From Lesion to Decision: AI for ARIA Detection and Predictive Imaging in Alzheimer’s Disease
Abstract
1. Introduction
2. Discussion
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s Disease |
| AI | Artificial Intelligence |
| APOE | Apolipoprotein E |
| Aβ | Amyloid-beta |
| ARIA | Amyloid-Related Imaging Abnormalities |
| ARIA-E | Amyloid-Related Imaging Abnormalities—Edema/Effusion |
| ARIA-H | Amyloid-Related Imaging Abnormalities—Hemosiderin-related |
| AUC | Area Under the Curve |
| BraTS | Brain Tumor Segmentation challenge |
| CAA | Cerebral Amyloid Angiopathy |
| CC BY | Creative Commons Attribution |
| CNN | Convolutional Neural Network |
| DWI | Diffusion-Weighted Imaging |
| FLAIR | Fluid-Attenuated Inversion Recovery |
| FP | False Positive |
| GRE | Gradient-Recalled Echo (MRI sequence) |
| IoMT | Internet of Medical Things |
| MAB(s) | Monoclonal Antibody(/ies) |
| MRI | Magnetic Resonance Imaging |
| NMDA | N-methyl-D-aspartate |
| QSM | Quantitative Susceptibility Mapping |
| SWI | Susceptibility Weighted Imaging |
| QSMnet | Quantitative Susceptibility Mapping neural network |
References
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- McDade, E.M. Alzheimer Disease. Continuum 2022, 28, 648–675. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Hong, F.; Yang, S. Amyloidosis in Alzheimer’s Disease: Pathogeny, Etiology, and Related Therapeutic Directions. Molecules 2022, 27, 1210. [Google Scholar] [CrossRef] [PubMed]
- Lacosta, A.-M.; Insua, D.; Badi, H.; Pesini, P.; Sarasa, M. Neurofibrillary Tangles of Aβx-40 in Alzheimer’s Disease Brains. J. Alzheimer’s Dis. 2017, 58, 661–667. [Google Scholar] [CrossRef]
- Bondi, M.W.; Edmonds, E.C.; Salmon, D.P. Alzheimer’s Disease: Past, Present, and Future. J. Int. Neuropsychol. Soc. 2017, 23, 818–831. [Google Scholar] [CrossRef]
- Raji, C.A.; Benzinger, T.L.S. The Value of Neuroimaging in Dementia Diagnosis. Continuum 2022, 28, 800–821. [Google Scholar] [CrossRef]
- Masdeu, J.C. Neuroimaging of Diseases Causing Dementia. Neurol. Clin. 2020, 38, 65–94. [Google Scholar] [CrossRef]
- Del Sole, A.; Malaspina, S.; Magenta Biasina, A. Magnetic resonance imaging and positron emission tomography in the diagnosis of neurodegenerative dementias. Funct. Neurol. 2016, 31, 205–215. [Google Scholar] [CrossRef]
- Jie, C.V.M.L.; Treyer, V.; Schibli, R.; Mu, L. TauvidTM: The First FDA-Approved PET Tracer for Imaging Tau Pathology in Alzheimer’s Disease. Pharmaceuticals 2021, 14, 110. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Bella, R.; Benussi, A.; Bologna, M.; Borroni, B.; Capone, F.; Chen, K.S.; Chen, R.; Chistyakov, A.V.; Classen, J.; et al. Diagnostic contribution and therapeutic perspectives of transcranial magnetic stimulation in dementia. Clin. Neurophysiol. 2021, 132, 2568–2607. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Nazzi, C.; Di Fazio, C.; Borgomaneri, S. The role of pre-supplementary motor cortex in action control with emotional stimuli: A repetitive transcranial magnetic stimulation study. Ann. N. Y. Acad. Sci. 2024, 1536, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Nazzi, C.; Fullana, M.A.; di Pellegrino, G.; Borgomaneri, S. ‘Nip it in the bud’: Low-frequency rTMS of the prefrontal cortex disrupts threat memory consolidation in humans. Behav. Res. Ther. 2024, 178, 104548. [Google Scholar] [CrossRef] [PubMed]
- Golde, T.E. Disease-Modifying Therapies for Alzheimer’s Disease: More Questions than Answers. Neurotherapeutics 2022, 19, 209–227. [Google Scholar] [CrossRef]
- Sperling, R.A.; Jack, C.R., Jr.; Black, S.E.; Frosch, M.P.; Greenberg, S.M.; Hyman, B.T.; Scheltens, P.; Carrillo, M.C.; Thies, W.; Bednar, M.M.; et al. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: Recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimer’s Dement. 2011, 7, 367–385. [Google Scholar] [CrossRef]
- Honig, L.S.; Sabbagh, M.N.; van Dyck, C.H.; Sperling, R.A.; Hersch, S.; Matta, A.; Giorgi, L.; Gee, M.; Kanekiyo, M.; Li, D.; et al. Updated safety results from phase 3 lecanemab study in early Alzheimer’s disease. Alzheimer’s Res. Ther. 2024, 16, 105, Erratum in Alzheimer’s Res. Ther. 2024, 16, 159. [Google Scholar] [CrossRef]
- Haeberlein, S.B.; Aisen, P.; Barkhof, F.; Chalkias, S.; Chen, T.; Cohen, S.; Dent, G.; Hansson, O.; Harrison, K.; von Hehn, C.; et al. Two Randomized Phase 3 Studies of Aducanumab in Early Alzheimer’s Disease. J. Prev. Alzheimer’s Dis. 2022, 9, 197–210. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, V.; Brahmbhatt, P.; Desai, A.; Vibhute, P.; Joseph-Mathurin, N.; Bathla, G. Amyloid-related Imaging Abnormalities in Alzheimer Disease Treated with Anti–Amyloid-β Therapy. RadioGraphics 2023, 43, e230009. [Google Scholar] [CrossRef]
- Doran, S.J.; Sawyer, R.P. Risk factors in developing amyloid related imaging abnormalities (ARIA) and clinical implications. Front. Neurosci. 2024, 18, 1326784. [Google Scholar] [CrossRef]
- Choi, K.S.; Sunwoo, L. Artificial Intelligence in Neuroimaging: Clinical Applications. Investig. Magn. Reson. Imaging 2022, 26, 1. [Google Scholar] [CrossRef]
- Jytzler, J.A.; Lysdahlgaard, S. Radiomics evaluation for the early detection of Alzheimer’s dementia using T1-weighted MRI. Radiography 2024, 30, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- Matsoukas, S.; Scaggiante, J.; Schuldt, B.R.; Smith, C.J.; Chennareddy, S.; Kalagara, R.; Majidi, S.; Bederson, J.B.; Fifi, J.T.; Mocco, J.; et al. Accuracy of artificial intelligence for the detection of intracranial hemorrhage and chronic cerebral microbleeds: A systematic review and pooled analysis. Radiol. Medica 2022, 127, 1106–1123. [Google Scholar] [CrossRef]
- Shafieioun, A.; Ghaffari, H.; Baradaran, M.; Rigi, A.; Eftekhar, M.S.; Shojaeshafiei, F.; Korani, M.A.; Hatami, B.; Shirdel, S.; Ghanbari, K.; et al. Predictive power of artificial intelligence for malignant cerebral edema in stroke patients: A CT-based systematic review and meta-analysis of prevalence and diagnostic performance. Neurosurg. Rev. 2025, 48, 318. [Google Scholar] [CrossRef]
- Marino, L.; Bilotta, F. Artificial intelligence in traumatic brain injury: Brain imaging analysis and outcome prediction: A mini review. World J. Crit. Care Med. 2025, 14, 107611. [Google Scholar] [CrossRef] [PubMed]
- Sima, D.M.; Phan, T.V.; Van Eyndhoven, S.; Vercruyssen, S.; Magalhães, R.; Liseune, A.; Brys, A.; Frenyo, P.; Terzopoulos, V.; Maes, C.; et al. Artificial Intelligence Assistive Software Tool for Automated Detection and Quantification of Amyloid-Related Imaging Abnormalities. JAMA Netw. Open 2024, 7, e2355800. [Google Scholar] [CrossRef] [PubMed]
- Roytman, M.; Mashriqi, F.; Al-Tawil, K.; Schulz, P.E.; Zaharchuk, G.; Benzinger, T.L.S.; Franceschi, A.M. Amyloid-Related Imaging Abnormalities: An Update. Am. J. Roentgenol. 2023, 220, 562–574. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Vernooij, M.W.; Cordonnier, C.; Viswanathan, A.; Salman, R.A.-S.; Warach, S.; Launer, L.J.; Van Buchem, M.A.; Breteler, M.M. Cerebral microbleeds: A guide to detection and interpretation. Lancet Neurol. 2009, 8, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.Y.; Suh, C.H.; Kim, S.J.; Lemere, C.A.; Lim, J.-S.; Lee, J.-H. Amyloid-Related Imaging Abnormalities in the Era of Anti-Amyloid Beta Monoclonal Antibodies for Alzheimer’s Disease: Recent Updates on Clinical and Imaging Features and MRI Monitoring. Korean J. Radiol. 2024, 25, 726–741. [Google Scholar] [CrossRef]
- Van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- Sims, J.R.; Zimmer, J.A.; Evans, C.D.; Lu, M.; Ardayfio, P.; Sparks, J.; Wessels, A.M.; Shcherbinin, S.; Wang, H.; Monkul Nery, E.S.; et al. Donanemab in Early Symptomatic Alzheimer Disease: The TRAILBLAZER-ALZ 2 Randomized Clinical Trial. JAMA 2023, 330, 512. [Google Scholar] [CrossRef]
- Cummings, J.; Apostolova, L.; Rabinovici, G.D.; Atri, A.; Aisen, P.; Greenberg, S.; Hendrix, S.; Selkoe, D.; Weiner, M.; Petersen, R.C.; et al. Lecanemab: Appropriate Use Recommendations. J. Prev. Alzheimer’s Dis. 2023, 10, 362–377. [Google Scholar] [CrossRef]
- Rabinovici, G.; Selkoe, D.; Schindler, S.; Aisen, P.; Apostolova, L.; Atri, A.; Greenberg, S.; Hendrix, S.; Petersen, R.; Weiner, M.; et al. Donanemab: Appropriate use recommendations. J. Prev. Alzheimer’s Dis. 2025, 12, 100150. [Google Scholar] [CrossRef] [PubMed]
- Barakos, J.; Purcell, D.; Suhy, J.; Chalkias, S.; Burkett, P.; Grassi, C.M.; Castrillo-Viguera, C.; Rubino, I.; Vijverberg, E. Detection and Management of Amyloid-Related Imaging Abnormalities in Patients with Alzheimer’s Disease Treated with Anti-Amyloid Beta Therapy. J. Prev. Alzheimer’s Dis. 2022, 9, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Chi, Y.; Zhang, Q.; Ma, Y. Safety and efficacy of lecanemab for Alzheimer’s disease: A systematic review and meta-analysis of randomized clinical trials. Front. Aging Neurosci. 2023, 15, 1169499. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Elhage, A.; Cho, M.; Apostolova, L.G.; Nicoll, J.A.R.; Atri, A. Amyloid-related imaging abnormalities (ARIA): Radiological, biological and clinical characteristics. Brain 2023, 146, 4414–4424. [Google Scholar] [CrossRef]
- Hsu, C.C.; Sethi, S.K.; Haacke, E.M. The Current State of Susceptibility-Weighted Imaging and Quantitative Susceptibility Mapping in Head Trauma. Neuroimaging Clin. N. Am. 2023, 33, 343–356. [Google Scholar] [CrossRef]
- Genc, O.; Morrison, M.A.; Villanueva-Meyer, J.E.; Burns, B.; Hess, C.P.; Banerjee, S.; Lupo, J.M. DeepSWI: Using Deep Learning to Enhance Susceptibility Contrast on T2*-Weighted MRI. J. Magn. Reson. Imaging 2023, 58, 1200–1210. [Google Scholar] [CrossRef]
- Liu, S.; Utriainen, D.; Chai, C.; Chen, Y.; Wang, L.; Sethi, S.K.; Xia, S.; Haacke, E.M. Cerebral microbleed detection using Susceptibility Weighted Imaging and deep learning. NeuroImage 2019, 198, 271–282. [Google Scholar] [CrossRef]
- Yoon, J.; Gong, E.; Chatnuntawech, I.; Bilgic, B.; Lee, J.; Jung, W.; Ko, J.; Jung, H.; Setsompop, K.; Zaharchuk, G.; et al. Quantitative susceptibility mapping using deep neural network: QSMnet. NeuroImage 2018, 179, 199–206. [Google Scholar] [CrossRef]
- Schmeel, F.C. Variability in quantitative diffusion-weighted MR imaging (DWI) across different scanners and imaging sites: Is there a potential consensus that can help reducing the limits of expected bias? Eur. Radiol. 2019, 29, 2243–2245. [Google Scholar] [CrossRef]
- Sundaresan, V.; Arthofer, C.; Zamboni, G.; Dineen, R.A.; Rothwell, P.M.; Sotiropoulos, S.N.; Auer, D.P.; Tozer, D.J.; Markus, H.S.; Miller, K.L.; et al. Automated Detection of Candidate Subjects with Cerebral Microbleeds Using Machine Learning. Front. Neurosci. 2022, 15, 777828. [Google Scholar] [CrossRef]
- Myung, M.J.; Lee, K.M.; Kim, H.-G.; Oh, J.; Lee, J.Y.; Shin, I.; Kim, E.J.; Lee, J.S. Novel Approaches to Detection of Cerebral Microbleeds: Single Deep Learning Model to Achieve a Balanced Performance. J. Stroke Cerebrovasc. Dis. 2021, 30, 105886. [Google Scholar] [CrossRef]
- Momeni, S.; Fazlollahi, A.; Lebrat, L.; Yates, P.; Rowe, C.; Gao, Y.; Liew, A.W.-C.; Salvado, O. Generative Model of Brain Microbleeds for MRI Detection of Vascular Marker of Neurodegenerative Diseases. Front. Neurosci. 2021, 15, 778767. [Google Scholar] [CrossRef] [PubMed]
- Aker, L.; Abandeh, L.; Abdelhady, M.; Aboughalia, H.; Vattoth, S. Susceptibility-weighted Imaging in Neuroradiology: Practical Imaging Principles, Pearls and Pitfalls. Curr. Probl. Diagn. Radiol. 2022, 51, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Al-Masni, M.A.; Kim, W.R.; Kim, E.Y.; Noh, Y.; Kim, D.H. Automated detection of cerebral microbleeds in MR images: A two-stage deep learning approach. NeuroImage Clin. 2020, 28, 102464. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Gao, K.; Fawaz, M.; Wu, B.; Zhong, Y.; Zhou, Y.; Haacke, E.M.; Dai, Y.; Liu, S. Automatic detection of cerebral microbleeds using susceptibility weighted imaging and artificial intelligence. Quant. Imaging Med. Surg. 2024, 14, 2640–2654. [Google Scholar] [CrossRef] [PubMed]
- Navab, N.; Hornegger, J.; Wells, W.M.; Frangi, A.F. (Eds.) Medical Image Computing and Computer-Assisted Intervention—MICCAI 2015. In Proceedings of the 18th International Conference, Munich, Germany, 5–9 October 2015; Springer International Publishing: Cham, Switzerland, 2015. Available online: http://link.springer.com/10.1007/978-3-319-24574-4 (accessed on 13 August 2025).
- Isensee, F.; Jaeger, P.F.; Kohl, S.A.A.; Petersen, J.; Maier-Hein, K.H. nnU-Net: A self-configuring method for deep learning-based biomedical image segmentation. Nat. Methods. 2021, 18, 203–211. [Google Scholar] [CrossRef]
- Beheshti, I.; Sone, D.; Leung, C.K.; Advances of Artificial Intelligence in Neuroimaging. Brain Sciences. 2025. Available online: https://www.embase.com/search/results?subaction=viewrecord&id=L2034339004&from=export (accessed on 13 August 2025).
- Maier, O.; Menze, B.H.; von der Gablentz, J.; Häni, L.; Heinrich, M.P.; Liebrand, M.; Winzeck, S.; Basit, A.; Bentley, P.; Chen, L.; et al. ISLES 2015—A public evaluation benchmark for ischemic stroke lesion segmentation from multispectral MRI. Med. Image Anal. 2017, 35, 250–269. [Google Scholar] [CrossRef]
- Bareja, R.; Ismail, M.; Martin, D.; Nayate, A.; Yadav, I.; Labbad, M.; Dullur, P.; Garg, S.; Tamrazi, B.; Salloum, R.; et al. nnU-Net–based Segmentation of Tumor Subcompartments in Pediatric Medulloblastoma Using Multiparametric MRI: A Multi-institutional Study. Radiol. Artif. Intell. 2024, 6, e230115. [Google Scholar] [CrossRef]
- Lee, H.; Kim, J.; Lee, S.; Jung, K.; Kim, W.; Noh, Y.; Kim, E.Y.; Kang, K.M.; Sohn, C.; Lee, D.Y.; et al. Detection of Cerebral Microbleeds in MR Images Using a Single-Stage Triplanar Ensemble Detection Network (TPE-Det). J. Magn. Reson. Imaging 2023, 58, 272–283. [Google Scholar] [CrossRef]
- Won, S.Y.; Kim, J.-H.; Woo, C.; Kim, D.-H.; Park, K.Y.; Kim, E.Y.; Baek, S.-Y.; Han, H.J.; Sohn, B. Real-world application of a 3D deep learning model for detecting and localizing cerebral microbleeds. Acta Neurochir. 2024, 166, 381. [Google Scholar] [CrossRef]
- Valverde, J.M.; Imani, V.; Abdollahzadeh, A.; De Feo, R.; Prakash, M.; Ciszek, R.; Tohka, J. Transfer Learning in Magnetic Resonance Brain Imaging: A Systematic Review. J. Imaging 2021, 7, 66. [Google Scholar] [CrossRef]
- Kim, H.E.; Cosa-Linan, A.; Santhanam, N.; Jannesari, M.; Maros, M.E.; Ganslandt, T. Transfer learning for medical image classification: A literature review. BMC Med. Imaging 2022, 22, 69. [Google Scholar] [CrossRef]
- Xie, L.; Yushkevich, P.A.; Das, S.R.; Wolk, D.A.; Gibson, E. Utilizing Advanced Artificial Intelligence for Automated Detection and Segmentation of Amyloid-Related Imaging Abnormality (ARIA). Alzheimer’s Dement. 2025, 20, e093974. [Google Scholar] [CrossRef]
- Sima, D.; Phan, T.V.; Van Eyndhoven, S.; Vercruyssen, S.; Magalhães, R.; Maes, C.; Khan, R.; Guo, J.; Hughes, R.; Gabr, R.; et al. Validation of Icobrain Aria—An AI-based Software Tool for Automated Detection and Quantification of Amyloidrelated Imaging Abnormalities. Neurology 2024, 102, 6102. [Google Scholar] [CrossRef]
- FDA. 510(k) Premarket Notification. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K240712 (accessed on 13 August 2025).
- Marco, M. FDA Clears Icobrain Aria, First AI Tool for Safer ARIA Detection in Alzheimer Treatment. Available online: https://www.neurologylive.com/view/fda-clears-icobrain-aria-first-ai-tool-safer-aria-detection-alzheimer-treatment (accessed on 13 August 2025).
- Cortechs. ai Nets FDA Clearance for NeuroQuant 5.0 with Improved ARIA Quantification. Available online: https://appliedradiology.com/articles/cortechs-ai-nets-fda-clearance-for-neuroquant-5-0-with-improved-aria-quantification (accessed on 13 August 2025).
- Petrella, J.R.; Liu, A.J.; Wang, L.A.; Doraiswamy, P.M. AI-Assisted Detection of Amyloid-related Imaging Abnormalities (ARIA): Promise and Pitfalls. AJNR Am 2025. [Google Scholar] [CrossRef] [PubMed]
- Salloway, S.; Wojtowicz, J.; Voyle, N.; Lane, C.A.; Klein, G.; Lyons, M.; Rossomanno, S.; Mazzo, F.; Bullain, S.; Barkhof, F.; et al. Amyloid-Related Imaging Abnormalities (ARIA) in Clinical Trials of Gantenerumab in Early Alzheimer Disease. JAMA Neurol. 2025, 82, 19. [Google Scholar] [CrossRef]
- Swanson, C.J.; Zhang, Y.; Dhadda, S.; Wang, J.; Kaplow, J.; Lai, R.Y.K.; Lannfelt, L.; Bradley, H.; Rabe, M.; Koyama, A.; et al. A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer’s disease with lecanemab, an anti-Aβ protofibril antibody. Alzheimer’s Res. Ther. 2021, 13, 80. [Google Scholar] [CrossRef]
- Wang, H.; Nery, E.S.M.; Ardayfio, P.; Khanna, R.; Svaldi, D.O.; Gueorguieva, I.; Shcherbinin, S.; Andersen, S.W.; Hauck, P.M.; Engle, S.E.; et al. Modified titration of donanemab reduces ARIA risk and maintains amyloid reduction. Alzheimer’s Dement. 2025, 21, e70062. [Google Scholar] [CrossRef]
- Oakden-Rayner, L.; Dunnmon, J.; Carneiro, G.; Re, C. Hidden stratification causes clinically meaningful failures in machine learning for medical imaging. In Proceedings of the ACM Conference on Health, Inference, and Learning, Toronto, ON, Canada, 2–4 April 2020; ACM: New York, NY, USA, 2020; pp. 151–159. Available online: https://dl.acm.org/doi/10.1145/3368555.3384468 (accessed on 14 August 2025).
- Guan, H.; Liu, M. Domain Adaptation for Medical Image Analysis: A Survey. IEEE Trans. Biomed. Eng. 2022, 69, 1173–1185. [Google Scholar] [CrossRef]
- Momeni, F.; Shahbazi-Gahrouei, D.; Mahmoudi, T.; Mehdizadeh, A. Transfer Learning and Neural Network-Based Approach on Structural MRI Data for Prediction and Classification of Alzheimer’s Disease. Diagnostics 2025, 15, 360. [Google Scholar] [CrossRef]
- Christodoulou, R.C.; Woodward, A.; Pitsillos, R.; Ibrahim, R.; Georgiou, M.F. Artificial Intelligence in Alzheimer’s Disease Diagnosis and Prognosis Using PET-MRI: A Narrative Review of High-Impact Literature Post-Tauvid Approval. J. Clin. Med. 2025, 14, 5913. [Google Scholar] [CrossRef]
- Christodoulou, R.; Vamvouras, G.; Lorentzen, L.; Vassiliou, E. Erythrocyte Load in Cerebrospinal Fluid Linked with Hippocampal Atrophy in Alzheimer’s Disease. J. Clin. Med. 2025, 14, 4670. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, R.C.; Vamvouras, G.; Petrou, V.; Papageorgiou, P.S.; Pitsillos, R.; Rivera, L.; Vassiliou, E.; Papageorgiou, S.G.; Solomou, E.E.; for the Alzheimer’s Disease Neuroimaging Initiative. Cerebrospinal Fluid Erythrocyte Burden Amplifies the Impact of PTAU on Entorhinal Degeneration in Alzheimer’s Disease. Biomolecules 2025, 15, 1300. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.Y.; Vaghari, D.; Burkhart, M.C.; Tino, P.; Montagnese, M.; Li, Z.; Zühlsdorff, K.; Giorgio, J.; Williams, G.; Chong, E.; et al. Robust and interpretable AI-guided marker for early dementia prediction in real-world clinical settings. eClinicalMedicine 2024, 74, 102725. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, R.; Christofi, G.; Pitsillos, R.; Ibrahim, R.; Papageorgiou, P.; Papageorgiou, S.G.; Vassiliou, E.; Georgiou, M.F. AI-Based Classification of Mild Cognitive Impairment and Cognitively Normal Patients. J. Clin. Med. 2025, 14, 5261. [Google Scholar] [CrossRef]
- Sundaresan, V.; Arthofer, C.; Zamboni, G.; Murchison, A.G.; Dineen, R.A.; Rothwell, P.M.; Auer, D.P.; Wang, C.; Miller, K.L.; Tendler, B.C.; et al. Automated detection of cerebral microbleeds on MR images using knowledge distillation framework. Front. Neurosci. 2023, 17, 1204186. [Google Scholar] [CrossRef]
- Ali, Z.; Naz, S.; Yasmin, S.; Bukhari, M.; Kim, M. Deep learning-assisted IoMT framework for cerebral microbleed detection. Heliyon 2023, 9, e22879. [Google Scholar] [CrossRef]
- Smucny, J.; Shi, G.; Davidson, I. Deep Learning in Neuroimaging: Overcoming Challenges with Emerging Approaches. Front. Psychiatry 2022, 13, 912600. [Google Scholar] [CrossRef]
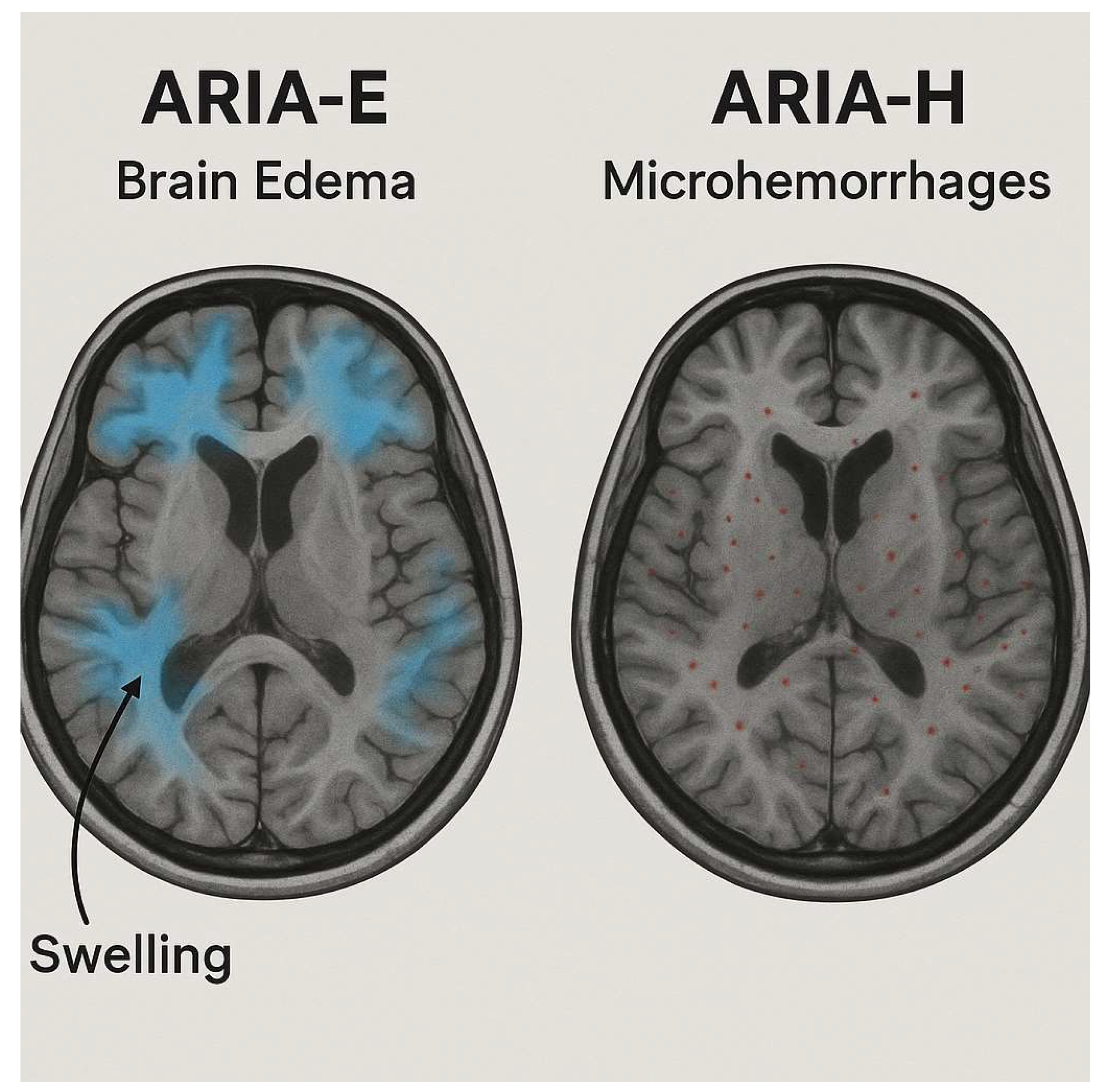

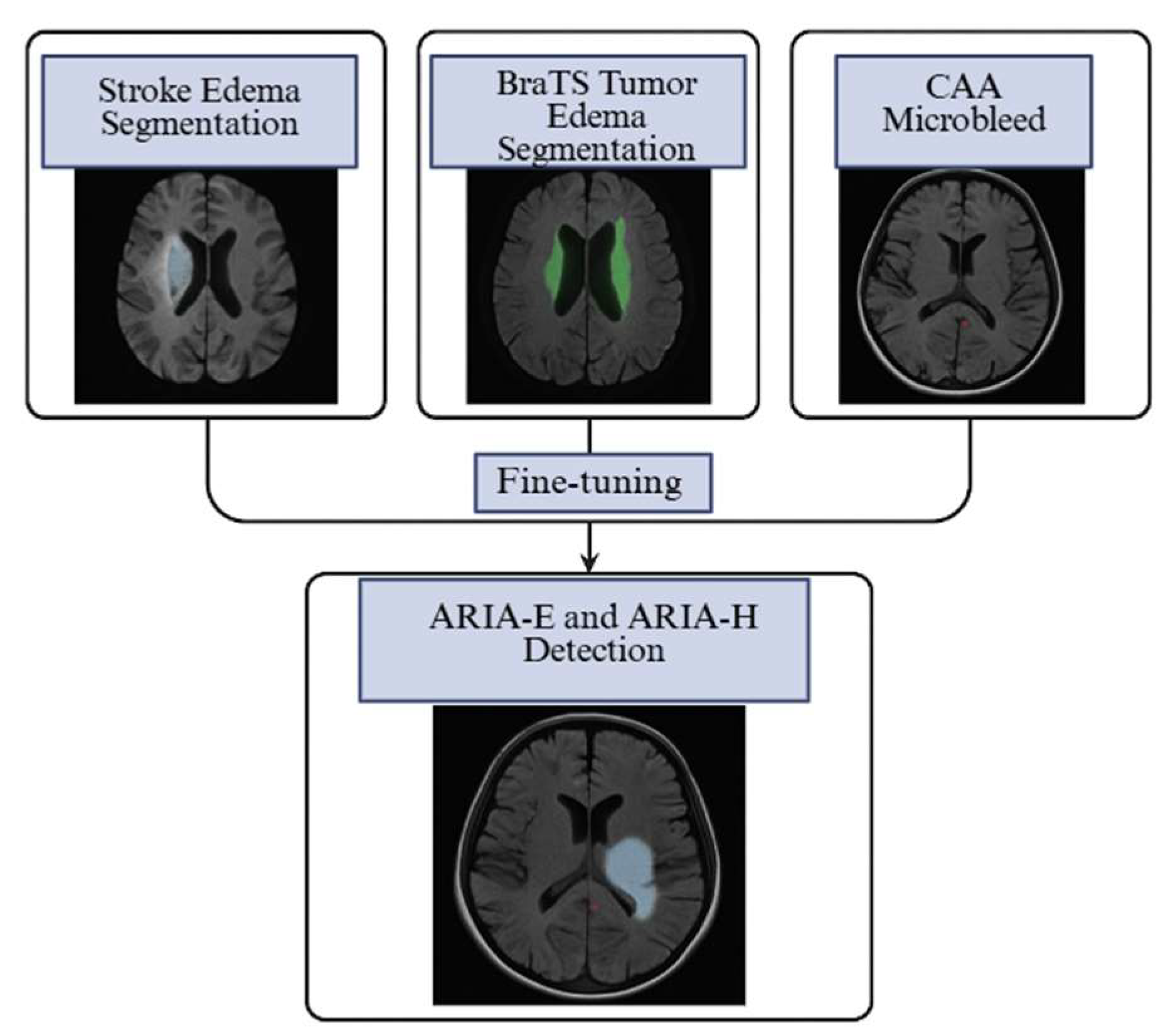
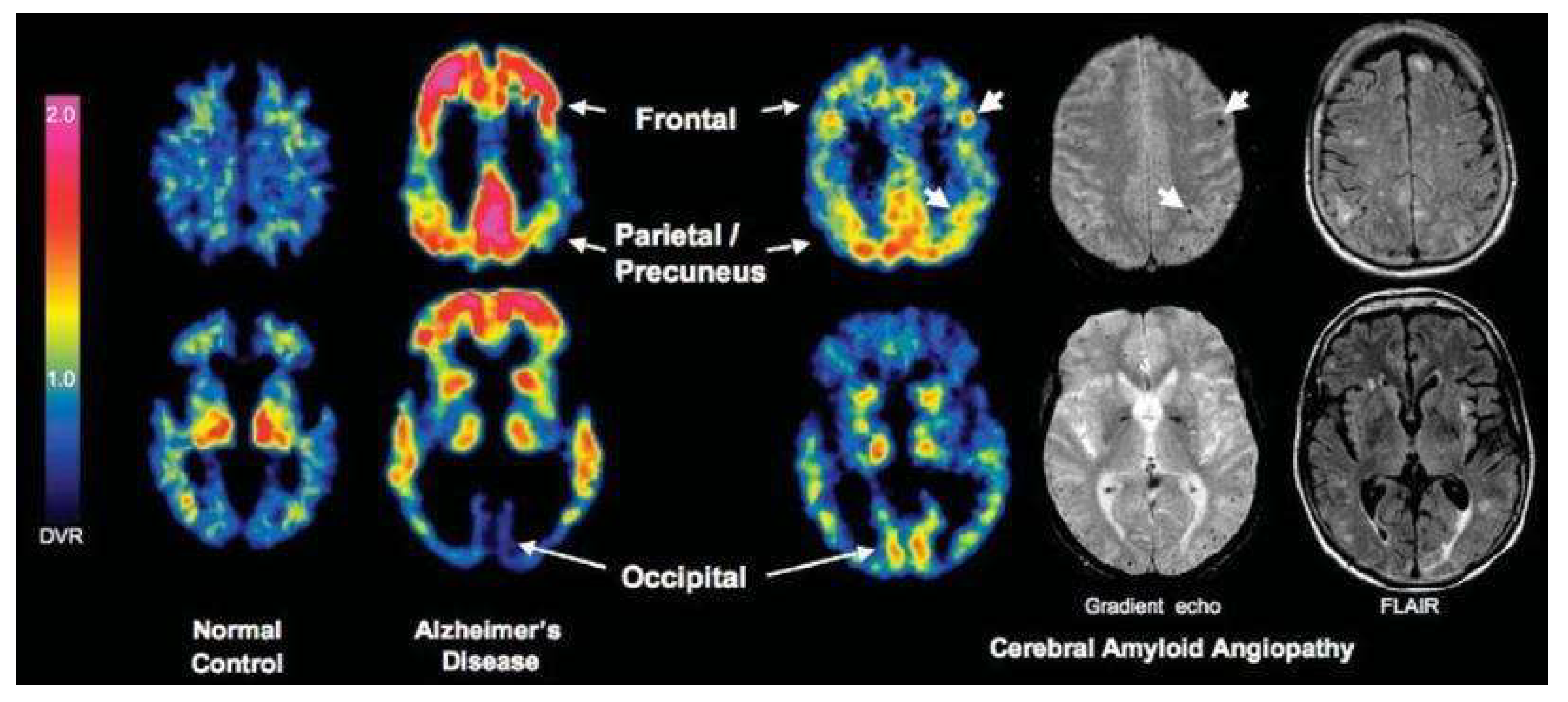
| Study | ARIA Type | Pathophysiology | Preferred MRI Sequence(s) | Typical Locations | Radiologic Signs | Trial-Based Definition (Examples) | Key Risk Factors | Key Clinical Impact | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Roytman et al., 2023 | ARIA-E (edema/effusion) | Vasogenic edema or sulcal effusion from increased vascular permeability after amyloid clearance | T2-FLAIR ± DWI (to exclude infarct) | Occipital and parietal lobes | Hyperintense cortical-subcortical signal, often asymmetric; may have mild mass effect | CLARITY-AD (2022): New or increased FLAIR hyperintensity consistent with vasogenic edema/sulcal effusion; TRAILBLAZER-ALZ2 (2023): Similar definitions, neuroradiologist adjudicated | APOE ε4 homozygosity, higher amyloid burden, high-dose regimens | May require temporary dose interruption or modification; linked to symptomatic confusion or headache in rare cases | [25] |
| Jeong et al., 2023 | ARIA-H (hemosiderin-related) | Microhemorrhages or superficial siderosis from vessel wall fragility after plaque clearance | SWI or GRE ± QSM (for quantification) | Cortical/subcortical regions, superficial sulci | Punctate hypointensities (microbleeds), linear cortical hypointensity (siderosis) | CLARITY-AD (2022): New micro/macrohemorrhages or superficial siderosis on SWI/GRE; TRAILBLAZER-ALZ2 (2023): Same lesion criteria, neuroradiologist adjudicated | APOE ε4, baseline microbleeds, anticoagulant use | Increases risk of symptomatic hemorrhage; may influence eligibility and anticoagulation management | [27] |
| Study | Source Domain/Pretrained Model | Target Task (ARIA-Related) | Imaging Modality | Transfer Learning Strategy | Reported Performance in Analogous Task | Potential Benefits for ARIA Detection | Limitations/Considerations | Reference |
|---|---|---|---|---|---|---|---|---|
| Isensee et al., 2021 | CNN models for acute stroke lesion segmentation (FLAIR/DWI) | ARIA-E segmentation | T2-FLAIR | Fine-tuning encoder–decoder weights on ARIA-E FLAIR data | Dice ≥ 0.85 in stroke edema segmentation | Captures vasogenic edema morphology; reduces need for large ARIA-E datasets | Edema appearance may differ in location and intensity between stroke and ARIA | [47] |
| Beheshti et al., 2025 | nnU-Net trained for peritumoral edema in brain tumors (BraTS challenge) | ARIA-E detection | T2-FLAIR | Full-network retraining with ARIA cases + augmentation | Top BraTS scores for peritumoral edema | Handles diffuse cortical-subcortical hyperintensities; strong generalization | Tumor edema more heterogeneous than ARIA-E; requires domain adaptation | [48] |
| Hsu et al., 2023 | SWI-based deep learning models for cerebral microbleed detection in CAA | ARIA-H microbleed detection | SWI/GRE | Transfer last layers; augment with ARIA-H SWI | Sensitivity 93–96%, FP ~1.5/case | Similar lesion morphology;robust small-lesion detection | Must adjust for distribution differences (location, number) between CAA and ARIA-H | [35] |
| Shafieioun et al., 2025 | Radiomics models predicting cerebral edema post-stroke | ARIA-E risk prediction | T2-FLAIR ± DWI | Feature selection + retraining classifier | AUC 0.94 for edema prediction | Integrates imaging and clinical features; interpretable | Requires harmonized imaging features; small ARIA datasets may limit stability | [22] |
| Yoon et al. (2018) | MRI-QSM enhancement models (QSMnet) trained on susceptibility mapping | ARIA-H quantification | SWI/QSM | Use as preprocessing stage for susceptibility normalization | Improved microbleed conspicuity in QSM | Reduces domain shift in susceptibility protocols | Needs QSM acquisition or synthetic generation for deployment | [38] |
| Drug | Trial | Dose Regimen | N (Treatment Arm) | ARIA-E Incidence | ARIA-H Incidence | Median Onset | Resolution Rate | Recurrence | Notes |
|---|---|---|---|---|---|---|---|---|---|
| Lecanemab | CLARITY-AD (2022) | 10 mg/kg biweekly | 898 | 12.6% | 17.3% | 3–6 months | Most resolved with monitoring | Rare | Higher rates in APOE ε4 carriers; MRI at 5, 7, 14 weeks recommended for high-risk patients |
| Donanemab | TRAILBLAZER-ALZ2 (2023) | Titration to high dose | 860 | 24% | 31% | 3–6 months | Most resolved | Rare | Modified titration in TRAILBLAZER-ALZ6 reduced ARIA-E while maintaining efficacy |
| Aducanumab | EMERGE/ENGAGE (2020) | 10 mg/kg high dose | 1105 combined High dose treatment group | Up to 35% | 15–20% | Mostly in first 8 doses | Most resolved | Uncommon | Discontinuation recommended for severe or symptomatic cases; careful monitoring in APOE ε4 carriers |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christodoulou, R.C.; Papageorgiou, P.S.; Sarquis, M.D.; Rivera, L.; Morales Gonzalez, C.; Eller, D.; Rivera, G.; Petrou, V.; Vamvouras, G.; Vassiliou, E.; et al. From Lesion to Decision: AI for ARIA Detection and Predictive Imaging in Alzheimer’s Disease. Biomedicines 2025, 13, 2739. https://doi.org/10.3390/biomedicines13112739
Christodoulou RC, Papageorgiou PS, Sarquis MD, Rivera L, Morales Gonzalez C, Eller D, Rivera G, Petrou V, Vamvouras G, Vassiliou E, et al. From Lesion to Decision: AI for ARIA Detection and Predictive Imaging in Alzheimer’s Disease. Biomedicines. 2025; 13(11):2739. https://doi.org/10.3390/biomedicines13112739
Chicago/Turabian StyleChristodoulou, Rafail C., Platon S. Papageorgiou, Maria Daniela Sarquis, Ludwing Rivera, Celimar Morales Gonzalez, Daniel Eller, Gipsany Rivera, Vasileia Petrou, Georgios Vamvouras, Evros Vassiliou, and et al. 2025. "From Lesion to Decision: AI for ARIA Detection and Predictive Imaging in Alzheimer’s Disease" Biomedicines 13, no. 11: 2739. https://doi.org/10.3390/biomedicines13112739
APA StyleChristodoulou, R. C., Papageorgiou, P. S., Sarquis, M. D., Rivera, L., Morales Gonzalez, C., Eller, D., Rivera, G., Petrou, V., Vamvouras, G., Vassiliou, E., Papageorgiou, S. G., & Georgiou, M. F. (2025). From Lesion to Decision: AI for ARIA Detection and Predictive Imaging in Alzheimer’s Disease. Biomedicines, 13(11), 2739. https://doi.org/10.3390/biomedicines13112739









