation. Front. Immunol. 2021, 12, 708770. [Google Scholar] [CrossRef]
- Calcagno, D.M.; Zhang, C.; Toomu, A.; Huang, K.; Ninh, V.K.; Miyamoto, S.; Aguirre, A.D.; Fu, Z.; Heller Brown, J.; King, K.R. SiglecF(HI) Marks Late-Stage Neutrophils of the Infarcted Heart: A Single-Cell Transcriptomic Analysis of Neutrophil Diversification. J. Am. Heart Assoc. 2021, 10, e019019. [Google Scholar] [CrossRef] [PubMed]
- Vafadarnejad, E.; Rizzo, G.; Krampert, L.; Arampatzi, P.; Arias-Loza, A.P.; Nazzal, Y.; Rizakou, A.; Knochenhauer, T.; Bandi, S.R.; Nugroho, V.A.; et al. Dynamics of Cardiac Neutrophil Diversity in Murine Myocardial Infarction. Circ. Res. 2020, 127, e232–e249. [Google Scholar] [CrossRef]
- Daseke, M.J., II; Chalise, U.; Becirovic-Agic, M.; Salomon, J.D.; Cook, L.M.; Case, A.J.; Lindsey, M.L. Neutrophil signaling during myocardial infarction wound repair. Cell. Signal. 2021, 77, 109816. [Google Scholar] [CrossRef]
- Cazares-Preciado, J.A.; Lopez-Arredondo, A.; Cruz-Cardenas, J.A.; Luevano-Martinez, L.A.; Garcia-Rivas, G.; Prado-Garcia, H.; Brunck, M.E.G. Metabolic features of neutrophilic differentiation of HL-60 cells in hyperglycemic environments. BMJ Open Diabetes Res. Care 2024, 12, e004181. [Google Scholar] [CrossRef]
- Dakir, E.H.; Mollinedo, F. Genome-wide miRNA profiling and pivotal roles of miRs 125a-5p and 17-92 cluster in human neutrophil maturation and differentiation of acute myeloid leukemia cells. Oncotarget 2019, 10, 5313–5331. [Google Scholar] [CrossRef] [PubMed]
- Rincon, E.; Rocha-Gregg, B.L.; Collins, S.R. A map of gene expression in neutrophil-like cell lines. BMC Genom. 2018, 19, 573. [Google Scholar] [CrossRef]
- Cecoltan, S.; Ciortan, L.; Macarie, R.D.; Vadana, M.; Mihaila, A.C.; Tucureanu, M.; Vlad, M.L.; Droc, I.; Gherghiceanu, M.; Simionescu, A.; et al. High Glucose Induced Changes in Human VEC Phenotype in a 3D Hydrogel Derived from Cell-Free Native Aortic Root. Front. Cardiovasc. Med. 2021, 8, 714573. [Google Scholar] [CrossRef] [PubMed]
- Preda, M.B.; Burlacu, A. Electrocardiography as a tool for validating myocardial ischemia-reperfusion procedures in mice. Comp. Med. 2010, 60, 443–447. [Google Scholar] [PubMed]
- Hama, N.; Itoh, H.; Shirakami, G.; Nakagawa, O.; Suga, S.; Ogawa, Y.; Masuda, I.; Nakanishi, K.; Yoshimasa, T.; Hashimoto, Y.; et al. Rapid ventricular induction of brain natriuretic peptide gene expression in experimental acute myocardial infarction. Circulation 1995, 92, 1558–1564. [Google Scholar] [CrossRef]
- Mavileti, S.K.; Bila, G.; Utka, V.; Bilyy, R., Jr.; Bila, E.; Butoi, E.; Gupta, S.; Balyan, P.; Kato, T.; Bilyy, R.; et al. Squaraine-Peptide Conjugates as Efficient Reporters of Neutrophil Extracellular Traps-Mediated Chronic Inflammation. ACS Appl. Mater. Interfaces 2025, 17, 9140–9154. [Google Scholar] [CrossRef]
- Rohr, S. Cardiac fibroblasts in cell culture systems: Myofibroblasts all along? J. Cardiovasc. Pharmacol. 2011, 57, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.D.; Hill, R.C.; Dzieciatkowska, M.; Nigam, V.; Behfar, A.; Christman, K.L.; Hansen, K.C. Quantification of decellularized human myocardial matrix: A comparison of six patients. Proteom. Clin. Appl. 2016, 10, 75–83. [Google Scholar] [CrossRef]
- Singh, D.; Rai, V.; Agrawal, D.K. Regulation of Collagen I and Collagen III in Tissue Injury and Regeneration. Cardiol. Cardiovasc. Med. 2023, 7, 5–16. [Google Scholar] [CrossRef]
- Mouton, A.J.; Ma, Y.; Rivera Gonzalez, O.J.; Daseke, M.J., II; Flynn, E.R.; Freeman, T.C.; Garrett, M.R.; DeLeon-Pennell, K.Y.; Lindsey, M.L. Fibroblast polarization over the myocardial infarction time continuum shifts roles from inflammation to angiogenesis. Basic Res. Cardiol. 2019, 114, 6. [Google Scholar] [CrossRef]
- Yoon, P.O.; Lee, M.A.; Cha, H.; Jeong, M.H.; Kim, J.; Jang, S.P.; Choi, B.Y.; Jeong, D.; Yang, D.K.; Hajjar, R.J.; et al. The opposing effects of CCN2 and CCN5 on the development of cardiac hypertrophy and fibrosis. J. Mol. Cell. Cardiol. 2010, 49, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Dorn, L.E.; Petrosino, J.M.; Wright, P.; Accornero, F. CTGF/CCN2 is an autocrine regulator of cardiac fibrosis. J. Mol. Cell. Cardiol. 2018, 121, 205–211. [Google Scholar] [CrossRef]
- Xu, H.; Li, P.; Liu, M.; Liu, C.; Sun, Z.; Guo, X.; Zhang, Y. CCN2 and CCN5 exerts opposing effect on fibroblast proliferation and transdifferentiation induced by TGF-β. Clin. Exp. Pharmacol. Physiol. 2015, 42, 1207–1219. [Google Scholar] [CrossRef]
- Fu, X.; Khalil, H.; Kanisicak, O.; Boyer, J.G.; Vagnozzi, R.J.; Maliken, B.D.; Sargent, M.A.; Prasad, V.; Valiente-Alandi, I.; Blaxall, B.C.; et al. Specialized fibroblast differentiated states underlie scar formation in the infarcted mouse heart. J. Clin. Investig. 2018, 128, 2127–2143. [Google Scholar] [CrossRef] [PubMed]
- Woodley, J.P.; Lambert, D.W.; Asencio, I.O. Understanding Fibroblast Behavior in 3D Biomaterials. Tissue Eng. Part B Rev. 2022, 28, 569–578. [Google Scholar] [CrossRef]
- Barbosa, M.A.G.; Xavier, C.P.R.; Pereira, R.F.; Petrikaite, V.; Vasconcelos, M.H. 3D Cell Culture Models as Recapitulators of the Tumor Microenvironment for the Screening of Anti-Cancer Drugs. Cancers 2021, 14, 190. [Google Scholar] [CrossRef]
- Molkentin, J.D.; Bugg, D.; Ghearing, N.; Dorn, L.E.; Kim, P.; Sargent, M.A.; Gunaje, J.; Otsu, K.; Davis, J. Fibroblast-Specific Genetic Manipulation of p38 Mitogen-Activated Protein Kinase In vivo Reveals Its Central Regulatory Role in Fibrosis. Circulation 2017, 136, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Tanada, Y.; Omiya, S.; Podaru, M.N.; Murakawa, T.; Ito, J.; Shah, A.M.; Conway, S.J.; Ono, M.; Otsu, K. NF-κB activation in cardiac fibroblasts results in the recruitment of inflammatory Ly6Chi monocytes in pressure-overloaded hearts. Sci. Signal. 2021, 14, eabe4932. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Chen, W.; Su, Y.; Rai, V.; Uche, O.U.; Li, N.; Frangogiannis, N.G. IL-1 induces proinflammatory leukocyte infiltration and regulates fibroblast phenotype in the infarcted myocardium. J. Immunol. 2013, 191, 4838–4848. [Google Scholar] [CrossRef]
- Kang, J.H.; Hwang, S.M.; Chung, I.Y. S100A8, S100A9 and S100A12 activate airway epithelial cells to produce MUC5AC via extracellular signal-regulated kinase and nuclear factor-κB pathways. Immunology 2015, 144, 79–90. [Google Scholar] [CrossRef] [PubMed]
- de Lemos, J.A.; Morrow, D.A.; Blazing, M.A.; Jarolim, P.; Wiviott, S.D.; Sabatine, M.S.; Califf, R.M.; Braunwald, E. Serial measurement of monocyte chemoattractant protein-1 after acute coronary syndromes: Results from the A to Z trial. J. Am. Coll. Cardiol. 2007, 50, 2117–2124. [Google Scholar] [CrossRef] [PubMed]
- de Lemos, J.A.; Morrow, D.A.; Sabatine, M.S.; Murphy, S.A.; Gibson, C.M.; Antman, E.M.; McCabe, C.H.; Cannon, C.P.; Braunwald, E. Association between plasma levels of monocyte chemoattractant protein-1 and long-term clinical outcomes in patients with acute coronary syndromes. Circulation 2003, 107, 690–695. [Google Scholar] [CrossRef]
- Horckmans, M.; Ring, L.; Duchene, J.; Santovito, D.; Schloss, M.J.; Drechsler, M.; Weber, C.; Soehnlein, O.; Steffens, S. Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur. Heart J. 2017, 38, 187–197. [Google Scholar] [CrossRef]
- Mihaila, A.C.; Ciortan, L.; Tucureanu, M.M.; Simionescu, M.; Butoi, E. Anti-Inflammatory Neutrophils Reprogram Macrophages toward a Pro-Healing Phenotype with Increased Efferocytosis Capacity. Cells 2024, 13, 208. [Google Scholar] [CrossRef] [PubMed]
- Novotny, J.; Oberdieck, P.; Titova, A.; Pelisek, J.; Chandraratne, S.; Nicol, P.; Hapfelmeier, A.; Joner, M.; Maegdefessel, L.; Poppert, H.; et al. Thrombus NET content is associated with clinical outcome in stroke and myocardial infarction. Neurology 2020, 94, e2346–e2360. [Google Scholar] [CrossRef]
- Traverse, J.H.; Henry, T.D.; Dib, N.; Patel, A.N.; Pepine, C.; Schaer, G.L.; DeQuach, J.A.; Kinsey, A.M.; Chamberlin, P.; Christman, K.L. First-in-Man Study of a Cardiac Extracellular Matrix Hydrogel in Early and Late Myocardial Infarction Patients. JACC Basic Transl. Sci. 2019, 4, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, C.; Hipler, U.C.; Elsner, P.; Tittelbach, J. Keratinocyte and Fibroblast Wound Healing In Vitro Is Repressed by Non-Optimal Conditions but the Reparative Potential Can Be Improved by Water-Filtered Infrared A. Biomedicines 2021, 9, 1802. [Google Scholar] [CrossRef]
- Zafeiriou, M.P.; Noack, C.; Zelarayan, L.C. Isolation and Primary Culture of Adult Mouse Cardiac Fibroblasts. Bio-Protoc. 2016, 6, e1860–e1865. [Google Scholar] [CrossRef]
- Macarie, R.D.; Vadana, M.; Ciortan, L.; Tucureanu, M.M.; Ciobanu, A.; Vinereanu, D.; Manduteanu, I.; Simionescu, M.; Butoi, E. The expression of MMP-1 and MMP-9 is up-regulated by smooth muscle cells after their cross-talk with macrophages in high glucose conditions. J. Cell. Mol. Med. 2018, 22, 4366–4376. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciortan, L.; Gan, A.-M.; Cecoltan, S.; Serbanescu, M.; Mihaila, A.C.; Macarie, R.D.; Tucureanu, M.M.; Naie, M.L.; Preda, M.B.; Cosman, B.-P.; et al. Factors Released by Polarized Neutrophil-like Cells Modulate Cardiac Fibroblast Phenotype and Limit the Inflammatory Response After Myocardial Infarction. Biomedicines 2025, 13, 2829. https://doi.org/10.3390/biomedicines13112829
Ciortan L, Gan A-M, Cecoltan S, Serbanescu M, Mihaila AC, Macarie RD, Tucureanu MM, Naie ML, Preda MB, Cosman B-P, et al. Factors Released by Polarized Neutrophil-like Cells Modulate Cardiac Fibroblast Phenotype and Limit the Inflammatory Response After Myocardial Infarction. Biomedicines. 2025; 13(11):2829. https://doi.org/10.3390/biomedicines13112829
Chicago/Turabian StyleCiortan, Letitia, Ana-Maria Gan, Sergiu Cecoltan, Mihaela Serbanescu, Andreea Cristina Mihaila, Razvan Daniel Macarie, Monica Madalina Tucureanu, Miruna Larisa Naie, Mihai Bogdan Preda, Bogdan-Paul Cosman, and et al. 2025. "Factors Released by Polarized Neutrophil-like Cells Modulate Cardiac Fibroblast Phenotype and Limit the Inflammatory Response After Myocardial Infarction" Biomedicines 13, no. 11: 2829. https://doi.org/10.3390/biomedicines13112829
APA StyleCiortan, L., Gan, A.-M., Cecoltan, S., Serbanescu, M., Mihaila, A. C., Macarie, R. D., Tucureanu, M. M., Naie, M. L., Preda, M. B., Cosman, B.-P., Bila, G., Bilyy, R., & Butoi, E. (2025). Factors Released by Polarized Neutrophil-like Cells Modulate Cardiac Fibroblast Phenotype and Limit the Inflammatory Response After Myocardial Infarction. Biomedicines, 13(11), 2829. https://doi.org/10.3390/biomedicines13112829






