Effect of Allogenic Mesenchymal Stem Cell Injection on Functional Repair Outcomes Following Skeletal Muscle Laceration Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Animal Model of Muscle Laceration
2.3. Rat Bone Marrow-Derived Mesenchymal Stromal Cell (BM-MSC) Preparation and Transplantation
2.4. Physiological Test: Muscle Force Measurement After Tetanic Contraction
2.5. Histology
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BM-MSCs | Bone marrow-derived mesenchymal stem cells |
| H | Hour |
| H&E | Hematoxylin and Eosin |
| MSCs | Mesenchymal stem cells |
| Hz | Hertz |
| PBS | Phosphate-buffered solution |
| SD | Standard deviation |
References
- Järvinen, T.A.; Järvinen, M.; Kalimo, H. Regeneration of injured skeletal muscle after the injury. Muscles Ligaments Tendons J. 2014, 3, 337. [Google Scholar] [CrossRef]
- Garg, K.; Ward, C.L.; Hurtgen, B.J.; Wilken, J.M.; Stinner, D.J.; Wenke, J.C.; Corona, B.T. Volumetric muscle loss: Persistent functional deficits beyond frank loss of tissue. J. Orthop. Res. 2015, 33, 40–46. [Google Scholar] [CrossRef]
- Sato, K.; Li, Y.; Foster, W.; Fukushima, K.; Badlani, N.; Adachi, N.; Huard, J. Improvement of muscle healing through enhancement of muscle regeneration and prevention of fibrosis. Muscle Nerve 2023, 28, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Baoge, L.; Van Den Steen, E.; Rimbaut, S.; Philips, N.; Witvrouw, E.; Almqvist, K.F.; Vanderstraeten, G.; Vanden Bossche, L.C. Treatment of skeletal muscle injury: A review. ISRN Orthop. 2012, 2012, 689012. [Google Scholar] [CrossRef] [PubMed]
- Edouard, P.; Reurink, G.; Mackey, A.L.; Lieber, R.L.; Pizzari, T.; Järvinen, T.A.; Hollander, K. Traumatic muscle injury. Nat. Rev. Dis. Primers 2023, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Mekkodathil, A.; El-Menyar, A.; Al-Thani, H. Occupational injuries in workers from different ethnicities. Int. J. Crit. Illn. Inj. Sci. 2016, 6, 25–32. [Google Scholar] [CrossRef]
- Khanghah, Y.S.; Foroutan, A.; Sherafat, A.; Fatemi, M.J.; Faradonbeh, H.B.; Akbari, H. Implementation of upper extremity trauma registry: A pilot study. World J. Plast. Surg. 2023, 12, 29. [Google Scholar] [CrossRef]
- Jivan, S.; Kumar, N.; Wiberg, M.; Kay, S. The influence of pre-surgical delay on functional outcome after reconstruction of brachial plexus injuries. J. Plast. Reconstr. Aesthet. Surg. 2009, 62, 472–479. [Google Scholar] [CrossRef]
- Ezzat, M.; Ezzat, M.M.; Tran, V.Q.; Aboseif, S.R. Repair of giant vesicovaginal fistulas. J. Urol. 2009, 181, 1184–1188. [Google Scholar] [CrossRef]
- Chichom-Mefire, A.; Palle-Ngunde, J.; Fokam, P.G.; Mokom-Awa, A.; Njock, R.; Ngowe-Ngowe, M. Injury patterns in road traffic victims comparing road user categories: Analysis of 811 consecutive cases in the emergency department of a level I institution in a low-income country. Int. J. Surg. Open 2018, 10, 30–36. [Google Scholar] [CrossRef]
- Ballard, D.H.; Campbell, K.J.; Hedgepeth, K.B.; Hollister, A.M.; Simoncini, A.A.; Pahilan, M.E.; Youssef, A.M. Anatomic guide and sonography for surgical repair of leg muscle lacerations. J. Surg. Res. 2013, 184, 178–182. [Google Scholar] [CrossRef]
- Kragh, J.F., Jr.; Svoboda, S.J.; Wenke, J.C.; Ward, J.A.; Walters, T.J. Epimysium and perimysium in suturing in skeletal muscle lacerations. J. Trauma 2005, 59, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Stulginski, A.; Vallurupalli, M.; Pakvasa, M.; Tang, C.J. Evaluating suturing methods for surgical repair of muscle belly lacerations: A scoping review of biomechanical studies. Eur. J. Plast. Surg. 2024, 47, 23. [Google Scholar] [CrossRef]
- Chance, J.R.; Kragh, J.F., Jr.; Agrawal, C.M.; Basamania, C.J. Pullout forces of sutures in muscle lacerations. Orthopedics 2005, 28, 1187–1190. [Google Scholar] [CrossRef]
- Oliva, F.; Giai Via, A.; Kiritsi, O.; Foti, C.; Maffulli, N. Surgical repair of muscle laceration: Biomechanical properties at 6 years follow-up. Muscles Ligaments Tendons J. 2014, 3, 313. [Google Scholar] [CrossRef]
- Musarò, A. The basis of muscle regeneration. Adv. Biol. 2014, 2014, 612471. [Google Scholar] [CrossRef]
- Southerland, K.W.; Xu, Y.; Peters, D.T.; Lin, X.; Wei, X.; Xiang, Y.; Diao, Y. Skeletal muscle regeneration failure in ischemic-damaged limbs is associated with pro-inflammatory macrophages and premature differentiation of satellite cells. Genome Med. 2023, 15, 95. [Google Scholar] [CrossRef] [PubMed]
- Laumonier, T.; Menetrey, J. Muscle injuries and strategies for improving their repair. J. Exp. Orthop. 2016, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.L.; Kamal, M.; Parise, G. The role of supporting cell populations in satellite cell mediated muscle repair. Cells 2023, 12, 1968. [Google Scholar] [CrossRef]
- Naldaiz-Gastesi, N.; Goicoechea, M.; Aragón, I.M.; Pérez-López, V.; Fuertes-Alvarez, S.; Herrera-Imbroda, B.; López de Munain, A.; de Luna-Diaz, R.; Baptista, P.M.; Fernández, M.A.; et al. Isolation and characterization of myogenic precursor cells from human cremaster muscle. Sci. Rep. 2019, 9, 3454. [Google Scholar] [CrossRef]
- Briggs, D.; Morgan, J.E. Recent progress in satellite cell/myoblast engraftment—Relevance for therapy. FEBS J. 2013, 280, 4281–4293. [Google Scholar] [CrossRef]
- Elster, J.L.; Rathbone, C.R.; Liu, Z.; Liu, X.; Barrett, H.H.; Rhoads, R.P.; Allen, R.E. Skeletal muscle satellite cell migration to injured tissue measured with 111In-oxine and high-resolution SPECT imaging. J. Muscle Res. Cell Motil. 2013, 34, 417–427. [Google Scholar] [CrossRef]
- Relaix, F.; Zammit, P.S. Satellite cells are essential for skeletal muscle regeneration: The cell on the edge returns centre stage. Development 2012, 139, 2845–2856. [Google Scholar] [CrossRef]
- Skuk, D.; Goulet, M.; Tremblay, J.P. Transplanted myoblasts can migrate several millimeters to fuse with damaged myofibers in nonhuman primate skeletal muscle. J. Neuropathol. Exp. Neurol. 2011, 70, 770–778. [Google Scholar] [CrossRef]
- Chiu, C.H.; Chang, T.H.; Chang, S.S.; Chang, G.J.; Chen, A.C.; Cheng, C.Y.; Chen, S.C.; Fu, J.F.; Wen, C.J.; Chan, Y.S. Application of bone marrow-derived mesenchymal stem cells for muscle healing after contusion injury in mice. Am. J. Sports Med. 2020, 48, 1226–1235. [Google Scholar] [CrossRef] [PubMed]
- Linard, C.; Brachet, M.; L’homme, B.; Strup-Perrot, C.; Busson, E.; Bonneau, M.; Lataillade, J.J.; Bey, E.; Benderitter, M. Long-term effectiveness of local BM-MSCs for skeletal muscle regeneration: A proof of concept obtained on a pig model of severe radiation burn. Stem Cell Res. Ther. 2018, 9, 299. [Google Scholar] [CrossRef] [PubMed]
- Brickson, S.; Meyer, P.; Saether, E.; Vanderby, R. Mesenchymal stem cells improve muscle function following single stretch injury: A preliminary study. J. Funct. Morphol. Kinesiol. 2016, 1, 396–406. [Google Scholar] [CrossRef]
- Helal, M.A.M.; Shaheen, N.E.M.; Abu Zahra, F.A. Immunomodulatory capacity of the local mesenchymal stem cells transplantation after severe skeletal muscle injury in female rats. Immunopharmacol. Immunotoxicol. 2016, 38, 414–422. [Google Scholar] [CrossRef]
- von Roth, P.; Duda, G.N.; Radojewski, P.; Preininger, B.; Strohschein, K.; Röhner, E.; Perka, C.; Winkler, T. Intra-Arterial MSC Transplantation Restores Functional Capacity After Skeletal Muscle Trauma. Open Orthop. J. 2012, 6, 352–356. [Google Scholar] [CrossRef]
- Elhussieny, A.; Nogami, K.; Sakai-Takemura, F.; Maruyama, Y.; Takemura, N.; Soliman, W.T.; Takeda, S.; Miyagoe-Suzuki, Y. Mesenchymal stem cells derived from human induced pluripotent stem cells improve the engraftment of myogenic cells by secreting urokinase-type plasminogen activator receptor (uPAR). Stem Cell Res. Ther. 2021, 12, 532. [Google Scholar] [CrossRef]
- de la Garza-Rodea, A.S.; van der Velde, I.; Boersma, H.; Gonçalves, M.A.; van Bekkum, D.W.; de Vries, A.A.; Knaän-Shanzer, S. Long-term contribution of human bone marrow mesenchymal stromal cells to skeletal muscle regeneration in mice. Cell Transplant. 2011, 20, 217–231. [Google Scholar] [CrossRef]
- García-Bernal, D.; García-Arranz, M.; Yáñez, R.M.; Hervás-Salcedo, R.; Cortés, A.; Fernández-García, M.; Hernando-Rodríguez, M.; Quintana-Bustamante, Ó.; Bueren, J.A.; García-Olmo, D.; et al. The Current Status of Mesenchymal Stromal Cells: Controversies, Unresolved Issues and Some Promising Solutions to Improve Their Therapeutic Efficacy. Front. Cell Dev. Biol. 2021, 9, 650664. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maldonado, V.V.; Patel, N.H.; Smith, E.E.; Barnes, C.L.; Gustafson, M.P.; Rao, R.R.; Samsonraj, R.M. Clinical utility of mesenchymal stem/stromal cells in regenerative medicine and cellular therapy. J. Biol. Eng. 2023, 17, 44. [Google Scholar] [CrossRef]
- Forcales, S.V. Potential of adipose-derived stem cells in muscular regenerative therapies. Front. Aging Neurosci. 2015, 7, 123. [Google Scholar] [CrossRef]
- Salemi, S.; Prange, J.A.; Baumgartner, V.; Mohr-Haralampieva, D.; Eberli, D. Adult stem cell sources for skeletal and smooth muscle tissue engineering. Stem Cell Res. Ther. 2022, 13, 156. [Google Scholar] [CrossRef]
- Pozzobon, M.; Franzin, C.; Piccoli, M.; De Coppi, P. Fetal stem cells and skeletal muscle regeneration: A therapeutic approach. Front. Aging Neurosci. 2014, 6, 222. [Google Scholar] [CrossRef]
- Huard, J. Stem cells, blood vessels, and angiogenesis as major determinants for musculoskeletal tissue repair. J. Orthop. Res. 2019, 37, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Caseiro, A.R.; Pereira, T.; Bártolo, P.J.; Santos, J.D.; Luís, A.L.; Maurício, A.C. Trends in mesenchymal stem cells’ applications for skeletal muscle repair and regeneration. In Progress in Stem Cell Transplantation; Demirer, T., Ed.; InTech Open: London, UK, 2015. [Google Scholar]
- Liu, X.; Zheng, L.; Zhou, Y.; Chen, Y.; Chen, P.; Xiao, W. BMSC transplantation aggravates inflammation, oxidative stress, and fibrosis and impairs skeletal muscle regeneration. Front. Physiol. 2019, 10, 87. [Google Scholar] [PubMed]
- Andrade, B.M.; Baldanza, M.R.; Ribeiro, K.C.; Porto, A.; Peçanha, R.; Fortes, F.S.; Zapata-Sudo, G.; Campos-de-Carvalho, A.C.; Goldenberg, R.C.; Werneck-de-Castro, J.P. Bone marrow mesenchymal cells improve muscle function in a skeletal muscle re-injury model. PLoS ONE 2015, 10, e0127561. [Google Scholar] [CrossRef] [PubMed]
- Merritt, E.K.; Cannon, M.V.; Hammers, D.W.; Le, L.N.; Gokhale, R.; Sarathy, A.; Song, T.J.; Tierney, M.T.; Suggs, L.J.; Walters, T.J.; et al. Repair of traumatic skeletal muscle injury with bone-marrow-derived mesenchymal stem cells seeded on extracellular matrix. Tissue Eng. Part A 2010, 16, 2871–2881. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Nam, H.Y.; Pingguan-Murphy, B.; Amir Abbas, A.; Mahmood Merican, A.; Kamarul, T. The proliferation and tenogenic differentiation potential of bone marrow-derived mesenchymal stromal cell are influenced by specific uniaxial cyclic tensile loading conditions. Biomech. Model Mechanobiol. 2015, 14, 649–663. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.Y.; Karunanithi, P.; Loo, W.C.; Naveen, S.; Chen, H.; Hussin, P.; Chan, L.; Kamarul, T. The effects of staged intra-articular injection of cultured autologous mesenchymal stromal cells on the repair of damaged cartilage: A pilot study in caprine model. Arthritis Res. Ther. 2013, 15, R129. [Google Scholar] [CrossRef]
- Holzer, N.; Hogendoorn, S.; Zürcher, L.; Garavaglia, G.; Yang, S.; König, S.; Laumonier, T.; Menetrey, J. Autologous transplantation of porcine myogenic precursor cells in skeletal muscle. Neuromuscul. Disord. 2005, 15, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Tanano, H.; Hasegawa, T.; Kimura, T.; Sasaki, T.; Kawahara, H.; Kubota, A.; Okada, A. Proposal of fibrosis index using image analyzer as a quantitative histological evaluation of liver fibrosis in biliary atresia. Pediatr. Surg. Int. 2003, 19, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Kragh, J.F., Jr.; Svoboda, S.J.; Wenke, J.C.; Ward, J.A.; Walters, T.J. Suturing of lacerations of skeletal muscle. J. Bone Jt. Surg. Br. 2005, 87, 1303–1305. [Google Scholar] [CrossRef]
- Natsu, K.; Ochi, M.; Mochizuki, Y.; Hachisuka, H.; Yanada, S.; Yasunaga, Y. Allogeneic bone marrow-derived mesenchymal stromal cells promote the regeneration of injured skeletal muscle without differentiation into myofibers. Tissue Eng. 2004, 10, 1093–1112. [Google Scholar] [CrossRef]
- Winkler, T.; von Roth, P.; Radojewski, P.; Urbanski, A.; Hahn, S.; Preininger, B.; Duda, G.N.; Perka, C. Immediate and delayed transplantation of mesenchymal stem cells improve muscle force after skeletal muscle injury in rats. J. Tissue Eng. Regen. Med. 2012, 6, s60–s67. [Google Scholar] [CrossRef]
- Winkler, T.; von Roth, P.; Matziolis, G.; Mehta, M.; Perka, C.; Duda, G.N. Dose–response relationship of mesenchymal stem cell transplantation and functional regeneration after severe skeletal muscle injury in rats. Tissue Eng. Part A 2009, 15, 487–492. [Google Scholar] [CrossRef]
- Matziolis, G.; Winkler, T.; Schaser, K.; Wiemann, M.; Krocker, D.; Tuischer, J.; Perka, C.; Duda, G.N. Autologous bone marrow-derived cells enhance muscle strength following skeletal muscle crush injury in rats. Tissue Eng. 2006, 12, 361–367. [Google Scholar] [CrossRef]
- von Roth, P.; Duda, G.N.; Radojewski, P.; Preininger, B.; Perka, C.; Winkler, T. Mesenchymal stem cell therapy following muscle trauma leads to improved muscular regeneration in both male and female rats. Gend. Med. 2012, 9, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Baraniak, P.R.; McDevitt, T.C. Stem cell paracrine actions and tissue regeneration. Regen. Med. 2010, 5, 121–143. [Google Scholar] [CrossRef]
- Binder-Markey, B.I.; Broda, N.M.; Lieber, R.L. Intramuscular anatomy drives collagen content variation within and between muscles. Front. Physiol. 2020, 11, 293. [Google Scholar] [CrossRef]
- Kowalski, K.; Kołodziejczyk, A.; Sikorska, M.; Płaczkiewicz, J.; Cichosz, P.; Kowalewska, M.; Stremińska, W.; Jańczyk-Ilach, K.; Koblowska, M.; Fogtman, A.; et al. Stem cells migration during skeletal muscle regeneration—The role of Sdf-1/Cxcr4 and Sdf-1/Cxcr7 axis. Cell Adhes. Migr. 2017, 11, 384–398. [Google Scholar] [CrossRef]
- Saeed, H.; Ahsan, M.; Saleem, Z.; Iqtedar, M.; Islam, M.; Danish, Z.; Khan, A.M. Mesenchymal stem cells (MSCs) as skeletal therapeutics—An update. J. Biomed. Sci. 2016, 23, 41. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Y.; Li, Y.; Cao, J.; Zhang, H.; Chen, M.; Wang, L.; Zhang, C. Long-term engraftment of myogenic progenitors from adipose-derived stem cells and muscle regeneration in dystrophic mice. Hum. Mol. Genet. 2015, 24, 6029–6040. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Reinecke, H.; Murry, C.E.; Torok-Storb, B. Myogenic fusion of human bone marrow stromal cells, but not hematopoietic cells. Blood 2004, 104, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Kasprzycka, P.; Archacka, K.; Kowalski, K.; Mierzejewski, B.; Zimowska, M.; Grabowska, I.; Piotrowski, M.; Rafałko, M.; Ryżko, A.; Irhashava, A.; et al. The factors present in regenerating muscles impact bone marrow-derived mesenchymal stromal/stem cell fusion with myoblasts. Stem Cell Res. Ther. 2019, 10, 343. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sassoli, C.; Zecchi-Orlandini, S.; Formigli, L. Trophic actions of bone marrow-derived mesenchymal stromal cells for muscle repair/regeneration. Cells 2012, 1, 832–850. [Google Scholar] [CrossRef]
- Nakamura, Y.; Miyaki, S.; Ishitobi, H.; Matsuyama, S.; Nakasa, T.; Kamei, N.; Akimoto, T.; Higashi, Y.; Ochi, M. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett. 2015, 589, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Qazi, T.H.; Duda, G.N.; Ort, M.J.; Perka, C.; Geissler, S.; Winkler, T. Cell therapy to improve regeneration of skeletal muscle injuries. J. Cachexia Sarcopenia Muscle 2019, 10, 501–516. [Google Scholar] [CrossRef]
- Hofer, H.R.; Tuan, R.S. Secreted trophic factors of mesenchymal stem cells support neurovascular and musculoskeletal therapies. Stem Cell Res. Ther. 2016, 7, 131. [Google Scholar] [CrossRef] [PubMed]
- Lukomska, B.; Stanaszek, L.; Zuba-Surma, E.; Legosz, P.; Sarzynska, S.; Drela, K. Challenges and controversies in human mesenchymal stem cell therapy. Stem Cells Int. 2019, 2019, 9628536. [Google Scholar] [CrossRef] [PubMed]
- Mahdy, M.A.A. Skeletal muscle fibrosis: An overview. Cell Tissue Res. 2019, 375, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Gnecchi, M.; He, H.; Liang, O.D.; Melo, L.G.; Morello, F.; Mu, H.; Noiseux, N.; Zhang, L.; Pratt, R.E.; Ingwall, J.S.; et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat. Med. 2005, 11, 367–368. [Google Scholar] [CrossRef] [PubMed]
- Schlosser, S.; Dennler, C.; Schweizer, R.; Eberli, D.; Stein, J.V.; Enzmann, V.; Giovanoli, P.; Erni, D.; Plock, J.A. Paracrine effects of mesenchymal stem cells enhance vascular regeneration in ischemic murine skin. Microvasc. Res. 2012, 83, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Iwasaki, K.; Peng, Y.; Honda, Y. Mesenchymal Stem Cell Extract Promotes Skin Wound Healing. Int. J. Mol. Sci. 2024, 25, 13745. [Google Scholar] [CrossRef]
- Shin, R.H.; Vathana, T.; Giessler, G.A.; Friedrich, P.F.; Bishop, A.T.; Shin, A.Y. Isometric tetanic force measurement method of the tibialis anterior in the rat. Microsurgery 2008, 2, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.R.; Valencia, A.P.; Hernández-Ochoa, E.O.; Lovering, R.M. In Vivo Assessment of Muscle Contractility in Animal Studies. Methods Mol. Biol. 2016, 1460, 293–307. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Durumutla, H.B.; Villa, C.; Panta, M.; Wintzinger, M.; Prabakaran, A.D.; Miz, K.; Quattrocelli, M. Comprehensive analyses of muscle function, lean and muscle mass, and myofiber typing in mice. Bio-Protocol 2023, 13, e4617. [Google Scholar] [CrossRef]
- Charles, J.P.; Kissane, R.W.P.; Askew, G.N. The impacts of muscle-specific force-velocity properties on predictions of mouse muscle function during locomotion. Front. Bioeng. Biotechnol. 2024, 23, 1436004. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Menetrey, J.; Kasemkijwattana, C.; Fu, F.H.; Moreland, M.S.; Huard, J. Suturing versus immobilization of a muscle laceration. A morphological and functional study in a mouse model. Am. J. Sports Med. 1999, 27, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Parry, S.M.; Puthucheary, Z.A. The impact of extended bed rest on the musculoskeletal system in the critical care environment. Extrem. Physiol. Med. 2015, 4, 16. [Google Scholar] [CrossRef] [PubMed]
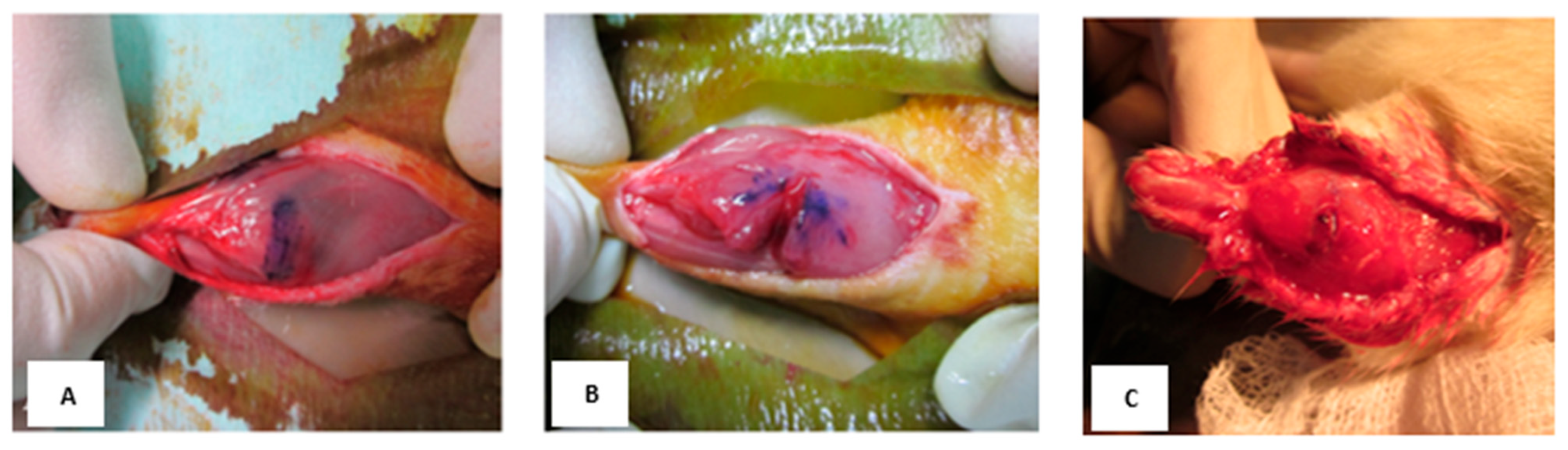
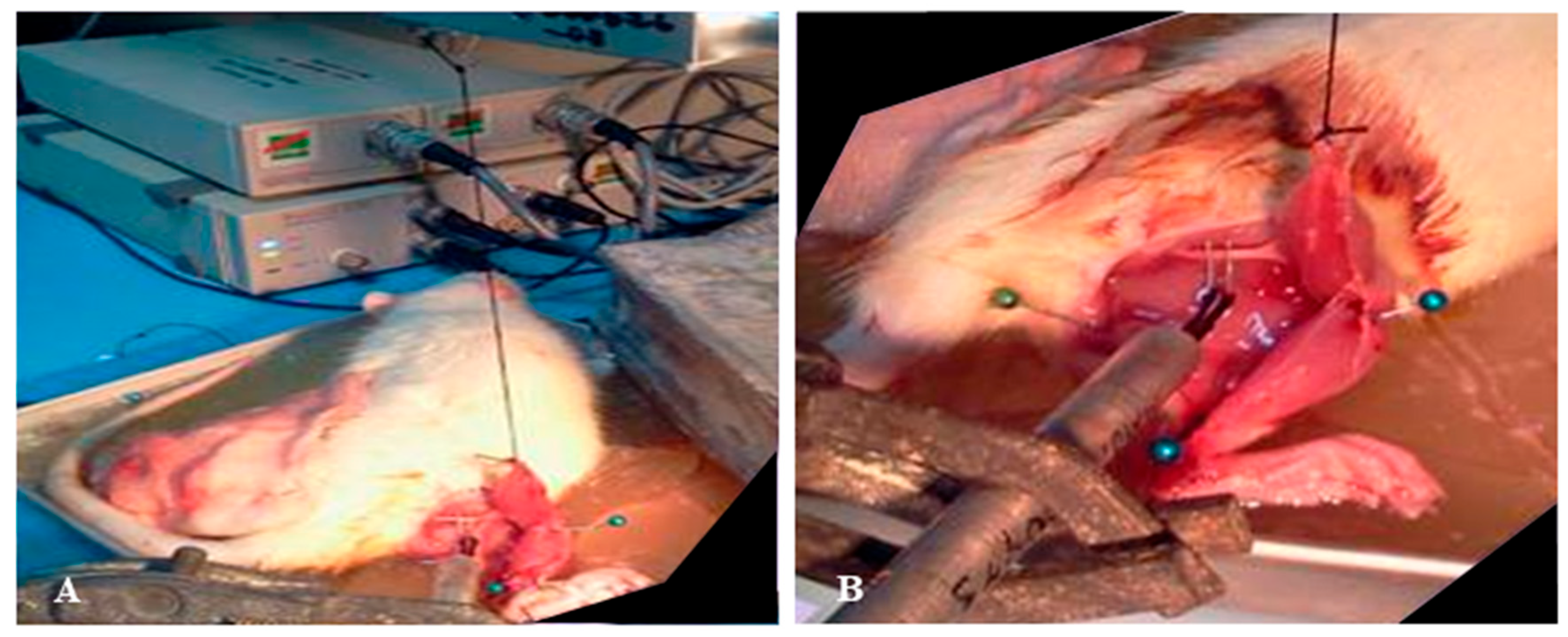
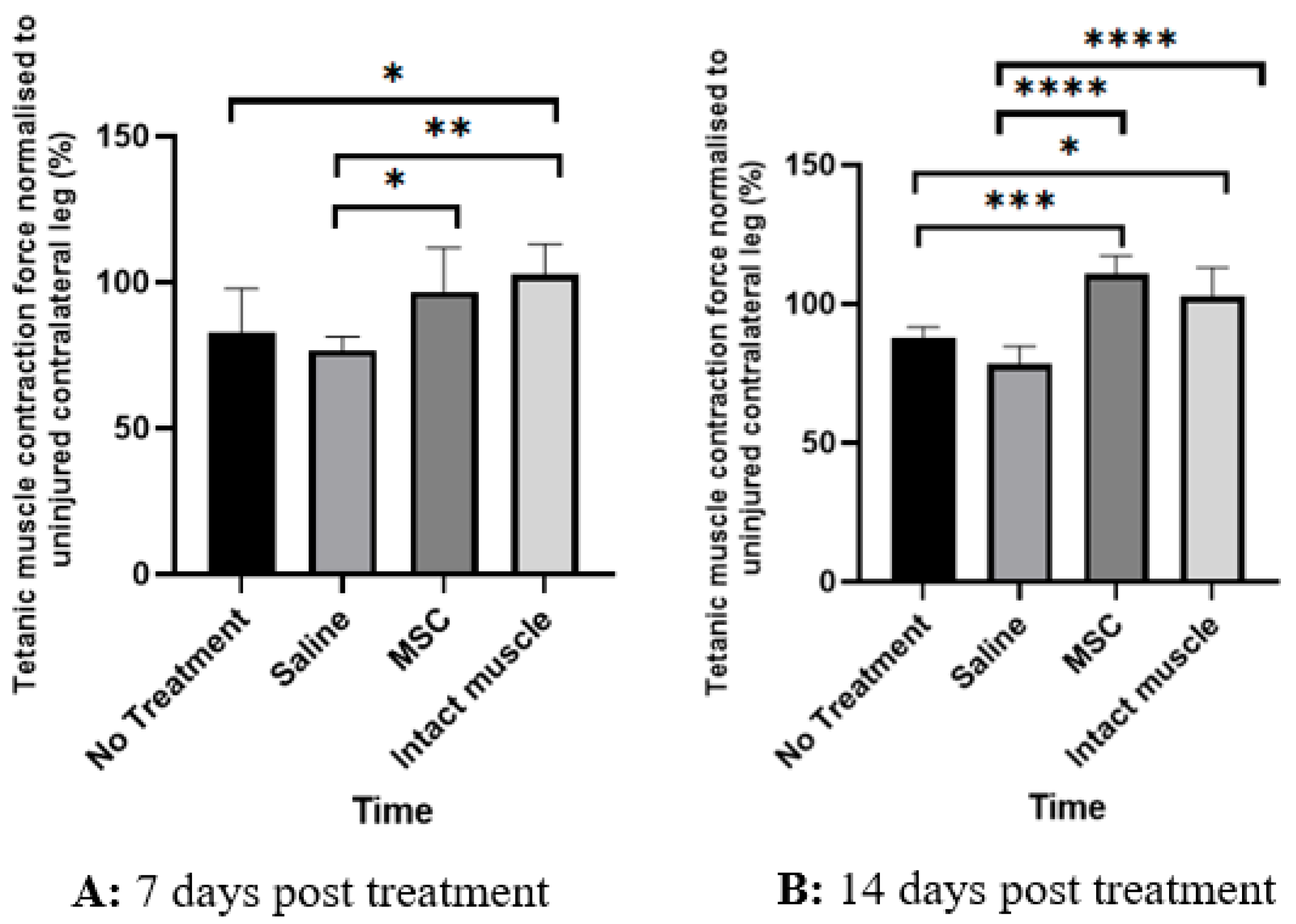
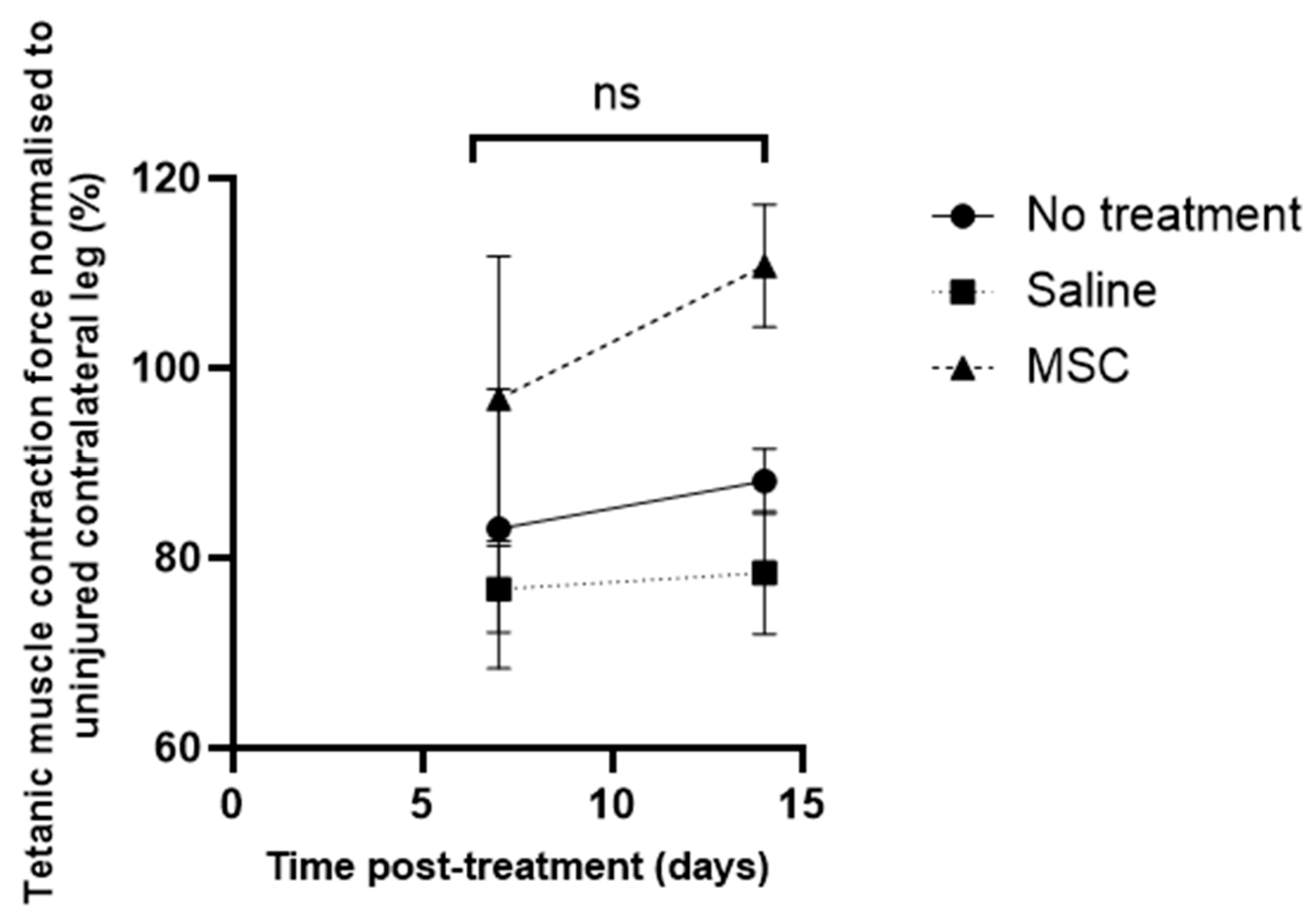

| Specimen No. | Muscle Force (g) in the Right Leg | Muscle Force (g) in the Left Leg | Ratio of Muscle Force in the Right Relative to the Left Leg (%) |
|---|---|---|---|
| 1 | 200.3 | 182.1 | 110.0 |
| 2 | 118.2 | 122 | 96.9 |
| 3 | 194.5 | 193.1 | 100.7 |
| 4 | 127.6 | 120.8 | 105.6 |
| 5 | 151.9 | 176 | 86.3 |
| 6 | 186.9 | 161.4 | 115.8 |
| Mean | 163.2 ± 35.6 | 159.2 ± 31.0 | 102.6 ± 10.4 |
| Study Group (6 Subjects per Group) | Force of Tetanic Muscle Contraction ± SD (g) | Ratio of Muscle Force of Injured Relative to Contralateral Uninjured Muscle (R/L leg) (%) | |
|---|---|---|---|
| Injured Muscle (Right Leg) | Uninjured Muscle (Left Leg) | ||
| No treatment | 162.7 ± 53.9 | 195.1 ± 33.1 | 83.1 ± 14.7 |
| Saline-treated | 121.4 ± 44.5 | 158.7 ± 45.5 | 76.7 ± 4.6 |
| MSCs-treated | 180.5 ± 27.2 | 187.2 ± 19.0 | 96.8 ± 15.0 |
| Uninjured Muscle | |||
| Right Leg (R) | Left Leg (L) | Ratio R/L (%) | |
| Intact muscle | 163.2 ± 35.6 | 159.2 ± 31.0 | 102.6 ± 10.4 |
| Study Group (6 Subjects per Group) | Force of Tetanic Muscle Contraction ± SD (g) | Ratio of Muscle Force of Injured Relative to Contralateral Uninjured Muscle (R/L leg) (%) | |
|---|---|---|---|
| Injured Muscle (Right Leg) | Uninjured Muscle (Left Leg) | ||
| No treatment | 180.2 ± 24.2 | 204.7 ± 6.8 | 88.1 ± 3.41 |
| Saline-treated | 114.4 ± 46.1 | 147.8 ± 56.7 | 78.4 ± 6.47 |
| MSCs-treated | 192.8 ± 39.6 | 178.6 ± 36.0 | 110.8 ± 6.46 |
| Uninjured Muscle | |||
| Right Leg (R) | Left Leg (L) | Ratio R/L (%) | |
| Intact muscle | 163.2 ± 35.6 | 159.2 ± 31.0 | 102.6 ± 10.4 |
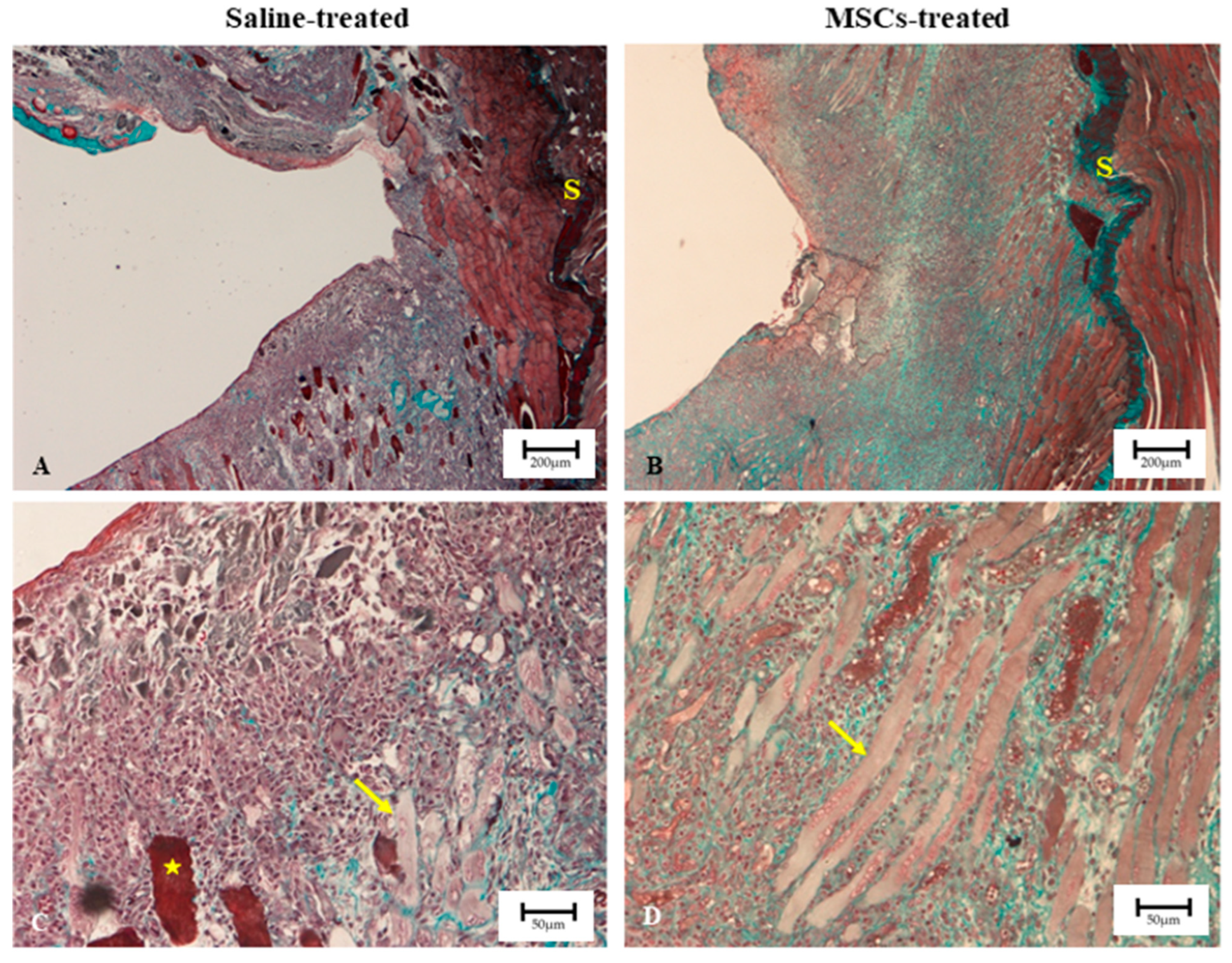
| Treatment Group | Mean Blue/Red Intensity Ratio | SD | Fibrosis Index (%) | SD | p Value |
|---|---|---|---|---|---|
| Saline | 0.865 | 0.032 | 31.77 | 0.43 | <0.0001 |
| MSCs | 0.779 | 0.014 | 29.30 | 0.29 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, R.E.; Mokhtar, A.H.; Mohamed Al-Fayyadh, M.Z.; Nam, H.Y.; Aziz, A.; Mansor, A.; Kamarul, T. Effect of Allogenic Mesenchymal Stem Cell Injection on Functional Repair Outcomes Following Skeletal Muscle Laceration Injury. Biomedicines 2025, 13, 2810. https://doi.org/10.3390/biomedicines13112810
Ahmad RE, Mokhtar AH, Mohamed Al-Fayyadh MZ, Nam HY, Aziz A, Mansor A, Kamarul T. Effect of Allogenic Mesenchymal Stem Cell Injection on Functional Repair Outcomes Following Skeletal Muscle Laceration Injury. Biomedicines. 2025; 13(11):2810. https://doi.org/10.3390/biomedicines13112810
Chicago/Turabian StyleAhmad, Raja Elina, Abdul Halim Mokhtar, Mohamed Zubair Mohamed Al-Fayyadh, Hui Yin Nam, Atiqah Aziz, Azura Mansor, and Tunku Kamarul. 2025. "Effect of Allogenic Mesenchymal Stem Cell Injection on Functional Repair Outcomes Following Skeletal Muscle Laceration Injury" Biomedicines 13, no. 11: 2810. https://doi.org/10.3390/biomedicines13112810
APA StyleAhmad, R. E., Mokhtar, A. H., Mohamed Al-Fayyadh, M. Z., Nam, H. Y., Aziz, A., Mansor, A., & Kamarul, T. (2025). Effect of Allogenic Mesenchymal Stem Cell Injection on Functional Repair Outcomes Following Skeletal Muscle Laceration Injury. Biomedicines, 13(11), 2810. https://doi.org/10.3390/biomedicines13112810







