Takotsubo Syndrome and Cancer: Pathophysiological Links and Clinical Perspectives
Abstract
1. Introduction
2. Materials and Methods
3. Physiopathological Mechanisms of Takotsubo Syndrome
4. Specific Triggers in Cancer Patients
5. Chemotherapy and Takotsubo Syndrome
5.1. Fluoropyrimidines
5.2. Targeted Agents
5.3. Immune Checkpoint Inhibitors
5.4. Other Agents
5.5. Radiotherapy
6. Clinical Presentation and Diagnosis
6.1. Clinical Presentation
6.2. Echocardiogram
6.3. Electrocardiogram
6.4. Biomarkers
6.5. Coronary Angiography and Ventriculography
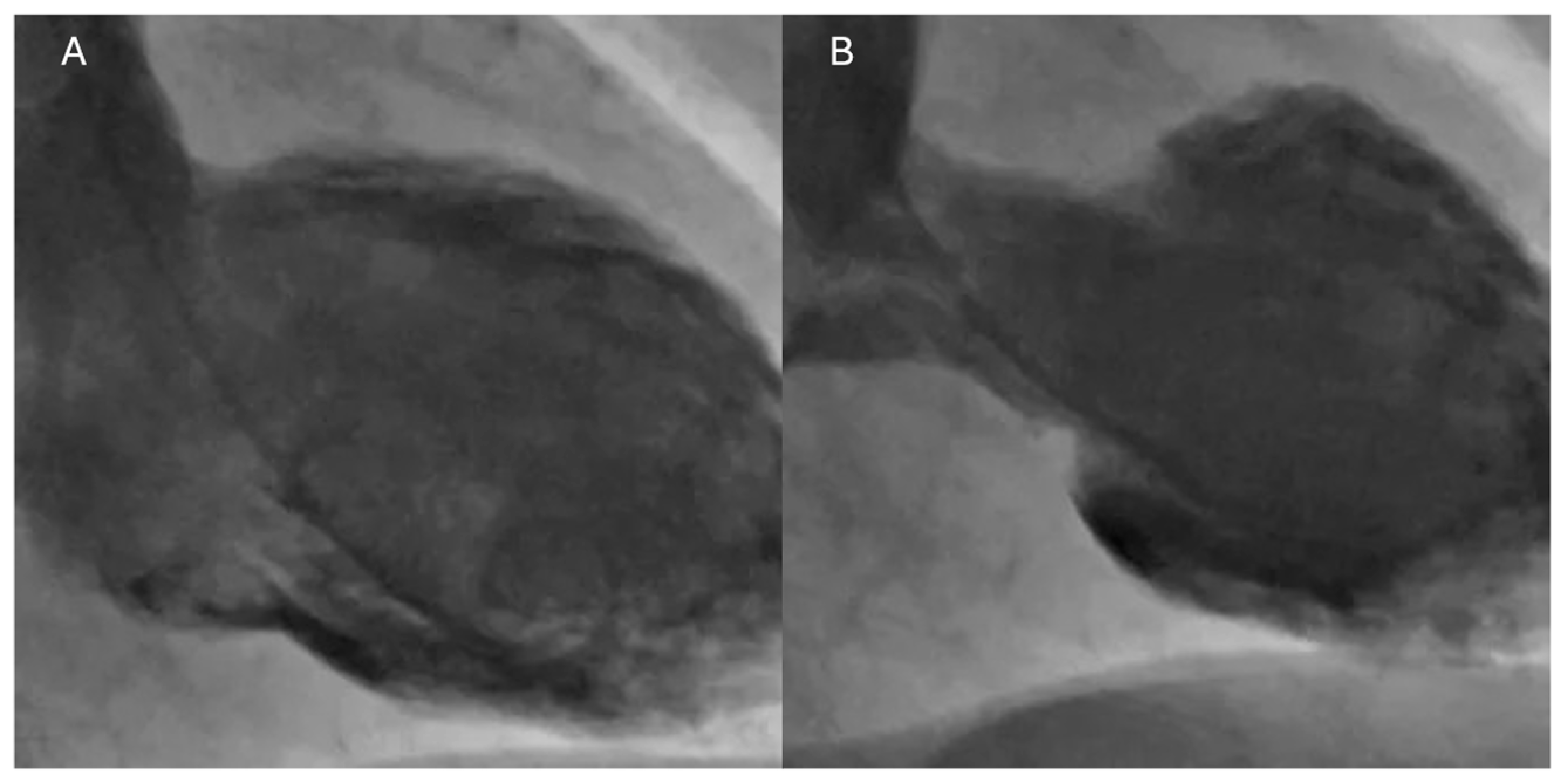
6.6. Cardiac Magnetic Resonance
- -
- T2-weighted imaging: In the acute phase of TTS, myocardial hyperemia and inflammation caused by excess catecholamines lead to an increase in tissue water content. T2-weighted images show myocardial edema that typically extends along the dyskinetic segments. This edema is a sign of acute and transient injury [64].
- -
- Late gadolinium enhancement (LGE): LGE is the standard technique for identifying myocardial fibrosis or irreversible necrosis. In TTS, the characteristic finding is the absence of LGE in the segments with ventricular dysfunction. This absence is an essential differentiator from myocardial infarction, where LGE is present in a subendocardial or transmural pattern corresponding to the territory of an occluded coronary artery. The absence of LGE in TTS reflects the lack of irreversible necrosis, supporting the benign and transient nature of the condition [65].
- -
- T1 mapping and T2 mapping: These quantitative techniques offer a more sensitive and specific assessment of diffuse edema and fibrosis. In acute TTS, T2 mapping values are elevated, reflecting the edema, while T1 mapping values are typically normal or only slightly elevated [66]. Table 3 summarizes the different features between TTS and ICI myocarditis.
6.7. Prognosis
6.8. Therapeutic Management
7. Future Perspective
- Pathophysiological distinction and diagnostic refinement: Further studies are required to definitively determine if cardiotoxicity induced by oncology drugs, especially ICIs, represents a true TTS, ICI-related myocarditis, or a distinct type of chemotherapy-related reversible non-ischemic cardiomyopathy. It is important to implement early diagnostic algorithms, including CMR and endomyocardial biopsy, to distinguish TTS from myocarditis in both clinically stable and unstable patients.
- Risk stratification and surveillance: Identifying cancer patient cohorts at the highest risk of TTS and late LV recovery (e.g., those with active malignancy, physical triggers, and high inflammatory markers) is paramount. Prospective baseline screening using biomarkers, such as troponin and neuropeptides, and cardiac imaging in high-risk patients may enable targeted cardioprotection strategies.
- Risk of recurrence: Most studies and registries report a recurrence rate that varies approximately between 1% and 6% of patients who have had a first episode. Recurrence can occur at variable time intervals, even several years after the initial event. Some studies suggest that the presence of neurological disorders and psychiatric disorders may be associated with a higher risk of recurrence, but there is no evidence regarding the role of cancer [82].
- Optimal management and re-challenge safety: There is an unmet need to establish the optimal management of ICI-related TTS, including the definitive role of immunosuppressive therapy, such as corticosteroids, when myocarditis is excluded. Furthermore, definitive data regarding the safety and timing of reintroducing antineoplastic therapy, especially ICIs, after an episode of TTS are urgently needed. Decisions must currently be individualized by a multidisciplinary cardio-oncology team.
- Prospective studies: Large, prospective multicenter registries are essential to gather high-granularity data on the long-term prognosis and true incidence of TTS in cancer survivors, particularly focusing on the adverse impact of delayed LV functional recovery.
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lyon, A.R.; Bossone, E.; Schneider, B.; Sechtem, U.; Citro, R.; Underwood, S.R.; Sheppard, M.N.; Figtree, G.A.; Parodi, G.; Akashi, Y.J.; et al. Current State of Knowledge on Takotsubo Syndrome: A Position Statement from the Taskforce on Takotsubo Syndrome of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2016, 18, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, J.; Brieger, D. Clinical Perspectives: Takotsubo Cardiomyopathy. Intern. Med. J. 2024, 54, 1785–1795. [Google Scholar] [CrossRef] [PubMed]
- Kurowski, V.; Kaiser, A.; von Hof, K.; Killermann, D.P.; Mayer, B.; Hartmann, F.; Schunkert, H.; Radke, P.W. Apical and Midventricular Transient Left Ventricular Dysfunction Syndrome (Tako-Tsubo Cardiomyopathy) Frequency, Mechanisms, and Prognosis. Chest 2007, 132, 809–816. [Google Scholar] [CrossRef]
- Templin, C.; Ghadri, J.R.; Diekmann, J.; Napp, L.C.; Bataiosu, D.R.; Jaguszewski, M.; Cammann, V.L.; Sarcon, A.; Geyer, V.; Neumann, C.A.; et al. Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. New Engl. J. Med. 2015, 373, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Carson, K.; Usmani, Z.; Sawhney, G.; Shah, R.; Horowitz, J. Systematic Review and Meta-Analysis of Incidence and Correlates of Recurrence of Takotsubo Cardiomyopathy. Int. J. Cardiol. 2014, 174, 696–701. [Google Scholar] [CrossRef]
- Matta, A.G.; Carrié, D. Epidemiology, Pathophysiology, Diagnosis, and Principles of Management of Takotsubo Cardiomyopathy: A Review. Med. Sci. Monit. 2023, 29, e939020-1. [Google Scholar] [CrossRef]
- Matsukawa, T.; Sugiyama, Y.; Watanabe, T.; Kobayashi, F.; Mano, T. Gender Difference in Age-Related Changes in Muscle Sympathetic Nerve Activity in Healthy Subjects. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1998, 275, R1600–R1604. [Google Scholar] [CrossRef]
- Parashar, R. Age Related Changes in Autonomic Functions. J. Clin. Diagn. Res. 2016, 10, CC11. [Google Scholar] [CrossRef]
- Brown, J.S.; Amend, S.R.; Austin, R.H.; Gatenby, R.A.; Hammarlund, E.U.; Pienta, K.J. Updating the Definition of Cancer. Mol. Cancer Res. 2023, 21, 1142–1147. [Google Scholar] [CrossRef]
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends—An Update. Cancer Epidemiol. Biomark. Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on Cardio-Oncology Developed in Collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Pelliccia, F.; Parodi, G.; Greco, C.; Antoniucci, D.; Brenner, R.; Bossone, E.; Cacciotti, L.; Capucci, A.; Citro, R.; Delmas, C.; et al. Comorbidities Frequency in Takotsubo Syndrome: An International Collaborative Systematic Review Including 1109 Patients. Am. J. Med. 2015, 128, 654.e11–654.e19. [Google Scholar] [CrossRef] [PubMed]
- Mauriello, A.; Giudice, C.D.; Vecchio, G.E.D.; Correra, A.; Maratea, A.C.; Grieco, M.; Amata, A.; Quagliariello, V.; Maurea, N.; Proietti, R.; et al. Takotsubo Syndrome and Oxidative Stress: Physiopathological Linkage and Future Perspectives. Antioxidants 2025, 14, 522. [Google Scholar] [CrossRef]
- Roqué, M.; Martínez-García, L.; Solà, I.; Alonso-Coello, P.; Bonfill, X.; Zamora, J. Toolkit of Methodological Resources to Conduct Systematic Reviews. F1000Res 2020, 9, 82. [Google Scholar] [CrossRef]
- Sharkey, S.W.; Windenburg, D.C.; Lesser, J.R.; Maron, M.S.; Hauser, R.G.; Lesser, J.N.; Haas, T.S.; Hodges, J.S.; Maron, B.J. Natural History and Expansive Clinical Profile of Stress (Tako-Tsubo) Cardiomyopathy. J. Am. Coll. Cardiol. 2010, 55, 333–341. [Google Scholar] [CrossRef]
- Viceconte, N.; Petrella, G.; Pelliccia, F.; Tanzilli, G.; Cicero, D.O. Unraveling Pathophysiology of Takotsubo Syndrome: The Emerging Role of the Oxidative Stress’s Systemic Status. J. Clin. Med. 2022, 11, 7515. [Google Scholar] [CrossRef]
- Sultana, S.S.; Nisar, S.; Kumar, F.M.; Khan, H.; Saeed, H.; Ahmed, G.; Malik, J. Role of Positive Emotions in Takotsubo Cardiomyopathy. Curr. Probl. Cardiol. 2023, 48, 101997. [Google Scholar] [CrossRef] [PubMed]
- Samuels, M.A. Neurogenic Heart Disease: A Unifying Hypothesis. Am. J. Cardiol. 1987, 60, J15–J19. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; Rees, P.S.; Prasad, S.; Poole-Wilson, P.A.; Harding, S.E. Stress (Takotsubo) Cardiomyopathy—A Novel Pathophysiological Hypothesis to Explain Catecholamine-Induced Acute Myocardial Stunning. Nat. Clin. Pract. Cardiovasc. Med. 2008, 5, 22–29. [Google Scholar] [CrossRef]
- Heubach, J.F.; Ravens, U.; Kaumann, A.J. Epinephrine Activates Both Gs and Gi Pathways, but Norepinephrine Activates Only the Gs Pathway through Human Β2-Adrenoceptors Overexpressed in Mouse Heart. Mol. Pharmacol. 2004, 65, 1313–1322. [Google Scholar] [CrossRef]
- Zhu, W.-Z.; Zheng, M.; Koch, W.J.; Lefkowitz, R.J.; Kobilka, B.K.; Xiao, R.-P. Dual Modulation of Cell Survival and Cell Death by β 2 -Adrenergic Signaling in Adult Mouse Cardiac Myocytes. Proc. Natl. Acad. Sci. USA 2001, 98, 1607–1612. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kang, M.-I.; Watai, Y.; Tong, K.I.; Shibata, T.; Uchida, K.; Yamamoto, M. Oxidative and Electrophilic Stresses Activate Nrf2 through Inhibition of Ubiquitination Activity of Keap1. Mol. Cell Biol. 2006, 26, 221–229. [Google Scholar] [CrossRef]
- Nef, H.M.; Möllmann, H.; Troidl, C.; Kostin, S.; Böttger, T.; Voss, S.; Hilpert, P.; Krause, N.; Weber, M.; Rolf, A.; et al. Expression Profiling of Cardiac Genes in Tako-Tsubo Cardiomyopathy: Insight into a New Cardiac Entity. J. Mol. Cell Cardiol. 2008, 44, 395–404. [Google Scholar] [CrossRef]
- Saito, H.; Itoh, T.; Itoh, M.; Kanaya, Y.; Suzuki, T.; Hiramori, K. Simultaneous Multivessel Coronary Spasm Causing Acute Myocardial Infarction. Angiology 2007, 58, 112–117. [Google Scholar] [CrossRef]
- Vitale, C.; Rosano, G.M.; Kaski, J.C. Role of Coronary Microvascular Dysfunction in Takotsubo Cardiomyopathy. Circ. J. 2016, 80, 299–305. [Google Scholar] [CrossRef] [PubMed]
- El Mahmoud, R.; Mansencal, N.; Pilliére, R.; Leyer, F.; Abbou, N.; Michaud, P.; Nallet, O.; Digne, F.; Lacombe, P.; Cattan, S.; et al. Prevalence and Characteristics of Left Ventricular Outflow Tract Obstruction in Tako-Tsubo Syndrome. Am. Heart J. 2008, 156, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Mauriello, A.; Marrazzo, G.; Del Vecchio, G.E.; Ascrizzi, A.; Roma, A.S.; Correra, A.; Sabatella, F.; Gioia, R.; Desiderio, A.; Russo, V.; et al. Echocardiography in Cardiac Arrest: Incremental Diagnostic and Prognostic Role during Resuscitation Care. Diagnostics 2024, 14, 2107. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, J.J.; Xu, D. The Role of Inflammation in Takotsubo Syndrome: A New Therapeutic Target? J. Cell Mol. Med. 2024, 28, e18503. [Google Scholar] [CrossRef]
- Ziegler, K.A.; Zeitler, M.; Meunier, S.; Sinicina, I.; Hasenbein, T.P.; Andergassen, D.; Bomhard, A.; van der Kwast, R.V.C.T.; Engelhardt, S. Ganglionic Inflammation in a Patient with Takotsubo Syndrome. Circulation 2025, 151, 890–892. [Google Scholar] [CrossRef]
- Desai, A.; Noor, A.; Joshi, S.; Kim, A.S. Takotsubo Cardiomyopathy in Cancer Patients. Cardio-Oncology 2019, 5, 7. [Google Scholar] [CrossRef]
- Almendro-Delia, M.; López-Flores, L.; Uribarri, A.; Vedia, O.; Blanco-Ponce, E.; López-Flores, M.D.C.; Rivas-García, A.P.; Fernández-Cordón, C.; Sionis, A.; Martín-García, A.C.; et al. Recovery of Left Ventricular Function and Long-Term Outcomes in Patients with Takotsubo Syndrome. J. Am. Coll. Cardiol. 2024, 84, 1163–1174. [Google Scholar] [CrossRef]
- Brunetti, N.D.; Tarantino, N.; Guastafierro, F.; De Gennaro, L.; Correale, M.; Stiermaier, T.; Möller, C.; Di Biase, M.; Eitel, I.; Santoro, F. Malignancies and Outcome in Takotsubo Syndrome: A Meta-Analysis Study on Cancer and Stress Cardiomyopathy. Heart Fail. Rev. 2019, 24, 481–488. [Google Scholar] [CrossRef]
- Aw, A.; de Jong, M.C.; Varghese, S.; Lee, J.; Foo, R.; Parameswaran, R. A Systematic Cohort Review of Pheochromocytoma-Induced Typical versus Atypical Takotsubo Cardiomyopathy. Int. J. Cardiol. 2023, 371, 287–292. [Google Scholar] [CrossRef]
- Keramida, K.; Farmakis, D.; Filippatos, G. Cancer and Takotsubo Syndrome: From Rarity to Clinical Practice. ESC Heart Fail. 2021, 8, 4365–4369. [Google Scholar] [CrossRef]
- Grunwald, M.R.; Howie, L.; Diaz, L.A. Takotsubo Cardiomyopathy and Fluorouracil: Case Report and Review of the Literature. J. Clin. Oncol. 2012, 30, e11–e14. [Google Scholar] [CrossRef]
- Cheriparambil, K.M.; Vasireddy, H.; Kuruvilla, A.; Gambarin, B.; Makan, M.; Saul, B.I. Acute Reversible Cardiomyopathy and Thromboembolism After Cisplatin and 5-Fluorouracil Chemotherapy. Angiology 2000, 51, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Franco, T. Takotsubo Cardiomyopathy in Two Men Receiving Bevacizumab for Metastatic Cancer. Ther. Clin. Risk Manag. 2008, 4, 1367–1370. [Google Scholar] [CrossRef] [PubMed]
- Ederhy, S.; Dolladille, C.; Thuny, F.; Alexandre, J.; Cohen, A. Takotsubo Syndrome in Patients with Cancer Treated with Immune Checkpoint Inhibitors: A New Adverse Cardiac Complication. Eur. J. Heart Fail. 2019, 21, 945–947. [Google Scholar] [CrossRef]
- Oberoi, M.; Garvin, R.; Christensen, C.; Porter, T.R. Abstract 15188: Mid-Cavity Takotsubo Cardiomyopathy Post CAR T-Cell Therapy. Circulation 2023, 148, A15188. [Google Scholar] [CrossRef]
- Gupta, R.; Sen, A.; Khosla, J.; Ranchal, P.; Aronow, W.S.; Shehu, M. Takotsubo Cardiomyopathy Secondary to Rituximab. Am. J. Ther. 2022, 29, 451–454. [Google Scholar] [CrossRef]
- Dobbin, S.J.H.; Petrie, M.C.; Myles, R.C.; Touyz, R.M.; Lang, N.N. Cardiotoxic Effects of Angiogenesis Inhibitors. Clin. Sci. 2021, 135, 71–100. [Google Scholar] [CrossRef]
- Camilli, M.; Cipolla, C.M.; Dent, S.; Minotti, G.; Cardinale, D.M. Anthracycline Cardiotoxicity in Adult Cancer Patients. Cardio Oncol. 2024, 6, 655–677. [Google Scholar] [CrossRef] [PubMed]
- Desai, R.; Desai, A.; Abbas, S.A.; Patel, U.; Bansod, S.; Damarlapally, N.; Doshi, R.; Savani, S.; Gangani, K.; Sachdeva, R.; et al. National Prevalence, Trends and Outcomes of Takotsubo Syndrome in Hospitalizations with Prior History of Mediastinal/Intrathoracic Cancer and Radiation Therapy. Int. J. Cardiol. 2020, 309, 14–18. [Google Scholar] [CrossRef]
- Trontzas, I.P.; Vathiotis, I.A.; Kyriakoulis, K.G.; Sofianidi, A.; Spyropoulou, Z.; Charpidou, A.; Kotteas, E.A.; Syrigos, K.N. Takotsubo Cardiomyopathy in Cancer Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Summary of Included Cases. Cancers 2023, 15, 2637. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Menger, J.; Collini, V.; Gröschel, J.; Adler, Y.; Brucato, A.; Christian, V.; Ferreira, V.M.; Gandjbakhch, E.; Heidecker, B.; Kerneis, M.; et al. 2025 ESC Guidelines for the Management of Myocarditis and Pericarditis. Eur. Heart J. 2025, 46, 3952–4041. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Khan, H.; Gamble, D.T.; Scally, C.; Newby, D.E.; Dawson, D. Takotsubo Syndrome: Pathophysiology, Emerging Concepts, and Clinical Implications. Circulation 2022, 145, 1002–1019. [Google Scholar] [CrossRef]
- Citro, R.; Lyon, A.R.; Meimoun, P.; Omerovic, E.; Redfors, B.; Buck, T.; Lerakis, S.; Parodi, G.; Silverio, A.; Eitel, I.; et al. Standard and Advanced Echocardiography in Takotsubo (Stress) Cardiomyopathy: Clinical and Prognostic Implications. J. Am. Soc. Echocardiogr. 2015, 28, 57–74. [Google Scholar] [CrossRef]
- Ludhwani, D.; Sheikh, B.; Patel, V.K.; Jhaveri, K.; Kizilbash, M.; Sura, P. Atypical Takotsubo Cardiomyopathy with Hypokinetic Left Mid-Ventricle and Apical Wall Sparing: A Case Report and Literature Review. Curr. Cardiol. Rev. 2020, 16, 241–246. [Google Scholar] [CrossRef]
- Tabira, A.; Misumi, I.; Sato, K.; Matsuda, H.; Iwasaki, T.; Usuku, H.; Tsujita, K. Mid-Ventricular Obstructive Cardiomyopathy after Takotsubo Cardiomyopathy. Intern. Med. 2023, 62, 2365–2373. [Google Scholar] [CrossRef]
- Awad, H.H.; McNeal, A.R.; Goyal, H. Reverse Takotsubo Cardiomyopathy: A Comprehensive Review. Ann. Transl. Med. 2018, 6, 460. [Google Scholar] [CrossRef]
- Tini, G.; Limite, L.R.; Arcari, L.; Cacciotti, L.; Russo, D.; Sclafani, M.; Brunelli, C.; Volpe, M.; Autore, C.; Musumeci, M.B. A Systematic Review on Focal Takotsubo Syndrome: A Not-so-Small Matter. Heart Fail. Rev. 2022, 27, 271–280. [Google Scholar] [CrossRef]
- Laumer, F.; Di Vece, D.; Cammann, V.L.; Würdinger, M.; Petkova, V.; Schönberger, M.; Schönberger, A.; Mercier, J.C.; Niederseer, D.; Seifert, B.; et al. Assessment of Artificial Intelligence in Echocardiography Diagnostics in Differentiating Takotsubo Syndrome from Myocardial Infarction. JAMA Cardiol. 2022, 7, 494. [Google Scholar] [CrossRef]
- Buckley, B.J.R.; Harrison, S.L.; Hill, A.; Underhill, P.; Lane, D.A.; Lip, G.Y.H. Stroke-Heart Syndrome: Incidence and Clinical Outcomes of Cardiac Complications Following Stroke. Stroke 2022, 53, 1759–1763. [Google Scholar] [CrossRef]
- Citro, R.; Rigo, F.; Ciampi, Q.; D’Andrea, A.; Provenza, G.; Mirra, M.; Giudice, R.; Silvestri, F.; Di Benedetto, G.; Bossone, E. Echocardiographic Assessment of Regional Left Ventricular Wall Motion Abnormalities in Patients with Tako-Tsubo Cardiomyopathy: Comparison with Anterior Myocardial Infarction. Eur. J. Echocardiogr. 2011, 12, 542–549. [Google Scholar] [CrossRef]
- Wittstein, I.S.; Thiemann, D.R.; Lima, J.A.C.; Baughman, K.L.; Schulman, S.P.; Gerstenblith, G.; Wu, K.C.; Rade, J.J.; Bivalacqua, T.J.; Champion, H.C. Neurohumoral Features of Myocardial Stunning Due to Sudden Emotional Stress. N. Engl. J. Med. 2005, 352, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, L. ST-Elevation in Takotsubo Cardiomyopathy. J. Electrocardiol. 2023, 77, 78–79. [Google Scholar] [CrossRef] [PubMed]
- Madias, J.E. Electrocardiogram Repolarization Markers and Ventricular Arrhythmias in Patients with Takotsubo Syndrome. Curr. Probl. Cardiol. 2024, 49, 102757. [Google Scholar] [CrossRef]
- Zeijlon, R.; Jha, S.; Le, V.; Chamat, J.; Shekka Espinosa, A.; Poller, A.; Thorleifsson, S.; Bobbio, E.; Mellberg, T.; Pirazzi, C.; et al. Temporal Electrocardiographic Changes in Anterior ST Elevation Myocardial Infarction versus the Takotsubo Syndrome. IJC Heart Vasc. 2023, 45, 101187. [Google Scholar] [CrossRef]
- Bakhshi, H.; Gibson, C.M. MINOCA: Myocardial Infarction No Obstructive Coronary Artery Disease. Am. Heart J. Plus Cardiol. Res. Pract. 2023, 33, 100312. [Google Scholar] [CrossRef] [PubMed]
- Vogiatzis, I.; Dapcevic, I.; Datsios, A.; Koutsambasopoulos, K.; Gontopoulos, A. Grigoriadis, and A Comparison of Prognostic Value of the Levels of ProBNP and Troponin T in Patients with Acute Coronary Syndrome (ACS). Med. Arch. 2016, 70, 269. [Google Scholar] [CrossRef]
- Pelliccia, F.; Kaski, J.C.; Crea, F.; Camici, P.G. Pathophysiology of Takotsubo Syndrome. Circulation 2017, 135, 2426–2441. [Google Scholar] [CrossRef] [PubMed]
- Misumida, N.; Ogunbayo, G.O.; Kim, S.M.; Abdel-Latif, A.; Ziada, K.M.; Sorrell, V.L. Clinical Outcome of Takotsubo Cardiomyopathy Diagnosed with or Without Coronary Angiography. Angiology 2019, 70, 56–61. [Google Scholar] [CrossRef]
- Padilla-Lopez, M.; Duran-Cambra, A.; Belmar-Cliville, D.; Soriano-Amores, M.; Arakama-Goikoetxea, S.; Vila-Perales, M.; Bragagnini, W.; Rodríguez-Sotelo, L.; Peña-Ortega, P.; Sánchez-Vega, J.; et al. Comparative Electrocardiographic Analysis of Midventricular and Typical Takotsubo Syndrome. Front. Cardiovasc. Med. 2023, 10, 1286975. [Google Scholar] [CrossRef]
- Ojha, V.; Khurana, R.; Ganga, K.P.; Kumar, S. Advanced Cardiac Magnetic Resonance Imaging in Takotsubo Cardiomyopathy. Br. J. Radiol. 2020, 93, 20200514. [Google Scholar] [CrossRef]
- Ghadri, J.-R.; Wittstein, I.S.; Prasad, A.; Sharkey, S.; Dote, K.; Akashi, Y.J.; Cammann, V.L.; Crea, F.; Galiuto, L.; Desmet, W.; et al. International Expert Consensus Document on Takotsubo Syndrome (Part II): Diagnostic Workup, Outcome, and Management. Eur. Heart J. 2018, 39, 2047–2062. [Google Scholar] [CrossRef]
- Arcari, L.; Camastra, G.; Ciolina, F.; Belmonte, E.; De Santis, D.; Danti, M.; Caruso, D.; Maestrini, V.; Santoro, F.; Brunetti, N.D.; et al. Cardiac Magnetic Resonance in Patients with Takotsubo Syndrome: Clinical Correlates of T2 Mapping. Int. J. Cardiol. 2025, 419, 132716. [Google Scholar] [CrossRef]
- Lu, S.-Y.; Chen, J.-J.; Pan, J.-I.; Fu, Z.-X.; Wu, J.-L.; Hsieh, T.-C. The Association Between Different Patterns of Traditional Chinese Medicine Treatment and All-Cause Mortality Among Cancer Patients. Integr. Cancer Ther. 2019, 18, 1534735418823273. [Google Scholar] [CrossRef] [PubMed]
- Givertz, M.M.; Mann, D.L. Epidemiology and Natural History of Recovery of Left Ventricular Function in Recent Onset Dilated Cardiomyopathies. Curr. Heart Fail. Rep. 2013, 10, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Lachmet-Thébaud, L.; Marchandot, B.; Matsushita, K.; Dagrenat, C.; Peillex, M.; Sato, C.; Trimaille, A.; Reydel, A.; Trinh, A.; Ohlmann, P.; et al. Systemic Inflammatory Response Syndrome Is a Major Determinant of Cardiovascular Outcome in Takotsubo Syndrome. Circ. J. 2020, 84, 592–600. [Google Scholar] [CrossRef]
- Núñez-Gil, I.J.; Vedia, O.; Almendro-Delia, M.; Raposeiras-Roubín, S.; Sionis, A.; Martin-García, A.C.; Martin-García, A.; Andrés, M.; Blanco, E.; Martín-de-Miguel, I.; et al. Implicaciones Clínicas y Pronósticas En Síndrome de Takotsubo y Cáncer: Percepciones Del Registro RETAKO. Med. Clin. 2020, 155, 521–528. [Google Scholar] [CrossRef]
- Sclafani, M.; Arcari, L.; Russo, D.; Tini, G.; Limite, L.R.; Cacciotti, L.; Volpe, M.; Autore, C.; Musumeci, M.B. Long-Term Management of Takotsubo Syndrome: A Not-so-Benign Condition. Rev. Cardiovasc. Med. 2021, 22, 597–611. [Google Scholar] [CrossRef]
- Curigliano, G.; Cardinale, D.; Dent, S.; Criscitiello, C.; Aseyev, O.; Lenihan, D.; Cipolla, C.M. Cardiotoxicity of Anticancer Treatments: Epidemiology, Detection, and Management. CA Cancer J. Clin. 2016, 66, 309–325. [Google Scholar] [CrossRef]
- Bybee, K.A.; Kara, T.; Prasad, A.; Lerman, A.; Barsness, G.W.; Wright, R.S.; Rihal, C.S. Systematic Review: Transient Left Ventricular Apical Ballooning: A Syndrome That Mimics ST-Segment Elevation Myocardial Infarction. Ann. Intern. Med. 2004, 141, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Mauriello, A.; Ascrizzi, A.; Roma, A.S.; Molinari, R.; Caturano, A.; Imbalzano, E.; D’Andrea, A.; Russo, V. Effects of Heart Failure Therapies on Atrial Fibrillation: Biological and Clinical Perspectives. Antioxidants 2024, 13, 806. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-K.; Sun, B.J.; Kim, D.-H.; Jung, S.H. Update on Left Ventricular Outflow Tract Obstruction. J. Cardiovasc. Imaging 2025, 33, 6. [Google Scholar] [CrossRef]
- Mauriello, A.; Correra, A.; Quagliariello, V.; Iovine, M.; Di Micco, P.; Imbalzano, E.; Giallauria, F.; Giordano, A.; Russo, V.; D’Andrea, A.; et al. Atrial Fibrillation and Cancer: Pathophysiological Mechanism and Clinical Implications. J. Clin. Med. 2025, 14, 5600. [Google Scholar] [CrossRef]
- Citro, R.; Bellino, M.; Merli, E.; Di Vece, D.; Sherrid, M.V. Obstructive Hypertrophic Cardiomyopathy and Takotsubo Syndrome: How to Deal with Left Ventricular Ballooning? J. Am. Heart Assoc. 2023, 12, e032028. [Google Scholar] [CrossRef] [PubMed]
- Santoro, F.; Stiermaier, T.; Guastafierro, F.; Tarantino, N.; Eitel, I.; Brunetti, N.D. Oral Anticoagulation in High Risk Takotsubo Syndrome: When Should It Be Considered and When Not? BMC Cardiovasc. Disord. 2018, 18, 205. [Google Scholar] [CrossRef]
- Santoro, F.; Stiermaier, T.; Tarantino, N.; De Gennaro, L.; Moeller, C.; Guastafierro, F.; Marchetti, M.F.; Montisci, R.; Carapelle, E.; Graf, T.; et al. Left Ventricular Thrombi in Takotsubo Syndrome: Incidence, Predictors, and Management: Results from the GEIST (German Italian Stress Cardiomyopathy) Registry. J. Am. Heart Assoc. 2017, 6, e006990. [Google Scholar] [CrossRef]
- Habib, M.; Aronson, D. Thromboembolic Complications in Takotsubo Cardiomyopathy. Semin. Thromb. Hemost. 2025, 51, 423–429. [Google Scholar] [CrossRef]
- Tocchetti, C.G.; Farmakis, D.; Koop, Y.; Andres, M.S.; Couch, L.S.; Formisano, L.; Ciardiello, F.; Pane, F.; Au, L.; Emmerich, M.; et al. Cardiovascular Toxicities of Immune Therapies for Cancer—A Scientific Statement of the Heart Failure Association (HFA) of the ESC and the ESC Council of Cardio-Oncology. Eur. J. Heart Fail. 2024, 26, 2055–2076. [Google Scholar] [CrossRef] [PubMed]
- Looi, J.-L.; Easton, A.; Webster, M.; To, A.; Lee, M.; Kerr, A.J. Recurrent Takotsubo Syndrome: How Frequent, and How Does It Present? Heart Lung Circ. 2024, 33, 1117–1122. [Google Scholar] [CrossRef] [PubMed]

| Risk Factor/Trigger | Antineoplastic Agents Implicated | Putative Mechanism | Clinical Notes |
|---|---|---|---|
| Emotional/Psychosocial Stress | Immune checkpoint inhibitors | Catecholamine excess, activation of the sympathetic nervous system. | TTS is associated with a higher incidence in females with cancer compared to the general population. |
| Chronic Inflammation | Rituximab, fluoropyrimidines, VEGF inhibitors and immune checkpoint inhibitors | Release of cytokines/paraneoplastic mediators modifying cardiac adrenoreceptors. | Elevated NT-proBNP and hs-CRP predict late recovery and worse outcomes. |
| Traditional Chemotherapy Targeted Therapies | 5-FU, capecitabine, cisplatin, daunorubicin. | Coronary vasospasm (most common theory for 5-FU), direct myocardial injury. | 5-FU-related TTS has a similar incidence across both sexes. Cardiotoxicity often occurs in the first cycle. |
| VEGF inhibitors, TKIs, trastuzumab. | Endothelial dysfunction, TKI-related cardiotoxicity, indirect toxicity. | Trastuzumab may induce the “reverse TTS” variant. | |
| Ipilimumab, pembrolizumab, nivolumab (monotherapy or combination). | Immune-mediated toxicity, overlap with myocarditis, and potentially T-cell cross-reactivity. | ICI exposure suggests a higher risk of TTS. Myocarditis exclusion (via CMR/biopsy) is essential. |
| ECG Features | STEMI | TTS |
|---|---|---|
| ST elevation | Regional ST elevation | Diffuse ST elevation |
| T-wave inversion | Regional T-wave inversion | Diffuse T-wave inversion |
| QTc evolution | Non-typical | Typical |
| Features | TTS | ICI Myocarditis |
|---|---|---|
| Symptoms/ECG | Diffuse T-wave inversion and QTc prolongation | High-grade AV block |
| Biomarkers | Troponin pattern | Troponin pattern and inflammatory markers |
| CMR | Edema in dysfunctional segments with absent or minimal LGE | Typical LGE patterns |
| Endomyocardial biopsy | If unstable or inconclusive | If unstable or inconclusive |
| Management | Avoid agents that worsen LVOTO in TTS | Use immunosuppression for suspected ICI myocarditis when appropriate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correra, A.; Mauriello, A.; Maratea, A.C.; Fonderico, C.; Di Peppo, M.; Russo, V.; D’Andrea, A.; Esposito, G.; Brunetti, N.D. Takotsubo Syndrome and Cancer: Pathophysiological Links and Clinical Perspectives. Biomedicines 2025, 13, 2718. https://doi.org/10.3390/biomedicines13112718
Correra A, Mauriello A, Maratea AC, Fonderico C, Di Peppo M, Russo V, D’Andrea A, Esposito G, Brunetti ND. Takotsubo Syndrome and Cancer: Pathophysiological Links and Clinical Perspectives. Biomedicines. 2025; 13(11):2718. https://doi.org/10.3390/biomedicines13112718
Chicago/Turabian StyleCorrera, Adriana, Alfredo Mauriello, Anna Chiara Maratea, Celeste Fonderico, Matilde Di Peppo, Vincenzo Russo, Antonello D’Andrea, Giovanni Esposito, and Natale Daniele Brunetti. 2025. "Takotsubo Syndrome and Cancer: Pathophysiological Links and Clinical Perspectives" Biomedicines 13, no. 11: 2718. https://doi.org/10.3390/biomedicines13112718
APA StyleCorrera, A., Mauriello, A., Maratea, A. C., Fonderico, C., Di Peppo, M., Russo, V., D’Andrea, A., Esposito, G., & Brunetti, N. D. (2025). Takotsubo Syndrome and Cancer: Pathophysiological Links and Clinical Perspectives. Biomedicines, 13(11), 2718. https://doi.org/10.3390/biomedicines13112718









