Measurement of Glutamate Suppression in a 6-OHDA-Induced Dopamine Deficiency Rat Model Following Acute Single-Dose L-DOPA Using GluCEST/MRS
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. 6-OHDA-Induced Unilateral PD Model
2.3. L-DOPA Administration
2.4. MRI Experiments
2.4.1. MRI Equipment
2.4.2. T2-Weighted Images
2.4.3. CEST
2.4.4. MRS
2.5. Histological Analysis
2.6. Statistical Analysis
3. Results
3.1. Model Induction Testing
3.2. CEST Imaging
3.3. MRS Study
3.4. Staining
4. Discussion
4.1. Quantitative Assessment of Glutamate Using CEST and MRS Techniques
4.2. Association Between Glial Cell Evaluation by GFAP Staining and Glutamate
4.3. Glutamate Dynamics Following L-DOPA Treatment in a Unilateral 6-OHDA Rat PD Model
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 6-OHDA | 6-hydroxydopamine |
| CEST | chemical exchange saturation transfer |
| GFAP | glial fibrillary acidic protein |
| GluCEST | glutamate chemical exchange saturation transfer |
| GSH | glutathione |
| L-DOPA | L-3,4-dihydroxyphenylalanine |
| MAPSHIM | mapping along projections shimming |
| MRI | magnetic resonance imaging |
| MRS | magnetic resonance spectroscopy |
| MTR | magnetization transfer ratio |
| MT | magnetization transfer |
| PD | Parkinson’s disease |
| RARE | rapid acquisition with relaxation enhancement |
| TE | echo time |
| TH | tyrosine hydroxylase |
| TR | repetition time |
| WASSR | water saturation shift referencing |
References
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol. 2021, 20, 385–397. [Google Scholar] [CrossRef]
- Prasad, E.M.; Hung, S.Y. Behavioral Tests in Neurotoxin-Induced Animal Models of Parkinson’s Disease. Antioxidants 2020, 9, 1007. [Google Scholar] [CrossRef]
- Weintraub, D.; Aarsland, D.; Chaudhuri, K.R.; Dobkin, R.D.; Leentjens, A.F.; Rodriguez-Violante, M.; Schrag, A. The neuropsychiatry of Parkinson’s disease: Advances and challenges. Lancet Neurol. 2022, 21, 89–102. [Google Scholar] [CrossRef]
- Lelos, M.J.; Morgan, R.J.; Kelly, C.M.; Torres, E.M.; Rosser, A.E.; Dunnett, S.B. Amelioration of non-motor dysfunctions after transplantation of human dopamine neurons in a model of Parkinson’s disease. Exp. Neurol. 2016, 278, 54–61. [Google Scholar] [CrossRef]
- Wang, J.; Wang, F.; Mai, D.; Qu, S. Molecular Mechanisms of Glutamate Toxicity in Parkinson’s Disease. Front. Neurosci. 2020, 14, 585584. [Google Scholar] [CrossRef]
- Ambrosi, G.; Cerri, S.; Blandini, F. A further update on the role of excitotoxicity in the pathogenesis of Parkinson’s disease. J. Neural Transm. 2014, 121, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Chuhma, N.; Zhang, H.; Masson, J.; Zhuang, X.; Sulzer, D.; Hen, R.; Rayport, S. Dopamine neurons mediate a fast excitatory signal via their glutamatergic synapses. J. Neurosci. 2004, 24, 972–981. [Google Scholar] [CrossRef] [PubMed]
- Golembiowska, K.; Dziubina, A. Effect of adenosine A2A receptor antagonists and L-DOPA on hydroxyl radical, glutamate and dopamine in the striatum of 6-OHDA-treated rats. Neurotox. Res. 2012, 21, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Luthra, P.M. Establishment of a 6-OHDA Induced Unilaterally Lesioned Male Wistar Rat Model of Parkinson’s Disease. Methods Mol. Biol. 2024, 2761, 491–498. [Google Scholar] [CrossRef]
- Taguchi, T.; Ikuno, M.; Yamakado, H.; Takahashi, R. Animal Model for Prodromal Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 1961. [Google Scholar] [CrossRef]
- Simola, N.; Morelli, M.; Carta, A.R. The 6-hydroxydopamine model of Parkinson’s disease. Neurotox. Res. 2007, 11, 151–167. [Google Scholar] [CrossRef]
- Schober, A. Classic toxin-induced animal models of Parkinson’s disease: 6-OHDA and MPTP. Cell Tissue Res. 2004, 318, 215–224. [Google Scholar] [CrossRef]
- Katzenschlager, R.; Lees, A.J. Treatment of Parkinson’s disease: Levodopa as the first choice. J. Neurol. 2002, 249 (Suppl. S2), II19–II24. [Google Scholar] [CrossRef]
- Morari, M.; Marti, M.; Sbrenna, S.; Fuxe, K.; Bianchi, C.; Beani, L. Reciprocal dopamine-glutamate modulation of release in the basal ganglia. Neurochem. Int. 1998, 33, 383–397. [Google Scholar] [CrossRef]
- Cai, K.; Haris, M.; Singh, A.; Kogan, F.; Greenberg, J.H.; Hariharan, H.; Detre, J.A.; Reddy, R. Magnetic resonance imaging of glutamate. Nat. Med. 2012, 18, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Cember, A.T.J.; Nanga, R.P.R.; Reddy, R. Glutamate-weighted CEST (gluCEST) imaging for mapping neurometabolism: An update on the state of the art and emerging findings from in vivo applications. NMR Biomed. 2023, 36, e4780. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A.; Nanga, R.P.R.; Hadar, P.; Chen, S.; Gibson, A.; Oechsel, K.; Elliott, M.A.; Stein, J.M.; Das, S.; Reddy, R.; et al. Mapping hippocampal glutamate in mesial temporal lobe epilepsy with glutamate weighted CEST (GluCEST) imaging. Hum. Brain Mapp. 2023, 44, 549–558. [Google Scholar] [CrossRef]
- Davis, K.A.; Nanga, R.P.; Das, S.; Chen, S.H.; Hadar, P.N.; Pollard, J.R.; Lucas, T.H.; Shinohara, R.T.; Litt, B.; Hariharan, H.; et al. Glutamate imaging (GluCEST) lateralizes epileptic foci in nonlesional temporal lobe epilepsy. Sci. Transl. Med. 2015, 7, 309ra161. [Google Scholar] [CrossRef] [PubMed]
- Neal, A.; Moffat, B.A.; Stein, J.M.; Nanga, R.P.R.; Desmond, P.; Shinohara, R.T.; Hariharan, H.; Glarin, R.; Drummond, K.; Morokoff, A.; et al. Glutamate weighted imaging contrast in gliomas with 7 Tesla magnetic resonance imaging. Neuroimage Clin. 2019, 22, 101694. [Google Scholar] [CrossRef]
- Li, C.; Peng, S.; Wang, R.; Chen, H.; Su, W.; Zhao, X.; Zhou, J.; Chen, M. Chemical exchange saturation transfer MR imaging of Parkinson’s disease at 3 Tesla. Eur. Radiol. 2014, 24, 2631–2639. [Google Scholar] [CrossRef]
- Li, C.; Wang, R.; Chen, H.; Su, W.; Li, S.; Zhao, X.; Zhou, J.; Qiao, J.; Lou, B.; Song, G.; et al. Chemical Exchange Saturation Transfer MR Imaging is Superior to Diffusion-Tensor Imaging in the Diagnosis and Severity Evaluation of Parkinson’s Disease: A Study on Substantia Nigra and Striatum. Front. Aging Neurosci. 2015, 7, 198. [Google Scholar] [CrossRef] [PubMed]
- Bagga, P.; Crescenzi, R.; Krishnamoorthy, G.; Verma, G.; Nanga, R.P.; Reddy, D.; Greenberg, J.; Detre, J.A.; Hariharan, H.; Reddy, R. Mapping the alterations in glutamate with GluCEST MRI in a mouse model of dopamine deficiency. J. Neurochem. 2016, 139, 432–439. [Google Scholar] [CrossRef]
- Momcilovic, M.; Shackelford, D.B. Imaging Cancer Metabolism. Biomol. Ther. 2018, 26, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Saito, S. 5. Advanced Imaging Technology-T1rho-CEST Imaging. Nihon Hoshasen Gijutsu Gakkai Zasshi 2022, 78, 95–100. [Google Scholar] [CrossRef]
- Chassain, C.; Bielicki, G.; Keller, C.; Renou, J.P.; Durif, F. Metabolic changes detected in vivo by 1H MRS in the MPTP-intoxicated mouse. NMR Biomed. 2010, 23, 547–553. [Google Scholar] [CrossRef]
- Saito, S.; Mori, Y.; Tanki, N.; Yoshioka, Y.; Murase, K. Factors affecting the chemical exchange saturation transfer of Creatine as assessed by 11.7 T MRI. Radiol. Phys. Technol. 2015, 8, 146–152. [Google Scholar] [CrossRef]
- DeBrosse, C.; Nanga, R.P.; Bagga, P.; Nath, K.; Haris, M.; Marincola, F.; Schnall, M.D.; Hariharan, H.; Reddy, R. Lactate Chemical Exchange Saturation Transfer (LATEST) Imaging in vivo A Biomarker for LDH Activity. Sci. Rep. 2016, 6, 19517. [Google Scholar] [CrossRef]
- Saito, S.; Takahashi, Y.; Ohki, A.; Shintani, Y.; Higuchi, T. Early detection of elevated lactate levels in a mitochondrial disease model using chemical exchange saturation transfer (CEST) and magnetic resonance spectroscopy (MRS) at 7T-MRI. Radiol. Phys. Technol. 2019, 12, 46–54. [Google Scholar] [CrossRef]
- Saito, S.; Ueda, J. Preclinical magnetic resonance imaging and spectroscopy in the fields of radiological technology, medical physics, and radiology. Radiol. Phys. Technol. 2024, 17, 47–59. [Google Scholar] [CrossRef]
- Kim, M.; Gillen, J.; Landman, B.A.; Zhou, J.; van Zijl, P.C. Water saturation shift referencing (WASSR) for chemical exchange saturation transfer (CEST) experiments. Magn. Reson. Med. 2009, 61, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Gilad, A.A.; Bulte, J.W.; van Zijl, P.C.; McMahon, M.T. High-throughput screening of chemical exchange saturation transfer MR contrast agents. Contrast Media Mol. Imaging 2010, 5, 162–170. [Google Scholar] [CrossRef]
- Provencher, S.W. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reson. Med. 1993, 30, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Bagga, P.; Pickup, S.; Crescenzi, R.; Martinez, D.; Borthakur, A.; D’Aquilla, K.; Singh, A.; Verma, G.; Detre, J.A.; Greenberg, J.; et al. In vivo GluCEST MRI: Reproducibility, background contribution and source of glutamate changes in the MPTP model of Parkinson’s disease. Sci. Rep. 2018, 8, 2883. [Google Scholar] [CrossRef]
- Zhou, J.; Heo, H.Y.; Knutsson, L.; van Zijl, P.C.M.; Jiang, S. APT-weighted MRI: Techniques, current neuro applications, and challenging issues. J. Magn. Reson. Imaging 2019, 50, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Henkelman, R.M.; Stanisz, G.J.; Graham, S.J. Magnetization transfer in MRI: A review. NMR Biomed. 2001, 14, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Cember, A.T.J.; Hariharan, H.; Kumar, D.; Nanga, R.P.R.; Reddy, R. Improved method for post-processing correction of B(1) inhomogeneity in glutamate-weighted CEST images of the human brain. NMR Biomed. 2021, 34, e4503. [Google Scholar] [CrossRef]
- Li, Y.; Xie, D.; Cember, A.; Nanga, R.P.R.; Yang, H.; Kumar, D.; Hariharan, H.; Bai, L.; Detre, J.A.; Reddy, R.; et al. Accelerating GluCEST imaging using deep learning for B(0) correction. Magn. Reson. Med. 2020, 84, 1724–1733. [Google Scholar] [CrossRef]
- Li, C.; Chen, M.; Zhao, X.; Wang, R.; Chen, H.; Su, W.; Li, S.; Lou, B.; Song, G.; Zhang, S.; et al. Chemical Exchange Saturation Transfer MRI Signal Loss of the Substantia Nigra as an Imaging Biomarker to Evaluate the Diagnosis and Severity of Parkinson’s Disease. Front. Neurosci. 2017, 11, 489. [Google Scholar] [CrossRef]
- Khlebnikov, V.; van der Kemp, W.J.M.; Hoogduin, H.; Klomp, D.W.J.; Prompers, J.J. Analysis of chemical exchange saturation transfer contributions from brain metabolites to the Z-spectra at various field strengths and pH. Sci. Rep. 2019, 9, 1089. [Google Scholar] [CrossRef]
- Lally, N.; An, L.; Banerjee, D.; Niciu, M.J.; Luckenbaugh, D.A.; Richards, E.M.; Roiser, J.P.; Shen, J.; Zarate, C.A., Jr.; Nugent, A.C. Reliability of 7T (1) H-MRS measured human prefrontal cortex glutamate, glutamine, and glutathione signals using an adapted echo time optimized PRESS sequence: A between- and within-sessions investigation. J. Magn. Reson. Imaging 2016, 43, 88–98. [Google Scholar] [CrossRef]
- Liu, D.; Zhou, J.; Xue, R.; Zuo, Z.; An, J.; Wang, D.J. Quantitative characterization of nuclear overhauser enhancement and amide proton transfer effects in the human brain at 7 tesla. Magn. Reson. Med. 2013, 70, 1070–1081. [Google Scholar] [CrossRef]
- Iovino, L.; Tremblay, M.E.; Civiero, L. Glutamate-induced excitotoxicity in Parkinson’s disease: The role of glial cells. J. Pharmacol. Sci. 2020, 144, 151–164. [Google Scholar] [CrossRef]
- Lee, H.G.; Wheeler, M.A.; Quintana, F.J. Function and therapeutic value of astrocytes in neurological diseases. Nat. Rev. Drug Discov. 2022, 21, 339–358. [Google Scholar] [CrossRef]
- Danbolt, N.C. Glutamate uptake. Prog. Neurobiol. 2001, 65, 1–105. [Google Scholar] [CrossRef]
- Feng, Y.; Zhou, S.; Sun, J. Exercise increases striatal Glu reuptake and improves motor dysfunction in 6-OHDA-induced Parkinson’s disease rats. Exp. Brain Res. 2021, 239, 3277–3287. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zheng, X.; Che, Y.; Zhang, Y.; Huang, Z.; Jia, L.; Zhu, Y.; Lei, W.; Guo, G.; Shao, C. Morphological changes in perisynaptic astrocytes induced by dopamine neuronal degeneration in the striatum of rats. Heliyon 2024, 10, e27637. [Google Scholar] [CrossRef]
- Rose, C.F.; Verkhratsky, A.; Parpura, V. Astrocyte glutamine synthetase: Pivotal in health and disease. Biochem. Soc. Trans. 2013, 41, 1518–1524. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, A.R.; Norenberg, M.D. Glutamine Synthetase: Role in Neurological Disorders. Adv. Neurobiol. 2016, 13, 327–350. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.B.; Shi, H.B.; Yin, S.K. Mechanisms underlying taurine protection against glutamate-induced neurotoxicity. Can. J. Neurol. Sci. 2013, 40, 628–634. [Google Scholar] [CrossRef]
- Bhat, M.A.; Ahmad, K.; Khan, M.S.A.; Bhat, M.A.; Almatroudi, A.; Rahman, S.; Jan, A.T. Expedition into Taurine Biology: Structural Insights and Therapeutic Perspective of Taurine in Neurodegenerative Diseases. Biomolecules 2020, 10, 863. [Google Scholar] [CrossRef]
- Abuirmeileh, A.N.; Abuhamdah, S.M.; Ashraf, A.; Alzoubi, K.H. Protective effect of caffeine and/or taurine on the 6-hydroxydopamine-induced rat model of Parkinson’s disease: Behavioral and neurochemical evidence. Restor. Neurol. Neurosci. 2021, 39, 149–157. [Google Scholar] [CrossRef]
- Garcia Dopico, J.; Perdomo Diaz, J.; Alonso, T.J.; Gonzalez Hernandez, T.; Castro Fuentes, R.; Rodriguez Diaz, M. Extracellular taurine in the substantia nigra: Taurine-glutamate interaction. J. Neurosci. Res. 2004, 76, 528–538. [Google Scholar] [CrossRef]
- Brenner, M. Role of GFAP in CNS injuries. Neurosci. Lett. 2014, 565, 7–13. [Google Scholar] [CrossRef]
- Brenner, M.; Messing, A. Regulation of GFAP Expression. ASN Neuro 2021, 13, 1759091420981206. [Google Scholar] [CrossRef] [PubMed]
- Jurga, A.M.; Paleczna, M.; Kadluczka, J.; Kuter, K.Z. Beyond the GFAP-Astrocyte Protein Markers in the Brain. Biomolecules 2021, 11, 1361. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.; Finn, D.P.; Dowd, E. Time-course of nigrostriatal neurodegeneration and neuroinflammation in the 6-hydroxydopamine-induced axonal and terminal lesion models of Parkinson’s disease in the rat. Neuroscience 2011, 175, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Lu, J.; Wei, K.; Wei, J.; Tian, P.; Yue, M.; Wang, Y.; Hong, D.; Li, F.; Wang, B.; et al. Neuroprotective Effect of Ceftriaxone on MPTP-Induced Parkinson’s Disease Mouse Model by Regulating Inflammation and Intestinal Microbiota. Oxid. Med. Cell. Longev. 2021, 2021, 9424582. [Google Scholar] [CrossRef]
- Cumming, P.; Gallinat, J. Invited commentary: Mapping the alteration in glutamate with GluCEST MRI in a mouse model of dopamine deficiency: An Editorial Highlight for ‘Mapping the alterations in glutamate with GluCEST MRI in a mouse model of dopamine deficiency’. J. Neurochem. 2016, 139, 346–348. [Google Scholar] [CrossRef]
- Liu, K.; Hui, Y.; Yang, Y.; Guo, Y.; Zhang, L. Blockade of mGluR1 and mGluR5 in the lateral habenula produces the opposite effects in the regulation of depressive-like behaviors in the hemiparkinsonian rats. Exp. Neurol. 2025, 386, 115154. [Google Scholar] [CrossRef]
- Knezovic, A.; Piknjac, M.; Osmanovic Barilar, J.; Babic Perhoc, A.; Virag, D.; Homolak, J.; Salkovic-Petrisic, M. Association of Cognitive Deficit with Glutamate and Insulin Signaling in a Rat Model of Parkinson’s Disease. Biomedicines 2023, 11, 683. [Google Scholar] [CrossRef]
- Touchon, J.C.; Holmer, H.K.; Moore, C.; McKee, B.L.; Frederickson, J.; Meshul, C.K. Apomorphine-induced alterations in striatal and substantia nigra pars reticulata glutamate following unilateral loss of striatal dopamine. Exp. Neurol. 2005, 193, 131–140. [Google Scholar] [CrossRef]
- Pierucci, M.; Di Matteo, V.; Benigno, A.; Crescimanno, G.; Esposito, E.; Di Giovanni, G. The unilateral nigral lesion induces dramatic bilateral modification on rat brain monoamine neurochemistry. Ann. N. Y. Acad. Sci. 2009, 1155, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Nandhu, M.S.; Paul, J.; Kuruvila, K.P.; Abraham, P.M.; Antony, S.; Paulose, C.S. Glutamate and NMDA receptors activation leads to cerebellar dysfunction and impaired motor coordination in unilateral 6-hydroxydopamine lesioned Parkinson’s rat: Functional recovery with bone marrow cells, serotonin and GABA. Mol. Cell. Biochem. 2011, 353, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Buard, I.; Lopez-Esquibel, N.; Carey, F.J.; Brown, M.S.; Medina, L.D.; Kronberg, E.; Martin, C.S.; Rogers, S.; Holden, S.K.; Greher, M.R.; et al. Does Prefrontal Glutamate Index Cognitive Changes in Parkinson’s Disease? Front. Hum. Neurosci. 2022, 16, 809905. [Google Scholar] [CrossRef]
- Buck, S.A.; Quincy Erickson-Oberg, M.; Logan, R.W.; Freyberg, Z. Relevance of interactions between dopamine and glutamate neurotransmission in schizophrenia. Mol. Psychiatry 2022, 27, 3583–3591. [Google Scholar] [CrossRef] [PubMed]
- Sesack, S.R.; Carr, D.B.; Omelchenko, N.; Pinto, A. Anatomical substrates for glutamate-dopamine interactions: Evidence for specificity of connections and extrasynaptic actions. Ann. N. Y. Acad. Sci. 2003, 1003, 36–52. [Google Scholar] [CrossRef]
- Eskenazi, D.; Malave, L.; Mingote, S.; Yetnikoff, L.; Ztaou, S.; Velicu, V.; Rayport, S.; Chuhma, N. Dopamine Neurons That Cotransmit Glutamate, From Synapses to Circuits to Behavior. Front. Neural Circuits 2021, 15, 665386. [Google Scholar] [CrossRef] [PubMed]
- Mingote, S.; Chuhma, N.; Kusnoor, S.V.; Field, B.; Deutch, A.Y.; Rayport, S. Functional Connectome Analysis of Dopamine Neuron Glutamatergic Connections in Forebrain Regions. J. Neurosci. 2015, 35, 16259–16271. [Google Scholar] [CrossRef]
- Ishida, Y.; Hashiguchi, H.; Todaka, K.; Ishizuka, Y.; Mitsuyama, Y. Repeated administration of high dose levodopa enhances hydroxyl radical production in the rat striatum denervated with 6-hydroxydopamine. Neurosci. Lett. 2000, 290, 33–36. [Google Scholar] [CrossRef]
- Lelos, M.J.; Murphy, E.M.; Lindgren, H.S.; Dunnett, S.B.; Lane, E.L. Impaired cognitive and motor function are coincident with L-DOPA-induced dyskinesia in a model of Parkinson’s disease. Sci. Rep. 2023, 13, 17697. [Google Scholar] [CrossRef]
- Morin, N.; Di Paolo, T. Pharmacological Treatments Inhibiting Levodopa-Induced Dyskinesias in MPTP-Lesioned Monkeys: Brain Glutamate Biochemical Correlates. Front. Neurol. 2014, 5, 144. [Google Scholar] [CrossRef]
- Pourmirbabaei, S.; Dolatshahi, M.; Rahmani, F. Pathophysiological clues to therapeutic applications of glutamate mGlu5 receptor antagonists in levodopa-induced dyskinesia. Eur. J. Pharmacol. 2019, 855, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Rosa, I.; Di Censo, D.; Ranieri, B.; Di Giovanni, G.; Scarnati, E.; Alecci, M.; Galante, A.; Florio, T.M. Comparison between Tail Suspension Swing Test and Standard Rotation Test in Revealing Early Motor Behavioral Changes and Neurodegeneration in 6-OHDA Hemiparkinsonian Rats. Int. J. Mol. Sci. 2020, 21, 2874. [Google Scholar] [CrossRef] [PubMed]
- Song, H.J.; Desai, J.N.; Norman, M.K.; Norman, A.B. Using cocaine and apomorphine self-administration in rats to measure the pharmacokinetics of competitive dopamine receptor antagonists administered by different routes. Sci. Rep. 2025, 15, 24087. [Google Scholar] [CrossRef] [PubMed]


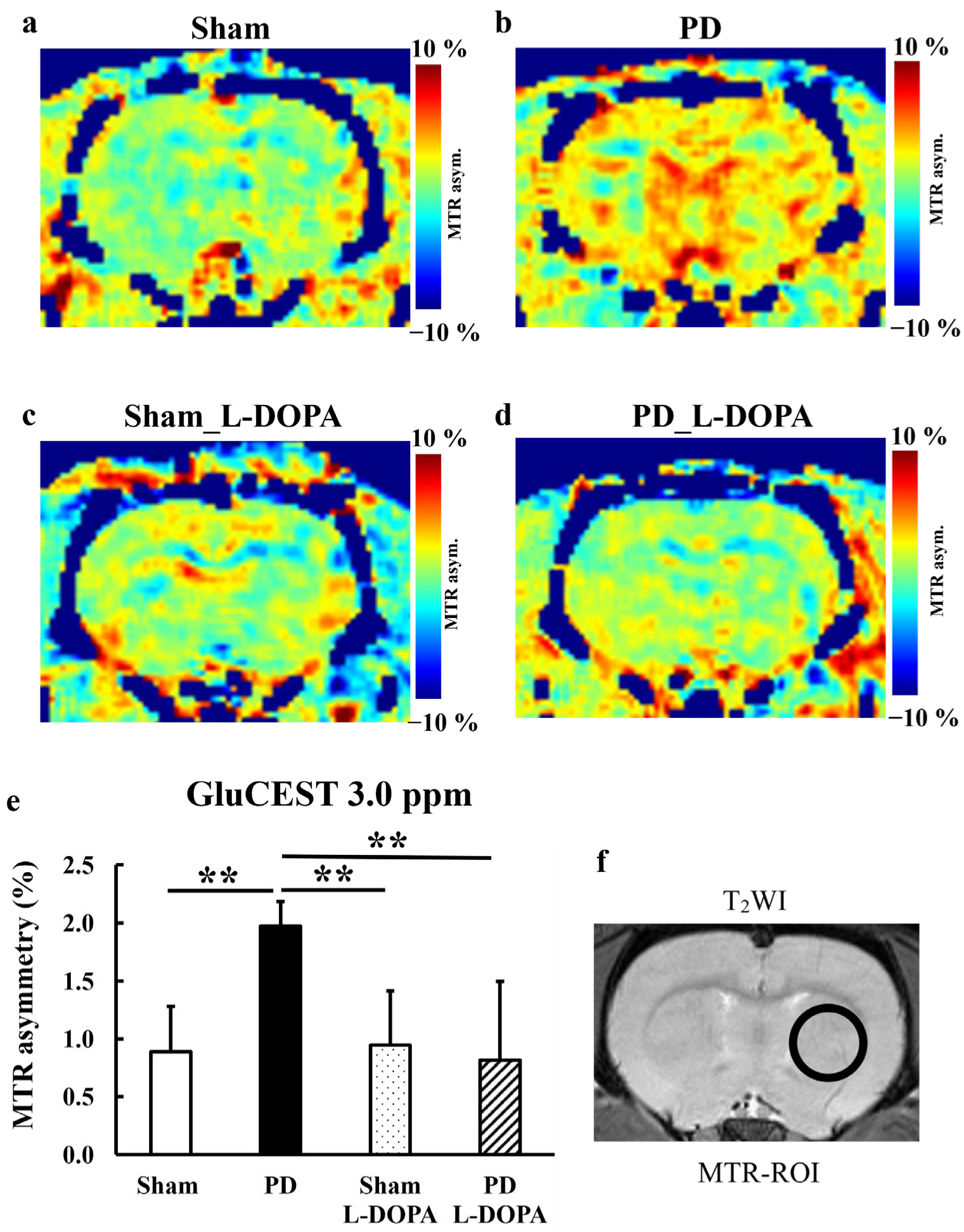
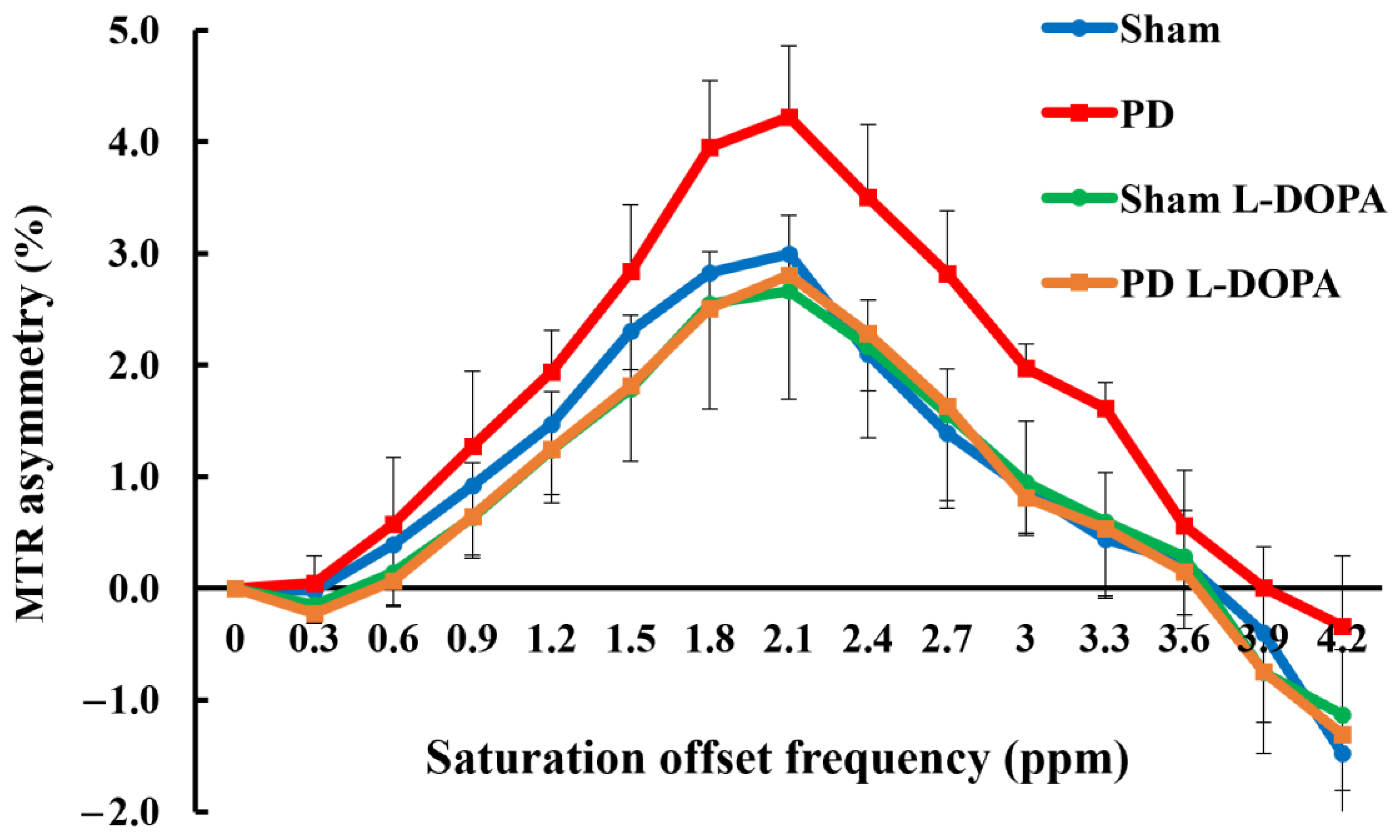

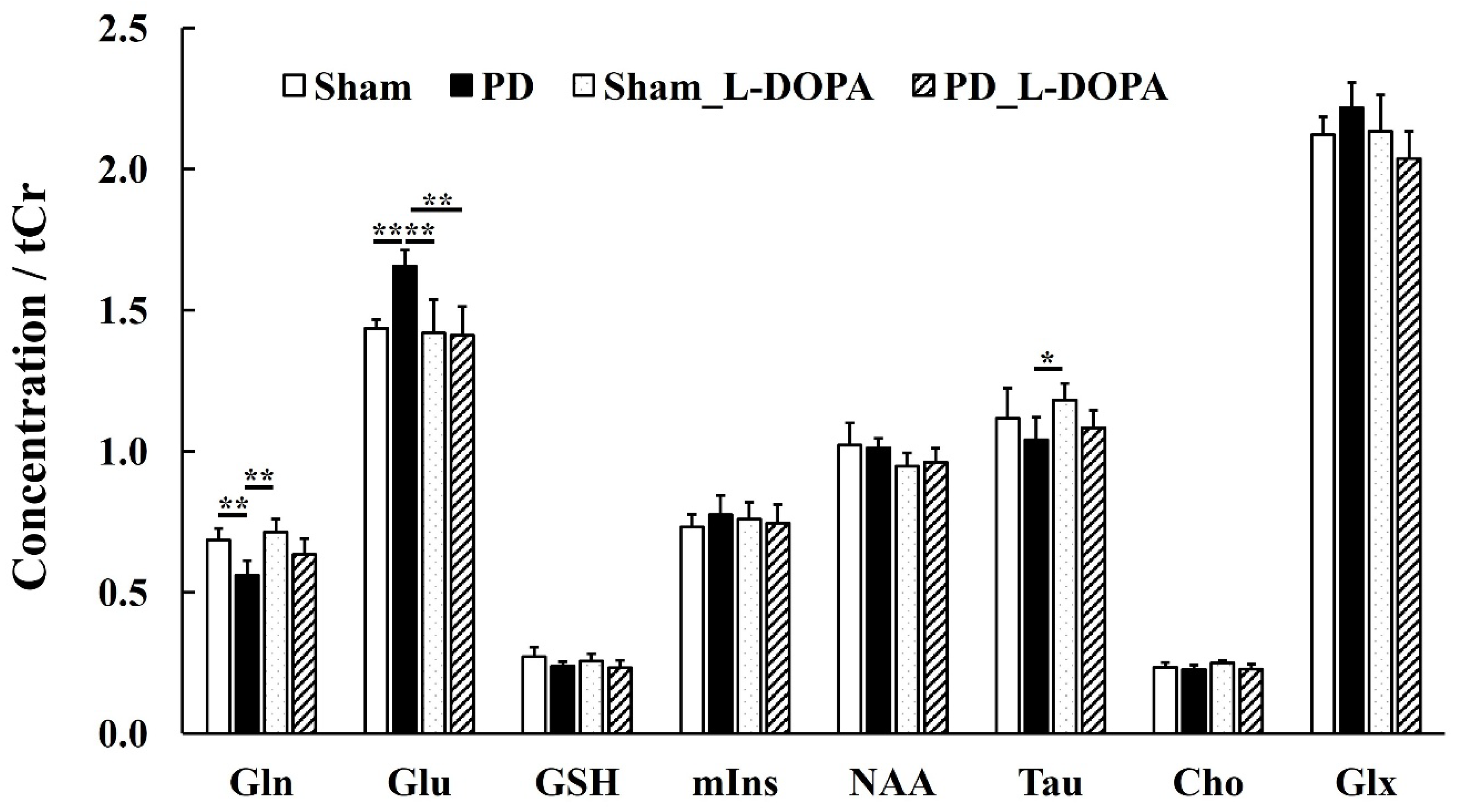
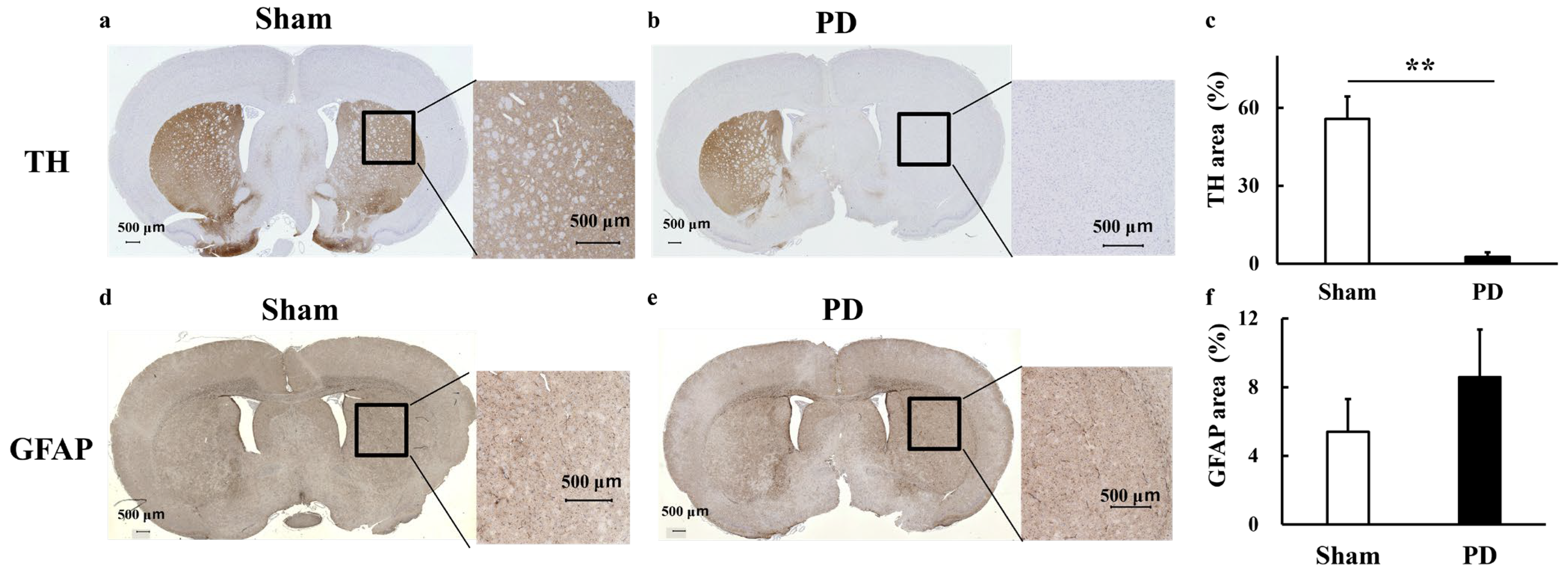
| /tCr | Sham | PD | Sham_L-DOPA | PD_L-DOPA |
|---|---|---|---|---|
| Gln | 0.7 ± 0.04 ** | 0.6 ± 0.05 | 0.7 ± 0.05 ** | 0.6 ± 0.06 |
| Glu | 1.4 ± 0.03 ** | 1.7 ± 0.06 | 1.4 ± 0.12 ** | 1.4 ± 0.10 ** |
| GSH | 0.3 ± 0.03 | 0.2 ± 0.01 | 0.3 ± 0.03 | 0.2 ± 0.03 |
| mIns | 0.7 ± 0.05 | 0.8 ± 0.07 | 0.8 ± 0.06 | 0.7 ± 0.07 |
| NAA | 1.0 ± 0.08 | 1.0 ± 0.03 | 0.9 ± 0.05 | 1.0 ± 0.05 |
| Tau | 1.1 ± 0.11 | 1.0 ± 0.08 | 1.2 ± 0.06 * | 1.1 ± 0.06 |
| Cho | 0.2 ± 0.02 | 0.2 ± 0.02 | 0.2 ± 0.01 | 0.2 ± 0.02 |
| Glx | 2.1 ± 0.06 | 2.2 ± 0.09 | 2.1 ± 0.13 | 2.0 ± 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakano, T.; Bono, K.; Ueda, J.; Ohmi, M.; Saito, S. Measurement of Glutamate Suppression in a 6-OHDA-Induced Dopamine Deficiency Rat Model Following Acute Single-Dose L-DOPA Using GluCEST/MRS. Biomedicines 2025, 13, 2761. https://doi.org/10.3390/biomedicines13112761
Nakano T, Bono K, Ueda J, Ohmi M, Saito S. Measurement of Glutamate Suppression in a 6-OHDA-Induced Dopamine Deficiency Rat Model Following Acute Single-Dose L-DOPA Using GluCEST/MRS. Biomedicines. 2025; 13(11):2761. https://doi.org/10.3390/biomedicines13112761
Chicago/Turabian StyleNakano, Tensei, Kazuma Bono, Junpei Ueda, Masato Ohmi, and Shigeyoshi Saito. 2025. "Measurement of Glutamate Suppression in a 6-OHDA-Induced Dopamine Deficiency Rat Model Following Acute Single-Dose L-DOPA Using GluCEST/MRS" Biomedicines 13, no. 11: 2761. https://doi.org/10.3390/biomedicines13112761
APA StyleNakano, T., Bono, K., Ueda, J., Ohmi, M., & Saito, S. (2025). Measurement of Glutamate Suppression in a 6-OHDA-Induced Dopamine Deficiency Rat Model Following Acute Single-Dose L-DOPA Using GluCEST/MRS. Biomedicines, 13(11), 2761. https://doi.org/10.3390/biomedicines13112761






