Immunohistochemical Expression of TNFR1, IL-6, and TGF-β1 in the Synovial Tissue of Patients with Hip Osteoarthritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Tissue Collection and Basic Staining Procedures
2.3. Immunofluorescence Staining
2.4. Data Acquisition and Quantitative Analysis
2.5. Differential Gene Expression
2.6. Statistical Analysis
3. Results
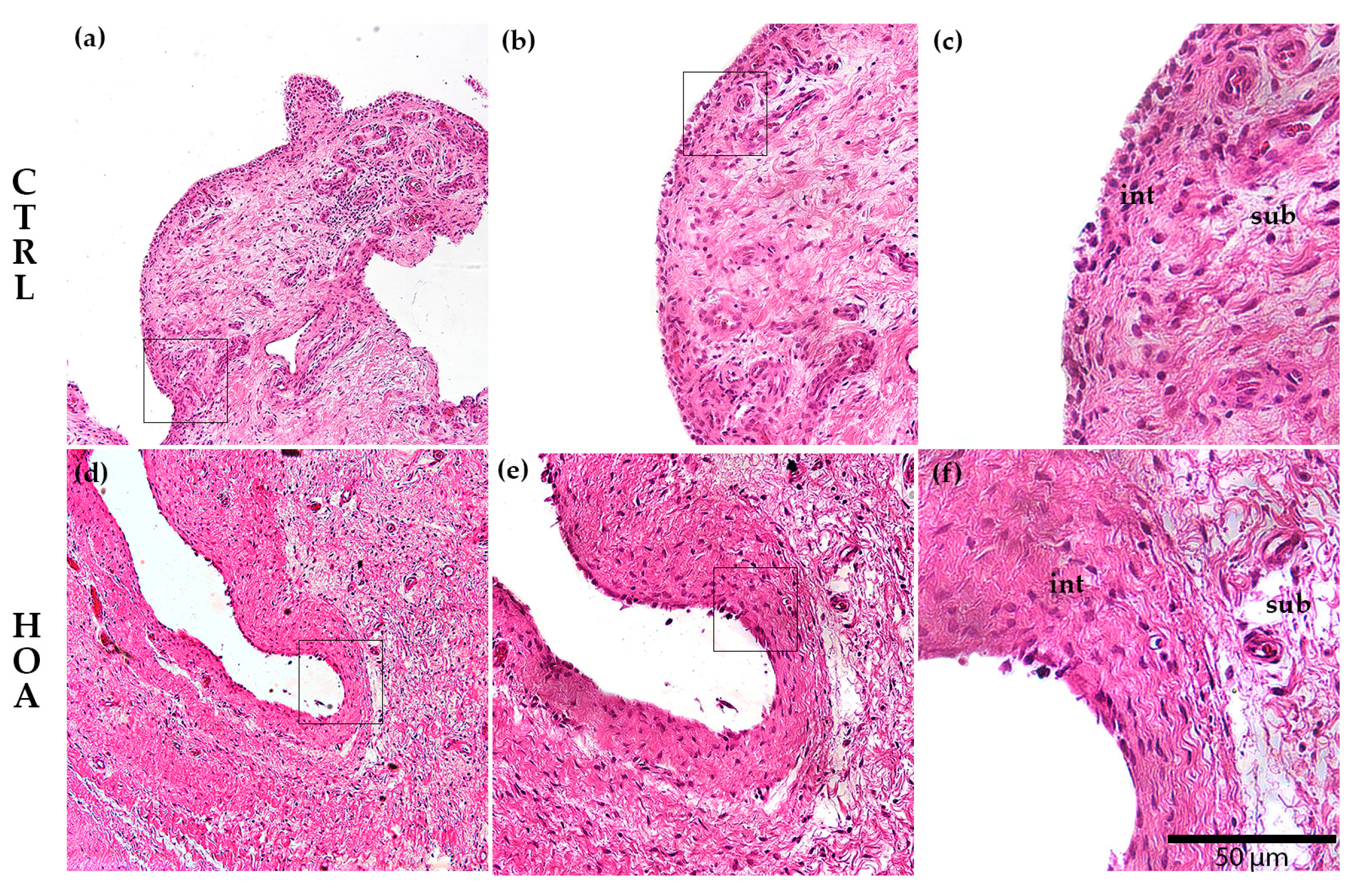
3.1. Hematoxylin and Eosin (H&E) Staining of the Synovial Membrane in Patients with Hip Osteoarthritis
3.2. TNFR1 Is Predominantly Upregulated in the Intima of Hip Osteoarthritis Synovium
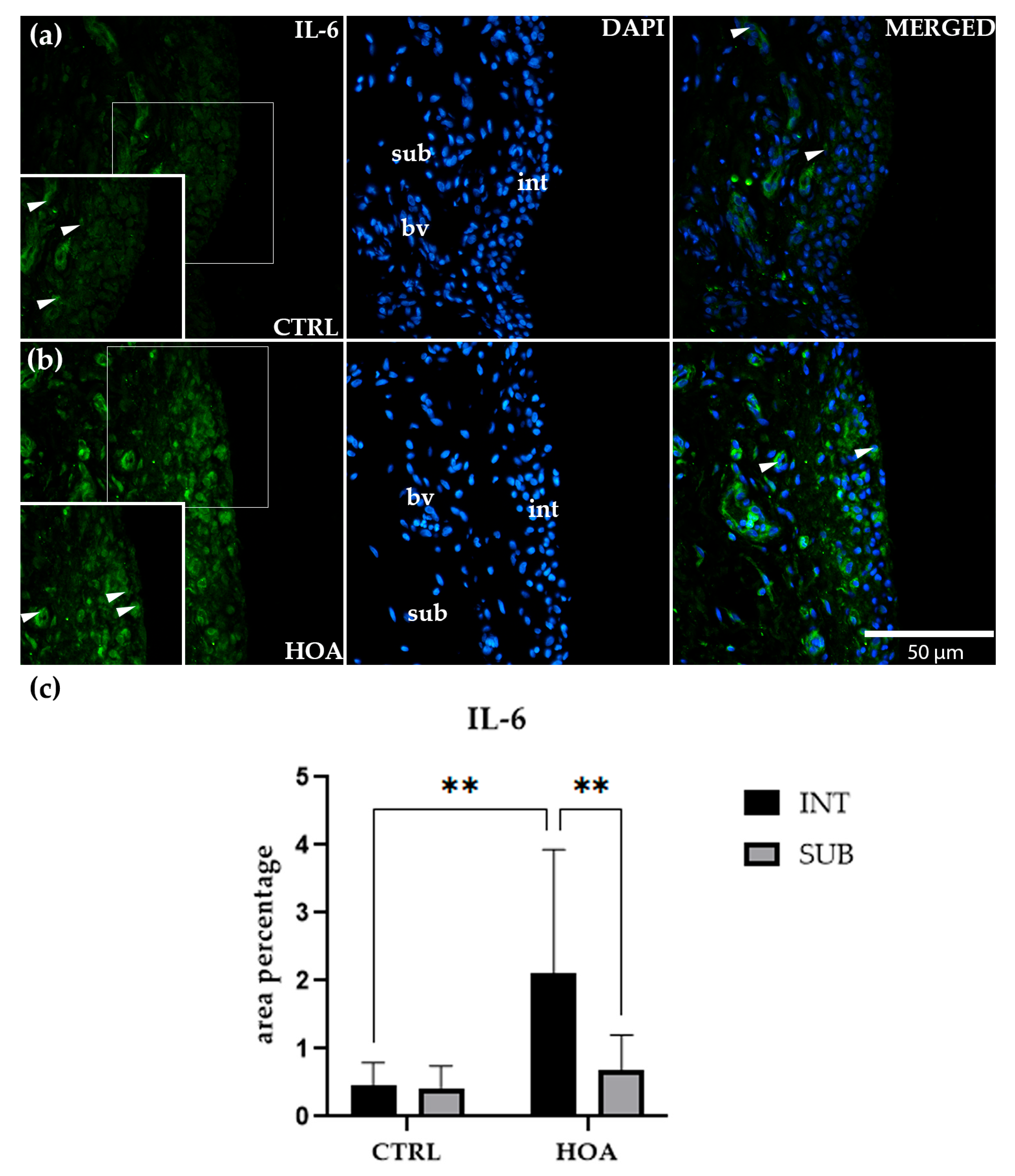
3.3. IL-6 Is Significantly Upregulated in the Intima of Hip Osteoarthritis Synovium
3.4. TGF-β1 Intimal Expression Is Reduced in Hip Osteoarthritis Compared with Controls
3.5. Transcriptomic Expression of IL-6, TNFR1, and TGF-β1 in Synovial Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TNFR1 | Tumor necrosis factor receptor 1 |
| IL-6 | Interleukin-6 |
| OA | Osteoarthritis |
| ANOVA | Analysis of variance |
| HOA | Hip osteoarthritis |
| TNF | Tumor necrosis factor |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| RA | Rheumatoid arthritis |
| JAK | Janus kinase |
| STAT | Signal transducer and activator of transcription |
| ECM | Extracellular matrix |
| SMAD2/3 | Mothers against decapentaplegic homologs 2 and 3 (canonical TGF-β pathway) |
| MAPK | Mitogen-activated protein kinase |
| SMAD1/5/9 | Mothers against decapentaplegic homologs 1, 5, and 9 (TGF-β pathway) |
| CCP | Cyclic citrullinated peptide |
| RF | Rheumatoid factor |
| HHS | Harris Hip Score |
| WOMAC | Western Ontario and McMaster Universities Osteoarthritis Index |
| VAS | Visual analogue scale |
| IQR | Interquartile range |
| BMI | Body mass index |
| BX | Olympus BX brightfield microscope |
| PBS | Phosphate-buffered saline |
| DAPI | 4′,6-diamidino-2-phenylindole |
| BX61 | Olympus BX61 fluorescence microscope |
| NIH | National Institutes of Health |
| SD | Standard deviation |
| GEO | Gene Expression Omnibus |
| NCBI | National Center for Biotechnology Information |
| GSE55235 | GEO dataset series 55235 |
| RNA | Ribonucleic acid |
| GEO2R | Gene Expression Omnibus online analysis tool 2R |
| FDR | False discovery rate |
| CTRL | Control |
| SAR44156 | TNFR1-selective inhibitor (drug candidate) |
| TNFR2 | Tumor necrosis factor receptor 2 |
| CD4 | Cluster of differentiation 4 |
| HLA-DRA | Human leukocyte antigen DR alpha |
| ACL | Anterior cruciate ligament |
| VEGF | Vascular endothelial growth factor |
| α-SMA | Alpha-smooth muscle actin |
| RUNX2 | Runt-related transcription factor 2 |
| COL10A1 | Collagen type X alpha 1 chain |
References
- Litwic, A.; Edwards, M.H.; Dennison, E.M.; Cooper, C. Epidemiology and Burden of Osteoarthritis. Br. Med. Bull. 2013, 105, 185–199. [Google Scholar] [CrossRef]
- He, Y.; Li, Z.; Alexander, P.G.; Ocasio-Nieves, B.D.; Yocum, L.; Lin, H.; Tuan, R.S. Pathogenesis of Osteoarthritis: Risk Factors, Regulatory Pathways in Chondrocytes, and Experimental Models. Biology 2020, 9, 1172–1183. [Google Scholar] [CrossRef]
- Courties, A.; Kouki, I.; Soliman, N.; Mathieu, S.; Sellam, J. Osteoarthritis Year in Review 2024: Epidemiology and Therapy. Osteoarthr. Cartil. 2024, 32, 1397–1404. [Google Scholar] [CrossRef]
- Long, H.; Liu, Q.; Yin, H.; Wang, K.; Diao, N.; Zhang, Y.; Lin, J.; Guo, A. Prevalence Trends of Site-Specific Osteoarthritis From 1990 to 2019: Findings from the Global Burden of Disease Study 2019. Arthritis Rheumatol. 2022, 74, 1172–1183. [Google Scholar] [CrossRef]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Casartelli, N.C.; Maffiuletti, N.A.; Valenzuela, P.L.; Grassi, A.; Ferrari, E.; van Buuren, M.M.A.; Nevitt, M.C.; Leunig, M.; Agricola, R. Is Hip Morphology a Risk Factor for Developing Hip Osteoarthritis? A Systematic Review with Meta-Analysis. Osteoarthr. Cartil. 2021, 29, 1252–1264. [Google Scholar] [CrossRef] [PubMed]
- Martel-Pelletier, J. Cytokines and Their Role in the Pathophysiology of Osteoarthritis. Front. Biosci. 1999, 4, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Mathiessen, A.; Conaghan, P.G. Synovitis in Osteoarthritis: Current Understanding with Therapeutic Implications. Arthritis Res. Ther. 2017, 19, 18. [Google Scholar] [CrossRef]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.P.; Fahmi, H. Role of Proinflammatory Cytokines in the Pathophysiology of Osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef]
- Wajant, H.; Scheurich, P. TNFR1-induced Activation of the Classical NF-κB Pathway. FEBS J. 2011, 278, 862–876. [Google Scholar] [CrossRef]
- Li, Y.; Ye, R.; Dai, H.; Lin, J.; Cheng, Y.; Zhou, Y.; Lu, Y. Exploring TNFR1: From Discovery to Targeted Therapy Development. J. Transl. Med. 2025, 23, 71. [Google Scholar] [CrossRef]
- Choi, M.C.; Jo, J.; Park, J.; Kang, H.K.; Park, Y. NF-Κb Signaling Pathways in Osteoarthritic Cartilage Destruction. Cells 2019, 8, 734. [Google Scholar] [CrossRef]
- Fazio, A.; Di Martino, A.; Brunello, M.; Traina, F.; Marvi, M.V.; Mazzotti, A.; Faldini, C.; Manzoli, L.; Evangelisti, C.; Ratti, S. The Involvement of Signaling Pathways in the Pathogenesis of Osteoarthritis: An Update. J. Orthop. Transl. 2024, 47, 116–124. [Google Scholar] [CrossRef]
- Huang, B.; Lang, X.; Li, X. The Role of IL-6/JAK2/STAT3 Signaling Pathway in Cancers. Front. Oncol. 2022, 12, 1023177. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, P.C.; Behrmann, I.; Müller-Newen, G.; Schaper, F.; Graeve, L. Interleukin-6-Type Cytokine Signalling through the Gp130/Jak/STAT Pathway. Biochem. J. 1998, 334, 297–314. [Google Scholar] [CrossRef]
- Wang, F.; Guo, Z.; Yuan, Y. STAT3 Speeds up Progression of Osteoarthritis through NF-κB Signaling Pathway. Exp. Ther. Med. 2020, 19, 722–728. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Thielen, N.; van der Kraan, P.; van Caam, A. TGFβ/BMP Signaling Pathway in Cartilage Homeostasis. Cells 2019, 8, 969. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Xu, L.; Li, Y.; Zhao, Z. Roles of TGF-Beta 1 Signaling in the Development of Osteoarthritis. Histol. Histopathol. 2016, 31, 1161–1167. [Google Scholar] [PubMed]
- Deng, Z.; Fan, T.; Xiao, C.; Tian, H.; Zheng, Y.; Li, C.; He, J. TGF-β Signaling in Health, Disease and Therapeutics. Signal Transduct. Target. Ther. 2024, 9, 61. [Google Scholar] [CrossRef]
- van der Kraan, P.M. Differential Role of Transforming Growth Factor-Beta in an Osteoarthritic or a Healthy Joint. J. Bone Metab. 2018, 25, 65. [Google Scholar] [CrossRef]
- Blaney Davidson, E.N.; van der Kraan, P.M.; van den Berg, W.B. TGF-β and Osteoarthritis. Osteoarthr. Cartil. 2007, 15, 597–604. [Google Scholar] [CrossRef]
- Kellgren, J.H.; Lawrence, J.S. Radiological Assessment of Osteo-Arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef]
- Harris, W.H. Traumatic Arthritis of the Hip after Dislocation and Acetabular Fractures: Treatment by Mold Arthroplasty. An End-Result Study Using a New Method of Result Evaluation. J. Bone Jt. Surg. Am. 1969, 51, 737–755. [Google Scholar] [CrossRef]
- Bellamy, N.; Buchanan, W.W.; Goldsmith, C.H.; Campbell, J.; Stitt, L.W. Validation Study of WOMAC: A Health Status Instrument for Measuring Clinically Important Patient Relevant Outcomes to Antirheumatic Drug Therapy in Patients with Osteoarthritis of the Hip or Knee. J. Rheumatol. 1988, 15, 1833–1840. [Google Scholar]
- Huskisson, E.C. Measurement of Pain. Lancet 1974, 304, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- Krenn, V.; Morawietz, L.; Burmester, G.; Kinne, R.W.; Mueller-Ladner, U.; Muller, B.; Haupl, T. Synovitis Score: Discrimination between Chronic Low-grade and High-grade Synovitis. Histopathology 2006, 49, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Pavic, B.; Ogorevc, M.; Boric, K.; Vukovic, D.; Saraga-Babic, M.; Mardesic, S. Connexin 37, 40, 43 and Pannexin 1 Expression in the Gastric Mucosa of Patients with Systemic Sclerosis. Biomedicines 2023, 11, 2487. [Google Scholar] [CrossRef]
- Komić, J.; Kelam, N.; Racetin, A.; Filipović, N.; Saraga-Babić, M.; Ihara, D.; Katsuyama, Y.; Vukojević, K. Spatial and Temporal Expression Patterns of EDA2R, PCDH9, and TRAF7 in Yotari (Dab1−/−) Mice: Implicationsfor Understanding CAKUT Pathogenesis. Int. J. Mol. Sci. 2025, 26, 6421. [Google Scholar] [CrossRef]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for Functional Genomics Data Sets—Update. Nucleic Acids Res. 2012, 41, D991–D995. [Google Scholar] [CrossRef] [PubMed]
- Woetzel, D.; Huber, R.; Kupfer, P.; Pohlers, D.; Pfaff, M.; Driesch, D.; Häupl, T.; Koczan, D.; Stiehl, P.; Guthke, R.; et al. Identification of Rheumatoid Arthritis and Osteoarthritis Patients by Transcriptome-Based Rule Set Generation. Arthritis Res. Ther. 2014, 16, R84. [Google Scholar] [CrossRef]
- Goldring, M.B.; Otero, M. Inflammation in Osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 471–478. [Google Scholar] [CrossRef]
- Benito, M.J.; Veale, D.J.; FitzGerald, O.; van den Berg, W.B.; Bresnihan, B. Synovial Tissue Inflammation in Early and Late Osteoarthritis. Ann. Rheum. Dis. 2005, 64, 1263–1267. [Google Scholar] [CrossRef]
- Scanzello, C.R.; Goldring, S.R. The Role of Synovitis in Osteoarthritis Pathogenesis. Bone 2012, 51, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Arntz, O.J.; Geurts, J.; Veenbergen, S.; Bennink, M.B.; van den Brand, B.T.; Abdollahi-Roodsaz, S.; van den Berg, W.B.; van de Loo, F.A. A Crucial Role for Tumor Necrosis Factor Receptor 1 in Synovial Lining Cells and the Reticuloendothelial System in Mediating Experimental Arthritis. Arthritis Res. Ther. 2010, 12, R61. [Google Scholar] [CrossRef] [PubMed]
- Semenistaja, S.; Sokolovska, L.; Svirskis, S.; Studers, P.; Groma, V.; Skuja, S. Distinct Late-Stage Osteoarthritis Profiles Identified through NF-ΚB, TNF-α, and TGF-β-Driven Synovial Inflammation and Pain. Sci. Rep. 2025, 15, 30288. [Google Scholar] [CrossRef]
- Chou, C.-H.; Jain, V.; Gibson, J.; Attarian, D.E.; Haraden, C.A.; Yohn, C.B.; Laberge, R.-M.; Gregory, S.; Kraus, V.B. Synovial Cell Cross-Talk with Cartilage Plays a Major Role in the Pathogenesis of Osteoarthritis. Sci. Rep. 2020, 10, 10868. [Google Scholar] [CrossRef] [PubMed]
- Webb, G.R.; Westacott, C.I.; Elson, C.J. Osteoarthritic Synovial Fluid and Synovium Supernatants Up-Regulate Tumor Necrosis Factor Receptors on Human Articular Chondrocytes. Osteoarthr. Cartil. 1998, 6, 167–176. [Google Scholar] [CrossRef][Green Version]
- Yu, H.; Li, M.; Wen, X.; Yang, J.; Liang, X.; Li, X.; Bao, X.; Shu, J.; Ren, X.; Chen, W.; et al. Elevation of α-1,3 Fucosylation Promotes the Binding Ability of TNFR1 to TNF-α and Contributes to Osteoarthritic Cartilage Destruction and Apoptosis. Arthritis Res. Ther. 2022, 24, 93. [Google Scholar] [CrossRef]
- Zhang, L.; Xing, R.; Huang, Z.; Ding, L.; Zhang, L.; Li, M.; Li, X.; Wang, P.; Mao, J. Synovial Fibrosis Involvement in Osteoarthritis. Front. Med. 2021, 8, 684389. [Google Scholar] [CrossRef]
- Stannus, O.; Jones, G.; Cicuttini, F.; Parameswaran, V.; Quinn, S.; Burgess, J.; Ding, C. Circulating Levels of IL-6 and TNF-α Are Associated with Knee Radiographic Osteoarthritis and Knee Cartilage Loss in Older Adults. Osteoarthr. Cartil. 2010, 18, 1441–1447. [Google Scholar] [CrossRef]
- Sullivan, B.; Stone, A.V.; Conley, C.E.W.; Hunt, E.R.; Lattermann, C.; Jacobs, C.A. Human Synovial Fluid Interleukin-6, but Not Type II Collagen Breakdown, Positively Correlated with Pain after Anterior Cruciate Ligament Injury and Reconstruction. J. Orthop. Res. 2023, 41, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Lambert, C.; Mathy-Hartert, M.; Dubuc, J.-E.; Montell, E.; Vergés, J.; Munaut, C.; Noël, A.; Henrotin, Y. Characterization of Synovial Angiogenesis in Osteoarthritis Patients and Its Modulation by Chondroitin Sulfate. Arthritis Res. Ther. 2012, 14, R58. [Google Scholar] [CrossRef] [PubMed]
- Muratovic, D.; Findlay, D.M.; Quarrington, R.D.; Cao, X.; Solomon, L.B.; Atkins, G.J.; Kuliwaba, J.S. Elevated Levels of Active Transforming Growth Factor Β1 in the Subchondral Bone Relate Spatially to Cartilage Loss and Impaired Bone Quality in Human Knee Osteoarthritis. Osteoarthr. Cartil. 2022, 30, 896–907. [Google Scholar] [CrossRef]
- Ciregia, F.; Deroyer, C.; Cobraiville, G.; Plener, Z.; Malaise, O.; Gillet, P.; Fillet, M.; Malaise, M.G.; de Seny, D. Modulation of AVβ6 Integrin in Osteoarthritis-Related Synovitis and the Interaction with VTN(381–397 a.a.) Competing for TGF-Β1 Activation. Exp. Mol. Med. 2021, 53, 210–222. [Google Scholar] [CrossRef]
- Thielen, N.G.M.; van Caam, A.P.M.; Beuningen, H.M.; Vitters, E.L.; van den Bosch, M.H.J.; Koenders, M.I.; van de Loo, F.A.J.; Blaney Davidson, E.N.; van der Kraan, P.M. Separating Friend from Foe: Inhibition of TGF-β-Induced Detrimental SMAD1/5/9 Phosphorylation While Maintaining Protective SMAD2/3 Signaling in OA Chondrocytes. Osteoarthr. Cartil. 2023, 31, 1481–1490. [Google Scholar] [CrossRef]
- Scharstuhl, A.; Glansbeek, H.L.; van Beuningen, H.M.; Vitters, E.L.; van der Kraan, P.M.; van den Berg, W.B. Inhibition of Endogenous TGF-β During Experimental Osteoarthritis Prevents Osteophyte Formation and Impairs Cartilage Repair. J. Immunol. 2002, 169, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Tasende, J.A.; Fernandez-Moreno, M.; Rego Perez, I.; Fernandez-Lopez, J.C.; Oreiro-Villar, N.; De Toro Santos, F.J.; Blanco-García, F.J. Higher Synovial Immunohistochemistry Reactivity of IL-17A, Dkk1, and TGF-Β1 in Patients with Early Psoriatic Arthritis and Rheumatoid Arthritis Could Predict the Use of Biologics. Biomedicines 2024, 12, 815. [Google Scholar] [CrossRef]




| Characteristic | Controls (n = 10) | HOA Krenn 0–2 (n = 10) | HOA Krenn ≥ 3 (n = 9) | p-Value |
|---|---|---|---|---|
| Age (years), median (IQR) | 74 (73.6–76.1) | 73 (63.7–75.9) | 73 (66–78) | 0.854 |
| Sex (Male/Female) | 6/4 | 6/4 | 5/4 | 0.732 |
| BMI (kg/m2), median (IQR) | 25.9 (24.0–26.6) | 24.7 (23.3–25.8) | 26.7 (25.5–29.4) | 0.054 |
| K–L grade, median (IQR) | 0.5 (0–1) | 2 (2–2) | 4 (3–4) | <0.0001 |
| Krenn synovitis score, median (IQR) | 0 (0–0) | 6.4 (5.6–9.0) | 9 (7–9) | <0.0001 |
| Harris Hip Score (HHS) | – | 48.7 (43.5–56.8) | 41 (33.5–49.6) | * |
| VAS (0–10) | – | 6 (4.6–6.8) | 6 (5–7) | * |
| WOMAC | – | 46.2 (40.2–56.4) | 47.3 (36.1–55.3) | * |
| Antibody Type | Antibody | Host | Dilution | Source |
|---|---|---|---|---|
| Primary | Anti-TNFR1/CD120a (21574-1-AP) | Rabbit | 1:300 | Proteintech, Rosemont, IL, USA |
| Anti-IL-6 (66146-1-Ig) | Mouse | 1:200 | ||
| Anti-TGF-β1 (21898-1-AP) | Rabbit | 1:300 | ||
| Secondary | Alexa Fluor® 488 Anti-Rabbit IgG (711-545-152) Alexa Fluor® 488 AffiniPure® Donkey Anti-Mouse IgG (H+L) (715-545-150) | Donkey | 1:300 | Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA, USA) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todorović, P.; Jurić, I.; Kelam, N.; Rošin, M.; Čarić, D.; Boban, D.; Kopilaš, A.; Vukojević, K. Immunohistochemical Expression of TNFR1, IL-6, and TGF-β1 in the Synovial Tissue of Patients with Hip Osteoarthritis. Biomedicines 2025, 13, 2732. https://doi.org/10.3390/biomedicines13112732
Todorović P, Jurić I, Kelam N, Rošin M, Čarić D, Boban D, Kopilaš A, Vukojević K. Immunohistochemical Expression of TNFR1, IL-6, and TGF-β1 in the Synovial Tissue of Patients with Hip Osteoarthritis. Biomedicines. 2025; 13(11):2732. https://doi.org/10.3390/biomedicines13112732
Chicago/Turabian StyleTodorović, Petar, Ivana Jurić, Nela Kelam, Matko Rošin, Davor Čarić, Danica Boban, Andrea Kopilaš, and Katarina Vukojević. 2025. "Immunohistochemical Expression of TNFR1, IL-6, and TGF-β1 in the Synovial Tissue of Patients with Hip Osteoarthritis" Biomedicines 13, no. 11: 2732. https://doi.org/10.3390/biomedicines13112732
APA StyleTodorović, P., Jurić, I., Kelam, N., Rošin, M., Čarić, D., Boban, D., Kopilaš, A., & Vukojević, K. (2025). Immunohistochemical Expression of TNFR1, IL-6, and TGF-β1 in the Synovial Tissue of Patients with Hip Osteoarthritis. Biomedicines, 13(11), 2732. https://doi.org/10.3390/biomedicines13112732









