β-Casomorphin-7 as a Potential Inflammatory Marker: How β-Casomorphin-7 Induces Endothelial Dysfunction in HUVEC/TERT2 Cell Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture Conditions
2.2. Determination of Cell Viability
2.3. Determination of Apoptosis
2.4. Determination of Necrosis
2.5. Measurement of ROS
2.6. PCR
2.7. Statistics
3. Results
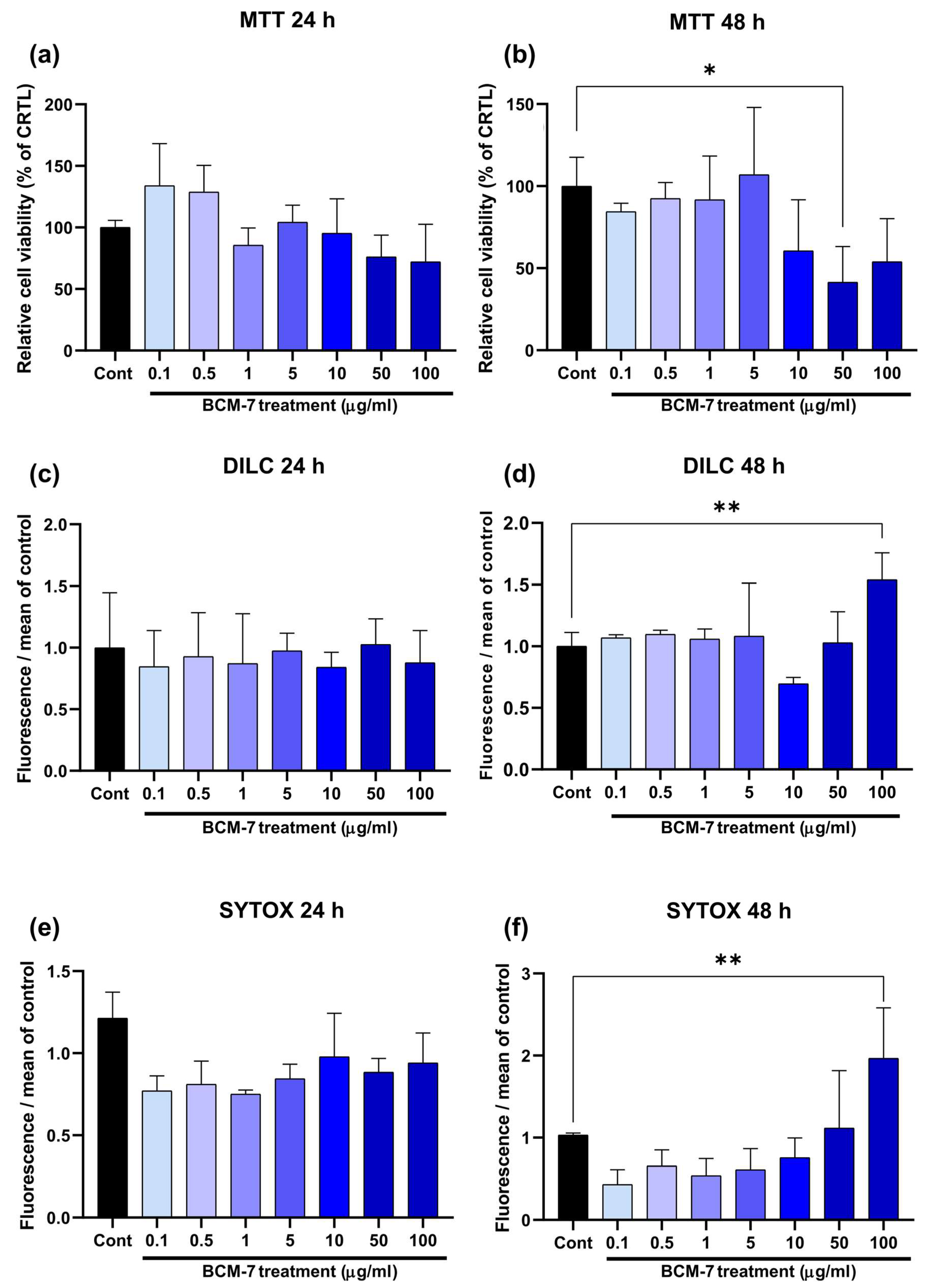
3.1. BCM-7 Effects on Endothelial Cell Viability
3.2. BCM-7 Treatment-Induced Intracellular ROS Production in HUVECs
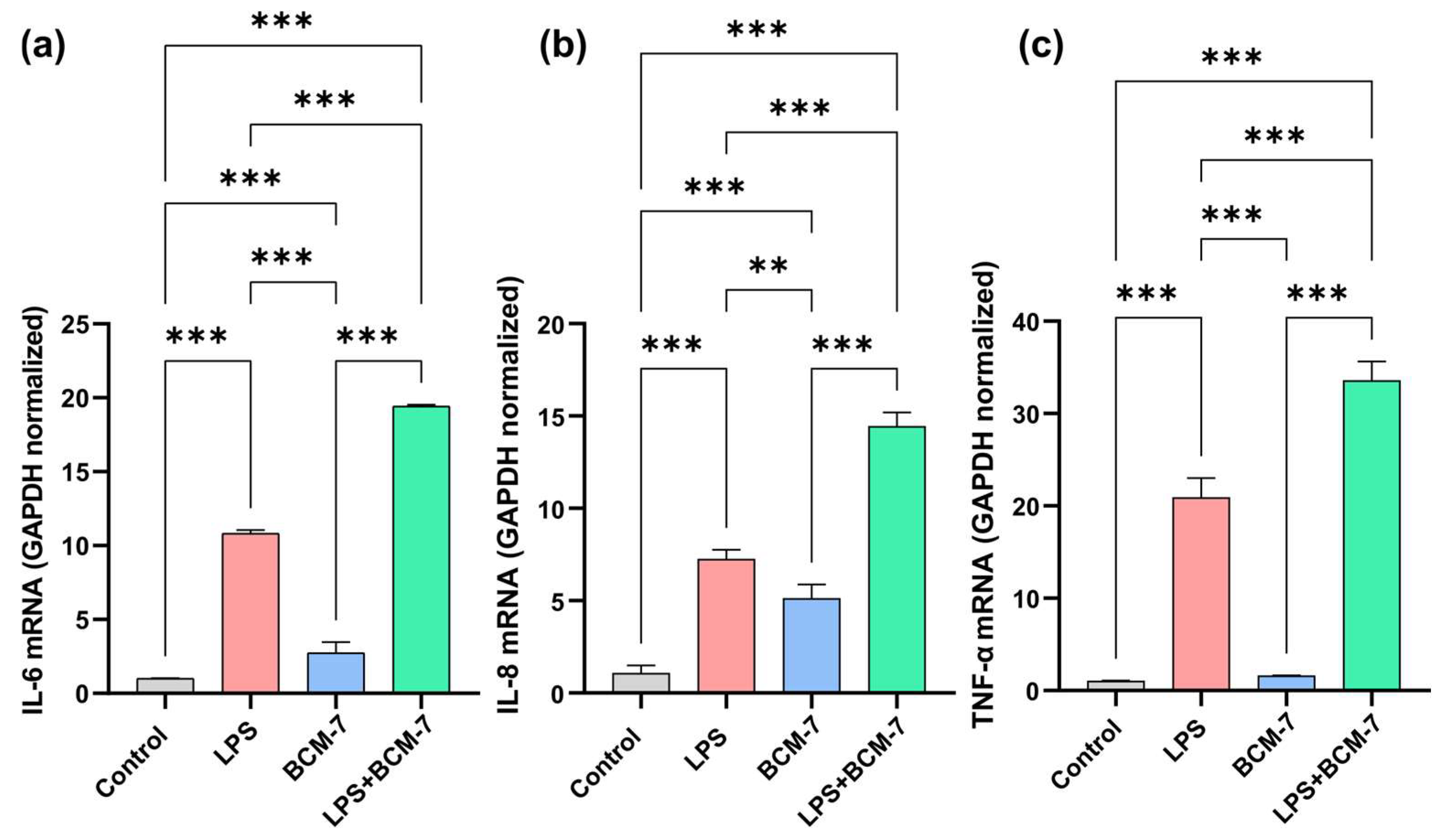
3.3. LPS-Induced Proinflammatory Cytokine Expression Was Increased by BCM-7 in Endothelial Cells
3.4. BCM-7 Remarkably Influences the Expression of Genes That Play an Important Role in Maintaining Endothelial Cell Homeostasis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muehlhoff, E.; FAO (Eds.) Milk and Dairy Products in Human Nutrition; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; ISBN 978-92-5-107864-8. [Google Scholar]
- Jost, T.; Lacroix, C.; Braegger, C.; Chassard, C. Impact of Human Milk Bacteria and Oligosaccharides on Neonatal Gut Microbiota Establishment and Gut Health. Nutr. Rev. 2015, 73, 426–437. [Google Scholar] [CrossRef]
- Kaur, L.; Mao, B.; Beniwal, A.S.; Abhilasha; Kaur, R.; Chian, F.M.; Singh, J. Alternative Proteins vs Animal Proteins: The Influence of Structure and Processing on Their Gastro-Small Intestinal Digestion. Trends Food Sci. Technol. 2022, 122, 275–286. [Google Scholar] [CrossRef]
- Hodgkinson, A.J.; Wallace, O.A.M.; Smolenski, G.; Prosser, C.G. Gastric Digestion of Cow and Goat Milk: Peptides Derived from Simulated Conditions of Infant Digestion. Food Chem. 2019, 276, 619–625. [Google Scholar] [CrossRef]
- Italia, 1 de Septiembre de 2023. Lactancia Materna. Fondo de Las Naciones Unidas Para La Infancia (UNICEF). 2023. Available online: https://globalcitieshub.org/en/united-nations-childrens-fund-unicef/ (accessed on 2 November 2025).
- Brüssow, H. Nutrition, Population Growth and Disease: A Short History of Lactose. Environ. Microbiol. 2013, 15, 2154–2161. [Google Scholar] [CrossRef]
- Durham, R.J. Bioactive Milk Protein and Peptide Functionality. In Dairy-Derived Ingredients; Elsevier: Amsterdam, The Netherlands, 2009; pp. 238–268. ISBN 978-1-84569-465-4. [Google Scholar]
- Farrell, H.M.; Jimenez-Flores, R.; Bleck, G.T.; Brown, E.M.; Butler, J.E.; Creamer, L.K.; Hicks, C.L.; Hollar, C.M.; Ng-Kwai-Hang, K.F.; Swaisgood, H.E. Nomenclature of the Proteins of Cows’ Milk—Sixth Revision. J. Dairy Sci. 2004, 87, 1641–1674. [Google Scholar] [CrossRef] [PubMed]
- Dai, R.; Fang, Y.; Zhao, W.; Liu, S.; Ding, J.; Xu, K.; Yang, L.; He, C.; Ding, F.; Meng, H. Identification of Alleles and Genotypes of Beta-Casein with DNA Sequencing Analysis in Chinese Holstein Cow. J. Dairy Res. 2016, 83, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Brooke-Taylor, S.; Dwyer, K.; Woodford, K.; Kost, N. Systematic Review of the Gastrointestinal Effects of A1 Compared with A2 β-Casein. Adv. Nutr. 2017, 8, 739–748. [Google Scholar] [CrossRef]
- Ng-Kwai-Hang, K.F.; Grosclaude, F. Genetic Polymorphism of Milk Proteins. In Advanced Dairy Chemistry; Springer: Boston, MA, USA, 2013; pp. 463–514. ISBN 978-1-4614-4713-9. [Google Scholar]
- Cieślińska, A.; Fiedorowicz, E.; Zwierzchowski, G.; Kordulewska, N.; Jarmołowska, B.; Kostyra, E. Genetic Polymorphism of β-Casein Gene in Polish Red Cattle—Preliminary Study of A1 and A2 Frequency in Genetic Conservation Herd. Animals 2019, 9, 377. [Google Scholar] [CrossRef] [PubMed]
- Jinsmaa, Y.; Yoshikawa, M. Enzymatic Release of Neocasomorphin and β-Casomorphin from Bovine β-Casein. Peptides 1999, 20, 957–962. [Google Scholar] [CrossRef]
- Kamiński, S.; Cieślińska, A.; Kostyra, E. Polymorphism of Bovine Beta-Casein and Its Potential Effect on Human Health. J. Appl. Genet. 2007, 48, 189–198. [Google Scholar] [CrossRef]
- Kumar, S.; Dahiya, S.P.; Kumar, S.; Magotra, A. Type of Milk (A1/A2) and Human Health Attributes: A Review. Indian J. Health Well-Being 2017, 8, 1268–1270. [Google Scholar]
- Ul Haq, M.R.; Kapila, R.; Kapila, S. Release of β-Casomorphin-7/5 during Simulated Gastrointestinal Digestion of Milk β-Casein Variants from Indian Crossbred Cattle (Karan Fries). Food Chem. 2015, 168, 70–79. [Google Scholar] [CrossRef]
- Meisel, H.; FitzGerald, R.J. Opioid Peptides Encrypted in Intact Milk Protein Sequences. Br. J. Nutr. 2000, 84, 27–31. [Google Scholar] [CrossRef]
- Nylund, G.; Pettersson, A.; Bengtsson, C.; Khorram-Manesh, A.; Nordgren, S.; Delbro, D.S. Functional Expression of μ-Opioid Receptors in the Human Colon Cancer Cell Line, HT-29, and Their Localization in Human Colon. Dig. Dis. Sci. 2008, 53, 461–466. [Google Scholar] [CrossRef]
- Bidlack, J.M. Detection and Function of Opioid Receptors on Cells from the Immune System. Clin. Diagn. Lab. Immunol. 2000, 7, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.; Fox, C.A.; Akil, H.; Watson, S.J. Opioid-Receptor mRNA Expression in the Rat CNS: Anatomical and Functional Implications. Trends Neurosci. 1995, 18, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Teschemacher, H. Opioid Receptor Ligands Derived from Food Proteins. Curr. Pharm. Des. 2003, 9, 1331–1344. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, M.S.; Shah, J.S.; Al-Mughairy, S.; Hodgson, N.W.; Simms, B.; Trooskens, G.A.; Van Criekinge, W.; Deth, R.C. Food-Derived Opioid Peptides Inhibit Cysteine Uptake with Redox and Epigenetic Consequences. J. Nutr. Biochem. 2014, 25, 1011–1018. [Google Scholar] [CrossRef]
- Crowley, E.; Williams, L.; Roberts, T.; Dunstan, R.; Jones, P. Does Milk Cause Constipation? A Crossover Dietary Trial. Nutrients 2013, 5, 253–266. [Google Scholar] [CrossRef]
- Deth, R.; Clarke, A.; Ni, J.; Trivedi, M. Clinical Evaluation of Glutathione Concentrations after Consumption of Milk Containing Different Subtypes of β-Casein: Results from a Randomized, Cross-over Clinical Trial. Nutr. J. 2015, 15, 82. [Google Scholar] [CrossRef]
- Semaan, H.B.; Gurbel, P.A.; Anderson, J.L.; Muhlestein, J.B.; Carlquist, J.F.; Horne, B.D.; Serebruany, V.L. Soluble VCAM-1 and E-Selectin, but Not ICAM-1 Discriminate Endothelial Injury in Patients with Documented Coronary Artery Disease. Cardiology 2000, 93, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Mundi, S.; Massaro, M.; Scoditti, E.; Carluccio, M.A.; Van Hinsbergh, V.W.M.; Iruela-Arispe, M.L.; De Caterina, R. Endothelial Permeability, LDL Deposition, and Cardiovascular Risk Factors—A Review. Cardiovasc. Res. 2018, 114, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Cieślińska, A.; Fiedorowicz, E.; Rozmus, D.; Sienkiewicz-Szłapka, E.; Jarmołowska, B.; Kamiński, S. Does a Little Difference Make a Big Difference? Bovine β-Casein A1 and A2 Variants and Human Health—An Update. Int. J. Mol. Sci. 2022, 23, 15637. [Google Scholar] [CrossRef] [PubMed]
- Tailford, K.A.; Berry, C.L.; Thomas, A.C.; Campbell, J.H. A Casein Variant in Cow’s Milk Is Atherogenic. Atherosclerosis 2003, 170, 13–19. [Google Scholar] [CrossRef]
- McLachlan, C.N.S. β-Casein A1, Ischaemic Heart Disease Mortality, and Other Illnesses. Med. Hypotheses 2001, 56, 262–272. [Google Scholar] [CrossRef]
- Elliott, R.B.; Harris, D.P.; Hill, J.P.; Bibby, N.J.; Wasmuth, H.E. Type I (Insulin-Dependent) Diabetes Mellitus and Cow Milk: Casein Variant Consumption. Diabetologia 1999, 42, 292–296. [Google Scholar] [CrossRef]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative Stress and Reactive Oxygen Species in Endothelial Dysfunction Associated with Cardiovascular and Metabolic Diseases. Vascul. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef]
- Gard, F.; Flad, L.M.; Weißer, T.; Ammer, H.; Deeg, C.A. Effects of A1 Milk, A2 Milk and the Opioid-like Peptide β-Casomorphin-7 on the Proliferation of Human Peripheral Blood Mononuclear Cells. Biomolecules 2024, 14, 690. [Google Scholar] [CrossRef]
- Fiedorowicz, E.; Markiewicz, L.H.; Sidor, K.; Świątecka, D.; Cieślińska, A.; Matysiewicz, M.; Piskorz-Ogórek, K.; Sienkiewicz-Szłapka, E.; Teodorowicz, M.; Świątecki, A.; et al. The Influence of Breast Milk and Infant Formulae Hydrolysates on Bacterial Adhesion and Caco-2 Cells Functioning. Food Res. Int. 2016, 89, 679–688. [Google Scholar] [CrossRef]
- Fiedorowicz, E.; Jarmołowska, B.; Iwan, M.; Kostyra, E.; Obuchowicz, R.; Obuchowicz, M. The Influence of μ-Opioid Receptor Agonist and Antagonist Peptides on Peripheral Blood Mononuclear Cells (PBMCs). Peptides 2011, 32, 707–712. [Google Scholar] [CrossRef]
- Boekholdt, S.M.; Peters, R.J.G.; Hack, C.E.; Day, N.E.; Luben, R.; Bingham, S.A.; Wareham, N.J.; Reitsma, P.H.; Khaw, K.-T. IL-8 Plasma Concentrations and the Risk of Future Coronary Artery Disease in Apparently Healthy Men and Women: The EPIC-Norfolk Prospective Population Study. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1503–1508. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Hirao, Y.; Oshima, S.; Yuasa, K.; Taniguchi, K.; Nagai, R.; Kobayashi, I. Interleukin-8 as a Sensitive Marker of Unstable Coronary Artery Disease. Am. J. Cardiol. 1996, 77, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Romuk, E.; Skrzep-Poloczek, B.; Wojciechowska, C.; Tomasik, A.; Birkner, E.; Wodniecki, J.; Gabrylewicz, B.; Ochala, A.; Tendera, M. Selectin-P and Interleukin-8 Plasma Levels in Coronary Heart Disease Patients. Eur. J. Clin. Investig. 2002, 32, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Idriss, H.T.; Naismith, J.H. TNFα and the TNF Receptor Superfamily: Structure-Function Relationship(s). Microsc. Res. Tech. 2000, 50, 184–195. [Google Scholar] [CrossRef]
- Zhang, H.; Park, Y.; Wu, J.; Chen, X.P.; Lee, S.; Yang, J.; Dellsperger, K.C.; Zhang, C. Role of TNF-α in Vascular Dysfunction. Clin. Sci. 2009, 116, 219–230. [Google Scholar] [CrossRef]
- Duan, H.O.; Simpson-Haidaris, P.J. Functional Analysis of Interleukin 6 Response Elements (IL-6REs) on the Human γ-Fibrinogen Promoter. J. Biol. Chem. 2003, 278, 41270–41281. [Google Scholar] [CrossRef]
- Teixeira, B.C.; Lopes, A.L.; Macedo, R.C.O.; Correa, C.S.; Ramis, T.R.; Ribeiro, J.L.; Reischak-Oliveira, A. Inflammatory Markers, Endothelial Function and Cardiovascular Risk. J. Vasc. Bras. 2014, 13, 108–115. [Google Scholar] [CrossRef]
- Kohlstedt, K.; Busse, R.; Fleming, I. Signaling via the Angiotensin-Converting Enzyme Enhances the Expression of Cyclooxygenase-2 in Endothelial Cells. Hypertension 2005, 45, 126–132. [Google Scholar] [CrossRef]
- Gibson, L.L.; Hahner, L.; Osborne-Lawrence, S.; German, Z.; Wu, K.K.; Chambliss, K.L.; Shaul, P.W. Molecular Basis of Estrogen-Induced Cyclooxygenase Type 1 Upregulation in Endothelial Cells. Circ. Res. 2005, 96, 518–525. [Google Scholar] [CrossRef]
- Haeggström, J.Z.; Funk, C.D. Lipoxygenase and Leukotriene Pathways: Biochemistry, Biology, and Roles in Disease. Chem. Rev. 2011, 111, 5866–5898. [Google Scholar] [CrossRef]
- Förstermann, U.; Münzel, T. Endothelial Nitric Oxide Synthase in Vascular Disease: From Marvel to Menace. Circulation 2006, 113, 1708–1714. [Google Scholar] [CrossRef]
- Zou, M.-H.; Cohen, R.A.; Ullrich, V. Peroxynitrite and Vascular Endothelial Dysfunction in Diabetes Mellitus. Endothelium 2004, 11, 89–97. [Google Scholar] [CrossRef]
- Caughey, G.E.; Cleland, L.G.; Penglis, P.S.; Gamble, J.R.; James, M.J. Roles of Cyclooxygenase (COX)-1 and COX-2 in Prostanoid Production by Human Endothelial Cells: Selective Up-Regulation of Prostacyclin Synthesis by COX-2. J. Immunol. 2001, 167, 2831–2838. [Google Scholar] [CrossRef]
- Coupland, A.P.; Thapar, A.; Qureshi, M.I.; Jenkins, H.; Davies, A.H. The Definition of Stroke. J. R. Soc. Med. 2017, 110, 9–12. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Homoki, J.R.; Szilágyi-Tolnai, E.; Kovács-Forgács, I.; Pesti-Asbóth, G.; Markovics, A.; Biró, A.; Dávid, P.; Lukács, J.; Stündl, L.; Remenyik, J.; et al. β-Casomorphin-7 as a Potential Inflammatory Marker: How β-Casomorphin-7 Induces Endothelial Dysfunction in HUVEC/TERT2 Cell Lines. Biomedicines 2025, 13, 2712. https://doi.org/10.3390/biomedicines13112712
Homoki JR, Szilágyi-Tolnai E, Kovács-Forgács I, Pesti-Asbóth G, Markovics A, Biró A, Dávid P, Lukács J, Stündl L, Remenyik J, et al. β-Casomorphin-7 as a Potential Inflammatory Marker: How β-Casomorphin-7 Induces Endothelial Dysfunction in HUVEC/TERT2 Cell Lines. Biomedicines. 2025; 13(11):2712. https://doi.org/10.3390/biomedicines13112712
Chicago/Turabian StyleHomoki, Judit Rita, Emese Szilágyi-Tolnai, Ildikó Kovács-Forgács, Georgina Pesti-Asbóth, Arnold Markovics, Attila Biró, Péter Dávid, János Lukács, László Stündl, Judit Remenyik, and et al. 2025. "β-Casomorphin-7 as a Potential Inflammatory Marker: How β-Casomorphin-7 Induces Endothelial Dysfunction in HUVEC/TERT2 Cell Lines" Biomedicines 13, no. 11: 2712. https://doi.org/10.3390/biomedicines13112712
APA StyleHomoki, J. R., Szilágyi-Tolnai, E., Kovács-Forgács, I., Pesti-Asbóth, G., Markovics, A., Biró, A., Dávid, P., Lukács, J., Stündl, L., Remenyik, J., & Kiss, A. P. (2025). β-Casomorphin-7 as a Potential Inflammatory Marker: How β-Casomorphin-7 Induces Endothelial Dysfunction in HUVEC/TERT2 Cell Lines. Biomedicines, 13(11), 2712. https://doi.org/10.3390/biomedicines13112712







