A Hot-Spring Water Improves Inflammatory Conditions in an Injury-Induced Atopic Dermatitis Mouse Model by Regulating Skin Barrier Function
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Generation of AD Mouse Model
2.3. Preparation and Treatment with Tap Water and Hot Spring Water
2.4. Measurement of Transepithelial Water Loss (TEWL)
2.5. Preparation of Skin Samples for Staining
2.6. Histological Examination
2.7. Immunostaining
2.8. Statistical Analysis
3. Results
3.1. Properties and Mineral Content of the Hot-Spring Water Used Are Described Below
3.2. Effects of Hot-Spring Water on the Skin Barrier Function in an Injury-Induced AD Model Mouse Are Described Below
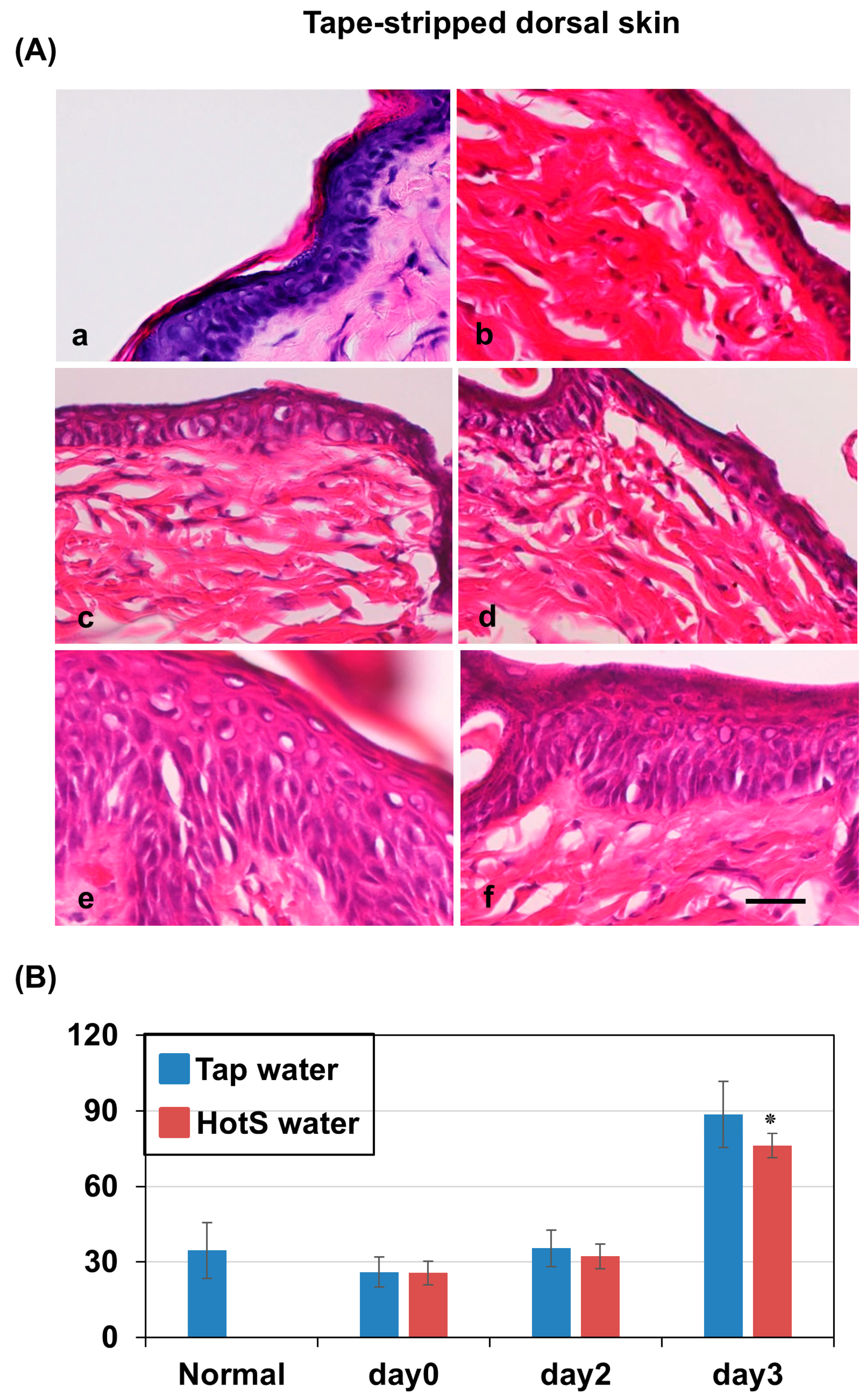
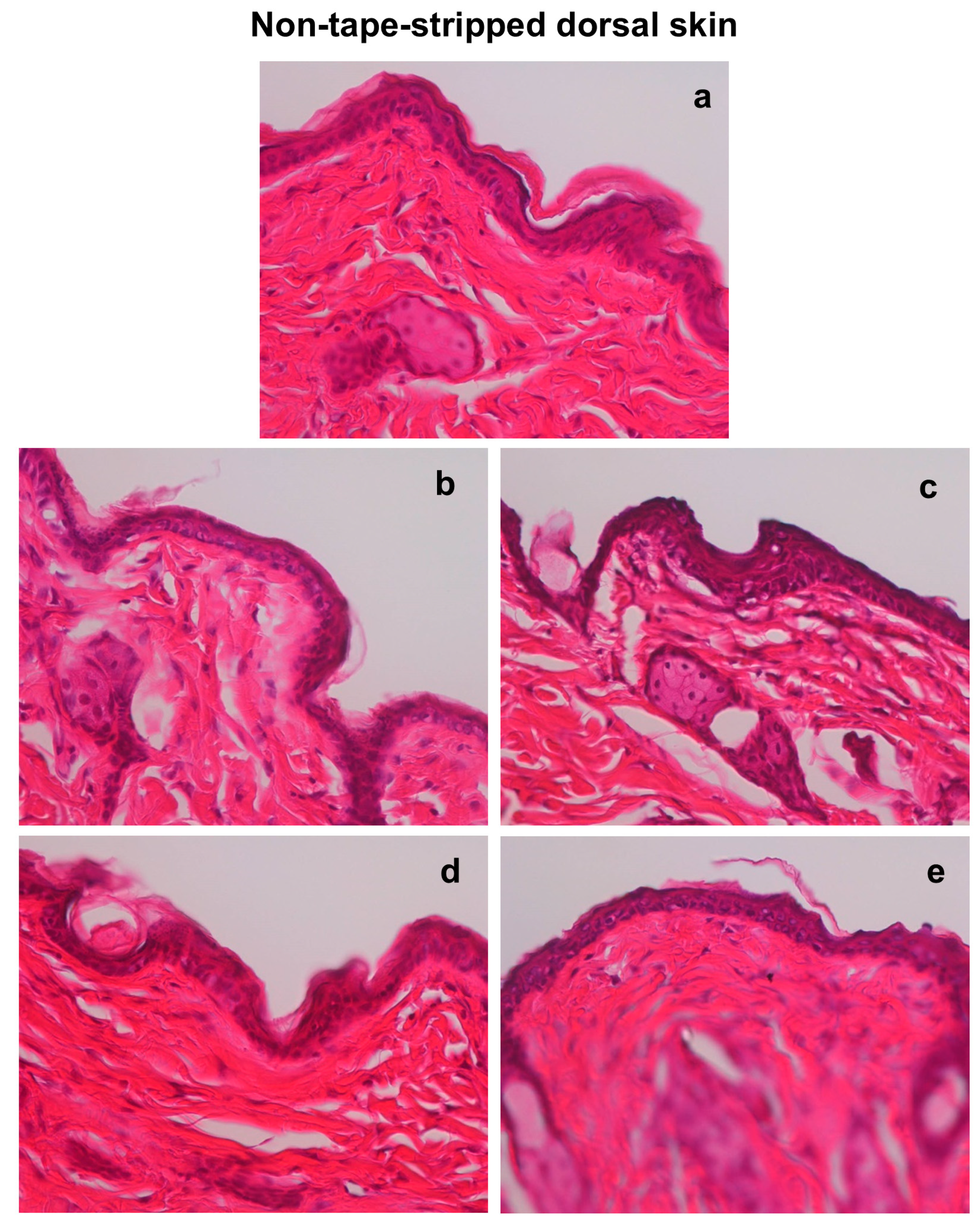
3.3. Effects of Hot-Spring Water on the Skin Histology in an AD Model Mouse
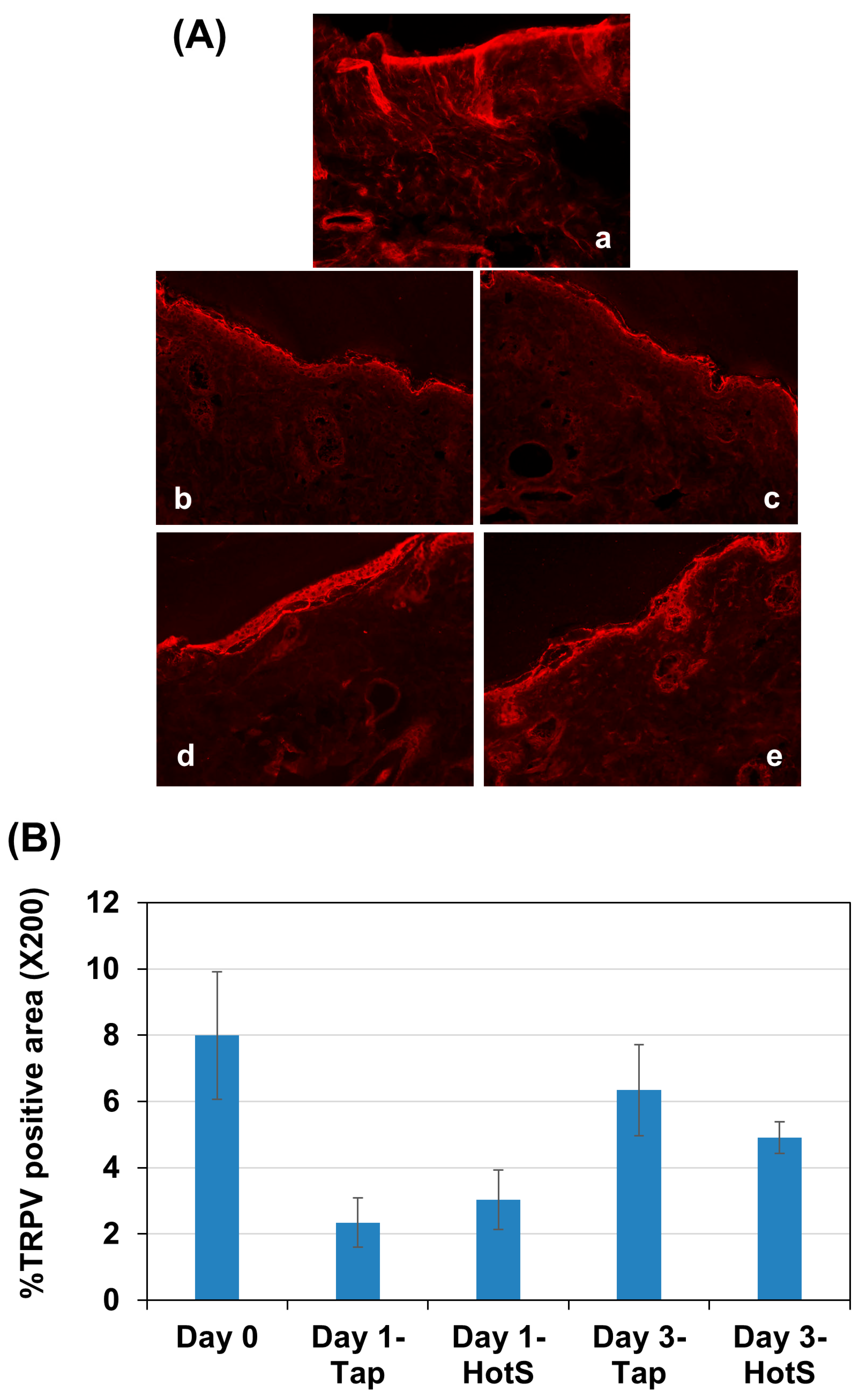
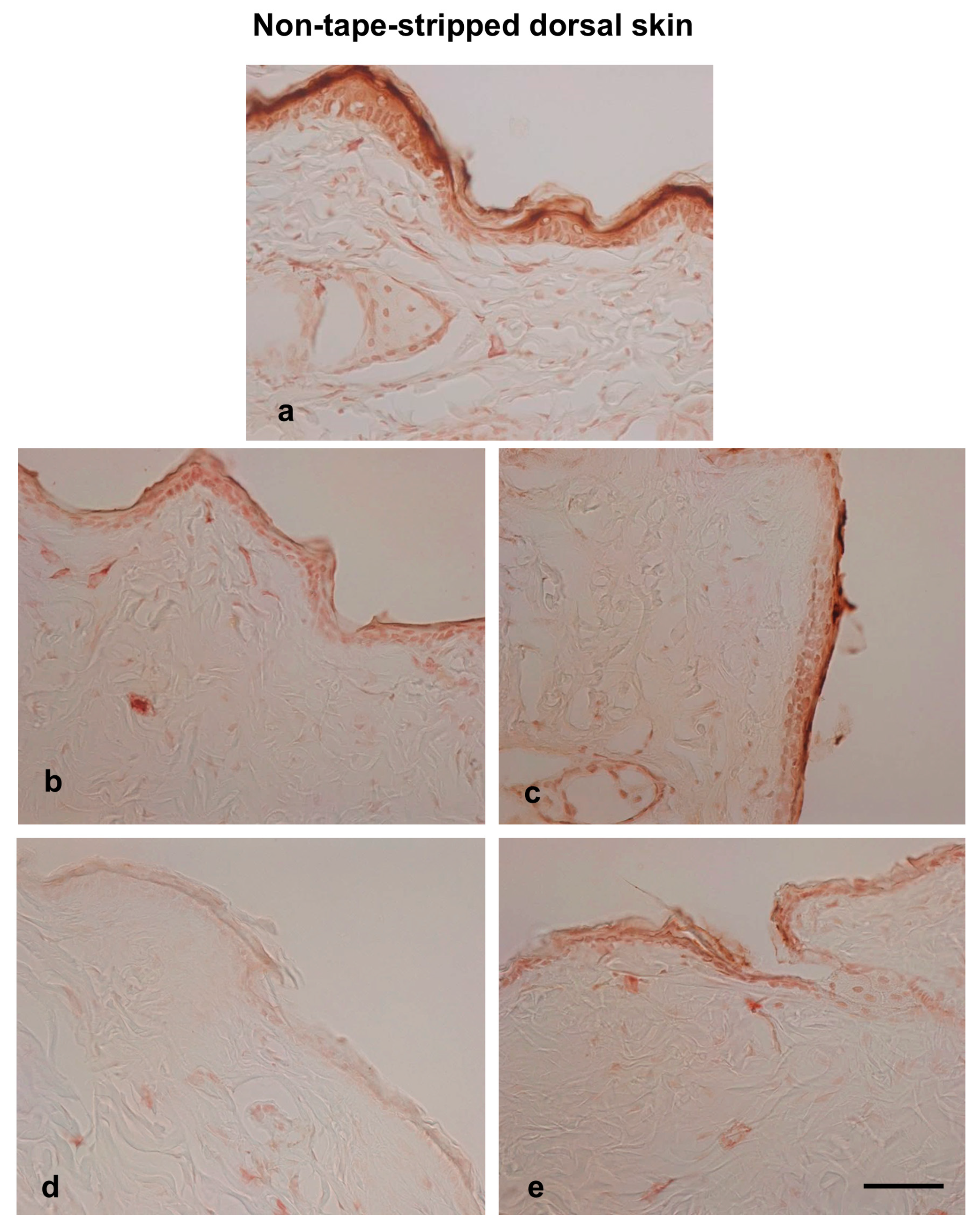
3.4. Effects of Hot-Spring Water on TRPV4 Protein Levels in an AD Model Mouse
3.5. Effects of Hot-Spring Water on Filaggrin Protein Levels in an AD Model Mouse
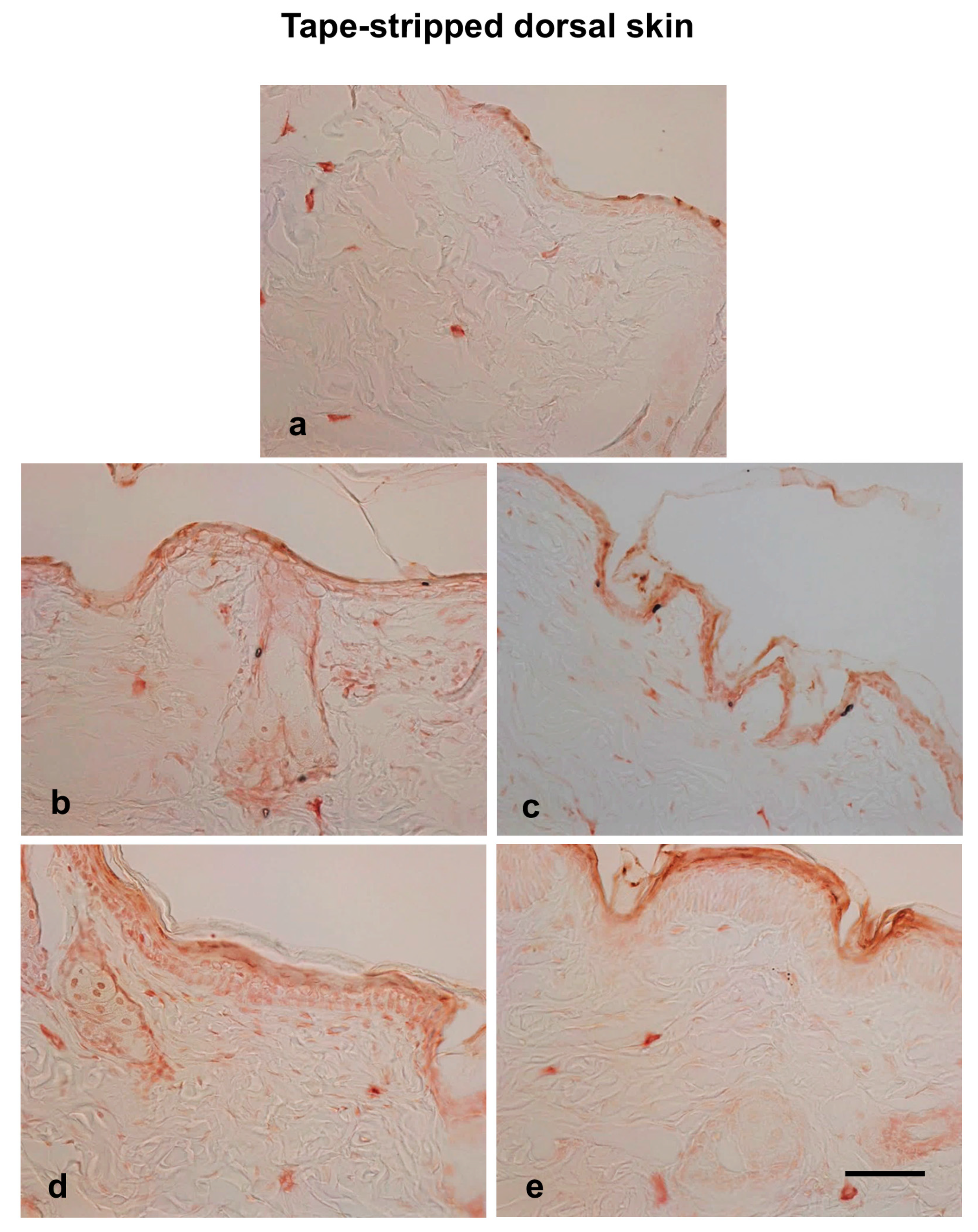
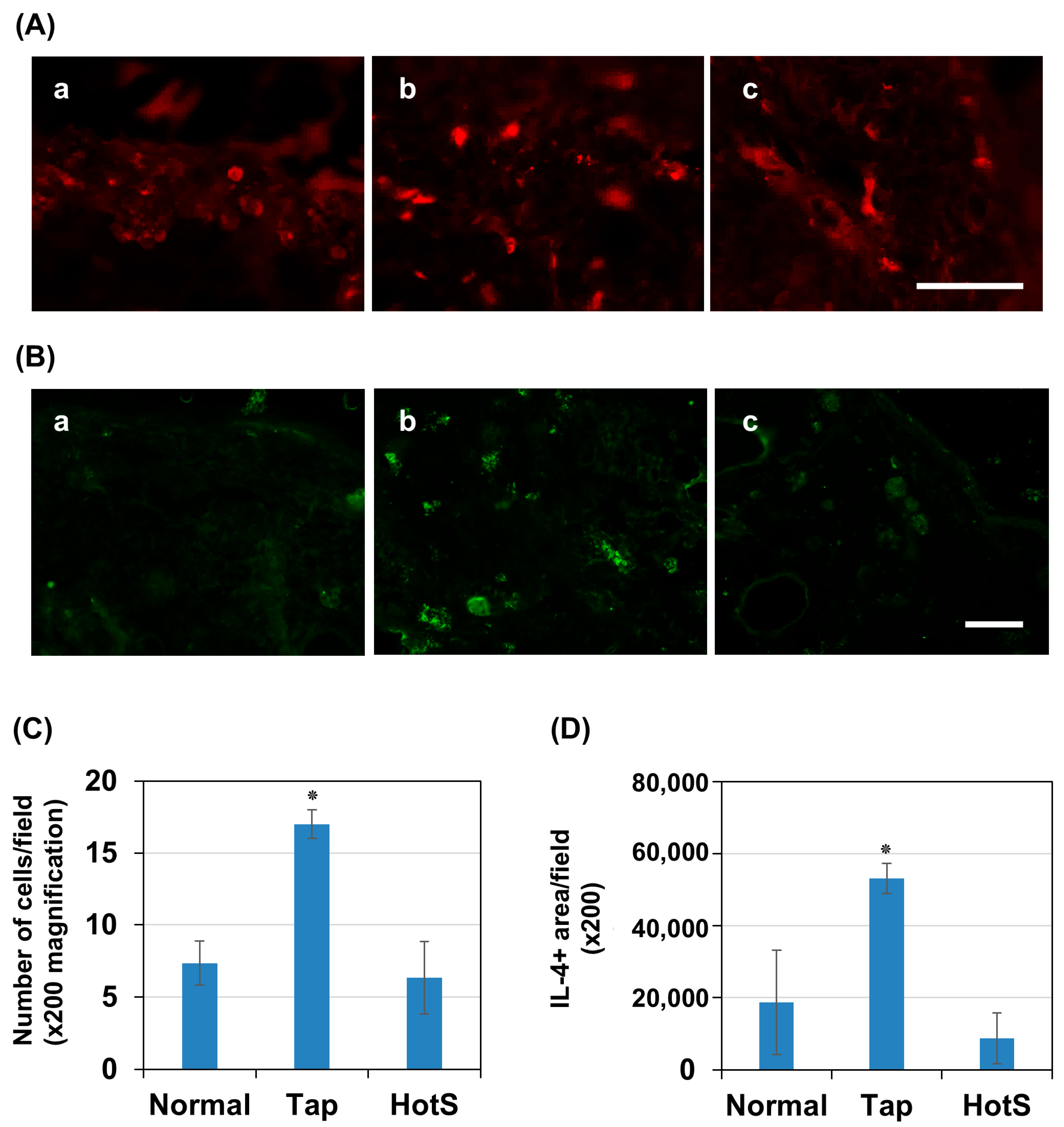
3.6. Effects of Hot Spring Water on Skin Inflammation in an AD Mouse Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torres, T.; Ferreira, E.O.; Gonçalo, M.; Mendes-Bastos, P.; Selores, M.; Filipe, P. Update on atopic dermatitis. Acta Medica Port. 2019, 32, 606–613. [Google Scholar] [CrossRef]
- Frazier, W.; Bhardwaj, N. Atopic dermatitis: Diagnosis and treatment. Am. Fam. Physician 2020, 101, 590–598. [Google Scholar]
- Lugović-Mihić, L.; Meštrović-Štefekov, J.; Potočnjak, I.; Cindrić, T.; Ilić, I.; Lovrić, I.; Skalicki, L.; Bešlić, I.; Pondeljak, N. Atopic dermatitis: Disease features, therapeutic options, and a multidisciplinary approach. Life 2023, 13, 1419. [Google Scholar] [CrossRef]
- Morar, N.; Willis-Owen, S.A.; Moffatt, M.F.; Cookson, W.O. The genetics of atopic dermatitis. J. Allergy Clin. Immunol. 2006, 118, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Pareek, A.; Kumari, L.; Pareek, A.; Chaudhary, S.; Ratan, Y.; Janmeda, P.; Chuturgoon, S.; Chuturgoon, A. Unraveling Atopic Dermatitis: Insights into Pathophysiology, Therapeutic Advances, and Future Perspectives. Cells 2024, 13, 425. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Jeong, Y.; Ashraf, M.U.; Bae, Y.-S. Dendritic cell-mediated Th2 immunity and immune disorders. Int. J. Mol. Sci. 2019, 20, 2159. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Li, Y.; Fischer, M.J.; Steinhoff, M.; Chen, W.; Wang, J. Th2 modulation of transient receptor potential channels: An unmet therapeutic intervention for atopic dermatitis. Front. Immunol. 2021, 12, 696784. [Google Scholar] [CrossRef]
- Furue, M. T helper type 2 signatures in atopic dermatitis. J. Cutan. Immunol. Allergy 2018, 1, 93–99. [Google Scholar] [CrossRef]
- Atsumi, Y.; Toriyama, M.; Kato, H.; Nakamura, M.; Morita, A.; Takaishi, M.; Saito, K.; Tanaka, M.; Okada, F.; Tominaga, M. Anti-inflammatory role of TRPV4 in human macrophages. ImmunoHorizons 2023, 7, 81–96. [Google Scholar] [CrossRef]
- Sandilands, A.; Sutherland, C.; Irvine, A.D.; McLean, W.I. Filaggrin in the frontline: Role in skin barrier function and disease. J. Cell Sci. 2009, 122, 1285. [Google Scholar] [CrossRef]
- Gittler, J.K.; Shemer, A.; Suárez-Fariñas, M.; Fuentes-Duculan, J.; Gulewicz, K.J.; Wang, C.Q.; Mitsui, H.; Cardinale, I.; de Guzman Strong, C.; Krueger, J.G. Progressive activation of TH2/TH22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J. Allergy Clin. Immunol. 2012, 130, 1344–1354. [Google Scholar] [CrossRef] [PubMed]
- Boudaka, A.; Al-Yazeedi, M.; Al-Lawati, I. Role of transient receptor potential vanilloid 4 channel in skin physiology and pathology. Sultan Qaboos Univ. Med. J. 2020, 20, e138. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fang, Q.; Wang, Z.; Zhang, J.Y.; MacLeod, A.S.; Hall, R.P.; Liedtke, W.B. Transient receptor potential vanilloid 4 ion channel functions as a pruriceptor in epidermal keratinocytes to evoke histaminergic itch. J. Biol. Chem. 2016, 291, 10252–10262. [Google Scholar] [CrossRef]
- Zhang, Q.; Dias, F.; Fang, Q.; Henry, G.; Wang, Z.; Suttle, A.; Chen, Y. Involvement of Sensory Neurone-TRPV4 in Acute and Chronic Itch Behaviours. Acta Derm. Venereol. 2022, 102, adv00651. [Google Scholar] [CrossRef]
- Kwatra, S.G.; Misery, L.; Clibborn, C.; Steinhoff, M. Molecular and cellular mechanisms of itch and pain in atopic dermatitis and implications for novel therapeutics. Clin. Transl. Immunol. 2022, 11, e1390. [Google Scholar] [CrossRef]
- Bouwstra, J.V.S.J.A. Stratum corneum lipids: Their role for the skin barrier function in healthy subjects and atopic dermatitis patients. Skin. Barrier Funct. 2016, 49, 8–26. [Google Scholar]
- Armengot-Carbo, M.; Hernández-Martín, Á.; Torrelo, A. The role of filaggrin in the skin barrier and disease development. Actas Dermo-Sifiliográficas (Engl. Ed.) 2015, 106, 86–95. [Google Scholar] [CrossRef]
- Kezic, S.; Jakasa, I. Filaggrin and skin barrier function. Curr. Probl. Dermatol. 2016, 49, 1–7. [Google Scholar] [PubMed]
- Honda, T.; Kabashima, K. Reconciling innate and acquired immunity in atopic dermatitis. J. Allergy Clin. Immunol. 2020, 145, 1136–1137. [Google Scholar] [CrossRef]
- Wallrapp, A.; Riesenfeld, S.J.; Burkett, P.R.; Kuchroo, V.K. Type 2 innate lymphoid cells in the induction and resolution of tissue inflammation. Immunol. Rev. 2018, 286, 53–73. [Google Scholar] [CrossRef]
- Palomares, O.; Akdis, M.; Martín-Fontecha, M.; Akdis, C.A. Mechanisms of immune regulation in allergic diseases: The role of regulatory T and B cells. Immunol. Rev. 2017, 278, 219–236. [Google Scholar] [CrossRef]
- Gröne, A. Keratinocytes and cytokines. Vet. Immunol. Immunopathol. 2002, 88, 1–12. [Google Scholar] [CrossRef]
- Chieosilapatham, P.; Kiatsurayanon, C.; Umehara, Y.; Trujillo-Paez, J.V.; Peng, G.; Yue, H.; Nguyen, L.T.H.; Niyonsaba, F. Keratinocytes: Innate immune cells in atopic dermatitis. Clin. Exp. Immunol. 2021, 204, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Huang, I.H.; Chung, W.H.; Wu, P.C.; Chen, C.B. JAK-STAT signaling pathway in the pathogenesis of atopic dermatitis: An updated review. Front. Immunol. 2022, 13, 1068260. [Google Scholar] [CrossRef]
- Furue, M. Regulation of filaggrin, loricrin, and involucrin by IL-4, IL-13, IL-17A, IL-22, AHR, and NRF2: Pathogenic implications in atopic dermatitis. Int. J. Mol. Sci. 2020, 21, 5382. [Google Scholar] [CrossRef]
- Humeau, M.; Boniface, K.; Bodet, C. Cytokine-mediated crosstalk between keratinocytes and T cells in atopic dermatitis. Front. Immunol. 2022, 13, 801579. [Google Scholar] [CrossRef]
- Mitamura, Y.; Nunomura, S.; Nanri, Y.; Ogawa, M.; Yoshihara, T.; Masuoka, M.; Tsuji, G.; Nakahara, T.; Hashimoto-Hachiya, A.; Conway, S.J. The IL-13/periostin/IL-24 pathway causes epidermal barrier dysfunction in allergic skin inflammation. Allergy 2018, 73, 1881–1891. [Google Scholar] [CrossRef] [PubMed]
- Hönzke, S.; Wallmeyer, L.; Ostrowski, A.; Radbruch, M.; Mundhenk, L.; Schäfer-Korting, M.; Hedtrich, S. Influence of Th2 cytokines on the cornified envelope, tight junction proteins, and β-defensins in filaggrin-deficient skin equivalents. J. Investig. Dermatol. 2016, 136, 631–639. [Google Scholar] [CrossRef]
- Di, Z.-H.; Ma, L.; Qi, R.-Q.; Sun, X.-D.; Huo, W.; Zhang, L.; Lyu, Y.-N.; Hong, Y.-X.; Chen, H.-D.; Gao, X.-H. T helper 1 and T helper 2 cytokines differentially modulate expression of filaggrin and its processing proteases in human keratinocytes. Chin. Med. J. 2016, 129, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.; Northstone, K.; Lee, S.P.; Liao, H.; Zhao, Y.; Pembrey, M.; Mukhopadhyay, S.; Smith, G.D.; Palmer, C.N.; McLean, W.I. The burden of disease associated with filaggrin mutations: A population-based, longitudinal birth cohort study. J. Allergy Clin. Immunol. 2008, 121, 872–877.e9. [Google Scholar] [CrossRef]
- Bantz, S.K.; Zhu, Z.; Zheng, T. The atopic march: Progression from atopic dermatitis to allergic rhinitis and asthma. J. Clin. Cell. Immunol. 2014, 5, 202. [Google Scholar] [PubMed]
- Alvarenga, J.M.; Bieber, T.; Torres, T. Emerging biologic therapies for the treatment of atopic dermatitis. Drugs 2024, 84, 1379–1394. [Google Scholar] [CrossRef]
- Chu, D.K.; Koplin, J.J.; Ahmed, T.; Islam, N.; Chang, C.-L.; Lowe, A.J. How to prevent atopic dermatitis (eczema) in 2024: Theory and evidence. J. Allergy Clin. Immunol. Pract. 2024, 12, 1695–1704. [Google Scholar] [CrossRef]
- Silverberg, N.B. Atopic dermatitis prevention and treatment. Cutis 2017, 100, 173. [Google Scholar]
- Schneider, L.; Tilles, S.; Lio, P.; Boguniewicz, M.; Beck, L.; LeBovidge, J.; Novak, N.; Bernstein, D.; Blessing-Moore, J.; Khan, D. Atopic dermatitis: A practice parameter update 2012. J. Allergy Clin. Immunol. 2013, 131, 295–299.e27. [Google Scholar] [CrossRef]
- Davis, D.M.; Drucker, A.M.; Alikhan, A.; Bercovitch, L.; Cohen, D.E.; Darr, J.M.; Eichenfield, L.F.; Frazer-Green, L.; Paller, A.S.; Schwarzenberger, K. Guidelines of care for the management of atopic dermatitis in adults with phototherapy and systemic therapies. J. Am. Acad. Dermatol. 2024, 90, e43–e56. [Google Scholar] [CrossRef]
- Le Floc’h, A.; Allinne, J.; Nagashima, K.; Scott, G.; Birchard, D.; Asrat, S.; Bai, Y.; Lim, W.K.; Martin, J.; Huang, T. Dual blockade of IL-4 and IL-13 with dupilumab, an IL-4Rα antibody, is required to broadly inhibit type 2 inflammation. Allergy 2020, 75, 1188–1204. [Google Scholar] [CrossRef] [PubMed]
- Fluhr, J.W.; Muguet, V.; Christen-Zaech, S. Restoring Skin Hydration and Barrier Function: Mechanistic Insights Into Basic Emollients for Xerosis Cutis. Int. J. Dermatol. 2025, 64, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Biliński, K.; Rakoczy, K.; Karwowska, A.; Cichy, O.; Wojno, A.; Wojno, A.; Kulbacka, J.; Ponikowska, M. Anti-inflammatory therapies for atopic dermatitis: A new era in targeted treatment. J. Clin. Med. 2025, 14, 5053. [Google Scholar] [CrossRef]
- Smith, R.; Russo, J.; Fiegel, J.; Brogden, N. Antibiotic delivery strategies to treat skin infections when innate antimicrobial defense fails. Antibiotics 2020, 9, 56. [Google Scholar] [CrossRef]
- Rawlings, A.; Canestrari, D.A.; Dobkowski, B. Moisturizer technology versus clinical performance. Dermatol. Ther. 2004, 17, 49–56. [Google Scholar] [CrossRef]
- Peris, K.; Valeri, P.; Altobelli, E.; Fargnoli, M.C.; Carrozzo, A.M.; Chimenti, S. Efficacy evaluation of an oil-in-water emulsion (Dermoflan) in atopic dermatitis. Acta Derm.-Venereol. 2002, 82, 465–466. [Google Scholar] [CrossRef]
- Verallo-Rowell, V.M.; Dillague, K.M.; Syah-Tjundawan, B.S. Novel antibacterial and emollient effects of coconut and virgin olive oils in adult atopic dermatitis. DERM 2008, 19, 308–315. [Google Scholar] [CrossRef]
- Cacciapuoti, S.; Luciano, M.A.; Megna, M.; Annunziata, M.C.; Napolitano, M.; Patruno, C.; Scala, E.; Colicchio, R.; Pagliuca, C.; Salvatore, P.; et al. The Role of Thermal Water in Chronic Skin Diseases Management: A Review of the Literature. J. Clin. Med. 2020, 9, 3047. [Google Scholar] [CrossRef]
- Huang, A.; Seité, S.; Adar, T. The use of balneotherapy in dermatology. Clin. Dermatol. 2018, 36, 363–368. [Google Scholar] [CrossRef]
- Fikri-Benbrahim, K.; Houti, A.; El Ouali Lalami, A.; Flouchi, R.; El Hachlafi, N.; Houti, M.; Rachiq, S. Main therapeutic uses of some moroccan hot springs’ waters. Evid.-Based Complement. Altern. Med. 2021, 2021, 5599269. [Google Scholar] [CrossRef]
- Kubota, K.; Machida, I.; Tamura, K.; Take, H.; Kurabayashi, H.; Akiba, T.; Tamura, J. Treatment of refractory cases of atopic dermatitis with acidic hot-spring bathing. Acta Derm.-Venereol. 1997, 77, 452–454. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Kang, D.; Wang, Y.; Yu, Y.; Fan, J.; Takashi, E. Carbonate ion-enriched hot spring water promotes skin wound healing in nude rats. PLoS ONE 2015, 10, e0117106. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Lee, H.J.; Woo, S.Y.; Lee, K.H.; Yun, S.T.; Kim, J.M.; Kim, H.J.; Kim, J.W. Therapeutic effects and immunomodulation of suanbo mineral water therapy in a murine model of atopic dermatitis. Ann. Dermatol. 2013, 25, 462–470. [Google Scholar] [CrossRef]
- Jobeili, L.; Rousselle, P.; Béal, D.; Blouin, E.; Roussel, A.-M.; Damour, O.; Rachidi, W. Selenium preserves keratinocyte stemness and delays senescence by maintaining epidermal adhesion. Aging 2017, 9, 2302. [Google Scholar] [CrossRef] [PubMed]
- Sivaprasad, U.; Warrier, M.R.; Gibson, A.M.; Chen, W.; Tabata, Y.; Bass, S.A.; Rothenberg, M.E.; Khurana Hershey, G.K. IL-13Rα2 has a protective role in a mouse model of cutaneous inflammation. J Immunol. 2010, 185, 6802–6808. [Google Scholar] [CrossRef]
- Yosipovitch, G.; Misery, L.; Proksch, E.; Metz, M.; Ständer, S.; Schmelz, M. Skin Barrier Damage and Itch: Review of Mechanisms, Topical Management and Future Directions. Acta Derm.-Venereol. 2019, 99, 1201–1209. [Google Scholar] [CrossRef]
- van den Bogaard, E.H.; Elias, P.M.; Goleva, E.; Berdyshev, E.; Smits, J.P.H.; Danby, S.G.; Cork, M.J.; Leung, D.Y.M. Targeting Skin Barrier Function in Atopic Dermatitis. J. Allergy Clin. Immunol. Pract. 2023, 11, 1335–1346. [Google Scholar] [CrossRef]
- Cork, M.J.; Danby, S.; Vasilopoulos, Y.; Moustafa, M.; MacGowan, A.; Varghese, J.; Duff, G.W.; Tazi-Ahnini, R.; Ward, S.J. Epidermal barrier dysfunction in atopic dermatitis. In Textbook of Atopic Dermatitis; CRC Press: Boca Raton, FL, USA, 2008; pp. 47–70. [Google Scholar]
- Hori, M.; Shozugawa, K.; Sugimori, K.; Watanabe, Y. A survey of monitoring tap water hardness in Japan and its distribution patterns. Sci. Rep. 2021, 11, 13546. [Google Scholar] [CrossRef]
- Hahn, G.S. Strontium is a potent and selective inhibitor of sensory irritation. Dermatol. Surg. 1999, 25, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Kruszewski, F.H.; Punnonen, K.; Tucker, R.W.; Yuspa, S.H.; Hennings, H. Strontium induces murine keratinocyte differentiation in vitro in the presence of serum and calcium. J. Cell. Physiol. 1993, 154, 643–653. [Google Scholar] [CrossRef]
- Pihl, C.; Togsverd-Bo, K.; Andersen, F.; Haedersdal, M.; Bjerring, P.; Lerche, C.M. Keratinocyte carcinoma and photoprevention: The protective actions of repurposed pharmaceuticals, phytochemicals and vitamins. Cancers 2021, 13, 3684. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Shi, Z.; Cao, X.; Dang, Z.; Zhang, Q.; Zhang, W.; Wu, L.; Zhang, Y.; Wang, T. Research progress on anti-inflammatory effects and related mechanisms of astragalin. Int. J. Mol. Sci. 2024, 25, 4476. [Google Scholar] [CrossRef]
- Ru, X.; Yang, L.; Shen, G.; Wang, K.; Xu, Z.; Bian, W.; Zhu, W.; Guo, Y. Microelement strontium and human health: Comprehensive analysis of the role in inflammation and non-communicable diseases (NCDs). Front. Chem. 2024, 12, 1367395. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, S.; Jafarian-Dehkordi, A.; Hajhashemi, V.; Asilian-Mahabadi, A.; Nasr-Esfahani, M.H. A comparison of the effect of certain inorganic salts on suppression acute skin irritation by human biometric assay: A randomized, double-blind clinical trial. J. Res. Med. Sci. 2016, 21, 102. [Google Scholar] [CrossRef]
- Zhai, H.; Hannon, W.; Hahn, G.S.; Pelosi, A.; Harper, R.A.; Maibach, H.I. Strontium nitrate suppresses chemically-induced sensory irritation in humans. Contact Dermat. 2000, 42, 98–100. [Google Scholar] [CrossRef]
- Fernandez, C.; Adamson, K.; Dale, M.; Cunliffe, W.J. A randomized double-blind study to assess the effects of silicic acid compared to placebo in patients with mild to moderate acne. J. Dermatol. Treat. 2005, 16, 287–294. [Google Scholar] [CrossRef]
- Chiller, K.; Selkin, B.A.; Murakawa, G.J. Skin microflora and bacterial infections of the skin. J. Investig. Dermatol. Symp. Proc. 2001, 6, 170–174. [Google Scholar] [CrossRef]
- Douladiris, N.; Vakirlis, E.; Vassilopoulou, E. Atopic Dermatitis and Water: Is There an Optimum Water Intake Level for Improving Atopic Skin? Children 2023, 10, 273. [Google Scholar] [CrossRef]
- Purnamawati, S.; Indrastuti, N.; Danarti, R.; Saefudin, T. The Role of Moisturizers in Addressing Various Kinds of Dermatitis: A Review. Clin. Med. Res. 2017, 15, 75–87. [Google Scholar] [CrossRef]
- Werner, Y. The water content of the stratum corneum in patients with atopic dermatitis. Measurement with the Corneometer CM 420. Acta Derm.-Venereol. 1986, 66, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.K.; Zhong, L.; Santiago, J.L. Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. Int. J. Mol. Sci. 2017, 19, 70. [Google Scholar] [CrossRef]
- Kono, T.; Miyachi, Y.; Kawashima, M. Clinical significance of the water retention and barrier function-improving capabilities of ceramide-containing formulations: A qualitative review. J. Dermatol. 2021, 48, 1807–1816. [Google Scholar] [CrossRef]
- Alexander, H.; Brown, S.; Danby, S.; Flohr, C. Research techniques made simple: Transepidermal water loss measurement as a research tool. J. Investig. Dermatol. 2018, 138, 2295–2300.e1. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Yoshihara, T.; Yamamoto, M.; Konno, K.; Momoi, Y.; Nishifuji, K.; Iwasaki, T. Transepidermal water loss (TEWL) reflects skin barrier function of dog. J. Vet. Med. Sci. 2008, 70, 841–843. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; He, R.; Oyoshi, M.; Geha, R.S. Animal models of atopic dermatitis. J. Investig. Dermatol. 2009, 129, 31–40. [Google Scholar] [CrossRef]
- Bakhshian Nik, A.; Alvarez-Argote, S.; O’Meara, C.C. Interleukin 4/13 signaling in cardiac regeneration and repair. Am. J. Physiol. Heart Circ. Physiol. 2022, 323, H833–H844. [Google Scholar] [CrossRef]
- Zheng, T.; Oh, M.H.; Oh, S.Y.; Schroeder, J.T.; Glick, A.B.; Zhu, Z. Transgenic expression of interleukin-13 in the skin induces a pruritic dermatitis and skin remodeling. J. Investig. Dermatol. 2009, 129, 742–751. [Google Scholar] [CrossRef]
- Kim, K.; Kim, H.; Sung, G.Y. An Interleukin-4 and Interleukin-13 Induced Atopic Dermatitis Human Skin Equivalent Model by a Skin-On-A-Chip. Int. J. Mol. Sci. 2022, 23, 2116. [Google Scholar] [CrossRef]
- Tu, C.L.; Bikle, D.D. Role of the calcium-sensing receptor in calcium regulation of epidermal differentiation and function. Best. Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 415–427. [Google Scholar] [CrossRef]
- Carrasco, M.A.; Jaimovich, E.; Kemmerling, U.; Hidalgo, C. Signal transduction and gene expression regulated by calcium release from internal stores in excitable cells. Biol. Res. 2004, 37, 701–712. [Google Scholar] [CrossRef]
- Tsuji, G.; Hashimoto-Hachiya, A.; Yumine, A.; Takemura, M.; Kido-Nakahara, M.; Ito, T.; Yamamura, K.; Nakahara, T. PDE4 inhibition by difamilast regulates filaggrin and loricrin expression via keratinocyte proline-rich protein in human keratinocytes. J. Dermatol. Sci. 2023, 110, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Howell, M.D.; Kim, B.E.; Gao, P.; Grant, A.V.; Boguniewicz, M.; DeBenedetto, A.; Schneider, L.; Beck, L.A.; Barnes, K.C.; Leung, D.Y. Cytokine modulation of atopic dermatitis filaggrin skin expression. J. Allergy Clin. Immunol. 2009, 124, R7–R12. [Google Scholar] [CrossRef]
- Yamazaki, T.; Ushikoshi-Nakayama, R.; Shakya, S.; Omagari, D.; Matsumoto, N.; Nukuzuma, C.; Komatsu, T.; Lee, M.C.-i.; Inoue, H.; Saito, I. The effects of bathing in neutral bicarbonate ion water. Sci. Rep. 2021, 11, 21789. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Grace, M.S.; Gondin, A.B.; Retamal, J.S.; Dill, L.; Darby, W.; Bunnett, N.W.; Abogadie, F.C.; Carbone, S.E.; Tigani, T.; et al. The transient receptor potential vanilloid 4 (TRPV4) ion channel mediates protease activated receptor 1 (PAR1)-induced vascular hyperpermeability. Lab. Investig. 2020, 100, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.; Siddiqui, G.; Veldhuis, N.A.; Poole, D.P. Diverse Roles of TRPV4 in Macrophages: A Need for Unbiased Profiling. Front. Immunol. 2021, 12, 828115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-T.; Fang, S.-G.; Wang, C.-Y. A novel nonsense mutation and polymorphisms in the mouse hairless gene. J. Investig. Dermatol. 2005, 124, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, Y.; Ogura, Y.; Ehama, R.; Amano, S.; Nishiyama, T.; Tagami, H. Establishment of a mouse skin model of the lichenification in human chronic eczematous dermatitis. Br. J. Dermatol. 2007, 156, 884–891. [Google Scholar] [CrossRef]
- Saito, S.; Aoki, A.; Arai, I.; Takaishi, S.; Ito, H.; Akiyama, N.; Kiyonari, H. Regulation of Th2 responses by different cell types expressing the interleukin-31 receptor. Allergy Asthma Clin. Immunol. 2017, 13, 23. [Google Scholar] [CrossRef] [PubMed]

| Components | mg | mval | mval% |
|---|---|---|---|
| Li+ | 1.8 | 0.26 | 0.25 |
| Na+ | 1710 | 74.38 | 70.51 |
| K+ | 72.5 | 1.85 | 1.75 |
| Mg2+ | 84.4 | 6.94 | 6.58 |
| Ca2+ | 433 | 21.61 | 20.49 |
| Sr2+ | 12.3 | 0.28 | 0.27 |
| Mn2+ | 0.4 | 0.01 | 0.01 |
| Fe2++Fe3+ | 4.1 | 0.15 | 0.14 |
| Total Cations | 2318.5 | 105.48 | 100.00 |
| Components | mg | mval | mval% |
|---|---|---|---|
| F− | 1.0 | 0.05 | 0.05 |
| Cl− | 2660 | 75.03 | 67.77 |
| Br− | 8.6 | 0.11 | 0.10 |
| I− | 0.7 | 0.01 | 0.01 |
| SO42− | 965 | 20.09 | 18.14 |
| HCO3− | 941 | 15.42 | 13.93 |
| Total Anions | 4576.3 | 110.71 | 100.00 |
| Components | mg | mmol |
|---|---|---|
| HAsO2 | 2.3 | 0.02 |
| H2SiO3 | 118 | 1.51 |
| HBO2 | 35.3 | 0.81 |
| Total non-dissociated components | 155.6 | 2.34 |
| Components | mg | mmol |
|---|---|---|
| CO2 | 484 | 11.00 |
| H2S | 0.0 | 0.00 |
| Total dissolved gas components | 484.0 | 11.00 |
| Ba2+ | 0.05 mg |
| Al3+ | 0.03 mg |
| Cu2+ | Not detected < 0.005 mg |
| Zn2+ | 0.088 mg |
| Cd2+ | 0.002 mg |
| Pb2+ | 0.073 mg |
| Hg | Not detected < 0.0005 mg |
| As | 1.59 mg |
| S2O32− | Not detected < 0.01 mg |
| CO32− | Not detected < 0.1 mg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naito, Y.; Sheikh, A.M.; Bhuiya, J.; Abdullah, F.B.; Fahmida, J.; Tabassum, S.; Tamegai, H.; Iwasa, K.; Yano, S.; Nagai, A. A Hot-Spring Water Improves Inflammatory Conditions in an Injury-Induced Atopic Dermatitis Mouse Model by Regulating Skin Barrier Function. Biomedicines 2025, 13, 2707. https://doi.org/10.3390/biomedicines13112707
Naito Y, Sheikh AM, Bhuiya J, Abdullah FB, Fahmida J, Tabassum S, Tamegai H, Iwasa K, Yano S, Nagai A. A Hot-Spring Water Improves Inflammatory Conditions in an Injury-Induced Atopic Dermatitis Mouse Model by Regulating Skin Barrier Function. Biomedicines. 2025; 13(11):2707. https://doi.org/10.3390/biomedicines13112707
Chicago/Turabian StyleNaito, Yoko, Abdullah Md. Sheikh, Jubo Bhuiya, Fatema Binte Abdullah, Jerin Fahmida, Shatera Tabassum, Hiro Tamegai, Kenichi Iwasa, Shozo Yano, and Atsushi Nagai. 2025. "A Hot-Spring Water Improves Inflammatory Conditions in an Injury-Induced Atopic Dermatitis Mouse Model by Regulating Skin Barrier Function" Biomedicines 13, no. 11: 2707. https://doi.org/10.3390/biomedicines13112707
APA StyleNaito, Y., Sheikh, A. M., Bhuiya, J., Abdullah, F. B., Fahmida, J., Tabassum, S., Tamegai, H., Iwasa, K., Yano, S., & Nagai, A. (2025). A Hot-Spring Water Improves Inflammatory Conditions in an Injury-Induced Atopic Dermatitis Mouse Model by Regulating Skin Barrier Function. Biomedicines, 13(11), 2707. https://doi.org/10.3390/biomedicines13112707






