Genetic Insights into Peripheral Artery Disease: A Narrative Review
Abstract
1. Introduction
2. Management of PAD in ESC 2024 Guidelines
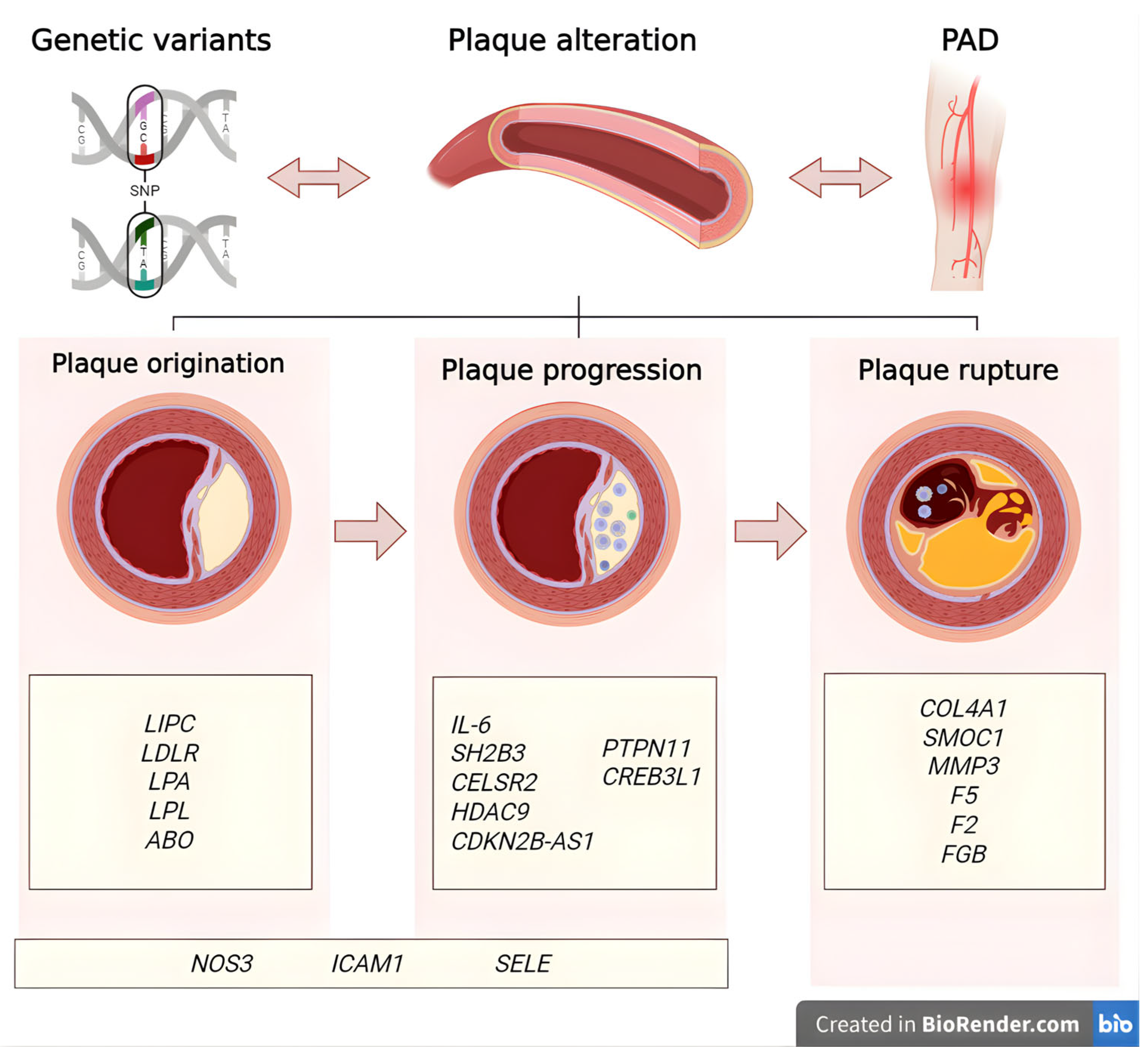
3. PAD Genetic Factors
3.1. Variants Associated with Plaque Origination in PAD
3.1.1. NOS3
3.1.2. ICAM1
3.1.3. SELE
3.1.4. LIPC
3.1.5. LDLR
3.1.6. LPA
3.1.7. LPL
3.1.8. ABO
3.2. Variants Associated with Plaque Progression in PAD
3.2.1. IL-6
3.2.2. SH2B3
3.2.3. CELSR2
3.2.4. HDAC9
3.2.5. CDKN2B-AS1
3.2.6. PTPN11
3.2.7. CREB3L1
3.3. Variants Associated with Plaque Rupture in PAD
3.3.1. COL4A1
3.3.2. SMOC1
3.3.3. MMP3
3.3.4. F5
3.3.5. F2
3.3.6. FGB
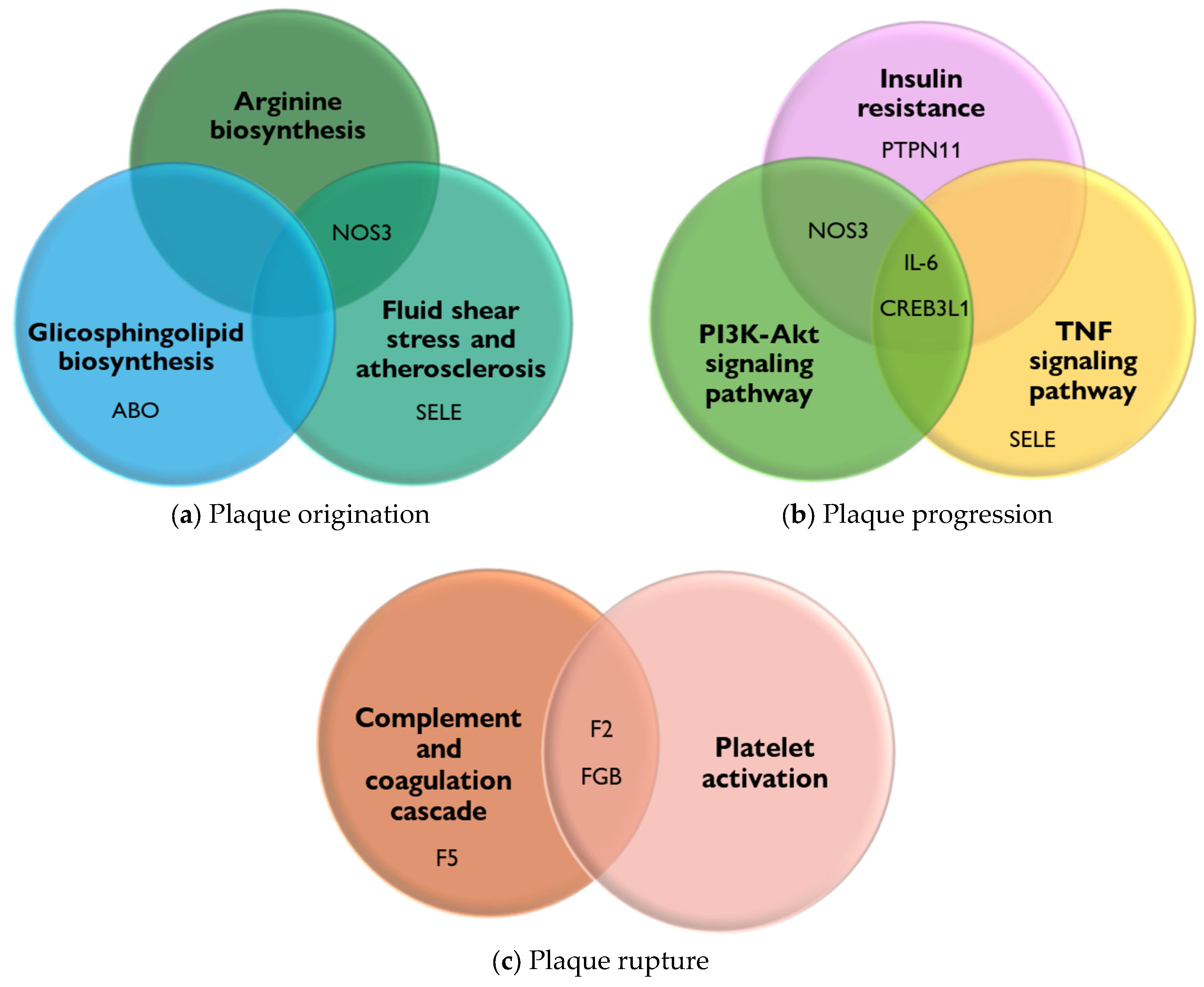
- (1).
- (2).
- The biosynthesis of glycosphingolipids and their metabolites, when altered, can lead to their accumulation in tissues. This accumulation has been linked to the development and progression of PAD. It is suggested that alterations in sphingolipid metabolism contribute to the cellular and tissue damage that occurs during the atherosclerotic process [58].
- (3).
- Fluid shear stress and atherosclerosis, due to the force exerted by the constant flow of blood on the walls of blood vessels, play an important role in atherogenesis by altering the integrity of the endothelium, increasing its permeability, and allowing the entry of lipoproteins and inflammatory cells, which initiates the process of plaque formation and thrombi in the arteries and causes PAD [59].
- (1).
- The PI3K-Akt signaling pathway is involved in regulating inflammation and oxidative stress, both of which are key factors in the pathogenesis of PAD [60].
- (2).
- The TNF signaling pathway promotes oxidative stress and decreases the bioavailability of NO, a crucial vasodilator, contributing to endothelial dysfunction. Furthermore, the TNF signaling pathway impacts vascular remodeling processes, leading to structural changes in the arteries of patients [61].
- (3).
- The insulin resistance pathway causes vascular damage through endothelial dysfunction via the inhibition of NO production. In addition, insulin resistance can activate pro-inflammatory molecular pathways, such as the MAP kinase (MAPK) pathway, which contribute to the disease [62].
4. Discussion
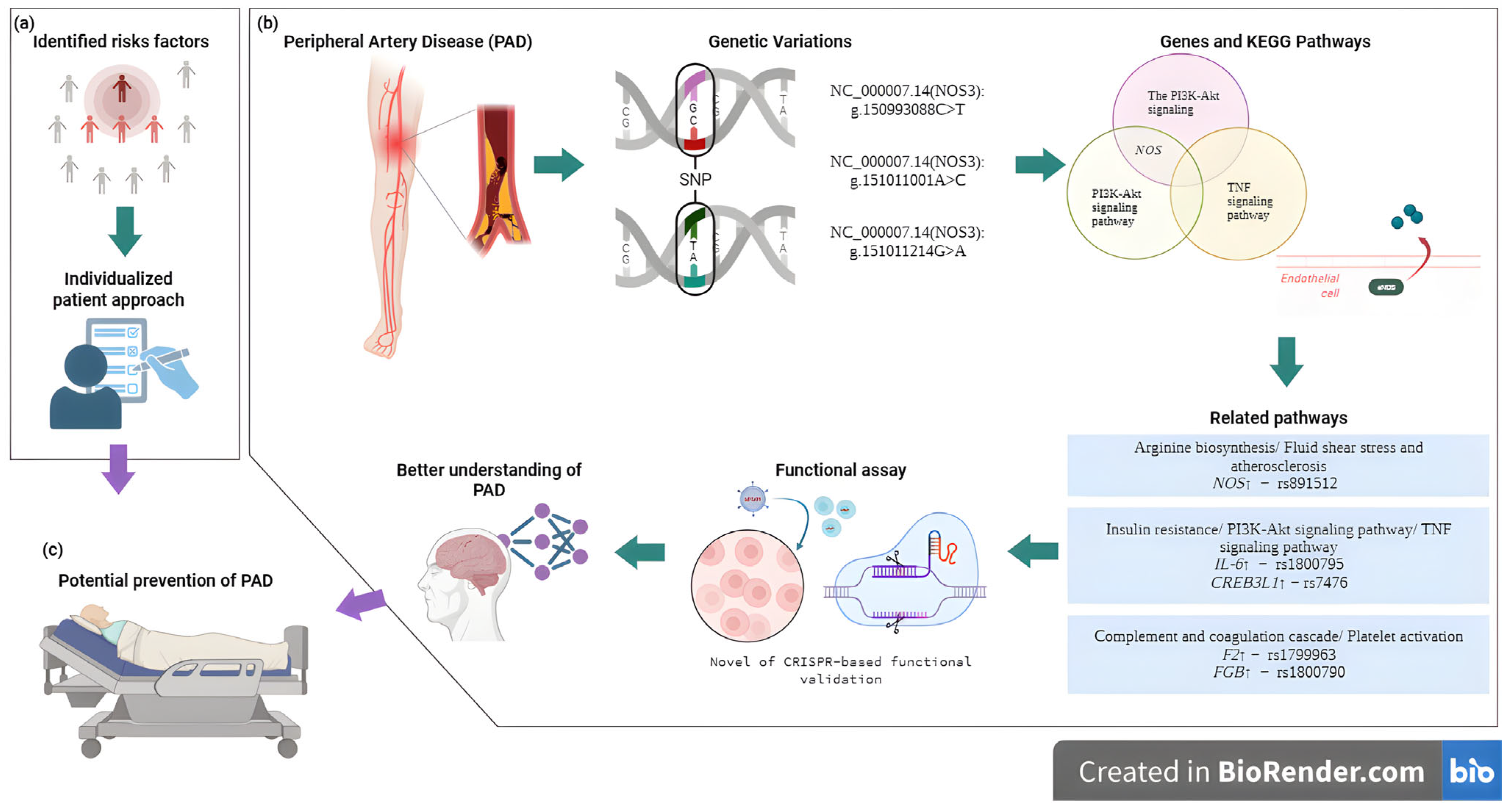
5. Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Treat-Jacobson, D.; McDermott, M.M.; Bronas, U.G.; Campia, U.; Collins, T.C.; Criqui, M.H.; Gardner, A.W.; Hiatt, W.R.; Regensteiner, J.G.; Rich, K.; et al. Optimal Exercise Programs for Patients with Peripheral Artery Disease: A Scientific Statement from the American Heart Association. Circulation 2019, 139, e10–e33. [Google Scholar] [CrossRef]
- Mazzolai, L.; Teixido-Tura, G.; Lanzi, S.; Boc, V.; Bossone, E.; Brodmann, M.; Bura-Rivière, A.; De Backer, J.; Deglise, S.; Della Corte, A.; et al. 2024 ESC Guidelines for the Management of Peripheral Arterial and Aortic Diseases. Eur. Heart J. 2024, 45, 3538–3700. [Google Scholar] [CrossRef]
- Conte, M.S.; Bradbury, A.W.; Kolh, P.; White, J.V.; Dick, F.; Fitridge, R.; Mills, J.L.; Ricco, J.-B.; Suresh, K.R.; Murad, M.H.; et al. Global Vascular Guidelines on the Management of Chronic Limb-Threatening Ischemia. J. Vasc. Surg. 2019, 69, 3S–125S.e40. [Google Scholar] [CrossRef]
- Campia, U.; Gerhard-Herman, M.; Piazza, G.; Goldhaber, S.Z. Peripheral Artery Disease: Past, Present, and Future. Am. J. Med. 2019, 132, 1133–1141. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primer 2019, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Gornik, H.L.; Aronow, H.D.; Goodney, P.P.; Arya, S.; Brewster, L.P.; Byrd, L.; Chandra, V.; Drachman, D.E.; Eaves, J.M.; Ehrman, J.K.; et al. 2024 ACC/AHA/AACVPR/APMA/ABC/SCAI/SVM/SVN/SVS/SIR/VESS Guideline for the Management of Lower Extremity Peripheral Artery Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1313–e1410. [Google Scholar] [CrossRef] [PubMed]
- Klarin, D.; Tsao, P.S.; Damrauer, S.M. Genetic Determinants of Peripheral Artery Disease. Circ. Res. 2021, 128, 1805–1817. [Google Scholar] [CrossRef]
- Erzinger, F.L.; Polimanti, A.C.; Pinto, D.M.; Murta, G.; Cury, M.V.; da Silva, R.B.; Biagioni, R.B.; Belckzac, S.Q.; Joviliano, E.E.; de Araujo, W.J.B.; et al. Brazilian Society of Angiology and Vascular Surgery Guidelines on Peripheral Artery Disease. J. Vasc. Bras. 2024, 23, e20230059. [Google Scholar] [CrossRef]
- Bonaca, M.P.; Hamburg, N.M.; Creager, M.A. Contemporary Medical Management of Peripheral Artery Disease. Circ. Res. 2021, 128, 1868–1884. [Google Scholar] [CrossRef] [PubMed]
- Sommerset, J.; Karmy-Jones, R.; Dally, M.; Feliciano, B.; Vea, Y.; Teso, D. Plantar Acceleration Time: A Novel Technique to Evaluate Arterial Flow to the Foot. Ann. Vasc. Surg. 2019, 60, 308–314. [Google Scholar] [CrossRef]
- Horváth, L.; Németh, N.; Fehér, G.; Kívés, Z.; Endrei, D.; Boncz, I. Epidemiology of Peripheral Artery Disease: Narrative Review. Life 2022, 12, 1041. [Google Scholar] [CrossRef]
- Kullo, I.J.; Leeper, N.J. The Genetic Basis of Peripheral Arterial Disease: Current Knowledge, Challenges, and Future Directions. Circ. Res. 2015, 116, 1551–1560. [Google Scholar] [CrossRef]
- Trudsø, L.C.; Andersen, J.D.; Jacobsen, S.B.; Christiansen, S.L.; Congost-Teixidor, C.; Kampmann, M.-L.; Morling, N. A Comparative Study of Single Nucleotide Variant Detection Performance Using Three Massively Parallel Sequencing Methods. PLoS ONE 2020, 15, e0239850. [Google Scholar] [CrossRef]
- Tam, V.; Patel, N.; Turcotte, M.; Bossé, Y.; Paré, G.; Meyre, D. Benefits and Limitations of Genome-Wide Association Studies. Nat. Rev. Genet. 2019, 20, 467–484. [Google Scholar] [CrossRef] [PubMed]
- Uffelmann, E.; Huang, Q.Q.; Munung, N.S.; de Vries, J.; Okada, Y.; Martin, A.R.; Martin, H.C.; Lappalainen, T.; Posthuma, D. Genome-Wide Association Studies. Nat. Rev. Methods Primer 2021, 1, 59. [Google Scholar] [CrossRef]
- Zschocke, J.; Byers, P.H.; Wilkie, A.O.M. Mendelian Inheritance Revisited: Dominance and Recessiveness in Medical Genetics. Nat. Rev. Genet. 2023, 24, 442–463. [Google Scholar] [CrossRef]
- Tomaiuolo, R. Genetics and Genomics of Reproductive Medicine. Genes 2021, 12, 1612. [Google Scholar] [CrossRef] [PubMed]
- David, S. A Current Guide to Candidate Gene Association Studies. Trends Genet. TIG 2021, 37, 1056–1059. [Google Scholar] [CrossRef] [PubMed]
- Klarin, D.; Lynch, J.; Aragam, K.; Chaffin, M.; Assimes, T.L.; Huang, J.; Lee, K.M.; Shao, Q.; Huffman, J.E.; Natarajan, P.; et al. Genome-Wide Association Study of Peripheral Artery Disease in the Million Veteran Program. Nat. Med. 2019, 25, 1274–1279. [Google Scholar] [CrossRef]
- Birney, E. The International Human Genome Project. Hum. Mol. Genet. 2021, 30, R161–R163. [Google Scholar] [CrossRef]
- Belsare, S.; Levy-Sakin, M.; Mostovoy, Y.; Durinck, S.; Chaudhuri, S.; Xiao, M.; Peterson, A.S.; Kwok, P.-Y.; Seshagiri, S.; Wall, J.D. Evaluating the Quality of the 1000 Genomes Project Data. BMC Genom. 2019, 20, 620. [Google Scholar] [CrossRef] [PubMed]
- Byrska-Bishop, M.; Evani, U.S.; Zhao, X.; Basile, A.O.; Abel, H.J.; Regier, A.A.; Corvelo, A.; Clarke, W.E.; Musunuri, R.; Nagulapalli, K.; et al. High-Coverage Whole-Genome Sequencing of the Expanded 1000 Genomes Project Cohort Including 602 Trios. Cell 2022, 185, 3426–3440.e19. [Google Scholar] [CrossRef] [PubMed]
- Leeper, N.J.; Kullo, I.J.; Cooke, J.P. Genetics of Peripheral Artery Disease. Circulation 2012, 125, 3220–3228. [Google Scholar] [CrossRef]
- Villamil, C. Created in BioRender. 2025. Available online: https://app.biorender.com/illustrations/6368c2ffb888492c0c82d0c8 (accessed on 30 May 2025).
- Adams, J.A.; Uryash, A.; Lopez, J.R.; Sackner, M.A. The Endothelium as a Therapeutic Target in Diabetes: A Narrative Review and Perspective. Front. Physiol. 2021, 12, 638491. [Google Scholar] [CrossRef]
- Bartoli-Leonard, F.; Zimmer, J.; Sonawane, A.R.; Perez, K.; Turner, M.E.; Kuraoka, S.; Pham, T.; Li, F.; Aikawa, M.; Singh, S.; et al. NLRP3 Inflammasome Activation in Peripheral Arterial Disease. J. Am. Heart Assoc. 2023, 12, e026945. [Google Scholar] [CrossRef]
- Aimo, A.; Botto, N.; Vittorini, S.; Emdin, M. Polymorphisms in the eNOS Gene and the Risk of Coronary Artery Disease: Making the Case for Genome-Wide Association Studies. Eur. J. Prev. Cardiol. 2019, 26, 157–159. [Google Scholar] [CrossRef]
- Trapé, Á.A.; Rodrigues, J.A.L.; Ferezin, L.P.; Ferrari, G.D.; Lizzi, E.A.d.S.; de Moraes, V.N.; da Silva, R.F.; Zago, A.S.; Brazo-Sayavera, J.; Bueno Júnior, C.R. NOS3 Polymorphisms Can Influence the Effect of Multicomponent Training on Blood Pressure, Nitrite Concentration and Physical Fitness in Prehypertensive and Hypertensive Older Adult Women. Front. Physiol. 2021, 12, 566023. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, S.; Zhu, Z.; Gatt, A.; Liu, J. E-Selectin in Vascular Pathophysiology. Front. Immunol. 2024, 15, 1401399. [Google Scholar] [CrossRef]
- Ochoa Chaar, C.I.; Kim, T.; Alameddine, D.; DeWan, A.; Guzman, R.; Dardik, A.; Grossetta Nardini, H.K.; Wallach, J.D.; Kullo, I.; Murray, M. Systematic Review and Meta-Analysis of the Genetics of Peripheral Arterial Disease. JVS-Vasc. Sci. 2024, 5, 100133. [Google Scholar] [CrossRef]
- Guerra-García, M.T.; Moreno-Macías, H.; Ochoa-Guzmán, A.; Ordoñez-Sánchez, M.L.; Rodríguez-Guillen, R.; Vázquez-Cárdenas, P.; Ortíz-Ortega, V.M.; Peimbert-Torres, M.; Aguilar-Salinas, C.A.; Tusié-Luna, M.T. The -514C>T Polymorphism in the LIPC Gene Modifies Type 2 Diabetes Risk through Modulation of HDL-Cholesterol Levels in Mexicans. J. Endocrinol. Investig. 2021, 44, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Braun, V.; Hantke, K. Lipoproteins: Structure, Function, Biosynthesis. Subcell. Biochem. 2019, 92, 39–77. [Google Scholar] [CrossRef] [PubMed]
- Porntadavity, S.; Jeenduang, N. Structure-Function Relationships of LDL Receptor Missense Mutations Using Homology Modeling. Protein J. 2019, 38, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Poredoš, P.; Šabovič, M.; Božič Mijovski, M.; Nikolajević, J.; Antignani, P.L.; Paraskevas, K.I.; Mikhailidis, D.P.; Blinc, A. Inflammatory and Prothrombotic Biomarkers, DNA Polymorphisms, MicroRNAs and Personalized Medicine for Patients with Peripheral Arterial Disease. Int. J. Mol. Sci. 2022, 23, 12054. [Google Scholar] [CrossRef] [PubMed]
- Lampsas, S.; Xenou, M.; Oikonomou, E.; Pantelidis, P.; Lysandrou, A.; Sarantos, S.; Goliopoulou, A.; Kalogeras, K.; Tsigkou, V.; Kalpis, A.; et al. Lipoprotein(a) in Atherosclerotic Diseases: From Pathophysiology to Diagnosis and Treatment. Molecules 2023, 28, 969. [Google Scholar] [CrossRef]
- Li, Q.; Wang, C.; Li, W.; Zhang, Z.; Wang, S.; Wupuer, A.; Hu, X.; Wumaier, K.; Zhu, Y.; Li, H.; et al. A Novel Mutation in COL4A1 Gene in a Chinese Family with Pontine Autosomal Dominant Microangiopathy and Leukoencephalopathy. Transl. Stroke Res. 2022, 13, 238–244. [Google Scholar] [CrossRef]
- Gudbjartsson, D.F.; Thorgeirsson, G.; Sulem, P.; Helgadottir, A.; Gylfason, A.; Saemundsdottir, J.; Bjornsson, E.; Norddahl, G.L.; Jonasdottir, A.; Jonasdottir, A.; et al. Lipoprotein(a) Concentration and Risks of Cardiovascular Disease and Diabetes. J. Am. Coll. Cardiol. 2019, 74, 2982–2994. [Google Scholar] [CrossRef]
- van Zuydam, N.R.; Stiby, A.; Abdalla, M.; Austin, E.; Dahlström, E.H.; McLachlan, S.; Vlachopoulou, E.; Ahlqvist, E.; Di Liao, C.; Sandholm, N.; et al. Genome-Wide Association Study of Peripheral Artery Disease. Circ. Genom. Precis. Med. 2021, 14, e002862. [Google Scholar] [CrossRef]
- Ni, X.; Bai, C.; Nie, C.; Qi, L.; Liu, Y.; Yuan, H.; Zhu, X.; Sun, L.; Zhou, Q.; Li, Y.; et al. Identification and Replication of Novel Genetic Variants of ABO Gene to Reduce the Incidence of Diseases and Promote Longevity by Modulating Lipid Homeostasis. Aging 2021, 13, 24655–24674. [Google Scholar] [CrossRef]
- Abubakar, M.; Rasool, H.F.; Javed, I.; Raza, S.; Abang, L.; Hashim, M.M.A.; Saleem, Z.; Abdullah, R.M.; Faraz, M.A.; Hassan, K.M.; et al. Comparative Roles of IL-1, IL-6, IL-10, IL-17, IL-18, 1L-22, IL-33, and IL-37 in Various Cardiovascular Diseases with Potential Insights for Targeted Immunotherapy. Cureus 2023, 15, e42494. [Google Scholar] [CrossRef]
- Levin, M.G.; Klarin, D.; Georgakis, M.K.; Lynch, J.; Liao, K.P.; Voight, B.F.; O’Donnell, C.J.; Chang, K.-M.; Assimes, T.L.; Tsao, P. S et al. A Missense Variant in the IL-6 Receptor and Protection from Peripheral Artery Disease. Circ. Res. 2021, 129, 968–970. [Google Scholar] [CrossRef]
- Alexander, M.R.; Hank, S.; Dale, B.L.; Himmel, L.; Zhong, X.; Smart, C.D.; Fehrenbach, D.J.; Chen, Y.; Prabakaran, N.; Tirado, B.; et al. A Single Nucleotide Polymorphism in SH2B3/LNK Promotes Hypertension Development and Renal Damage. Circ. Res. 2022, 131, 731–747, Erratum in Circ Res. 2023, 132, e95. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, T.; Zhou, Y.; Han, W. Association of Serum Lipoprotein-Associated Phospholipase A2 and A379V Gene Polymorphisms with Carotid Plaques. Genet. Test. Mol. Biomark. 2020, 24, 131–137. [Google Scholar] [CrossRef]
- Zhao, J.; Sun, B.; Huang, S.; Chen, Y.; Yan, J. Causal Association between Circulating Inflammatory Proteins and Peripheral Artery Disease: A Bidirectional Two-Sample Mendelian Randomization Study. Front. Immunol. 2024, 15, 1432041. [Google Scholar] [CrossRef]
- Ali, M.W.; Patro, C.P.K.; Devall, M.; Dampier, C.H.; Plummer, S.J.; Kuscu, C.; Adli, M.; Lai, R.K.; Casey, G. A Functional Variant on 9p21.3 Related to Glioma Risk Affects Enhancer Activity and Modulates Expression of CDKN2B-AS1. Hum. Mutat. 2021, 42, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Trejo-Reveles, V.; Owen, N.; Ching Chan, B.H.; Toms, M.; Schoenebeck, J.J.; Moosajee, M.; Rainger, J.; Genomics England Research Consortium. Identification of Novel Coloboma Candidate Genes through Conserved Gene Expression Analyses across Four Vertebrate Species. Biomolecules 2023, 13, 293. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, J.; Du, A.; Zhang, S.; Deng, M.; Zhao, R.; Lu, Y.; Ji, Y.; Shao, Y.; Sun, W.; et al. SPARC-Related Modular Calcium Binding 1 Regulates Aortic Valve Calcification by Disrupting BMPR-II/p-P38 Signalling. Cardiovasc. Res. 2022, 118, 913–928. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Xu, M.; Lu, F.; He, Y. Development of Matrix Metalloproteinases-Mediated Extracellular Matrix Remodeling in Regenerative Medicine: A Mini Review. Tissue Eng. Regen. Med. 2023, 20, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Butnariu, L.I.; Gorduza, E.V.; Florea, L.; Țarcă, E.; Moisă, Ș.M.; Tradafir, L.M.; Cojocaru, E.; Luca, A.-C.; Stătescu, L.; Bădescu, M.C. The Genetic Architecture of the Etiology of Lower Extremity Peripheral Artery Disease: Current Knowledge and Future Challenges in the Era of Genomic Medicine. Int. J. Mol. Sci. 2022, 23, 10481. [Google Scholar] [CrossRef]
- Ryu, J.; Rämö, J.T.; Jurgens, S.J.; Niiranen, T.; Sanna-Cherchi, S.; Bauer, K.A.; Haj, A.; Choi, S.H.; Palotie, A.; Daly, M.; et al. Thrombosis Risk in Single- and Double-Heterozygous Carriers of Factor V Leiden and Prothrombin G20210A in FinnGen and the UK Biobank. Blood 2024, 143, 2425–2432. [Google Scholar] [CrossRef]
- Fowkes, F.G.; Connor, J.M.; Smith, F.B.; Wood, J.; Donnan, P.T.; Lowe, G.D. Fibrinogen Genotype and Risk of Peripheral Atherosclerosis. Lancet 1992, 339, 693–696. [Google Scholar] [CrossRef]
- Lasek-Bal, A.; Kula, D.; Urbanek, T.; Puz, P.; Szymszal, J.; Jarzab, M.; Halczok, M.; Cyplinska, R.; Bal, W.; Łabuz-Roszak, B.; et al. The Association of SNPs Located in the CDKN2B-AS1 and LPA Genes with Carotid Artery Stenosis and Atherogenic Stroke. Front. Neurol. 2019, 10, 1170. [Google Scholar] [CrossRef]
- Pavlatos, N.; Kalra, D.K. The Role of Lipoprotein(a) in Peripheral Artery Disease. Biomedicines 2024, 12, 1229. [Google Scholar] [CrossRef] [PubMed]
- Kullo, I.J.; Shameer, K.; Jouni, H.; Lesnick, T.G.; Pathak, J.; Chute, C.G.; de Andrade, M. The ATXN2-SH2B3 Locus Is Associated with Peripheral Arterial Disease: An Electronic Medical Record-Based Genome-Wide Association Study. Front. Genet. 2014, 5, 166. [Google Scholar] [CrossRef]
- Ewald, J.; Zhou, G.; Lu, Y.; Xia, J. Using ExpressAnalyst for Comprehensive Gene Expression Analysis in Model and Non-Model Organisms. Curr. Protoc. 2023, 3, e922. [Google Scholar] [CrossRef]
- Kurhaluk, N.; Tkaczenko, H. L-Arginine and Nitric Oxide in Vascular Regulation-Experimental Findings in the Context of Blood Donation. Nutrients 2025, 17, 665. [Google Scholar] [CrossRef]
- Iannuzzi, A.; Iannuzzo, G.; Sapio, C.; Pauciullo, P.; Iorio, D.; Spampinato, N.; Mancini, M.; Rubba, P. L-Arginine Improves Post-Ischemic Vasodilation in Coronary Heart Disease Patients Taking Vasodilating Drugs. J. Cardiovasc. Pharmacol. Ther. 2001, 6, 121–127. [Google Scholar] [CrossRef]
- Gomez-Larrauri, A.; Larrea-Sebal, A.; Martín, C.; Gomez-Muñoz, A. The Critical Roles of Bioactive Sphingolipids in In-flammation. J. Biol. Chem. 2025, 301, 110475. [Google Scholar] [CrossRef]
- Gaffori, O.; de Wied, D. Further Evidence for a Dissociation of Peripheral and Central Effects of Vasopressin. Psychoneuroen-Docrinology 1985, 10, 439–444. [Google Scholar] [CrossRef]
- Linton, M.F.; Moslehi, J.J.; Babaev, V.R. Akt Signaling in Macrophage Polarization, Survival, and Atherosclerosis. Int. J. Mol. Sci. 2019, 20, 2703. [Google Scholar] [CrossRef] [PubMed]
- Lamb, F.S.; Choi, H.; Miller, M.R.; Stark, R.J. TNFα and Reactive Oxygen Signaling in Vascular Smooth Muscle Cells in Hypertension and Atherosclerosis. Am. J. Hypertens. 2020, 33, 902–913. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M.M.; Ameneiros-Rodríguez, E.; Fernández-Fernández, C.; Domínguez-Montero, A.; Funcasta-Calderón, R. Insulin Resistance Is Associated with Subclinical Vascular Disease in Humans. World J. Diabetes 2019, 10, 63–77. [Google Scholar] [CrossRef]
- Miceli, G.; Basso, M.G.; Rizzo, G.; Pintus, C.; Tuttolomondo, A. The Role of the Coagulation System in Peripheral Arterial Disease: Interactions with the Arterial Wall and Its Vascular Microenvironment and Implications for Rational Therapies. Int. J. Mol. Sci. 2022, 23, 14914. [Google Scholar] [CrossRef] [PubMed]
- Lebas, H.; Yahiaoui, K.; Martos, R.; Boulaftali, Y. Platelets Are at the Nexus of Vascular Diseases. Front. Cardiovasc. Med. 2019, 6, 132. [Google Scholar] [CrossRef] [PubMed]
- Jain, I.; Oropeza, B.P.; Huang, N.F. Multiomics Analyses of Peripheral Artery Disease Muscle Biopsies. Circ. Res. 2023, 132, 1444–1446. [Google Scholar] [CrossRef]
- Ferrucci, L.; Candia, J.; Ubaida-Mohien, C.; Lyashkov, A.; Banskota, N.; Leeuwenburgh, C.; Wohlgemuth, S.; Guralnik, J.M.; Kaileh, M.; Zhang, D.; et al. Transcriptomic and Proteomic of Gastrocnemius Muscle in Peripheral Artery Disease. Circ. Res. 2023, 132, 1428–1443. [Google Scholar] [CrossRef]
- Pass, C.G.; Palzkill, V.; Tan, J.; Kim, K.; Thome, T.; Yang, Q.; Fazzone, B.; Robinson, S.T.; O’Malley, K.A.; Yue, F.; et al. Single-Nuclei RNA-Sequencing of the Gastrocnemius Muscle in Peripheral Artery Disease. Circ. Res. 2023, 133, 791–809. [Google Scholar] [CrossRef]
- Matsukura, M.; Ozaki, K.; Takahashi, A.; Onouchi, Y.; Morizono, T.; Komai, H.; Shigematsu, H.; Kudo, T.; Inoue, Y.; Kimura, H.; et al. Genome-Wide Association Study of Peripheral Arterial Disease in a Japanese Population. PLoS ONE 2015, 10, e0139262. [Google Scholar] [CrossRef]
- Zhabin, S.; Lazarenko, V.; Azarova, I.; Klyosova, E.; Bashkatov, D.; Kononov, S.; Dolgintsev, M.; Churnosov, M.; Solodilova, M.; Polonikov, A. The Promoter Polymorphism Rs3918226 of the Endothelial Nitric Oxide Synthase Gene as a Novel Susceptibility Marker for Peripheral Artery Disease. Ann. Vasc. Surg. 2024, 108, 557–563. [Google Scholar] [CrossRef]
- Kardia, S.L.; Greene, M.T.; Boerwinkle, E.; Turner, S.T.; Kullo, I.J. Investigating the Complex Genetic Architecture of Ankle-Brachial Index, a Measure of Peripheral Arterial Disease, in Non-Hispanic Whites. BMC Med. Genom. 2008, 1, 16. [Google Scholar] [CrossRef]
- Casas, J.P.; Cavalleri, G.L.; Bautista, L.E.; Smeeth, L.; Humphries, S.E.; Hingorani, A.D. Endothelial Nitric Oxide Synthase Gene Polymorphisms and Cardiovascular Disease: A HuGE Review. Am. J. Epidemiol. 2006, 164, 921–935. [Google Scholar] [CrossRef]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef] [PubMed]
- Zintzaras, E.; Zdoukopoulos, N. A Field Synopsis and Meta-Analysis of Genetic Association Studies in Peripheral Arterial Disease: The CUMAGAS-PAD Database. Am. J. Epidemiol. 2009, 170, 1–11. [Google Scholar] [CrossRef]
- Libby, P. Inflammation in Atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Barresi, V.; Signorelli, S.S.; Musso, N.; Anzaldi, M.; Fiore, V.; Alberghina, M.; Condorelli, D.F. ICAM-1 and SRD5A1 Gene Polymorphisms in Symptomatic Peripheral Artery Disease. Vasc. Med. 2014, 19, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Shaker, O.; Zahra, A.; Sayed, A.; Refaat, A.; El-Khaiat, Z.; Hegazy, G.; El-Hindawi, K.; Ay-El Deen, M. Role of ICAM-1 and E-Selectin Gene Polymorphisms in Pathogenesis of PAOD in Egyptian Patients. Vasc. Health Risk Manag. 2010, 6, 9–15. [Google Scholar] [CrossRef]
- Flex, A.; Gaetani, E.; Angelini, F.; Sabusco, A.; Chillà, C.; Straface, G.; Biscetti, F.; Pola, P.; Castellot, J.J.; Pola, R. Pro-Inflammatory Genetic Profiles in Subjects with Peripheral Arterial Occlusive Disease and Critical Limb Ischemia. J. Intern. Med. 2007, 262, 124–130. [Google Scholar] [CrossRef]
- Teslovich, T.M.; Musunuru, K.; Smith, A.V.; Edmondson, A.C.; Stylianou, I.M.; Koseki, M.; Pirruccello, J.P.; Ripatti, S.; Chasman, D.I.; Willer, C.J.; et al. Biological, Clinical and Population Relevance of 95 Loci for Blood Lipids. Nature 2010, 466, 707–713. [Google Scholar] [CrossRef]
- Verdier, C.; Ruidavets, J.-B.; Genoux, A.; Combes, G.; Bongard, V.; Taraszkiewicz, D.; Galinier, M.; Elbaz, M.; Ferrières, J.; Martinez, L.O.; et al. Common P2y13 Polymorphisms Are Associated with Plasma Inhibitory Factor 1 and Lipoprotein(a) Concentrations, Heart Rate and Body Fat Mass: The GENES Study. Arch. Cardiovasc. Dis. 2019, 112, 124–134. [Google Scholar] [CrossRef]
- Valdivielso, P.; Ariza, M.J.; de la Vega-Román, C.; González-Alegre, T.; Rioja, J.; Ulzurrun, E.; González-Santos, P. Association of the -250G/A Promoter Polymorphism of the Hepatic Lipase Gene with the Risk of Peripheral Arterial Disease in Type 2 Diabetic Patients. J. Diabetes Complicat. 2008, 22, 273–277. [Google Scholar] [CrossRef]
- Hopewell, J.C.; Clarke, R.; Parish, S.; Armitage, J.; Lathrop, M.; Hager, J.; Collins, R.; Heart Protection Study Collaborative Group. Lipoprotein(a) Genetic Variants Associated with Coronary and Peripheral Vascular Disease but Not with Stroke Risk in the Heart Protection Study. Circ. Cardiovasc. Genet. 2011, 4, 68–73. [Google Scholar] [CrossRef]
- Laschkolnig, A.; Kollerits, B.; Lamina, C.; Meisinger, C.; Rantner, B.; Stadler, M.; Peters, A.; Koenig, W.; Stöckl, A.; Dähnhardt, D.; et al. Lipoprotein (a) Concentrations, Apolipoprotein (a) Phenotypes, and Peripheral Arterial Disease in Three Independent Cohorts. Cardiovasc. Res. 2014, 103, 28–36. [Google Scholar] [CrossRef]
- Kronenberg, F.; Mora, S.; Stroes, E.S.G.; Ference, B.A.; Arsenault, B.J.; Berglund, L.; Dweck, M.R.; Koschinsky, M.; Lambert, G.; Mach, F.; et al. Lipoprotein(a) in Atherosclerotic Cardiovascular Disease and Aortic Stenosis: A European Atherosclerosis Society Consensus Statement. Eur. Heart J. 2022, 43, 3925–3946. [Google Scholar] [CrossRef]
- Erqou, S.; Thompson, A.; Di Angelantonio, E.; Saleheen, D.; Kaptoge, S.; Marcovina, S.; Danesh, J. Apolipoprotein(a) Isoforms and the Risk of Vascular Disease: Systematic Review of 40 Studies Involving 58,000 Participants. J. Am. Coll. Cardiol. 2010, 55, 2160–2167. [Google Scholar] [CrossRef]
- Zhabin, S.N.; Lazarenko, V.A.; Azarova, I.E.; Klyosova, E.Y.; Bykanova, M.A.; Churnosov, M.I.; Solodilova, M.A.; Polonikov, A.V. Lipid-Associated GWAS Loci as Important Markers of the Risk, Severity, and Clinical Course of Peripheral Artery Disease. Expert Rev. Mol. Diagn. 2024, 24, 1033–1044. [Google Scholar] [CrossRef]
- Danielsson, P.; Truedsson, L.; Eriksson, K.F.; Norgren, L. Inflammatory Markers and IL-6 Polymorphism in Peripheral Arterial Disease with and without Diabetes Mellitus. Vasc. Med. 2005, 10, 191–198. [Google Scholar] [CrossRef]
- Ridker, P.M.; Hennekens, C.H.; Buring, J.E.; Rifai, N. C-Reactive Protein and Other Markers of Inflammation in the Prediction of Cardiovascular Disease in Women. N. Engl. J. Med. 2000, 342, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Libra, M.; Signorelli, S.S.; Bevelacqua, Y.; Navolanic, P.M.; Bevelacqua, V.; Polesel, J.; Talamini, R.; Stivala, F.; Mazzarino, M.C.; Malaponte, G. Analysis of G(-174)C IL-6 Polymorphism and Plasma Concentrations of Inflammatory Markers in Patients with Type 2 Diabetes and Peripheral Arterial Disease. J. Clin. Pathol. 2006, 59, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Tragante, V.; Doevendans, P.A.F.M.; Nathoe, H.M.; van der Graaf, Y.; Spiering, W.; Algra, A.; de Borst, G.J.; de Bakker, P.I.W.; Asselbergs, F.W.; SMART Study Group. The Impact of Susceptibility Loci for Coronary Artery Disease on Other Vascular Domains and Recurrence Risk. Eur. Heart J. 2013, 34, 2896–2904. [Google Scholar] [CrossRef]
- Cluett, C.; McDermott, M.M.; Guralnik, J.; Ferrucci, L.; Bandinelli, S.; Miljkovic, I.; Zmuda, J.M.; Li, R.; Tranah, G.; Harris, T.; et al. The 9p21 Myocardial Infarction Risk Allele Increases Risk of Peripheral Artery Disease in Older People. Circ. Cardiovasc. Genet. 2009, 2, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Willeit, J.; Kiechl, S.; Oberhollenzer, F.; Rungger, G.; Egger, G.; Bonora, E.; Mitterer, M.; Muggeo, M. Distinct Risk Profiles of Early and Advanced Atherosclerosis: Prospective Results from the Bruneck Study. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 529–537. [Google Scholar] [CrossRef]
- Bérard, A.M.; Bedel, A.; Le Trequesser, R.; Freyburger, G.; Nurden, A.; Colomer, S.; Guérin, V.; Vergnes, M.-C.; Becker, F.; Camelot, G.; et al. Novel Risk Factors for Premature Peripheral Arterial Occlusive Disease in Non-Diabetic Patients: A Case-Control Study. PLoS ONE 2013, 8, e37882. [Google Scholar] [CrossRef] [PubMed]
- Reny, J.L.; Alhenc-Gelas, M.; Fontana, P.; Bissery, A.; Julia, P.L.; Fiessinger, J.N.; Aiach, M.; Emmerich, J. The Factor II G20210A Gene Polymorphism, but Not Factor V Arg506Gln, Is Associated with Peripheral Arterial Disease: Results of a Case-Control Study. J. Thromb. Haemost. JTH 2004, 2, 1334–1340. [Google Scholar] [CrossRef]
- Sofi, F.; Lari, B.; Rogolino, A.; Marcucci, R.; Pratesi, G.; Dorigo, W.; Pratesi, C.; Gensini, G.F.; Abbate, R.; Prisco, D. Thrombophilic Risk Factors for Symptomatic Peripheral Arterial Disease. J. Vasc. Surg. 2005, 41, 255–260. [Google Scholar] [CrossRef][Green Version]
- Vazquez, F.; Rodger, M.; Carrier, M.; Le Gal, G.; Reny, J.-L.; Sofi, F.; Mueller, T.; Nagpal, S.; Jetty, P.; Gandara, E. Prothrombin G20210A Mutation and Lower Extremity Peripheral Arterial Disease: A Systematic Review and Meta-Analysis. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2015, 50, 232–240. [Google Scholar] [CrossRef][Green Version]
- Murakami, T. Atherosclerosis and Arteriosclerosis. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2023, 46, 1810–1811. [Google Scholar] [CrossRef]
- Lee, A.J.; Fowkes, F.G.; Lowe, G.D.; Connor, J.M.; Rumley, A. Fibrinogen, Factor VII and PAI-1 Genotypes and the Risk of Coronary and Peripheral Atherosclerosis: Edinburgh Artery Study. Thromb. Haemost. 1999, 81, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.B.; Connor, J.M.; Lee, A.J.; Cooke, A.; Lowe, G.D.O.; Rumley, A.; Fowkes, F.G. Relationship of the Platelet Glycoprotein PlA and Fibrinogen T/G+1689 Polymorphisms with Peripheral Arterial Disease and Ischaemic Heart Disease. Thromb. Res. 2003, 112, 209–216. [Google Scholar] [CrossRef]
- Aday, A.W.; Duncan, M.S.; Patterson, O.V.; DuVall, S.L.; Alba, P.R.; Alcorn, C.W.; Tindle, H.A.; Creager, M.A.; Bonaca, M.P.; Damrauer, S.M.; et al. Association of Sex and Race with Incident Peripheral Artery Disease Among Veterans with Normal Ankle-Brachial Indices. JAMA Netw. Open 2022, 5, e2240188. [Google Scholar] [CrossRef]
- Barnes, J.A.; Eid, M.A.; Creager, M.A.; Goodney, P.P. Epidemiology and Risk of Amputation in Patients with Diabetes Mellitus and Peripheral Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1808–1817. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.; Nead, K.T.; Olin, J.W.; Cooke, J.P.; Leeper, N.J. Clinical and Socioeconomic Factors Associated with Unrecognized Peripheral Artery Disease. Vasc. Med. 2014, 19, 289–296. [Google Scholar] [CrossRef]
- Rebuffet, C.; Gillois, P.; Joly, M.; Satger, B.; Seinturier, C.; Pernod, G. Evaluation of Socio-Economic Insecurity in Peripheral Artery Disease Patients. J. Med. Vasc. 2021, 46, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Giannoli, J.-M.; Vassault, A.; Carobene, A.; Liaudet, A.P.; Blasutig, I.M.; Dabla, P.K.; Lin, J.; Thomas, A.; Tesser Poloni, J.A.; Meng, Q.H.; et al. Ensuring Internal Quality Control Practices in Medical Laboratories: IFCC Recommendations for Practical Applications Based on ISO 15189:2022. Clin. Chim. Acta Int. J. Clin. Chem. 2025, 571, 120240. [Google Scholar] [CrossRef]
- Wassel, C.L.; Loomba, R.; Ix, J.H.; Allison, M.A.; Denenberg, J.O.; Criqui, M.H. Family History of Peripheral Artery Disease Is Associated with Prevalence and Severity of Peripheral Artery Disease: The San Diego Population Study (SDPS). J. Am. Coll. Cardiol. 2011, 58, 1386–1392. [Google Scholar] [CrossRef]
- Villamil, C. Created in BioRender. 2025. Available online: https://app.biorender.com/illustrations/6904eab05a145cdd59b382d8 (accessed on 30 May 2025).
- Paynter, N.P.; Ridker, P.M.; Chasman, D.I. Are Genetic Tests for Atherosclerosis Ready for Routine Clinical Use? Circ. Res. 2016, 118, 607–619. [Google Scholar] [CrossRef]
- Mitsis, A.; Khattab, E.; Kyriakou, M.; Sokratous, S.; Sakellaropoulos, S.G.; Tzikas, S.; Kadogou, N.P.E.; Kassimis, G. Genomic and Precision Medicine Approaches in Atherosclerotic Cardiovascular Disease: From Risk Prediction to Therapy-A Review. Biomedicines 2025, 13, 1723. [Google Scholar] [CrossRef]
- Morales, A.; Goehringer, J.; Sanoudou, D. Evolving Cardiovascular Genetic Counseling Needs in the Era of Precision Medicine. Front. Cardiovasc. Med. 2023, 10, 1161029. [Google Scholar] [CrossRef] [PubMed]
- Khachigian, L.M.; Varcoe, R.L.; Suoranta, T.; Laham-Karam, N.; Ylä-Herttuala, S. Gene Therapeutic Strategies for Peripheral Artery Disease and New Opportunities Provided by Adeno-Associated Virus Vectors. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 836–851. [Google Scholar] [CrossRef]
- Li, J.-M.; Huang, J.; Liao, Y.; Hu, T.; Wang, C.-L.; Zhang, W.-Z.; Huang, C.-W. Gene and RNA Editing: Revolutionary Approaches to Treating Diseases. MedComm 2025, 6, e70389. [Google Scholar] [CrossRef]
- Forster, R.; Liew, A.; Bhattacharya, V.; Shaw, J.; Stansby, G. Gene Therapy for Peripheral Arterial Disease. Cochrane Database Syst. Rev. 2018, 10, CD012058. [Google Scholar] [CrossRef]
- Wahbeh, M.H.; Boyd, R.J.; Yovo, C.; Rike, B.; McCallion, A.S.; Avramopoulos, D. A Functional Schizophrenia-Associated Genetic Variant near the TSNARE1 and ADGRB1 Genes. HGG Adv. 2024, 5, 100303. [Google Scholar] [CrossRef] [PubMed]
| Gene | SNP | Variant * | Role in PAD |
|---|---|---|---|
| Plaque origination | |||
| NOS3 | rs3918226 | NC_000007.14(NOS3):g.150993088C>T | Variants in the NOS3 gene modulate gene expression and enzymatic activity, ultimately affecting NO production, disrupting vascular tone regulation, and contributing to cardiovascular dysfunction. These variants influence NOS3 at multiple levels. The rs3918226, located in the promoter region, reduces its transcriptional activity, resulting in reduced NOS3 expression and diminished NO bioavailability. In contrast, the rs891512 variant potentially enhances transcription; however, this increased expression, under pro-oxidative conditions, might produce nitrosative stress and endothelial damage. The rs1808593 missense mutation results in a functional change in NOS3, decreasing its enzymatic activity and further limiting NO synthesis [25,27,28]. |
| rs891512 | NC_000007.14(NOS3):g.151011001A>C | ||
| rs1808593 | NC_000007.14(NOS3):g.151011214G>A | ||
| ICAM1 | rs5498 | NC_000019.10(ICAM1):g.10285007A>G | This variant alters splicing and increases the expression of ICAM-1, enhancing leukocyte adhesion and endothelial activation. The G allele has been linked to increased levels of the soluble form of ICAM-1, promoting inflammation and plaque formation [27,28]. |
| SELE | rs5361 | NC_000001.11(SELE):g.169731919T>A | This variant is a missense mutation that alters the EGF-like domain of E-selectin, impacting leukocyte binding. This change contributes to increased endothelial activation, promoting vascular dysfunction and atheroma plaque formation [30]. |
| LIPC | rs2070895 | NC_000015.10(LIPC):g.58431740G>A | Variants such as rs2070895 and rs1800588, located in the promoter region of the LIPC gene, affect transcriptional regulation by altering binding sites, resulting in reduced hepatic lipase expression. This leads to higher LDL levels and lower HDL concentrations, ultimately impacting vascular health [30]. |
| rs1800588 | NC_000015.10(LIPC):g.58431476C>A | ||
| LDLR | rs138294113 | NC_000019.10(LDLR): g.11081053C>T | This variant causes a nonsense change in the LDLR gene, leading to a truncated, non-functional LDL receptor or degradation of the mutant mRNA through nonsense-mediated decay. As a result, receptor availability on the cell surface decreases, impairing LDL clearance and promoting the formation of atherosclerotic plaques [7]. |
| LPA | rs118039278 | NC_000006.12(LPA):g.160564494G>A | Variants in the LPA gene, including rs118039278, rs3798220, rs10455872, and rs7452960, affect regulatory and coding regions, leading to altered mRNA splicing, transcript stability, or amino acid changes in apolipoprotein(a). These changes enhance the production or alter the structure of Lp(a), contributing to its accumulation and reduced clearance. This overexpression of LPA disrupts vascular homeostasis and facilitates atherosclerotic plaque development [38,52,53]. |
| rs3798220 | NC_000006.12(LPA):g.160540105T>C | ||
| rs10455872 | NC_000006.12:g.160589086A>G | ||
| rs7452960 | NC_000006.12(LPA):g.160520609G>A | ||
| LPL | rs322 | NC_000008.11(LPL):g.19961706A>C | Variants of this gene affect the activity of lipoprotein lipase, potentially leading to altered triglyceride levels favoring their accumulation in the vascular system. The rs322 variant is located within an intronic region, which may influence LPL gene expression or splicing, thus modulating enzyme availability and contributing to lipid dysregulation [19,35]. |
| ABO | rs505922 | NC_000009.12(ABO):g.133273813C>T | The rs505922 variant in the ABO gene is found within a noncoding region and plays a regulatory role that may influence the expression of nearby genes involved in vascular biology. This polymorphism has been linked to changes in circulating levels of pro-inflammatory and pro-thrombotic factors, providing a possible genetic basis for the heightened cardiovascular risk observed in individuals with certain blood groups [7]. |
| Plaque progression | |||
| IL-6 | rs1800795 | NC_000007.13(IL6):g.22766645C>G | Polymorphisms of this gene are linked to elevated IL-6 in plasma, indicating high inflammatory activity. Among them, rs1800795, located in the promoter region, modulates IL-6 transcriptional activity. The G allele is associated with increased promoter activity and high IL-6 expression, enhancing vascular inflammation and atherogenesis. The variant rs4722172 is located in an intronic region and may influence IL-6 expression through regulatory mechanisms [7,30]. |
| rs4722172 | NC_000007.13(IL6): g.22786532G>A | ||
| SH2B3 | rs653178 rs3184504 | NC_000012.12:g.111569952C>A NC_000012.12:g.111446804T>A | The variant rs653178 in the ATXN2-SH2B3 locus is significantly associated with an increased risk of PAD independent of traditional cardiovascular factors. It is in near-complete linkage disequilibrium with the missense variant rs3184504 in SH2B3, which affects immune and inflammatory pathways critical for vascular health. This variant influences endothelial function and inflammation, contributing to PAD development and related cardiovascular conditions such as myocardial infarction [54]. |
| CELSR2 | rs7528419 | NC_000012.12:g.111446804T>A | Variants of this gene cause elevated levels of lipoprotein-associated phospholipase A2, contributing to the formation of atherosclerotic plaques, leading to arterial narrowing and reduced blood flow. The variant rs7528419 is in strong linkage disequilibrium with regulatory variants that enhance the hepatic expression of SORT1. This upregulation alters LDL metabolism, promoting lipid accumulation, vascular inflammation, and atherogenesis [7]. |
| HDAC9 | rs2107595 | NC_000007.13(HDAC9): g.19049388G>A | This gene encodes histone deacetylase 9, an enzyme that removes acetyl groups from histones, leading to a more compact chromatin structure that represses gene transcription. This variant is located in a regulatory region and may alter transcription factor binding, indirectly modulating nearby genes and promoting vascular inflammation. In particular, the A allele is associated with the increased expression of HDAC9, which can enhance the transcriptional repression of anti-inflammatory genes while facilitating the expression of pro-inflammatory cytokines [7,44]. |
| CDKN2B-AS1 | rs1537372 | NC_000009.11(CDKN2B-AS1):g.22103183G>A | Variants of this gene are associated with the increased expression of CDKN2B-AS1, leading to the enhanced repression of cell cycle regulatory genes. Variants such as rs1537372, located in an enhancer region, modulate transcription, while rs4977574 and rs785734 have been linked to altered chromatin states and increased CDKN2B-AS1 activity. These mechanisms facilitate vascular remodeling and promote a pro-inflammatory environment [7,38]. |
| rs4977574 | NC_000009.11(CDKN2B-AS1):g.22098574A>G | ||
| rs7857345 | NC_000009.11(CDKN2B-AS1):g.22087473T>A | ||
| PTPN11 | rs11066301 | NC_000012.12(PTPN11):g.112433568A>G | This variant can influence alternative splicing or transcription efficiency, resulting in changes in PTPN11 levels. Altered PTPN11 expression impacts signaling cascades that regulate vascular cell proliferation and inflammatory responses, ultimately contributing to endothelial function and inflammation [7,38]. |
| CREB3L1 | rs7476 | NC_000011.10(CREB3L1):g.46321284A>C | rs7476 is associated with the increased expression of CREB3L1 by affecting mRNA stability and microRNA binding. This overexpression may influence lipid accumulation within vascular tissues, disrupt endothelial integrity, and promote vascular inflammation [7,38]. |
| Plaque rupture | |||
| COL4A1 | rs1975514 | NC_000013.11(COL4A1):g.110176544T>C | The rs1975514 variant may alter collagen stability, weakening the integrity of the vascular walls, and is an intronic variant located near a splice site in COL4A1 that may influence mRNA processing or transcript stability, subtly affecting the production or quality of type IV collagen. This can compromise basement membrane integrity and increase vascular vulnerability [7]. |
| SMOC1 | rs55784307 | NC_000014.9(SMOC1): g.70034647C>A | The polymorphism rs55784307, located in the genomic region of SMOC1, may influence matrix remodeling and cellular adhesion by altering regulation in gene expression. Such changes can disrupt the transcriptional control of SMOC1, modifying cellular responses to vascular injury and promoting a pro-inflammatory environment within the vascular wall [19]. |
| MMP3 | rs566125 | NC_000011.10(MMP3):g.102839740C>T | This intronic variant may alter MMP3 gene regulation, potentially enhancing enzyme expression. The resulting increase in the proteolytic activity of MMP3 can accelerate extracellular matrix degradation within the vascular wall, weakening plaque stability and increasing the risk of rupture and thrombosis [30,44]. |
| F5 | rs6025 | NC_000001.11(F5):g.169549811C>A | The polymorphism rs6025 causes a single amino acid substitution in Factor V, making it resistant to cleavage by activated protein C. As a result, the anticoagulant pathway is impaired, promoting sustained thrombin generation and elevating the risk of abnormal clot formation [30,49]. |
| F2 | rs1799963 | NC_000011.10(F2):g.46739505G>A | The rs1799963 variant can result in an altered prothrombin protein, often due to changes in the 3’ untranslated region of the gene, which may affect the regulation of gene expression. These changes can boost prothrombin production, leading to higher thrombin levels. This, in turn, disrupts the normal anticoagulation process, making abnormal blood clots more likely. On top of that, genetic variants of F2 can also increase the expression of pro-inflammatory cytokines, which intensify inflammation in the blood vessels, speeding up the progression of PAD [50]. |
| FGB | rs1800790 | NC_000004.12:g.154562556G>A | The rs1800790 variant enhances fibrinogen beta gene expression, leading to elevated plasma fibrinogen levels. Increased fibrinogen promotes thrombosis and vascular inflammation, thereby increasing the risk of PAD. Studies such as the Edinburgh Artery Study have associated the A allele with higher fibrinogen concentrations and a greater risk of PA [49]. |
| Gene | Relevant Finding/ Clinic Relevance | Direction | Interaction with Environment/Lifestyle/Metabolic Risks/Drugs | SNP ID of Genetic Variants | Association with PAD | Reference |
|---|---|---|---|---|---|---|
| NOS3 | Polymorphisms are associated with endothelial dysfunction and reduced NO bioavailability. | ↓NO: ↑PAD risk | Yes: oxidative stress, diet, smoking, DM, hypertension. | rs891512 rs1808593 rs3918226 | Contradictory results | [12,19,27,28,34,38,49,68,69,70,71] |
| ICAM1 | There is an overexpression in endothelial cells and high circulating levels, contributing to the initiation and progression of atherosclerotic plaque. | ↑ICAM1: ↑PAD risk | Yes: chronic inflammation, smoking, DM, and obesity. | rs5498 * rs5030352 * | Yes | [30,70,72,73,74,75,76] |
| SELE | Endothelial activation marker: its overexpression is associated with vascular dysfunction and atherosclerosis. | ↑SELE: ↑PAD risk | Yes: smoking, inflammation, obesity, and insulin resistance. | rs5368 rs5356 rs5361 | Yes | [29,70,73,77] |
| LIPC | Affects small LDL and HDL. | ↑Activity: ↑sdLDL-C or ↓HDL and ↑PAD risk | Yes: diet, alcohol consumption, obesity, and exercise. | rs2070895 * rs1800588 | Yes | [30,31,78,79,80] |
| LDLR | Genetic variants with loss of function increase LDL levels and promote accelerated atherosclerosis. | LDLR defective ↑LDL: ↑PAD risk | Yes: diet, smoking, alcohol consumption, and exercise. | rs651172 rs1122608 rs138294113 * | Yes | [7,19,30,33,49] |
| LPA | Elevated levels of Lp(a) are associated with acute atherothrombotic events, aortic stenosis, and PAD. | ↑Lp(a): ↑PAD risk | Yes: diet, smoking, alcohol consumption, obesity, and statins. | rs10455872 * rs7452960 * rs3798220 rs118039278 * | Yes | [30,34,35,38,53,81,82,83,84] |
| LPL | Genetic variants that favor loss/gain of function increase/decrease triglycerides. | ↓LDL function: ↑PAD risk ↑LDL function: ↓PAD risk | Yes: diet, alcohol consumption, exercise, and DM2. | rs328 rs322 * | Yes | [19,30,34] |
| ABO | Regulates levels of lipids, adhesion molecules, vWF, and FVIII; affects lipid metabolism, inflammation, and thrombosis. In non-O groups, a prothrombotic state is favored, and it is associated with the presence and severity of PAD. | ↑vWF and FVIII: ↑Thrombosis risk | Yes: smoking, hypertension, and coagulation factors. | rs505922 * rs616154 * rs635634 rs8176719 | Yes | [30,34,39,85] |
| IL-6 | The increase in IL-6 is associated with increased inflammation. | ↑IL-6: ↑PAD and adverse cardiovascular events risk | Yes: chronic inflammation, smoking, alcohol consumption, obesity, sedentary lifestyle, and DM2. | rs4722172 * rs1800795 rs2228145 rs2069827 * | Contradictory results | [12,30,41,70,73,76,77,86,87,88] |
| SH2B3 | Participates in the signaling of immune and hematopoietic cells, promotes inflammation, vascular tone, and PAD. | ↑ PAD risk with risk alleles | Yes: hypertension and obesity. | rs3184504 * rs7528419 rs653178 | Yes | [7,12,30,38,41,42,54,85,89] |
| CELSR2 | Alters LDL metabolism, promoting lipid accumulation, inflammation and atherosclerosis. | ↑LDL: ↑Atherosclerosis risk | Yes: diet, dyslipidemia, and obesity. | rs12740374 | No | [12,19,38,85] |
| HDAC9 | Modulates inflammation and the VSMC phenotype and is associated with vascular calcification and atherosclerosis. | ↑Expression/activity: ↑PAD risk | Yes: smoking, hypertension, and DM2. | rs2107595 rs2074633 * | Yes | [7,30,38,49,68] |
| CDKN2B-AS1 | Regulates the cell cycle, promotes inflammation and VSMC proliferation. | ↑CDKN2B-AS1 expression: ↑Atherosclerosis and PAD risk | Yes: smoking and DM2. | rs1537372 * rs10738610 * rs1333049 rs10757278 rs10757269 | Yes | [30,34,38,49,52,54,90] |
| PTPN11 | Participates in endothelial signaling, promotes inflammation, and is associated with pro-atherogenesis | ↑Function: ↑PAD risk | Yes: hypertension and DM2. | rs11066301 * rs10774624 | Yes | [12,19,30,34,38] |
| CREB3L1 | Modulates the stress response, is associated with vascular calcification, and promotes cellular remodeling and inflammation. | Depends on the context | Yes: obesity and DM2. | rs7476 * | Contradictory results | [7,19,30,38,48] |
| COL4A1 | Participates in the integrity of the basement membrane; its genetic variants cause microvascular fragility. | Defect: ↑Fragility | Yes: smoking and hypertension. | rs1975514 * | Contradictory results | [7,12,30,36,91] |
| SMOC1 | Regulates cellular homeostasis, cell migration and proliferation, calcification, tissue fibrosis, and angiogenesis. | ↑SMOC1: ↑Calcification/cell remodeling | Yes: DM2. | rs55784307 * | Contradictory results | [12,30,34,38,47] |
| MMP-3 | Overexpression is associated with extracellular matrix degradation and vulnerability of the atherosclerotic plaque. | ↑MMP3: Instability/rupture of the atherosclerotic plaque | Yes: smoking, obesity, and DM2. | rs566125 * rs5030352 | Yes | [7,30,48,70,77] |
| F5 | The Factor V Leiden mutation causes resistance to protein C, which promotes a prothrombotic state in PAD. | ↑Hypercoagulability | Yes: smoking, obesity, pregnancy, and contraceptives. | rs6025 * | Contradictory results | [7,12,19,30,49,50,73,92] |
| F2 | Mutations in prothrombin increase the risk of clots forming on the atherosclerotic plaque. | ↑Activity: ↑Thrombotic risk | Yes: smoking, obesity, pregnancy, and contraceptives. | rs1799963 | Contradictory results | [50,73,92,93,94,95] |
| FGB | Overexpression is associated with elevated plasma fibrinogen, inflammation, thrombosis and worse prognosis. | ↑Expression: ↑Fibrinogen levels and this, ↑PAD and CVDs risk | Yes: smoking, alcohol consumption, inflammation, obesity, and DM2. | rs4220 rs1800790 | Contradictory results | [5,49,51,96,97,98] |
| Factor | How the Result Could Be Affected | How To Avoid It | Factor | Reference |
|---|---|---|---|---|
| Sample size | Published research demonstrates substantial variability in sample sizes across PAD studies. In many cases, sample sizes were limited and determined by convenience, thereby restricting the statistical power to detect significant associations. | Use power analysis before starting the study (based on expected variant frequency, estimated OR, alpha). Ensure that the sample has ≥80% power to detect significant associations. | Sample size | Low (<100) [10,13,26,28] High (>500) [22,38,54,99] |
| Heterogeneity in diagnostic criteria/Definition of PAD | The diagnostic criteria for PAD varied considerably across studies. In some cohorts, diagnosis was established solely by ABI, claudication symptoms, history of revascularization, chronic ischemia, or non-traumatic amputation. In others, diagnosis relied on a more comprehensive assessment that combined medical history, detailed physical examination, and confirmatory tests such as imaging. This heterogeneity in case definition may influence both the magnitude and the direction of the reported associations. | Use consensus definitions from international guidelines (e.g., ABI ≤ 0.90 for PAD, CLTI criteria from the Global Vascular Guidelines). Avoid diagnoses based solely on nonspecific symptoms or poorly validated medical records. Include symptoms and procedures (claudication, revascularization, amputation) only as additional criteria. Validate and, if possible, integrate emerging tools (e.g., PAT) in patients where the ABI may be unreliable. | Heterogeneity in diagnostic criteria/Definition of PAD | [1,2,3,6,99] |
| Study of PAD alone or associated with classic cardiovascular risk factors or environmental factors | Some studies focused exclusively on patients diagnosed with PAD, whereas others included individuals with PAD in combination with at least one traditional cardiovascular risk factor, such as DM2 or hypertension. This variability in inclusion criteria may introduce heterogeneity and affect the comparability of results across studies. | Analysis stratification (conduct separate analyses for PAD alone and for PAD with comorbidities). Include classic risk factors as covariates to reduce bias. | Study of PAD alone or associated with classic cardiovascular risk factors or environmental factors | [99,100] |
| Type of analysis/study power | Some studies performed analyses adjusting for potential confounders, including hypertension, DM2, sex, age, and smoking, and in some cases incorporated interaction models. In contrast, others did not, which may obscure true effects within specific subgroups. Furthermore, several studies reported statistical power below 50%, limiting the reliability of their findings. | Perform a stratified design and analysis (e.g., severity, ancestry), harmonizing data, and, use matching and predefined interaction models. Increase the number of cases by working with cohorts from different centers. | Type of analysis/study power | [14,15] |
| Study design | Population selection varied across studies and included differences in outpatient versus hospitalized cohorts, as well as demographic and socioeconomic characteristics such as age, sex, ancestry, ethnicity, and socioeconomic status. These factors may significantly influence disease prevalence and outcomes, thereby affecting comparability across studies. | Clearly define the research question, hypothesis, and objectives (use the PICO/PECO framework). Standardize the definition of variables. | Study design | [68,101,102] |
| Standardization of the technique and determinations | Rigorous standardization and the meticulous execution of laboratory measurements are essential to ensure accuracy, reproducibility, and comparability across studies. Inadequate standardization can introduce systematic error, bias results, and hinder the validity of cross-study comparisons. | Adhere to the CLSI and ISO 15189:2022 guidelines. Ensure the training and certification of operational personnel. | Standardization of the technique and determinations | [103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Hernández, N.; Rodríguez-Pérez, J.M.; Nicanor-Juárez, L.E.; Torres-Machorro, A.; García-Alva, J.R.; Villamil-Castañeda, C.; Borgonio-Cuadra, V.M.; Flores-García, M. Genetic Insights into Peripheral Artery Disease: A Narrative Review. Biomedicines 2025, 13, 2723. https://doi.org/10.3390/biomedicines13112723
Pérez-Hernández N, Rodríguez-Pérez JM, Nicanor-Juárez LE, Torres-Machorro A, García-Alva JR, Villamil-Castañeda C, Borgonio-Cuadra VM, Flores-García M. Genetic Insights into Peripheral Artery Disease: A Narrative Review. Biomedicines. 2025; 13(11):2723. https://doi.org/10.3390/biomedicines13112723
Chicago/Turabian StylePérez-Hernández, Nonanzit, José Manuel Rodríguez-Pérez, Luis Eduardo Nicanor-Juárez, Adriana Torres-Machorro, José Ramón García-Alva, Clara Villamil-Castañeda, Verónica Marusa Borgonio-Cuadra, and Mirthala Flores-García. 2025. "Genetic Insights into Peripheral Artery Disease: A Narrative Review" Biomedicines 13, no. 11: 2723. https://doi.org/10.3390/biomedicines13112723
APA StylePérez-Hernández, N., Rodríguez-Pérez, J. M., Nicanor-Juárez, L. E., Torres-Machorro, A., García-Alva, J. R., Villamil-Castañeda, C., Borgonio-Cuadra, V. M., & Flores-García, M. (2025). Genetic Insights into Peripheral Artery Disease: A Narrative Review. Biomedicines, 13(11), 2723. https://doi.org/10.3390/biomedicines13112723







