Longitudinal Cerebral Structural, Microstructural, and Functional Alterations After Brain Tumor Surgery for Early Detection of Recurrent Tumors
Abstract
1. Introduction
2. Methods
2.1. Subjects and Inclusion Criteria
2.2. MRI Scanning Protocols
2.2.1. T1w
2.2.2. T2w FLAIR
2.2.3. DTI
2.2.4. rsfMRI
2.3. Data Analysis
2.3.1. Preprocessing: Alignment of Individual Longitudinal Scans and Parameterizations
2.3.2. Post-Processing: Region of Interest (ROI) Analyses
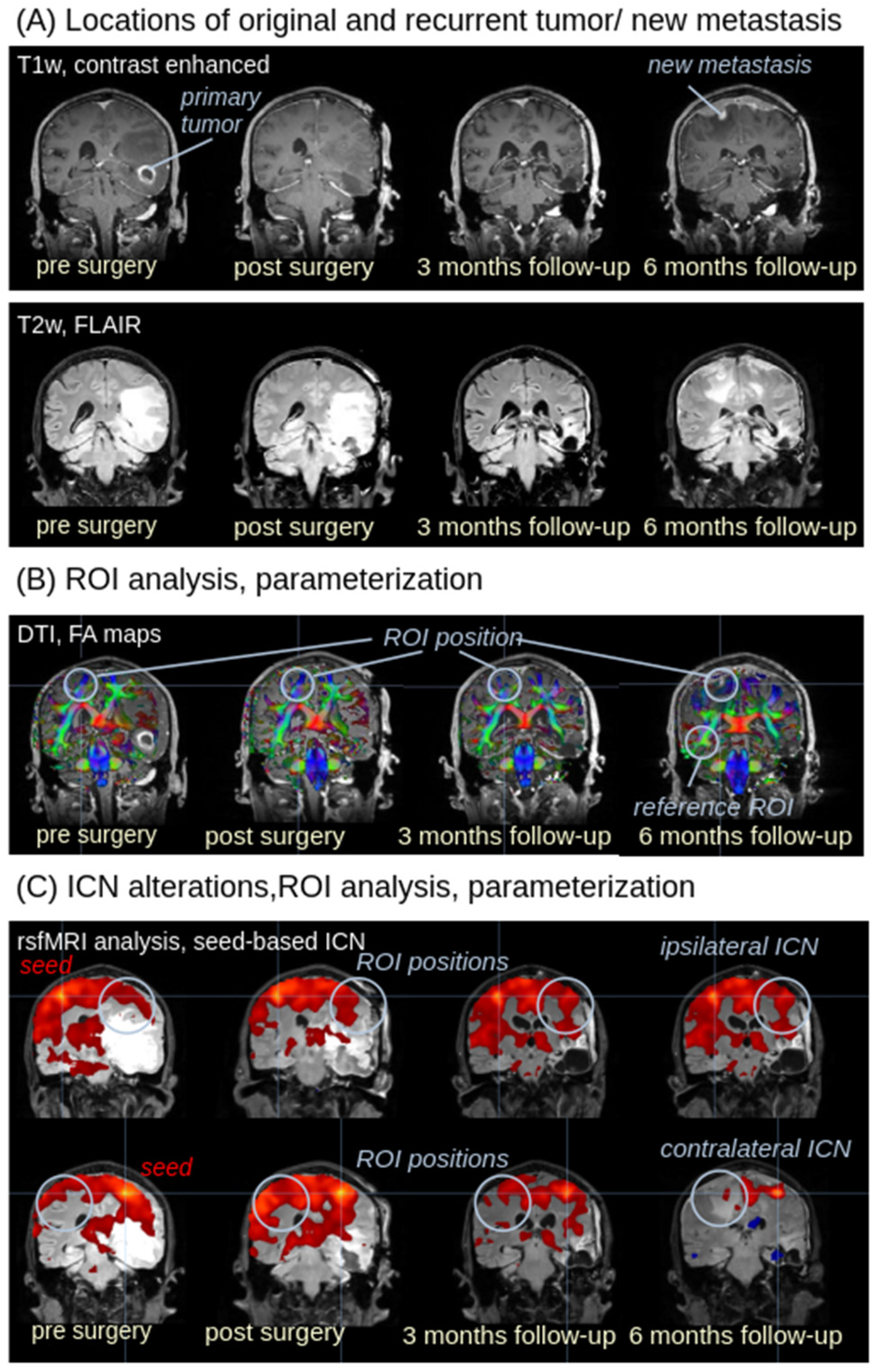
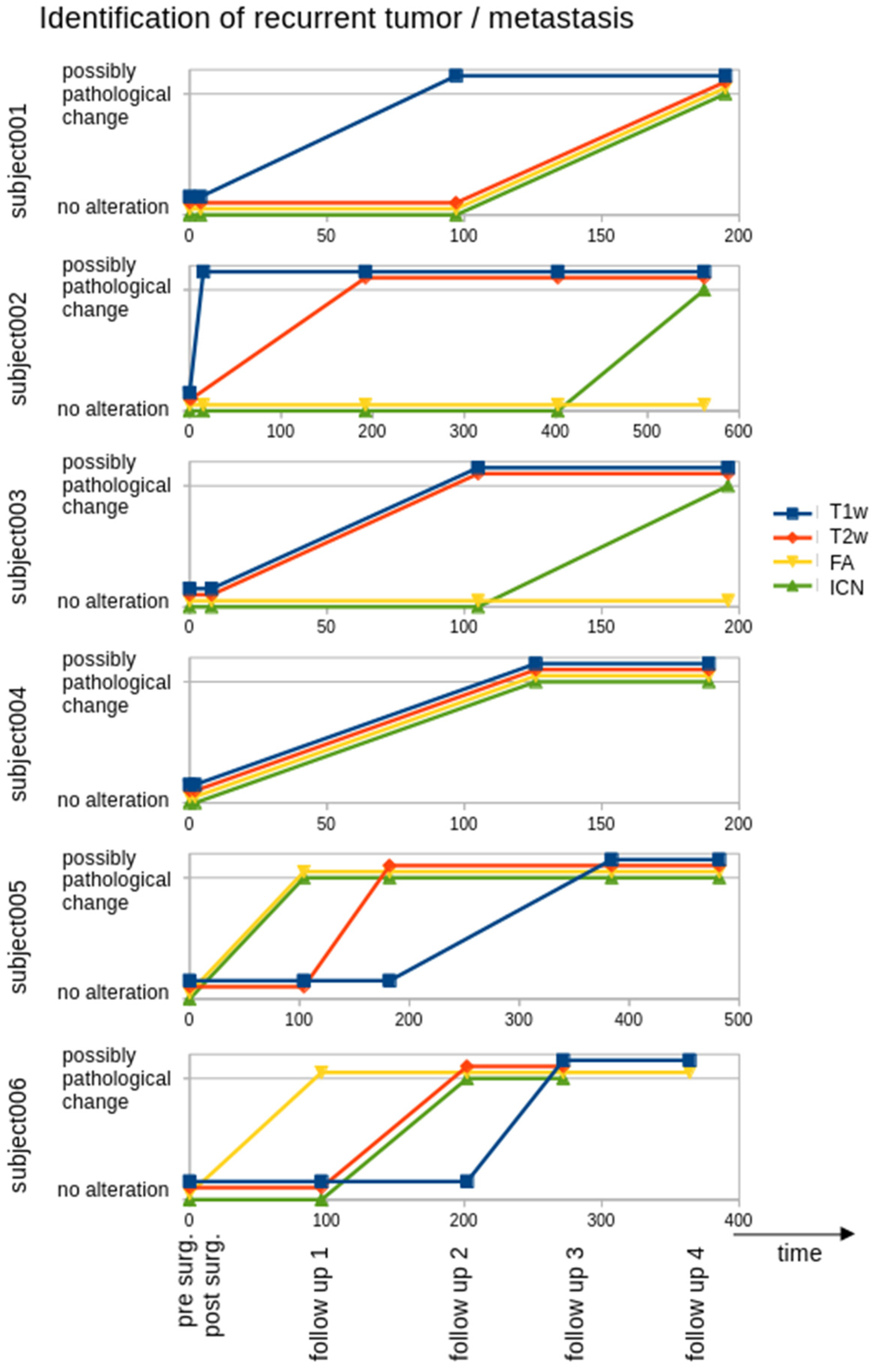
3. Results
3.1. Recurrent Tumor in T1w and T2w Images
3.2. ROI Analysis in FA Maps
3.3. ROI Analysis in ICNs
4. Discussion
4.1. Multiparametric MRI in the Assessment of (Recurrent) Tumors
4.2. Limitations
4.3. Summary and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DTI/DWI | diffusion tensor imaging/diffusion weighted imaging |
| FA | fractional anisotropy |
| FLAIR | fluid attenuated inversion recovery |
| GD | gradient directions |
| ifc | intrinsic functional connectivity |
| ICN | intrinsic functional connectivity network |
| ML | machine-learning |
| MRI | magnetic resonance imaging |
| rsfMRI | Resting state functional |
| ROI | region of interes |
| T1w/T2w | T1-weighted/T2-weighted |
| TE/TR | echo time/repetition time |
| TAT | total acquisition time |
| TIFT | Tensor Imaging and Fiber Tracking |
References
- Weller, M.; Wen, P.Y.; Chang, S.M.; Dirven, L.; Lim, M.; Monje, M.; Reifenberger, G. Glioma. Nat. Rev. Dis. Primers 2024, 10, 33. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Kassubek, R.; Müller, H.P.; Thiele, A.; Kassubek, J.; Niessen, H.G. Advanced magnetic resonance imaging to support clinical drug development for malignant glioma. Drug Discov. Today 2021, 26, 429–441. [Google Scholar] [CrossRef]
- Smits, M. Update on neuroimaging in brain tumours. Curr. Opin. Neurol. 2021, 34, 497–504. [Google Scholar] [CrossRef]
- Guzzino, J.; Crompton, D.J.; Agarwal, S.; Kern, S.; Bogan, A.; Vora, S.A.; Quinones-Hinojosa, A.; Burns, T.C.; Sherman, W.J.; Brown, P.D.; et al. Rapid early progression of glioblastoma: Evaluation of a novel prognostic radiologic biomarker. J. Neurooncol. 2025, 176, 5. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, W.; Zhao, C.; Zhang, J.; Zhang, Z.; Shen, P.; Wang, X.; Yang, G.; Du, J.; Zhang, H.; et al. Survival risk stratification of 2021 WHO glioblastoma by MRI radiomics and biological exploration. BMC Cancer 2025, 25, 1505. [Google Scholar] [CrossRef] [PubMed]
- Ozsahin, D.U.; Onakpojeruo, E.P.; Uzun, B.; Mustapha, M.T.; Ozsahin, I. Mathematical Assessment of Machine Learning Models Used for Brain Tumor Diagnosis. Diagnostics 2023, 13, 618. [Google Scholar] [CrossRef]
- Salvalaggio, A.; Pini, L.; Bertoldo, A.; Corbetta, M. Glioblastoma and Brain Connectivity: The Need for a Paradigm Shift. Lancet Neurol. 2024, 23, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Shukla, G.; Alexander, G.S.; Bakas, S.; Nikam, R.; Talekar, K.; Palmer, J.D.; Shi, W. Advanced Magnetic Resonance Imaging in Glioblastoma: A Review. Chin. Clin. Oncol. 2017, 6, 40. [Google Scholar] [CrossRef]
- Nakagawa, M.; Nakaura, T.; Namimoto, T.; Kitajima, M.; Uetani, H.; Tateishi, M.; Oda, S.; Utsunomiya, D.; Makino, K.; Nakamura, H.; et al. Machine Learning Based on Multi-Parametric Magnetic Resonance Imaging to Differentiate Glioblastoma Multiforme from Primary Cerebral Nervous System Lymphoma. Eur. J. Radiol. 2018, 108, 147–154. [Google Scholar] [CrossRef]
- Takeguchi, T.; Miki, H.; Shimizu, T.; Kikuchi, K.; Mochizuki, T.; Ohue, S.; Ohnishi, T. Evaluation of the Tumor-Brain Interface of Intracranial Meningiomas on MR Imaging Including FLAIR Images. Magn. Reson. Med. Sci. 2003, 2, 165–169. [Google Scholar] [CrossRef]
- Ellingson, B.M.; Bendszus, M.; Boxerman, J.; Barboriak, D.; Erickson, B.J.; Smits, M.; Nelson, S.J.; Gerstner, E.; Alexander, B.; Goldmacher, G.; et al. Jumpstarting Brain Tumor Drug Development Coalition Imaging Standardization Steering Committee. Consensus Recommendations for a Standardized Brain Tumor Imaging Protocol in Clinical Trials. Neuro Oncol. 2015, 17, 1188–1198. [Google Scholar] [CrossRef]
- Kassubek, R.; Gorges, M.; Westhoff, M.A.; Ludolph, A.C.; Kassubek, J.; Müller, H.P. Cerebral Microstructural Alterations after Radiation Therapy in High-Grade Glioma: A Diffusion Tensor Imaging-Based Study. Front. Neurol. 2017, 8, 286. [Google Scholar] [CrossRef] [PubMed]
- Kassubek, R.; Lulé, D.; Ludolph, A.C.; Kassubek, J.; Müller, H.P. Bevacizumab Is Associated with Cerebral Microstructural Alterations: A DTI Study in High-Grade Glioma. Front. Neurol. 2023, 14, 1191226. [Google Scholar] [CrossRef]
- Liheng, M.; Guofan, X.; Balzano, R.F.; Yuying, L.; Weifeng, H.; Ning, Y.; Yayun, J.; Mouyuan, L.; Guglielmi, G. The Value of DTI: Achieving High Diagnostic Performance for Brain Metastasis. Radiol. Med. 2021, 126, 291–298. [Google Scholar] [CrossRef]
- Latysheva, A.; Emblem, K.E.; Brandal, P.; Vik-Mo, E.O.; Pahnke, J.; Røysland, K.; Hald, J.K.; Server, A. Dynamic Susceptibility Contrast and Diffusion MR Imaging Identify Oligodendroglioma as Defined by the 2016 WHO Classification for Brain Tumors: Histogram Analysis Approach. Neuroradiology 2019, 61, 545–555. [Google Scholar] [CrossRef]
- Maesawa, S.; Bagarinao, E.; Fujii, M.; Futamura, M.; Wakabayashi, T. Use of Network Analysis to Establish Neurosurgical Parameters in Gliomas and Epilepsy. Neurol. Med. Chir. 2016, 56, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Park, K.Y.; Snyder, A.Z.; Olufawo, M.; Trevino, G.; Luckett, P.H.; Lamichhane, B.; Xie, T.; Lee, J.J.; Shimony, J.S.; Leuthardt, E.C. Glioblastoma Induces Whole-Brain Spectral Change in Resting State fMRI: Associations with Clinical Comorbidities and Overall Survival. Neuroimage Clin. 2023, 39, 103476. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.S.; Pendem, S.; Menon, G.R.; Sampathila, N.; Koteshwar, P. Quality Assessment of MRI-Radiomics-Based Machine Learning Methods in Classification of Brain Tumors: Systematic Review. Diagnostics 2024, 14, 2741. [Google Scholar] [CrossRef]
- Sighinolfi, G.; Mitolo, M.; Testa, C.; Martinoni, M.; Evangelisti, S.; Rochat, M.J.; Zoli, M.; Mazzatenta, D.; Lodi, R.; Tonon, C. What Can Resting-State fMRI Data Analysis Explain about the Functional Brain Connectivity in Glioma Patients? Tomography 2022, 8, 267–280. [Google Scholar] [CrossRef]
- Müller, H.P.; Unrath, A.; Ludolph, A.C.; Kassubek, J. Preservation of Diffusion Tensor Properties during Spatial Normalization by Use of Tensor Imaging and Fibre Tracking on a Normal Brain Database. Phys. Med. Biol. 2007, 52, N99–N109. [Google Scholar] [CrossRef]
- Müller, H.P.; Kassubek, J. Diffusion tensor magnetic resonance imaging in the analysis of neurodegenerative diseases. J. Vis. Exp. 2013, 77, 50427. [Google Scholar] [CrossRef]
- Papageorgiou, T.S.; Chourmouzi, D.; Drevelengas, A.; Kouskouras, K.; Siountas, A. Diffusion Tensor Imaging in Brain Tumors: A Study on Gliomas and Metastases. Phys. Med. 2015, 31, 767–773. [Google Scholar] [CrossRef]
- Gorges, M.; Müller, H.P.; Ludolph, A.C.; Rasche, V.; Kassubek, J. Intrinsic Functional Connectivity Networks in Healthy Elderly Subjects: A Multiparametric Approach with Structural Connectivity Analysis. Biomed. Res. Int. 2014, 2014, 947252. [Google Scholar] [CrossRef]
- Kunimatsu, A.; Aoki, S.; Masutani, Y.; Abe, O.; Hayashi, N.; Mori, H.; Masumoto, T.; Ohtomo, K. The Optimal Trackability Threshold of Fractional Anisotropy for Diffusion Tensor Tractography of the Corticospinal Tract. Magn. Reson. Med. Sci. 2004, 3, 11–17. [Google Scholar] [CrossRef]
- Soares, J.M.; Marques, P.; Alves, V.; Sousa, N. A Hitchhiker’s Guide to Diffusion Tensor Imaging. Front. Neurosci. 2013, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Kosztyla, R.; Reinsberg, S.A.; Moiseenko, V.; Toyota, B.; Nichol, A. Interhemispheric Difference Images from Postoperative Diffusion Tensor Imaging of Gliomas. Cureus 2016, 8, e817. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Du, F.Z.; He, C.; Gu, M.; Ke, Z.W.; Li, J.H. The Value of Diffusion Tensor Imaging in Differentiating High-Grade Gliomas from Brain Metastases: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e112550. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Martinez-Lage, M.; Sakai, Y.; Chawla, S.; Kim, S.G.; Alonso-Basanta, M.; Lustig, R.A.; Brem, S.; Mohan, S.; Wolf, R.L.; et al. Differentiating Tumor Progression from Pseudoprogression in Patients with Glioblastomas Using Diffusion Tensor Imaging and Dynamic Susceptibility Contrast MRI. AJNR Am. J. Neuroradiol. 2016, 37, 28–36. [Google Scholar] [CrossRef]
- Won, Y.I.; Chung, C.K.; Kim, C.H.; Park, C.K.; Koo, B.B.; Lee, J.M.; Jung, H.W. White Matter Change Revealed by Diffusion Tensor Imaging in Gliomas. Brain Tumor Res. Treat. 2016, 4, 100–106. [Google Scholar] [CrossRef]
- Ozsahin, D.U.; Ozsahin, I. A Comparative Analysis of the Novel Conditional Deep Convolutional Neural Network Model, Using Conditional Deep Convolutional Generative Adversarial Network-Generated Synthetic and Augmented Brain Tumor Datasets for Image Classification. Brain Sci. 2024, 14, 559. [Google Scholar] [CrossRef] [PubMed]
- Onakpojeruo, E.P.; Mustapha, M.T.; Ozsahin, D.U.; Ozsahin, I. Enhanced MRI-Based Brain Tumour Classification with a Novel Pix2pix Generative Adversarial Network Augmentation Framework. Brain Commun. 2024, 6, fcae372. [Google Scholar] [CrossRef]
- Kizilirmak, J.M.; Soch, J.; Schütze, H.; Düzel, E.; Feldhoff, H.; Fischer, L.; Knopf, L.; Maass, A.; Raschick, M.; Schult, A.; et al. The Relationship between Resting-State Amplitude Fluctuations and Memory-Related Deactivations of the Default Mode Network in Young and Older Adults. Hum. Brain Mapp. 2023, 44, 3586–3609. [Google Scholar] [CrossRef]
- Stoecklein, V.M.; Stoecklein, S.; Galiè, F.; Ren, J.; Schmutzer, M.; Unterrainer, M.; Albert, N.L.; Kreth, F.W.; Thon, N.; Liebig, T.; et al. Resting-State fMRI Detects Alterations in Whole Brain Connectivity Related to Tumor Biology in Glioma Patients. Neuro Oncol. 2020, 22, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- De Simone, M.; Iaconetta, G.; Palermo, G.; Fiorindi, A.; Schaller, K.; De Maria, L. Clustering Functional Magnetic Resonance Imaging Time Series in Glioblastoma Characterization: A Review of the Evolution, Applications, and Potentials. Brain Sci. 2024, 14, 296. [Google Scholar] [CrossRef] [PubMed]
- Zijlmans, M.; de Kort, G.A.; Witkamp, T.D.; Huiskamp, G.M.; Seppenwoolde, J.H.; van Huffelen, A.C.; Leijten, F.S. 3T versus 1.5T phased-array MRI in the presurgical work-up of patients with partial epilepsy of uncertain focus. J. Magn. Reson. Imaging 2009, 30, 256–262. [Google Scholar] [CrossRef]
- Polders, D.L.; Leemans, A.; Hendrikse, J.; Donahue, M.J.; Luijten, P.R.; Hoogduin, J.M. Signal to noise ratio and uncertainty in diffusion tensor imaging at 1.5, 3.0, and 7.0 Tesla. J. Magn. Reson. Imaging 2011, 33, 1456–1463. [Google Scholar] [CrossRef]
- De Simone, M.; Choucha, A.; Ranalli, C.; Pecoraro, G.; Appay, R.; Chinot, O.L.; Dufour, H.; Iaconetta, G. Astrocytomas IDH-mutant of posterior cranial fossa, clinical presentation, imaging features and onco-functional balance in surgical management. Neurosurg. Rev. 2025, 48, 271. [Google Scholar] [CrossRef]

| Patient (Age/Sex) | Scans | Site of Tumor | Site of Tumor |
|---|---|---|---|
| 69/m | 4 | right temporal lobe | right temporal lobe |
| 41/f | 5 | right temporal lobe | right temporal lobe |
| 65/m | 4 | right temporal lobe | right temporal lobe |
| 57/f | 4 | left frontal lobe | left frontal lobe |
| 76/m | 5 | right parietal lobe | right parietal lobe |
| 61/m | 5 | right parietal lobe | right parietal lobe |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kassubek, R.; Amend, M.; Niessen, H.; Schmitz, B.; Engelke, J.; Grübel, N.; Weishaupt, J.; Haeusler, K.G.; Kassubek, J.; Müller, H.-P. Longitudinal Cerebral Structural, Microstructural, and Functional Alterations After Brain Tumor Surgery for Early Detection of Recurrent Tumors. Biomedicines 2025, 13, 2811. https://doi.org/10.3390/biomedicines13112811
Kassubek R, Amend M, Niessen H, Schmitz B, Engelke J, Grübel N, Weishaupt J, Haeusler KG, Kassubek J, Müller H-P. Longitudinal Cerebral Structural, Microstructural, and Functional Alterations After Brain Tumor Surgery for Early Detection of Recurrent Tumors. Biomedicines. 2025; 13(11):2811. https://doi.org/10.3390/biomedicines13112811
Chicago/Turabian StyleKassubek, Rebecca, Mario Amend, Heiko Niessen, Bernd Schmitz, Jens Engelke, Nadja Grübel, Jochen Weishaupt, Karl Georg Haeusler, Jan Kassubek, and Hans-Peter Müller. 2025. "Longitudinal Cerebral Structural, Microstructural, and Functional Alterations After Brain Tumor Surgery for Early Detection of Recurrent Tumors" Biomedicines 13, no. 11: 2811. https://doi.org/10.3390/biomedicines13112811
APA StyleKassubek, R., Amend, M., Niessen, H., Schmitz, B., Engelke, J., Grübel, N., Weishaupt, J., Haeusler, K. G., Kassubek, J., & Müller, H.-P. (2025). Longitudinal Cerebral Structural, Microstructural, and Functional Alterations After Brain Tumor Surgery for Early Detection of Recurrent Tumors. Biomedicines, 13(11), 2811. https://doi.org/10.3390/biomedicines13112811






