Potential Causal Effects of Cystatin C on Age-Related Macular Degeneration: A Two-Sample Mendelian Randomization Study
Abstract
1. Introduction
2. Materials and Methods
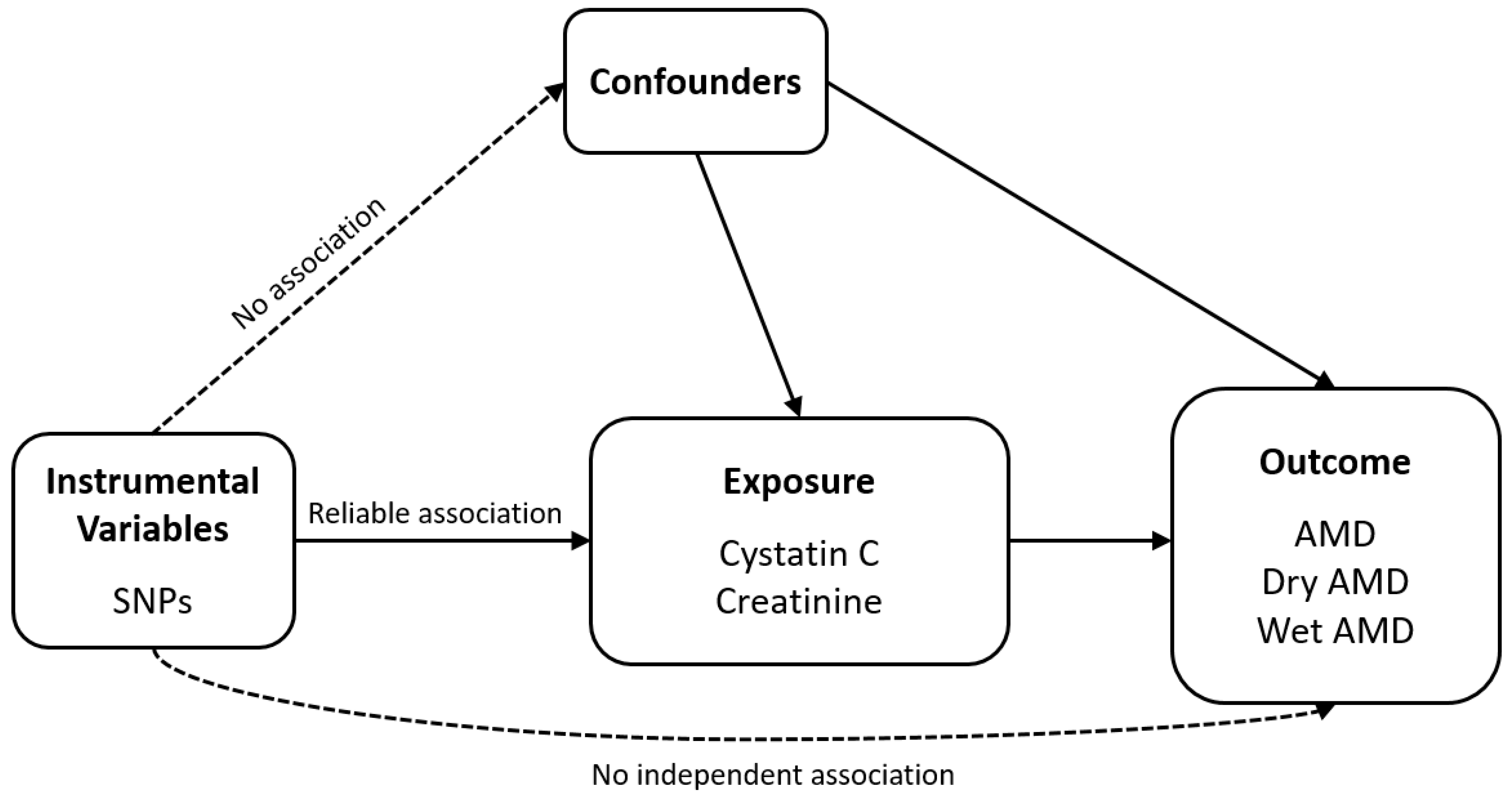
2.1. Study Design
2.2. Data Sources
2.3. Selection of the Genetic Instrumental Variables
2.4. Mendelian Randomization
3. Results
3.1. Selection of Instrumental Variables
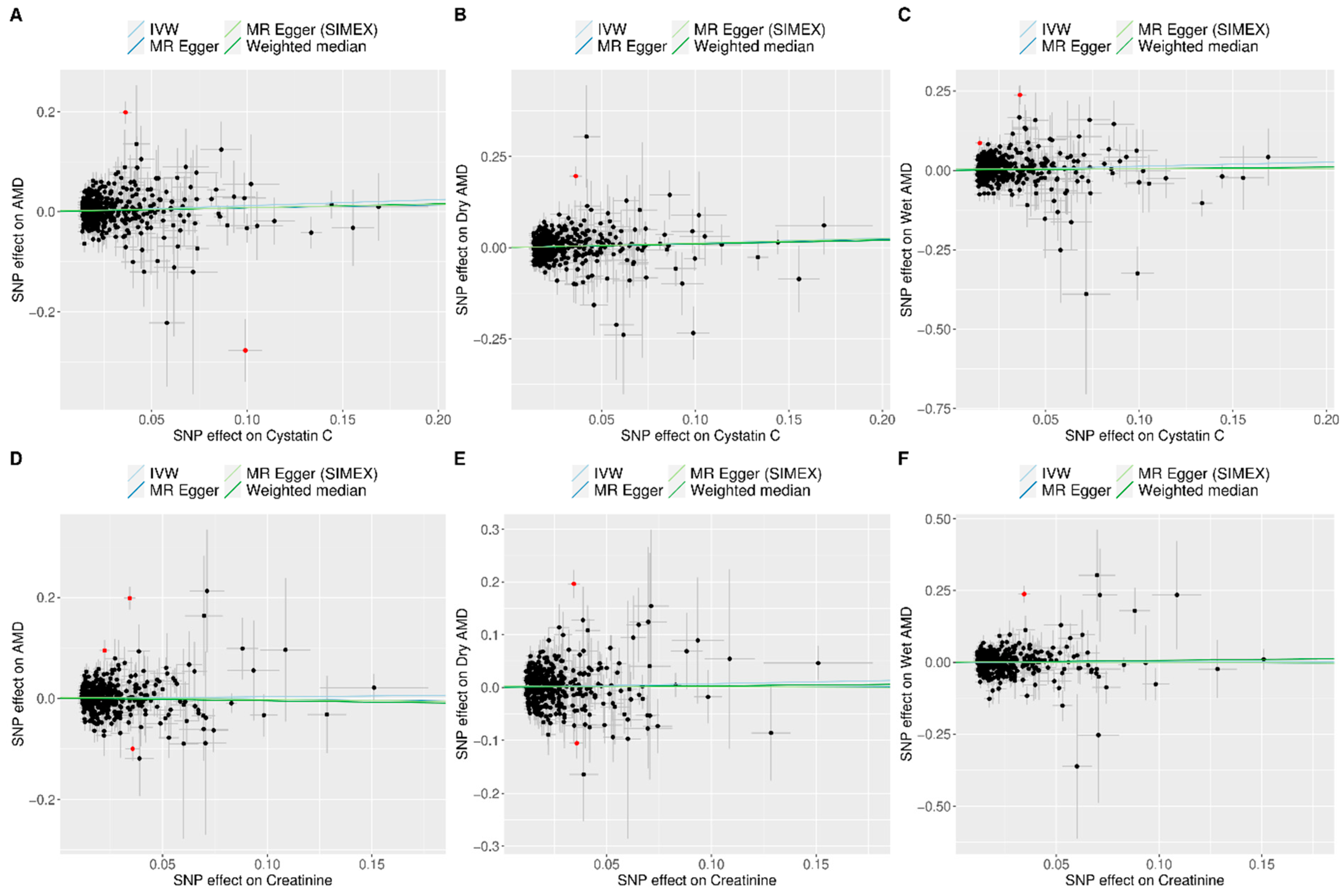
3.2. Heterogeneity and Horizontal Pleiotropy of the Instrumental Variables
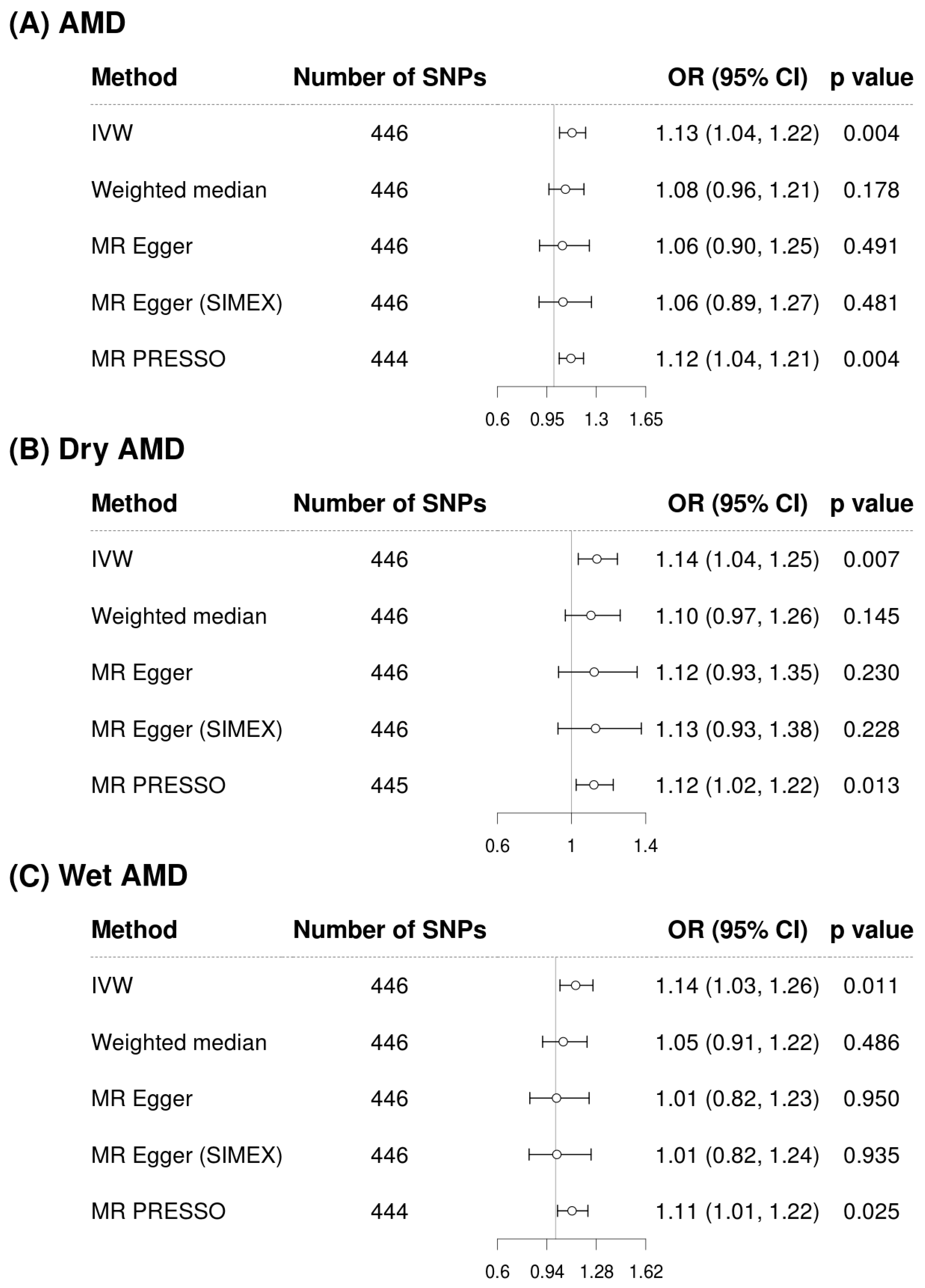
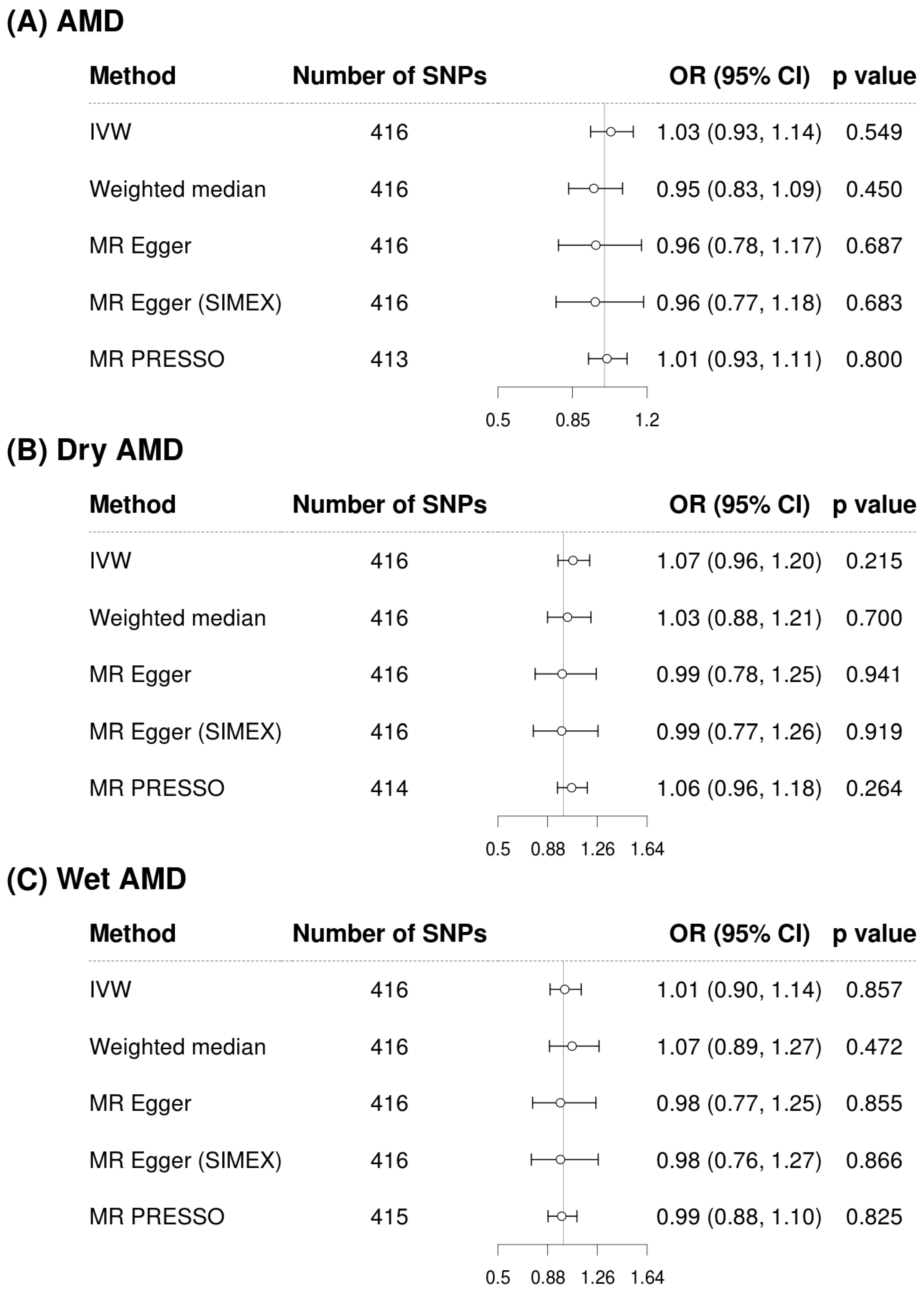
3.3. Mendelian Randomization
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fleckenstein, M.; Keenan, T.D.L.; Guymer, R.H.; Chakravarthy, U.; Schmitz-Valckenberg, S.; Klaver, C.C.; Wong, W.T.; Chew, E.Y. Age-related macular degeneration. Nat. Rev. Dis. Primers 2021, 7, 31. [Google Scholar] [CrossRef]
- Gottlieb, J.L. Age-related macular degeneration. JAMA 2002, 288, 2233–2236. [Google Scholar] [CrossRef]
- de Jong, P.T. Age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1474–1485. [Google Scholar] [CrossRef]
- Lim, L.S.; Mitchell, P.; Seddon, J.M.; Holz, F.G.; Wong, T.Y. Age-related macular degeneration. Lancet 2012, 379, 1728–1738. [Google Scholar] [CrossRef]
- Ferris, F.L., 3rd; Wilkinson, C.P.; Bird, A.; Chakravarthy, U.; Chew, E.; Csaky, K.; Sadda, S.R.; Beckman Initiative for Macular Research Classification Committee. Clinical classification of age-related macular degeneration. Ophthalmology 2013, 120, 844–851. [Google Scholar] [CrossRef]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Wong, T.Y.; Chakravarthy, U.; Klein, R.; Mitchell, P.; Zlateva, G.; Buggage, R.; Fahrbach, K.; Probst, C.; Sledge, I. The natural history and prognosis of neovascular age-related macular degeneration: A systematic review of the literature and meta-analysis. Ophthalmology 2008, 115, 116–126. [Google Scholar] [CrossRef]
- Colijn, J.M.; Buitendijk, G.H.S.; Prokofyeva, E.; Alves, D.; Cachulo, M.L.; Khawaja, A.P.; Cougnard-Gregoire, A.; Merle, B.M.J.; Korb, C.; Erke, M.G.; et al. Prevalence of Age-Related Macular Degeneration in Europe: The Past and the Future. Ophthalmology 2017, 124, 1753–1763. [Google Scholar] [CrossRef]
- Kawasaki, R.; Yasuda, M.; Song, S.J.; Chen, S.J.; Jonas, J.B.; Wang, J.J.; Mitchell, P.; Wong, T.Y. The prevalence of age-related macular degeneration in Asians: A systematic review and meta-analysis. Ophthalmology 2010, 117, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Kwon, K.E.; Choi, N.K.; Park, K.H.; Woo, S.J. Prevalence and Incidence of Exudative Age-Related Macular Degeneration in South Korea: A Nationwide Population-Based Study. Ophthalmology 2015, 122, 2063–2070.e2061. [Google Scholar] [CrossRef]
- Keenan, T.D.L.; Cukras, C.A.; Chew, E.Y. Age-Related Macular Degeneration: Epidemiology and Clinical Aspects. Adv. Exp. Med. Biol. 2021, 1256, 1–31. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kambhampati, S.P.; Bhutto, I.A.; McLeod, D.S.; Lutty, G.A.; Kannan, R.M. Evolution of oxidative stress, inflammation and neovascularization in the choroid and retina in a subretinal lipid induced age-related macular degeneration model. Exp. Eye Res. 2021, 203, 108391. [Google Scholar] [CrossRef]
- Shin, M.J.; Song, S.H.; Kwak, I.S.; Lee, S.B.; Lee, D.W.; Seong, E.Y.; Kim, I.Y.; Rhee, H.; Lee, N. Serum cystatin C as a predictor for cardiovascular events in end-stage renal disease patients at the initiation of dialysis. Clin. Exp. Nephrol. 2012, 16, 456–463. [Google Scholar] [CrossRef]
- Nowroozpoor, A.; Gutterman, D.; Safdar, B. Is microvascular dysfunction a systemic disorder with common biomarkers found in the heart, brain, and kidneys?—A scoping review. Microvasc. Res. 2021, 134, 104123. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Knudtson, M.D.; Lee, K.E.; Klein, B.E. Serum cystatin C level, kidney disease markers, and incidence of age-related macular degeneration: The Beaver Dam Eye Study. Arch. Ophthalmol. 2009, 127, 193–199. [Google Scholar] [CrossRef]
- Chong, E.W.; Guymer, R.H.; Klein, R.; Klein, B.E.; Cotch, M.F.; Wang, J.J.; Shlipak, M.G.; Wong, T.Y. Is renal function associated with early age-related macular degeneration? Optom. Vis. Sci. 2014, 91, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.C.; Sim, R.; Chee, M.L.; Yu, M.; Wang, Y.X.; Rim, T.H.; Hyung, P.K.; Woong, K.S.; Song, S.J.; Nangia, V.; et al. Is Kidney Function Associated with Age-Related Macular Degeneration?: Findings from the Asian Eye Epidemiology Consortium. Ophthalmology 2024, 131, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Li, Z.; Zhao, J.; Dong, H.; Tong, L.; Dou, J. Factors associated with aspirin resistance in diabetic patients: A metabolic and inflammatory profile analysis. PLoS ONE 2025, 20, e0332323. [Google Scholar] [CrossRef]
- Kwon, H.C.; Ha, J.W.; Kwon, O.C.; Park, Y.B.; Lee, S.W. Utility of serum cystatin C measured at diagnosis in evaluating cross-sectional activity and predicting all-cause mortality in patients with antineutrophil cytoplasmic antibody-associated vasculitis. Clin. Rheumatol. 2025, 44, 5003–5012. [Google Scholar] [CrossRef]
- Zyla, A.; Martel, A.; Jurczak, P.; Molinski, A.; Szymanska, A.; Kozak, M. Human cystatin C induces the disaggregation process of selected amyloid beta peptides: A structural and kinetic view. Sci. Rep. 2023, 13, 20833. [Google Scholar] [CrossRef]
- Kay, P.; Yang, Y.C.; Hiscott, P.; Gray, D.; Maminishkis, A.; Paraoan, L. Age-related changes of cystatin C expression and polarized secretion by retinal pigment epithelium: Potential age-related macular degeneration links. Investig. Ophthalmol. Vis. Sci. 2014, 55, 926–934. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, Y.; Steinle, J.J. Loss of cystatin C regulates permeability and inflammatory pathways in retina. Microvasc. Res. 2023, 148, 104510. [Google Scholar] [CrossRef]
- Carlsson, E.; Supharattanasitthi, W.; Jackson, M.; Paraoan, L. Increased Rate of Retinal Pigment Epithelial Cell Migration and Pro-Angiogenic Potential Ensuing From Reduced Cystatin C Expression. Investig. Ophthalmol. Vis. Sci. 2020, 61, 9. [Google Scholar] [CrossRef]
- Lawlor, D.A.; Harbord, R.M.; Sterne, J.A.; Timpson, N.; Davey Smith, G. Mendelian randomization: Using genes as instruments for making causal inferences in epidemiology. Stat. Med. 2008, 27, 1133–1163. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G. Multivariable Mendelian randomization: The use of pleiotropic genetic variants to estimate causal effects. Am. J. Epidemiol. 2015, 181, 251–260. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 2017, 32, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Chen, Y.; Chen, J.; Wang, L.; Cheng, H.; Hu, B.; Gong, J. The causal effects of lifestyle, circulating, pigment, and metabolic factors on early age-related macular degeneration: A comprehensive Mendelian randomization study. J. Transl. Med. 2024, 22, 988. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Seo, J.H. The Potential Causal Association of Apolipoprotein A and B and Age-Related Macular Degeneration: A Mendelian Randomisation Study. Biomedicines 2024, 12, 2828. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Ong, J.S.; An, J.; Hewitt, A.W.; Gharahkhani, P.; MacGregor, S. Using Mendelian randomization to evaluate the causal relationship between serum C-reactive protein levels and age-related macular degeneration. Eur. J. Epidemiol. 2020, 35, 139–146. [Google Scholar] [CrossRef]
- Yoon, B.W.; Lee, Y.; Seo, J.H. Potential Causal Association between C-Reactive Protein Levels in Age-Related Macular Degeneration: A Two-Sample Mendelian Randomization Study. Biomedicines 2024, 12, 807. [Google Scholar] [CrossRef]
- Burgess, S.; Butterworth, A.; Thompson, S.G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 2013, 37, 658–665. [Google Scholar] [CrossRef]
- Bowden, J.; Del Greco, M.F.; Minelli, C.; Davey Smith, G.; Sheehan, N.; Thompson, J. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat. Med. 2017, 36, 1783–1802. [Google Scholar] [CrossRef]
- Burgess, S.; Davey Smith, G.; Davies, N.M.; Dudbridge, F.; Gill, D.; Glymour, M.M.; Hartwig, F.P.; Kutalik, Z.; Holmes, M.V.; Minelli, C.; et al. Guidelines for performing Mendelian randomization investigations: Update for summer 2023. Wellcome Open Res. 2019, 4, 186. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Verbanck, M.; Chen, C.Y.; Neale, B.; Do, R. Publisher Correction: Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 1196. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Del Greco, M.F.; Minelli, C.; Davey Smith, G.; Sheehan, N.A.; Thompson, J.R. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: The role of the I2 statistic. Int. J. Epidemiol. 2016, 45, 1961–1974. [Google Scholar] [CrossRef]
- Bowden, J.; Holmes, M.V. Meta-analysis and Mendelian randomization: A review. Res. Synth. Methods 2019, 10, 486–496. [Google Scholar] [CrossRef]
- Jin, H.; Lee, S.; Won, S. Causal Evaluation of Laboratory Markers in Type 2 Diabetes on Cancer and Vascular Diseases Using Various Mendelian Randomization Tools. Front. Genet. 2020, 11, 597420. [Google Scholar] [CrossRef]
- Liew, G.; Mitchell, P.; Wong, T.Y.; Iyengar, S.K.; Wang, J.J. CKD increases the risk of age-related macular degeneration. J. Am. Soc. Nephrol. 2008, 19, 806–811. [Google Scholar] [CrossRef]
- Choi, J.; Moon, J.W.; Shin, H.J. Chronic kidney disease, early age-related macular degeneration, and peripheral retinal drusen. Ophthalmic Epidemiol. 2011, 18, 259–263. [Google Scholar] [CrossRef]
- Weiner, D.E.; Tighiouart, H.; Reynolds, R.; Seddon, J.M. Kidney function, albuminuria and age-related macular degeneration in NHANES III. Nephrol. Dial. Transpl. 2011, 26, 3159–3165. [Google Scholar] [CrossRef] [PubMed]
- Canney, M.; Sexton, D.J.; O’Leary, N.; Healy, M.; Kenny, R.A.; Little, M.A.; O’Seaghdha, C.M. Examining the utility of cystatin C as a confirmatory test of chronic kidney disease across the age range in middle-aged and older community-dwelling adults. J. Epidemiol. Community Health 2018, 72, 287–293. [Google Scholar] [CrossRef]
- Hou, Y.; Liu, Q.; Xiao, Z.; Li, Y.; Tian, X.; Wang, Z. Association between chronic kidney disease and age-related macular degeneration: A Mendelian randomization study. Front. Aging Neurosci. 2024, 16, 1399666. [Google Scholar] [CrossRef]
- Wong, C.W.; Lamoureux, E.L.; Cheng, C.Y.; Cheung, G.C.; Tai, E.S.; Wong, T.Y.; Sabanayagam, C. Increased Burden of Vision Impairment and Eye Diseases in Persons with Chronic Kidney Disease—A Population-Based Study. EBioMedicine 2016, 5, 193–197. [Google Scholar] [CrossRef]
- Dave, A.D.; Hess, K.; Chen, K.G.; Wiley, H.; Keenan, T.D.L.; Agron, E.; Chew, E.Y.; Cukras, C.A. Investigations of Renal Function and Age-Related Macular Degeneration Phenotypes. Transl. Vis. Sci. Technol. 2022, 11, 11. [Google Scholar] [CrossRef]
- Ali, M.; Ciebiera, M.; Vafaei, S.; Alkhrait, S.; Chen, H.Y.; Chiang, Y.F.; Huang, K.C.; Feduniw, S.; Hsia, S.M.; Al-Hendy, A. Progesterone Signaling and Uterine Fibroid Pathogenesis; Molecular Mechanisms and Potential Therapeutics. Cells 2023, 12, 1117. [Google Scholar] [CrossRef] [PubMed]
- Ambati, J.; Fowler, B.J. Mechanisms of age-related macular degeneration. Neuron 2012, 75, 26–39. [Google Scholar] [CrossRef]
- Ferreras, M.; Felbor, U.; Lenhard, T.; Olsen, B.R.; Delaisse, J. Generation and degradation of human endostatin proteins by various proteinases. FEBS Lett. 2000, 486, 247–251. [Google Scholar] [CrossRef]
- Paraoan, L.I.; Carlsson, E. AMD-linked variant B cystatin C interacts with mitochondrial proteins in retinal pigment epithelium cells. Investig. Ophthalmol. Vis. Sci. 2023, 64, 426. [Google Scholar]
- Carlsson, E.; Sharif, U.; Supharattanasitthi, W.; Paraoan, L. Analysis of Wild Type and Variant B Cystatin C Interactome in Retinal Pigment Epithelium Cells Reveals Variant B Interacting Mitochondrial Proteins. Cells 2023, 12, 713. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Schmid, C.H.; Tighiouart, H.; Eckfeldt, J.H.; Feldman, H.I.; Greene, T.; Kusek, J.W.; Manzi, J.; Van Lente, F.; Zhang, Y.L.; et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N. Engl. J. Med. 2012, 367, 20–29. [Google Scholar] [CrossRef] [PubMed]
| Traits | Data Source | No. of Participants | Population | No. of Variants | URL |
|---|---|---|---|---|---|
| Cystatin C | UKB | 400,940 | European | 23,012,599 | https://pan.ukbb.broadinstitute.org/downloads/index.html |
| Creatinine | UKB | 400,761 | European | 23,011,696 | |
| AMD | FinnGen | 357,849 (8913 cases + 348,936 controls) | European | 20,169,869 | https://finngen.gitbook.io/documentation/data-download |
| Dry AMD | FinnGen | 257,107 (6065 cases + 251,042 controls) | European | 20,165,949 | |
| Wet AMD | FinnGen | 257,125 (4848 cases + 252,277 controls) | European | 20,165,938 |
| Exposure | Outcome | Heterogeneity | Horizontal Pleiotropy | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MR-Egger | MR-Egger (SIMEX) | ||||||||||
| N | F | I2 (%) | p * | p † | p ‡ | Intercept, β (SE) | p | Intercept, β (SE) | p | ||
| Cystatin C | AMD | 446 | 89.92 | 95.20 | <0.001 | <0.001 | <0.001 | 0.002 (0.002) | 0.395 | 0.002 (0.002) | 0.435 |
| Creatinine | 416 | 92.83 | 94.61 | <0.001 | <0.001 | <0.001 | 0.002 (0.003) | 0.431 | 0.002 (0.003) | 0.436 | |
| Cystatin C | Dry AMD | 446 | 89.92 | 95.20 | <0.001 | <0.001 | <0.001 | 0.000 (0.003) | 0.876 | 0.000 (0.003) | 0.926 |
| Creatinine | 416 | 92.83 | 94.61 | <0.001 | <0.001 | <0.001 | 0.002 (0.003) | 0.444 | 0.002 (0.003) | 0.447 | |
| Cystatin C | Wet AMD | 446 | 89.92 | 95.19 | <0.001 | <0.001 | <0.001 | 0.004 (0.003) | 0.169 | 0.004 (0.003) | 0.189 |
| Creatinine | 416 | 92.83 | 94.61 | <0.001 | <0.001 | <0.001 | 0.001 (0.003) | 0.759 | 0.001 (0.003) | 0.771 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.; Seo, J.H. Potential Causal Effects of Cystatin C on Age-Related Macular Degeneration: A Two-Sample Mendelian Randomization Study. Biomedicines 2025, 13, 2827. https://doi.org/10.3390/biomedicines13112827
Lee Y, Seo JH. Potential Causal Effects of Cystatin C on Age-Related Macular Degeneration: A Two-Sample Mendelian Randomization Study. Biomedicines. 2025; 13(11):2827. https://doi.org/10.3390/biomedicines13112827
Chicago/Turabian StyleLee, Young, and Je Hyun Seo. 2025. "Potential Causal Effects of Cystatin C on Age-Related Macular Degeneration: A Two-Sample Mendelian Randomization Study" Biomedicines 13, no. 11: 2827. https://doi.org/10.3390/biomedicines13112827
APA StyleLee, Y., & Seo, J. H. (2025). Potential Causal Effects of Cystatin C on Age-Related Macular Degeneration: A Two-Sample Mendelian Randomization Study. Biomedicines, 13(11), 2827. https://doi.org/10.3390/biomedicines13112827






