1. Introduction
Pancreatic cancer (PC) ranks among the most lethal malignancies, exhibiting one of the highest mortality rates and a poor 5-year overall survival (OS) of only 13% [
1,
2]. Pancreatic ductal adenocarcinoma is the most prevalent subtype of PC. The poor prognosis is partly attributed to difficulties in diagnosing the disease at an early stage, which limits opportunities for curative surgical intervention [
3,
4]. Furthermore, the lack of effective molecular targets impedes therapeutic progress. Current treatment options, including surgery, chemotherapy, and radiotherapy, remain largely ineffective [
5]. Therefore, identifying effective biomarkers and therapeutic targets for early-stage PC has become an urgent need.
The specific etiology of PC remains unclear. However, growing evidence indicates that patients with chronic pancreatitis (CP) have a significantly increased risk of developing PC [
6]. Approximately 5 years after a CP diagnosis, the risk of PC increases by nearly eightfold [
7]. The mechanisms by which CP promotes PC development are not fully understood, but it is hypothesized that a series of sequential events leads to progressive DNA damage, ultimately resulting in pancreatic intraepithelial neoplasia [
8,
9,
10]. The persistent inflammatory and fibrotic microenvironment created by CP provides a crucial “soil” for PC development by activating key signaling pathways such as Kirsten rat sarcoma viral oncogene homolog and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) [
11,
12]. Numerous studies have shown that exacerbated pancreatic inflammation activates pancreatic stellate cells, potentially promoting carcinogenesis in genetically susceptible individuals [
13,
14]. Moreover, pathological studies have revealed a significantly higher incidence of high-grade pancreatic intraepithelial neoplasia (PanIN) in CP tissue adjacent to PC [
15]. Several consensus guidelines have therefore classified patients with CP as a high-risk group for PC and recommend regular monitoring. Although these two conditions are clinically difficult to distinguish, recent research shows they are fundamentally distinct at the molecular level. For example, Wu et al. used machine learning to identify DNA methylation signatures in tissue and circulating cell-free DNA that accurately differentiate PC from CP [
16]. In this complex transition from CP to PC, many molecular targets and signaling pathways remain unidentified, yet they may have significant implications for patient survival and prognosis.
S100P, a calcium-binding protein comprising 95 amino acids, belongs to the S100 family of proteins [
17]. It is overexpressed in various malignancies, including colorectal [
18,
19], breast [
20], lung [
21], and liver cancers [
22]. S100P plays a multifaceted role in tumor development, and its expression has been associated with poor outcomes in several gastrointestinal cancers. It contributes to tumor cell proliferation, survival, migration, invasion, and metastasis [
18,
19,
22]. However, contradictory findings exist. A 2019 meta-analysis reported that S100P overexpression is associated with poor prognosis in hepatocellular carcinoma and cholangiocarcinoma but not consistently in pancreatic, gastric, colorectal, or gallbladder cancers [
23]. Existing studies have shown that S100P can promote invasion and metastasis of PC through both intracellular and extracellular mechanisms. For instance, S100P secreted by PC cells can bind extracellularly to the receptor for advanced glycation end products (RAGE), activating the mitogen-activated protein kinase and NF-κB pathways to enhance invasion [
24,
25]. Intracellularly, S100P can activate Ezrin, thereby promoting the transendothelial migration of tumor cells [
26]. S100P also plays a role in the epithelial–mesenchymal transition (EMT); studies have confirmed that it promotes EMT, migration, and invasion in colon cancer cells [
27]. However, the relationship between S100P and EMT in PC remains unclear and warrants further investigation.
Cathepsin E (CTSE) is an intracellular aspartic protease that, along with pepsin A and cathepsin D, forms the peptidase A1 family [
28]. Found in a limited range of cell types, CTSE is predominantly expressed in immune system cells such as lymphocytes, macrophages, microglia, and dendritic cells [
29]. Its precise physiological functions remain unclear, as most studies have focused on its normal biological roles. For instance, Tsukuba et al. reported that CTSE-deficient mice spontaneously develop atopic dermatitis-like lesions, associated with increased bacterial infection and reduced conversion of interleukin (IL)-18 and IL-1β [
30]. Moreover, CTSE-deficient mice on a high-fat diet exhibit impaired adipose tissue development due to reduced macrophage infiltration [
31]. CTSE also plays an important role in antigen processing through the major histocompatibility complex (MHC) class II pathway [
32]. Previous studies have shown that CTSE is selectively expressed in several malignant tumors, including lung, bladder, and esophageal cancers [
33,
34,
35]. In PC, CTSE expression and proteolytic activity have been utilized to develop imaging probes for precise detection and activatable 5-ALA prodrugs [
36]. Furthermore, suppression of CTSE expression has been linked to abnormal activation of the EMT pathway and poor prognosis in patients with breast cancer [
37]. Although CTSE is believed to influence tumor invasion and EMT, its role and specific mechanisms in PC have not yet been elucidated.
Despite an incomplete understanding of the underlying cellular and molecular mechanisms, tumor invasion and metastasis remain major contributors to PC-related mortality. These processes are primarily driven by interactions between tumor cells and stromal components—a mechanism referred to as EMT [
38]. During EMT, epithelial cells acquire a mesenchymal phenotype, resulting in enhanced motility and invasiveness that enable tumor cells to breach the basement membrane, invade surrounding tissues, and disseminate to distant sites. In individual cells, complete EMT occurs, while partial EMT is observed in cells migrating collectively [
39]. Multiple signaling pathways regulate EMT, among which the phosphoinositide 3-kinase (PI3K)–protein kinase B (AKT) pathway is aberrantly activated during PC progression. This pathway is crucial in modulating malignant cell behavior and promoting EMT [
40]. However, although the PI3K-AKT pathway’s involvement in EMT has been reported, it remains unclear whether S100P and CTSE regulate EMT via this pathway, underscoring the aim of this study.
In this study, we quantified differentially expressed proteins in formalin-fixed, paraffin-embedded (FFPE) tissues from PC and patients with CP, focusing on S100P and CTSE expression levels. We further validated their associations with clinicopathological features and patient prognosis, and assessed their effects on PC cell invasiveness to identify predictive biological markers for diagnosis and treatment.
2. Materials and Methods
2.1. Tissue Sample Preparation
Formalin-fixed, paraffin-embedded (FFPE) tissue sections from 15 pancreatic cancer (PC) and 10 patients with chronic pancreatitis (CP) were obtained from the Third Affiliated Hospital of Soochow University, Jiangsu, China. All cases were histologically confirmed as PC or CP. Initially, 15 PC and 10 CP FFPE samples were included. Due to limited tissue availability and concerns about protein yield and detection sensitivity when analyzed individually, sample pooling was employed. The 10 CP samples were randomly combined in groups of three to form five CP pools (A1–A5), and the 15 PC samples were randomly combined in groups of three to form five PC pools (B1–B5). Thus, the effective number of biological replicates was n = 5 for both CP and PC pools. For validation, patient tissue microarrays (TMAs) were purchased from Shanghai Outdo Biotech Company (Shanghai, China; product number: HPan-Ade180Sur-01), comprising 90 PC tissues and paired adjacent non-cancerous tissues. All procedures were conducted in accordance with the Declaration of Helsinki and were approved by the Ethics Committee of the Third Affiliated Hospital of Soochow University (Approval Number: [2024] Science No. 214).
2.2. Liquid Chromatography-Tandem Mass Spectrometry and Data Analysis
FFPE samples were deparaffinized in xylene and rehydrated through graded ethanol solutions. Proteins were extracted using SDT buffer (4% sodium dodecyl sulfate; Sangon Biotech, Shanghai, China) and 100 mM tris(hydroxymethyl)aminomethane hydrochloride (Tris-HCl) [pH 7.6]) and quantified using a bicinchoninic acid assay kit (Beyotime, Shanghai, China). For quality control, 20 μg of total protein from each sample was mixed with 6× sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer (Beyotime), boiled for 5 min, and resolved on 12% SDS-PAGE gels. Coomassie Brilliant Blue staining was used to evaluate electrophoretic band integrity. Only samples exhibiting clear, well-defined protein bands and sufficient total protein yield for at least two experimental replicates were used for further analysis. For mass spectrometry analysis, 80 μg of protein from quality-controlled samples was digested with trypsin using the filter-aided sample preparation method. The procedure included reduction with 100 mM dithiothreitol (Sigma-Aldrich, St. Louis, MO, USA) (boiling, 5 min), detergent removal using UA buffer (8 M urea [Bio-Rad, Hercules, CA, USA] in 0.1 M Tris-HCl, pH 8.5), alkylation with 100 mM iodoacetamide (Sigma-Aldrich) in UA buffer (30 min, room temperature, dark), and trypsin digestion using 4 μg sequencing-grade modified trypsin (Promega, Madison, WI, USA) at 37 °C for 16–18 h. Peptides were separated using an Easy nano-Liquid Chromatography 1200 Ultra-High-Performance Liquid Chromatography system (Thermo Fisher Scientific, Waltham, MA, USA) and analyzed on a Q Exactive Plus Hybrid Quadrupole-Orbitrap mass spectrometer (Thermo Fisher Scientific). Protein identification and label-free quantification were performed using MaxQuant (version 1.5.5.1). Statistical analyses were conducted in R (version 3.3.1) using the limma package. Proteins were considered significantly regulated when they met both criteria: adjusted p < 0.05 (FDR correction) and |log2 fold change| ≥ 1.5. Missing values were imputed using the “missing not at random” approach, randomly sampling from a left-shifted Gaussian distribution (1.8 standard deviation shift, 0.3 width).
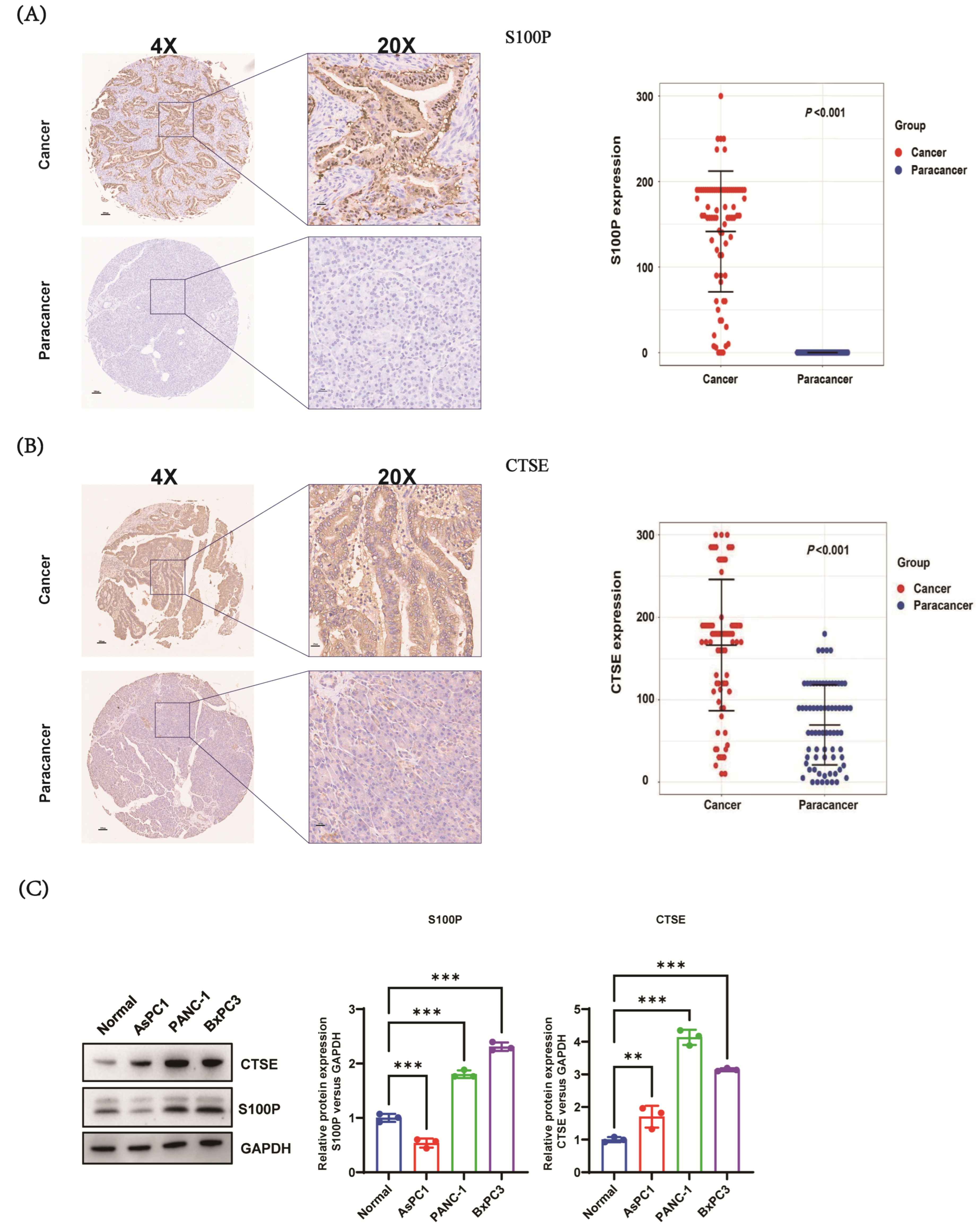
2.3. Immunohistochemistry and Data Analysis
TMAs (HPan-Ade180Sur-01, Shanghai Outdo Biotech Co.) containing 90 paired PC and adjacent non-cancerous tissues were used for immunohistochemistry (IHC) analysis. TMAs were baked, deparaffinized, and subjected to antigen retrieval, followed by IHC staining according to standard procedures. Sections were incubated with primary antibodies: anti-
cathepsin E (CTSE) (AF1294, R&D Systems, Minneapolis, MN, USA; dilution 1:50) and anti-S100P (AB133554, Abcam, Cambridge, UK; dilution 1:10,000). Corresponding secondary antibodies and diaminobenzidine (DAB) chromogen were used for visualization. Slides were independently evaluated by two blinded pathologists without access to clinical data. S100P and CTSE expression levels were quantified using H-scores (range: 0–300), calculated as:
For discordant evaluations, a third blinded pathologist re-scored the slides, and final scores were determined by consensus. Of the 90 tissue pairs, 12 were excluded from S100P analysis and 8 from CTSE analysis due to suboptimal staining or insufficient tumor content, yielding 78 evaluable S100P and 82 evaluable CTSE pairs. Optimal cut-off values for survival analysis were determined via iterative Kaplan–Meier analysis to maximize discrimination in survival outcomes: S100P expression was classified as low (<180) or high (≥180), and CTSE expression as low (≤170) or high (>170).
2.4. Cell Sources and Culture
The PC cell lines PANC-1, BxPC-3, and AsPC-1 were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The human pancreatic ductal epithelial cell line (HPDE) was kindly provided by the Second Affiliated Hospital of Sun Yat-sen University. BxPC-3 cells were cultured in RPMI 1640 medium (Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, Thermo Fisher Scientific, Waltham, MA, USA) and 1% penicillin-streptomycin (Gibco). PANC-1 and HPDE cells were maintained in complete Dulbecco’s Modified Eagle Medium (DMEM; Hyclone) containing 10% FBS and 1% penicillin-streptomycin. All cell lines were routinely tested for mycoplasma contamination using a polymerase chain reaction (PCR)-based method targeting the 16S rRNA gene and were confirmed to be negative before use. The recommended passage ratios were as follows: PANC-1, 1:2–1:4; BxPC-3, 1:2–1:3 (or 1:3–1:6); and HPDE, 1:2–1:3. Cell line authentication for PANC-1 and BxPC-3 was verified using short tandem repeat (STR) profiling. All cell cultures were incubated at 37 °C in a humidified atmosphere containing 5% CO2.
2.5. Lentiviral Vector Construction and Transfection
Shanghai Junli Biotechnology Company (Shanghai, China) designed and constructed the S100P and CTSE overexpression plasmids, short hairpin RNA (shRNA) constructs targeting S100P and CTSE, and the corresponding negative control plasmids. The shRNA sequences used in the S100P and CTSE experiments are listed in
Table 1. Transfection was performed following the manufacturer’s instructions using Lipofectamine 3000 (Thermo Fisher Scientific).
2.6. Quantitative Real-Time PCR
Total RNA was extracted from PC cells using TRIzol reagent (Thermo Fisher Scientific). Complementary DNA (cDNA) was generated through reverse transcription with the use of a PrimeScript RT kit from Beyotime. The cDNA was diluted and analyzed using quantitative real-time PCR (qRT-PCR) to assess messenger ribonucleic acid expression levels, with GAPDH used as the reference gene. The primer sequences used in the analysis are provided in
Table 2.
2.7. Western Blot Analysis
Cells were lysed with lysis buffer (Beyotime) to extract total protein. Protein concentration was determined using the bicinchoninic acid assay (Beyotime), and equal amounts of protein were separated using SDS-PAGE. After electrophoresis, proteins were transferred onto membranes, blocked, and incubated sequentially with primary and secondary antibodies. Detection and visualization were performed using a Tanon-4600 automated chemiluminescence imaging system (Tanon, Shanghai, China). Quantitative analysis of protein band intensity was carried out using ImageJ software (version 1.53k). Details of the antibodies used are listed in
Table 3.
2.8. Transwell Invasion Assay
The upper chamber of the Transwell insert was coated with diluted Matrigel (BD Biosciences, San Jose, CA, USA). A 100 µL cell suspension containing trypsinized and counted cells was added to the upper chamber, while 600 µL of culture medium supplemented with 20% FBS (Gibco) was placed in the lower chamber to serve as a chemoattractant. After 48 h of incubation at 37 °C in a humidified 5% CO2 atmosphere, non-migrated cells were gently removed with cotton swabs. The invaded cells were fixed with 4% paraformaldehyde (Beyotime), stained with 0.1% crystal violet (Beyotime), and counted under an inverted microscope (Leica Microsystems, Wetzlar, Germany).
2.9. Statistical Analysis
Differences in S100P and CTSE expression were analyzed using the Wilcoxon rank-sum test. The chi-squared test was used to assess associations between protein expression and clinicopathological characteristics. Kaplan–Meier survival curves were generated using the R packages “survival” and “survminer.” Univariate and multivariate Cox proportional hazards regression analyses were performed, with statistical significance set at p < 0.05. One-way analysis of variance was used to compare differences among groups. Statistical analyses were conducted using SPSS (version 26.0), and graphical outputs were generated using GraphPad Prism (version 9.5.1). Statistical significance was denoted as * p < 0.05, ** p < 0.01, and *** p < 0.001.
4. Discussion
Pancreatic cancer (PC) is an exceedingly aggressive malignancy with a high tendency for early metastasis. Despite ongoing advancements in therapeutic strategies, survival outcomes for patients with PC remain unsatisfactory [
1,
41]. Consequently, elucidating the molecular mechanisms underlying tumor progression and identifying novel biomarkers for diagnosis, therapy, and prognosis are crucial for improving overall survival (OS) in patients with PC.
In this study, we conducted a comprehensive proteomic analysis of PC and chronic pancreatitis (CP) tissues. Our results revealed that S100P and cathepsin E (CTSE) were significantly overexpressed in PC tissues relative to CP tissues, suggesting their potential roles in the pathogenesis and progression of PC. To validate the expression and clinical significance of S100P and CTSE in PC, we analyzed tissue microarrays (TMAs) containing samples from 90 patients with PC and paired adjacent non-cancerous tissues, performing immunohistochemistry (IHC) to assess the expression levels of these proteins.
S100P was localized within the cytoplasm, cell membrane, and nucleus of PC cells, whereas it was undetectable in adjacent non-cancerous tissues. Further analysis revealed that S100P expression in PC tissues was significantly higher than in adjacent tissues. These findings align with previous research indicating that S100P is not only markedly overexpressed in PC but also closely associated with the progression of pancreatic intraepithelial neoplasia (PanIN) grades [
42]. IHC analysis of CTSE revealed predominant cytoplasmic expression in PC cells, with lower levels in the cell membrane and nucleus, while adjacent non-cancerous tissues exhibited weak cytoplasmic and membranous staining. Data analysis confirmed that CTSE expression was elevated in PC tissues compared with that in adjacent non-cancerous tissues. Our findings corroborate those of Cruz-Monserrate et al., who reported that CTSE is significantly overexpressed in PC and serves as an early biological marker for PanIN [
43]. Collectively, these results suggest that the progression from CP to PanIN and subsequently to PC may involve multiple stages, during which the concentrations of S100P and CTSE accumulate, potentially playing important biological roles in PC development. Correlation analysis demonstrated a significant association between S100P expression and tumor size in patients with PC. CTSE expression was also correlated with tumor size, as well as T and M stages.
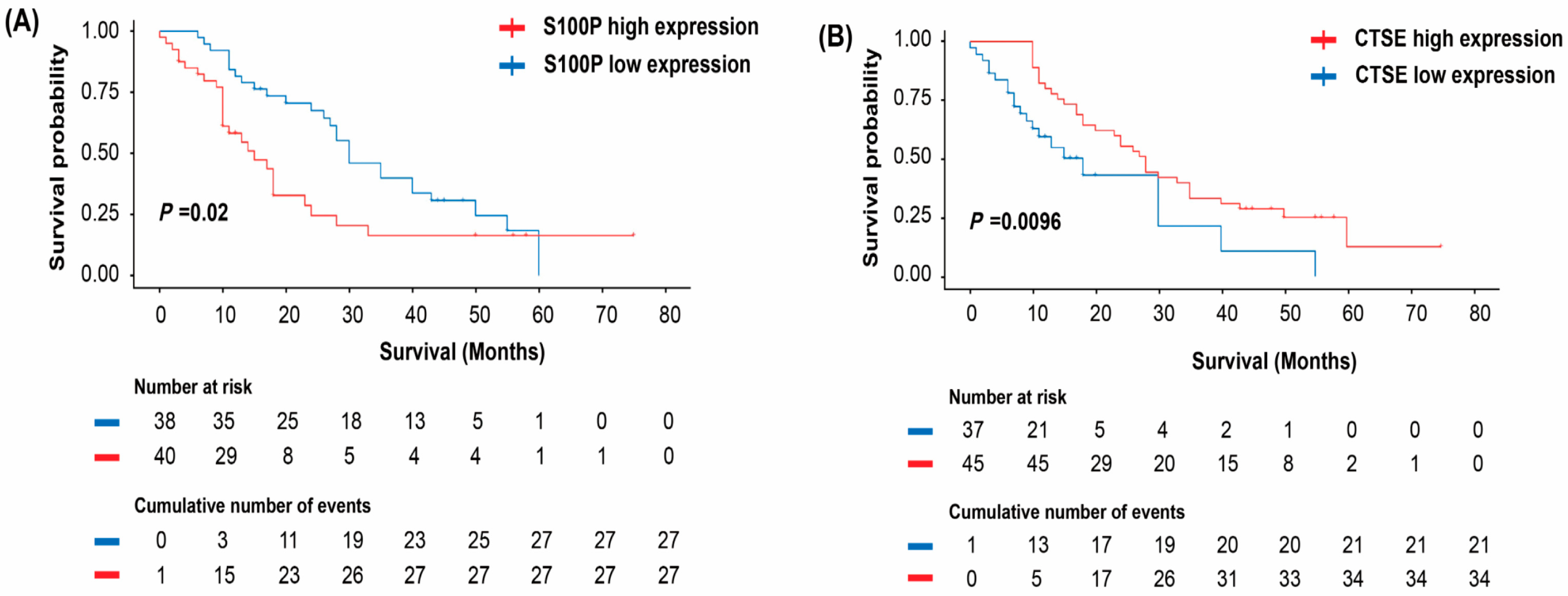
Building on these findings, we evaluated the prognostic significance of S100P and CTSE expression in patients with PC. Elevated S100P expression was significantly associated with poorer OS, while tumor size, N stage, TNM stage, and S100P expression emerged as key prognostic factors. Multivariate analysis identified tumor size and S100P expression as independent prognostic indicators. Conversely, patients with positive CTSE expression showed significantly better survival than those with negative expression. Univariate analysis further demonstrated significant associations among N stage, TNM stage, CTSE expression, and prognosis, while multivariate analysis confirmed tumor size and CTSE expression as independent prognostic factors. Collectively, these results indicate that S100P and CTSE are strongly associated with the prognosis of PC. Nevertheless, the precise mechanisms through which they contribute to PC pathogenesis and progression remain to be clarified.
During tumor progression, epithelial–mesenchymal transition (EMT) serves as a critical early event that promotes the invasion and dissemination of cancer cells. This process involves molecular and phenotypic changes, notably reduced epithelial marker expression and increased mesenchymal marker levels [
44]. EMT is characterized by several hallmark features, including the loss of cell polarity, morphological alterations, cytoskeleton remodeling, reduced cell adhesion, and the acquisition of invasive and migratory capabilities [
45]. In various malignancies, including PC, the phosphoinositide 3-kinase (PI3K)–protein kinase B (AKT) signaling pathway is activated by multiple components such as cytokines, chemokines, and growth factors. Activation of this pathway plays a key role in regulating essential cellular processes, including growth, survival, proliferation, metabolism, and motility [
46]. Numerous studies have established a strong association between PI3K-AKT pathway activation and EMT. AKT can modulate the cell cycle during EMT by downregulating E-cadherin [
47]. Moreover, AKT functions as a crucial downstream effector of methylsterol monooxygenase 1, influencing EMT through its interactions with epidermal growth factor receptor and various cross-talk proteins within the PI3K/AKT/mTOR signaling cascade [
48].
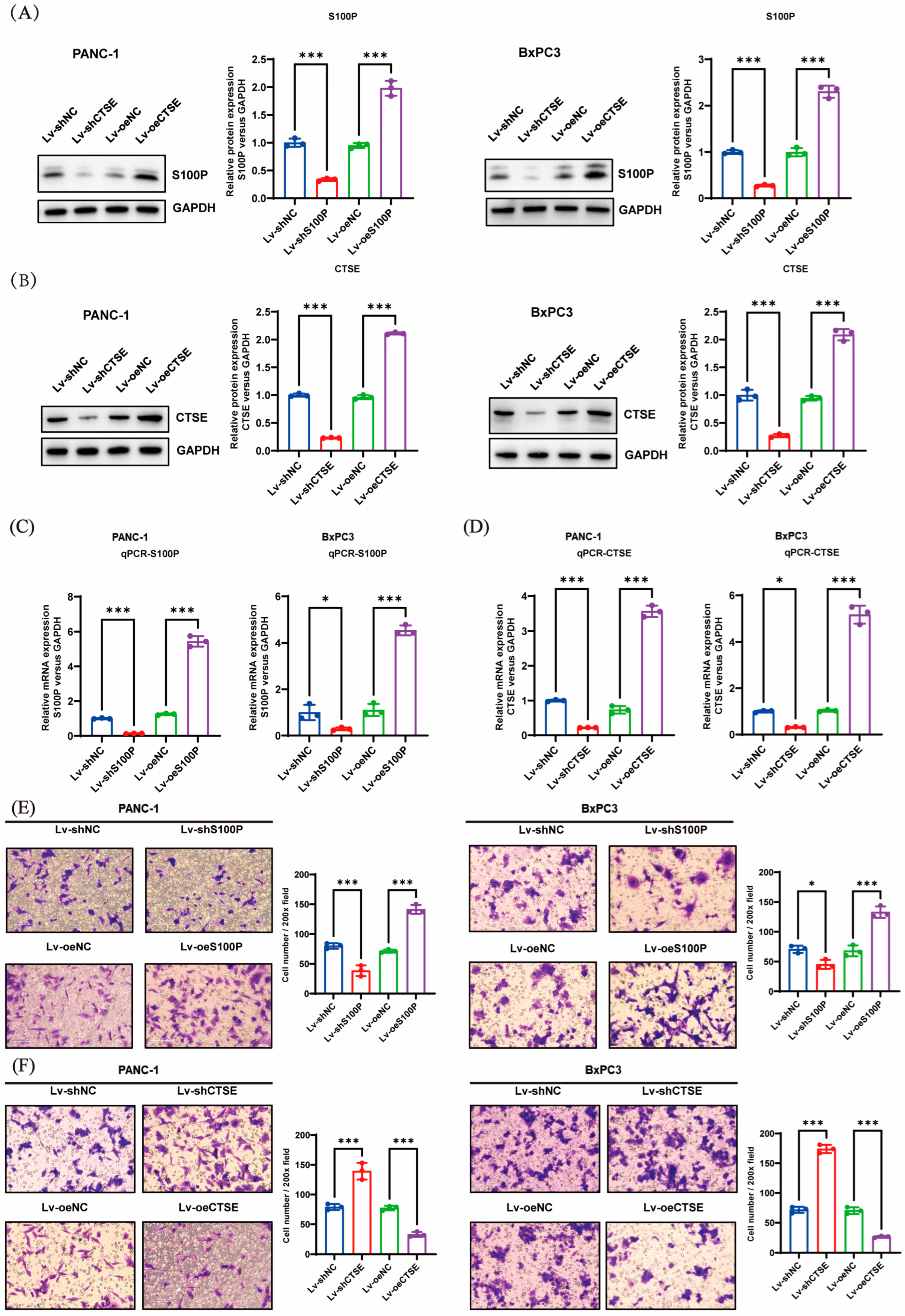
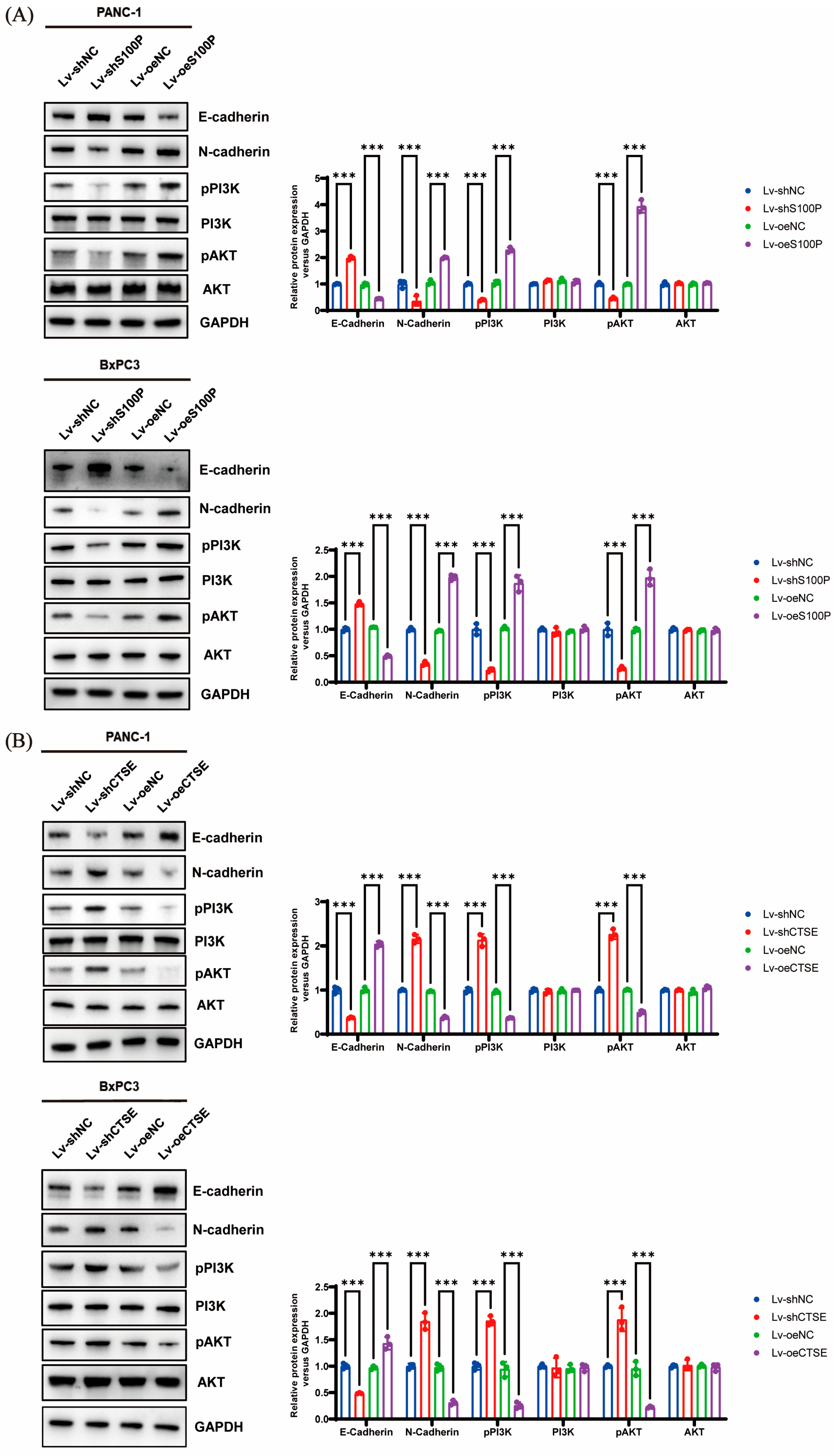
We knocked down or overexpressed S100P and CTSE to examine their effects on the invasive capacity of PC cells. Our experimental results showed that S100P downregulation inhibited invasion, whereas its overexpression enhanced invasive ability. In contrast, CTSE knockdown promoted PC cell invasion, while overexpression suppressed it. This contrasting behavior may be attributed to distinct underlying signaling mechanisms. Further mechanistic investigations demonstrated that S100P overexpression promoted EMT and invasion via activation of the PI3K–AKT pathway, whereas elevated CTSE expression inhibited both EMT and invasion by suppressing this same pathway. Although these results appear contradictory, we propose that they highlight the multifaceted role of CTSE within the complex tumor microenvironment of PC. Increasing evidence suggests that protein expression levels in tumor tissues represent a composite signal, and their prognostic implications are highly dependent on cellular origin. Our IHC and survival data reflect the relationship between total CTSE levels within tumor tissue and patient prognosis. We hypothesize that CTSE is primarily expressed and secreted by infiltrating immune cells (such as tumor-associated macrophages and lymphocytes), meaning that high CTSE expression reflects a more anti-tumor immune state that effectively suppresses tumor progression and metastasis, leading to improved OS. Thus, while CTSE expression within cancer cells may have inhibitory effects on invasion, its overall high tissue level correlates with a favorable prognosis. This tumor microenvironment-defined prognostic biomarker pattern is gaining increasing recognition [
49]. High CTSE expression (likely derived mainly from immune cells) signifies an active anti-tumor immune response and is associated with better prognosis. Moreover, CTSE acts as an intrinsic regulator within cancer cells, exerting cell-autonomous effects that inhibit invasion and metastasis. Taken together, we propose that CTSE plays a context-dependent role in the pathogenesis of PC: in the early stages, CTSE within cancer cells may limit invasion and migration by suppressing the PI3K-AKT pathway and EMT, and in later stages, CTSE expression by immune cells in the tumor microenvironment may increase total CTSE levels, thereby serving as a favorable prognostic indicator.
We speculate that CTSE and S100P establish a delicate “balance” within the tumor microenvironment. This dynamic equilibrium enables PC cells to survive and adapt under varying microenvironmental and physiological conditions by flexibly modulating the relative expression of CTSE and S100P, thereby influencing tumor progression and metastasis. Although both S100P and CTSE are highly expressed in pancreatic tissue, they predict different prognostic outcomes, suggesting distinct responses to cancer treatment that may ultimately affect patient survival. This phenomenon is also commonly observed in clinical practice. In recent years, immunotherapy—particularly immune checkpoint inhibitors—has achieved major breakthroughs in oncology. However, its efficacy in PC remains unsatisfactory, primarily due to the unique immunosuppressive microenvironment of this malignancy [
50]. Notably, the EMT process is closely linked to the formation of an immunosuppressive microenvironment: tumor cells undergoing EMT often exhibit characteristics of “immune-cold tumors,” including poor infiltration of effector T cells and accumulation of immunosuppressive cells such as myeloid-derived suppressor cells (MDSCs) [
51]. The heterogeneity of the tumor microenvironment and mechanisms of immune escape are central reasons for the marked variability in immunotherapy responses [
52], making the selection of appropriate “treatment timing” a critical issue in precision oncology. A global informatics study showed that since 2020, research focus has shifted notably toward neoadjuvant immunotherapy [
53]. Drawing from the experience in chronic myeloid leukemia, where early-stage (chronic phase) treatment with imatinib yields near-curative outcomes, whereas late-stage (blast crisis) therapy has limited efficacy, early intervention in solid tumors such as PC—before extensive clonal evolution and establishment of an immunosuppressive microenvironment—may be key to improving therapeutic success [
54]. Based on this, we propose a significant scientific hypothesis: the balance between S100P and CTSE may influence the immune microenvironment of PC by regulating the EMT process. Specifically, S100P-driven EMT may foster an immunosuppressive milieu by recruiting MDSCs and promoting M2 macrophage polarization, leading to resistance to immunotherapy. Conversely, CTSE may counteract this process, maintaining a more favorable immune environment, which could explain its association with a good prognosis.
Although neither our study nor available public databases (such as The Cancer Genome Atlas [TCGA]) revealed a significant linear correlation between the messenger ribonucleic acid expression levels of S100P and CTSE—implying no direct transcriptional regulation—this does not preclude the possibility of more complex, indirect interactions at the protein-functional level. For instance, S100P has been shown to act as a secreted protein that binds to the cell surface receptor RAGE, thereby activating downstream pathways such as PI3K/AKT. CTSE, an aspartic protease, likely has intracellular substrates and signaling regulatory networks that remain incompletely understood. These two proteins may operate as a finely tuned “balance,” maintaining dynamic equilibrium in PC progression by independently modulating shared downstream pathways, such as PI3K/AKT, or interacting nodes within this axis, ultimately determining the tumor’s invasive phenotype. S100P, as a secreted oncoprotein, represents a potential target for therapeutic intervention (e.g., monoclonal antibodies). Blocking its function may effectively inhibit the PI3K/AKT pathway and reverse EMT. Conversely, high CTSE expression is associated with a favorable prognosis and exhibits inhibitory effects on both the PI3K/AKT pathway and cellular invasion, making it a reliable prognostic biomarker. In the future, the expression ratio of CTSE to S100P could be incorporated into prognostic assessment models to enhance the accuracy of patient outcome prediction.
Currently, carbohydrate antigen 19-9 (CA19-9) is the only serum biomarker approved by the U.S. FDA for managing PC. However, its specificity is limited, as it can also be elevated in benign conditions such as cholangitis and pancreatitis; hence, it is not recommended for early screening in asymptomatic individuals [
55]. Our study demonstrated that both S100P and CTSE are significantly overexpressed in PC tissues, with IHC analysis confirming their diagnostic value in distinguishing malignant from adjacent non-cancerous tissues. In terms of prognosis, high S100P expression correlated with poorer OS, whereas high CTSE expression correlated with improved survival. Multivariate analysis confirmed both as independent prognostic factors. Notably, a recent study on intrahepatic cholangiocarcinoma revealed co-localization of CTSE
+ tumor cells with specific tumor-associated macrophages, jointly contributing to poor patient prognosis. This finding suggests that CTSE serves not only as a diagnostic marker but also as a prognostic indicator intricately linked to the tumor immune microenvironment [
56]. Such features make CTSE functionally complementary to CA19-9, which is primarily used for monitoring treatment response.
Modern oncology is shifting from reliance on single biomarkers toward integrated, multi-parameter diagnostic models. A promising approach is to combine novel biomarkers with established indicators like CA19-9 to construct predictive algorithms. Yang et al. developed a liquid biopsy technique that simultaneously detected CA19-9 and Kirsten rat sarcoma viral oncogene homolog mutations, achieving a diagnostic accuracy of 92% for pancreatic ductal adenocarcinoma—substantially outperforming single-marker assays [
57]. This provides a clear translational direction for our work: combining S100P and/or CTSE with CA19-9 could yield a highly sensitive and specific diagnostic panel. Furthermore, comprehensive scoring systems developed using logistic regression or machine learning algorithms may further enhance diagnostic performance.
This study has some limitations. First, we employed PC TMAs to examine the expression of S100P and CTSE and their associations with clinicopathological parameters. However, the sample size was relatively small, and some IHC slices were excluded due to poor quality or absence of tumor tissue, thereby reducing the number of analyzable samples. To strengthen and validate our findings, it would be valuable to assess additional public cohorts (e.g., TCGA-PAAD) or employ a second TMA for independent validation. Future studies should therefore include larger, multicenter cohorts to obtain more robust conclusions. Second, in our cellular experiments, we primarily investigated the effects of S100P and CTSE on invasion and EMT in relation to the PI3K–AKT signaling pathway. Given the complexity of tumor biology, future studies should explore additional regulatory mechanisms. Specifically, future research should involve PI3K inhibitors (such as LY294002 or Wortmannin) and AKT inhibitors (such as MK-2206) in cell models with S100P/CTSE overexpression or knockdown to confirm the upstream role of this signaling axis. Another limitation of this study lies in the incomplete assessment of the EMT process. While we examined the expression changes of E-cadherin and N-cadherin, we did not cover other key EMT markers such as Vimentin, Snail, Slug, and ZEB1, nor did we analyze the subcellular localization of β-catenin. Future research should systematically evaluate these markers and, in conjunction with techniques such as immunofluorescence, clarify their cellular distribution to more comprehensively reveal the roles of S100P and CTSE in EMT regulation. In the functional experiments of this study, although multiple independent shRNAs were used and consistent phenotypes were observed, future rescue experiments will further confirm the specificity of S100P and CTSE function. This will be one of the key directions of our subsequent research. Future research can further utilize wound healing experiments and 3D spheroid culture models to investigate the effects of S100P and CTSE on cell migration and multicellular invasive behaviors across different dimensions, which will help comprehensively reveal their complex roles in pancreatic cancer metastasis. Finally, due to limited tissue availability and concerns about low yield, insufficient detection sensitivity, and potential interference from tissue heterogeneity when performing protein extraction from small formalin-fixed, paraffin-embedded samples, we used a pooling strategy—combining tissues from multiple patients for proteomic analysis. While this approach improved protein detection, it also introduced potential confounding of inter-patient biological variability. Therefore, the proteomic differences identified between PC and chronic pancreatitis in this study should be regarded as preliminary and hypothesis-generating rather than confirmatory. The core value of this study lies in providing a valuable list of candidate proteins for further investigation. Future studies should rigorously validate these candidate biomarkers in larger, independent cohorts, using patient-specific analyses and more refined sampling methods such as laser microdissection.