Evaluation of Oral Mucosa Capillaries in Fibromyalgia Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
- Inclusion criteria: certified diagnosis of fibromyalgia established by a specialist using American College of Rheumatology (ACR) criteria for fibromyalgia.
- Exclusion criteria: conditions known to affect the microcirculation, including pregnancy, tobacco use, and alcohol or substance abuse.
2.2. Demographics and Pathological/Pharmacological Anamnesis
2.3. Data Collection and Videocapillaroscopy Analysis
- Right buccal mucosa
- Left buccal mucosa
- Lower labial mucosa
- Upper labial mucosa
2.4. Parameters Analyzed
- Parametric data:
- ○
- Loop density: capillaries per mm2.
- ○
- Loop caliber: mean of arteriolar and venular diameters in µm.
- ○
- Loop length in µm.
- Non-parametric data:
- ○
- Capillary visibility: clearly visible (1), poorly visible (2), or not visible (3).
- ○
- Orientation in relation to the surface: parallel (A), perpendicular (B), or mixed (AB).
- ○
- Presence of microhemorrhages and/or microaneurysms.
2.5. Statistical Analysis
3. Results
3.1. Caliber
3.2. Density
3.3. Length
3.4. Visibility
3.5. Orientation
3.6. Microhemorrhages
4. Discussion
Limitations of This Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhargava, J.; Goldin, J. Fibromyalgia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Iannuccelli, C.; Favretti, M.; Dolcini, G.; Di Carlo, M.; Pellegrino, G.; Bazzichi, L.; Atzeni, F.; Lucini, D.; Varassi, G.; Leoni, M.L.G.; et al. Fibromyalgia: One Year in Review 2025. Clin. Exp. Rheumatol. 2025, 43, 957–969. [Google Scholar] [CrossRef]
- García Rodríguez, D.F.; Abud Mendoza, C. Physiopathology of Fibromyalgia. Reumatol. Clin. 2020, 16, 191–194. [Google Scholar] [CrossRef]
- Jurado-Priego, L.N.; Cueto-Ureña, C.; Ramírez-Expósito, M.J.; Martínez-Martos, J.M. Fibromyalgia: A Review of the Pathophysiological Mechanisms and Multidisciplinary Treatment Strategies. Biomedicines 2024, 12, 1543. [Google Scholar] [CrossRef]
- Toda, K. What Is the Purpose of the Diagnostic Criterion for Fibromyalgia? Rheumatol. Int. 2023, 43, 193–194. [Google Scholar] [CrossRef]
- Pontes-Silva, A. Fibromyalgia: A New Set of Diagnostic Criteria Based on the Biopsychosocial Model. Rheumatol. Oxf. Engl. 2024, 63, 2037–2039. [Google Scholar] [CrossRef]
- Salaffi, F.; Farah, S.; Bianchi, B.; Lommano, M.G.; Di Carlo, M. Delay in Fibromyalgia Diagnosis and Its Impact on the Severity and Outcome: A Large Cohort Study. Clin. Exp. Rheumatol. 2024, 42, 1198–1204. [Google Scholar] [CrossRef]
- Mertoglu, C.; Gunay, M.; Yerligok, O. Could Endocan, a Marker of Inflammation and Endothelial Dysfunction, Be a New Diagnostic Marker for Fibromyalgia? Clin. Lab. 2018, 64, 405. [Google Scholar] [CrossRef] [PubMed]
- Ghoneim, F.M.; Abo-Elkhair, S.M.; Elsamanoudy, A.Z.; Shabaan, D.A. Evaluation of Endothelial Dysfunction and Autophagy in Fibromyalgia-Related Vascular and Cerebral Cortical Changes and the Ameliorative Effect of Fisetin. Cells 2022, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- García-Domínguez, M. Fibromyalgia and Inflammation: Unrevealing the Connection. Cells 2025, 14, 271. [Google Scholar] [CrossRef] [PubMed]
- Coskun Benlidayi, I. Role of Inflammation in the Pathogenesis and Treatment of Fibromyalgia. Rheumatol. Int. 2019, 39, 781–791. [Google Scholar] [CrossRef]
- Kim, S.-K.; Kim, K.S.; Lee, Y.S.; Park, S.-H.; Choe, J.-Y. Arterial Stiffness and Proinflammatory Cytokines in Fibromyalgia Syndrome. Clin. Exp. Rheumatol. 2010, 28, S71–S77. [Google Scholar]
- Sánchez-Domínguez, B.; Bullón, P.; Román-Malo, L.; Marín-Aguilar, F.; Alcocer-Gómez, E.; Carrión, A.M.; Sánchez-Alcazar, J.A.; Cordero, M.D. Oxidative Stress, Mitochondrial Dysfunction and, Inflammation Common Events in Skin of Patients with Fibromyalgia. Mitochondrion 2015, 21, 69–75. [Google Scholar] [CrossRef]
- Littlejohn, G.; Guymer, E. Neurogenic Inflammation in Fibromyalgia. Semin. Immunopathol. 2018, 40, 291–300. [Google Scholar] [CrossRef]
- Rodriguez-Pintó, I.; Agmon-Levin, N.; Howard, A.; Shoenfeld, Y. Fibromyalgia and cytokines. Immunol. Lett. 2014, 161, 200–203. [Google Scholar] [CrossRef]
- Nah, S.-S.; Lee, H.; Hong, Y.; Im, J.; Won, H.; Chang, S.-H.; Kim, H.-K.; Kwon, J.-T.; Kim, H.-J. Association between Endothelin-1 and Fibromyalgia Syndrome. Mol. Med. Rep. 2017, 16, 6234–6239. [Google Scholar] [CrossRef]
- Shukla, V.; Kumar, D.S.; Ali, M.A.; Agarwal, S.; Khandpur, S. Nitric Oxide, Lipid Peroxidation Products, and Antioxidants in Primary Fibromyalgia and Correlation with Disease Severity. J. Med. Biochem. 2020, 39, 165–170. [Google Scholar] [CrossRef]
- Rus, A.; Coca-Guzmán, B.; Casas-Barragán, A.; Molina, F.; Correa-Rodríguez, M.; Aguilar-Ferrándiz, M.E. Skin Temperature and Nitric Oxide in Premenopausal and Postmenopausal Women with Fibromyalgia. Climacteric J. Int. Menopause Soc. 2025, 28, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Lambova, S.N.; Muller-Ladner, U. Capillaroscopic Findings in Primary Fibromyalgia. Curr. Rheumatol. Rev. 2018, 14, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, G.Y.; Emekli, D.T.; Sahutoglu, E.; Kocyigit, B.F. Evaluation of Ophthalmic Vascular and Neuroretinal Alterations in Fibromyalgia Syndrome: A Cross-Sectional Comparative Study. Rheumatol. Int. 2024, 44, 1757–1765. [Google Scholar] [CrossRef] [PubMed]
- Contaldo, M. Oral Mucosa Capillaroscopy: A Narrative Review. Cancers 2024, 16, 3774. [Google Scholar] [CrossRef]
- Scardina, G.A.; Messina, P. Microvascular Abnormalities in Patients with Rheumatoid Arthritis. Ann. Anat. Anat. Anz. Off. Organ Anat. Ges. 2006, 188, 425–429. [Google Scholar] [CrossRef]
- Scardina, G.A.; Picone, V.; Cacioppo, A.; Messina, P. Study of Microcirculation in Oral Lichen Planus by Video-Capillaroscopy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 103, e30–e34. [Google Scholar] [CrossRef]
- Scardina, G.A.; Guercio, G.; Valenti, C.F.; Tegolo, D.; Messina, P. Videocapillaroscopy of the Oral Mucosa in Patients with Diabetic Foot: Possible Diagnostic Role of Microangiopathic Damage? J. Clin. Med. 2020, 9, 3641. [Google Scholar] [CrossRef]
- Nigliaccio, S.; Cumbo, E.; Fontana, D.A.; Valenti, C.F.; Tegolo, D.; Tocco, A.; Messina, P.; Scardina, G.A. Comparison of Manual and Automated Capillary Morphometry Measurements in Oral Mucosa: A Pilot Study. Appl. Sci. 2025, 15, 5904. [Google Scholar] [CrossRef]
- XLSTAT. Statistical Software for Excel. Available online: https://www.xlstat.com/ (accessed on 16 October 2025).
- Zieliński, G.; Gawda, P. Defining Effect Size Standards in Temporomandibular Joint and Masticatory Muscle Research. Med. Sci. Monit. 2025, 31, e948365. [Google Scholar] [CrossRef]
- Ayouni, I.; Chebbi, R.; Hela, Z.; Dhidah, M. Comorbidity between fibromyalgia and temporomandibular disorders: A systematic review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 128, 33–42. [Google Scholar] [CrossRef]
- De Stefano, R.; Bruno, A.; Muscatello, M.R.A.; Cedro, C.; Cicciù, A.; Rullo, R.; Gaeta, M.; Fiorillo, L. Oral Health and Fibromyalgia Syndrome: A Systemic Review. J. Funct. Morphol. Kinesiol. 2020, 5, 7. [Google Scholar] [CrossRef]
- Gau, S.-Y.; Leong, P.-Y.; Lin, C.-L.; Tsou, H.-K.; Wei, J.C.-C. Higher Risk for Sjögren’s Syndrome in Patients With Fibromyalgia: A Nationwide Population-Based Cohort Study. Front. Immunol. 2021, 12, 640618. [Google Scholar] [CrossRef]
- De Porras-Carrique, T.; Ramos-García, P.; Aguilar-Diosdado, M.; Warnakulasuriya, S.; González-Moles, M.Á. Autoimmune Disorders in Oral Lichen Planus: A Systematic Review and Meta-Analysis. Oral Dis. 2023, 29, 1382–1394. [Google Scholar] [CrossRef]
- Rhodus, N.L.; Fricton, J.; Carlson, P.; Messner, R. Oral Symptoms Associated with Fibromyalgia Syndrome. J. Rheumatol. 2003, 30, 1841–1845. [Google Scholar]
- Scardina, G.A.; Casella, S.; Bilello, G.; Messina, P. Photobiomodulation Therapy in the Management of Burning Mouth Syndrome: Morphological Variations in the Capillary Bed. Dent. J. 2020, 8, 99. [Google Scholar] [CrossRef] [PubMed]

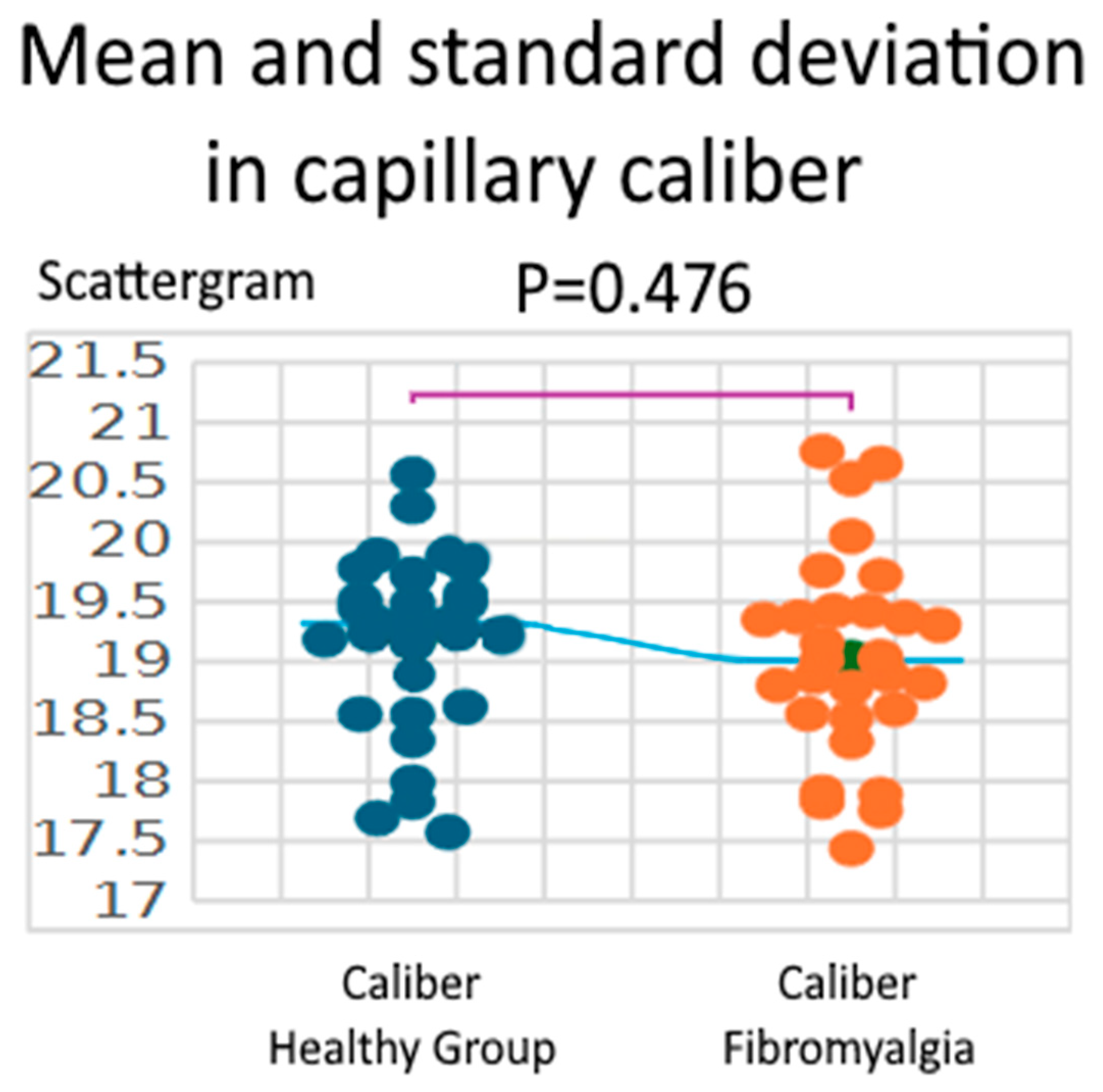
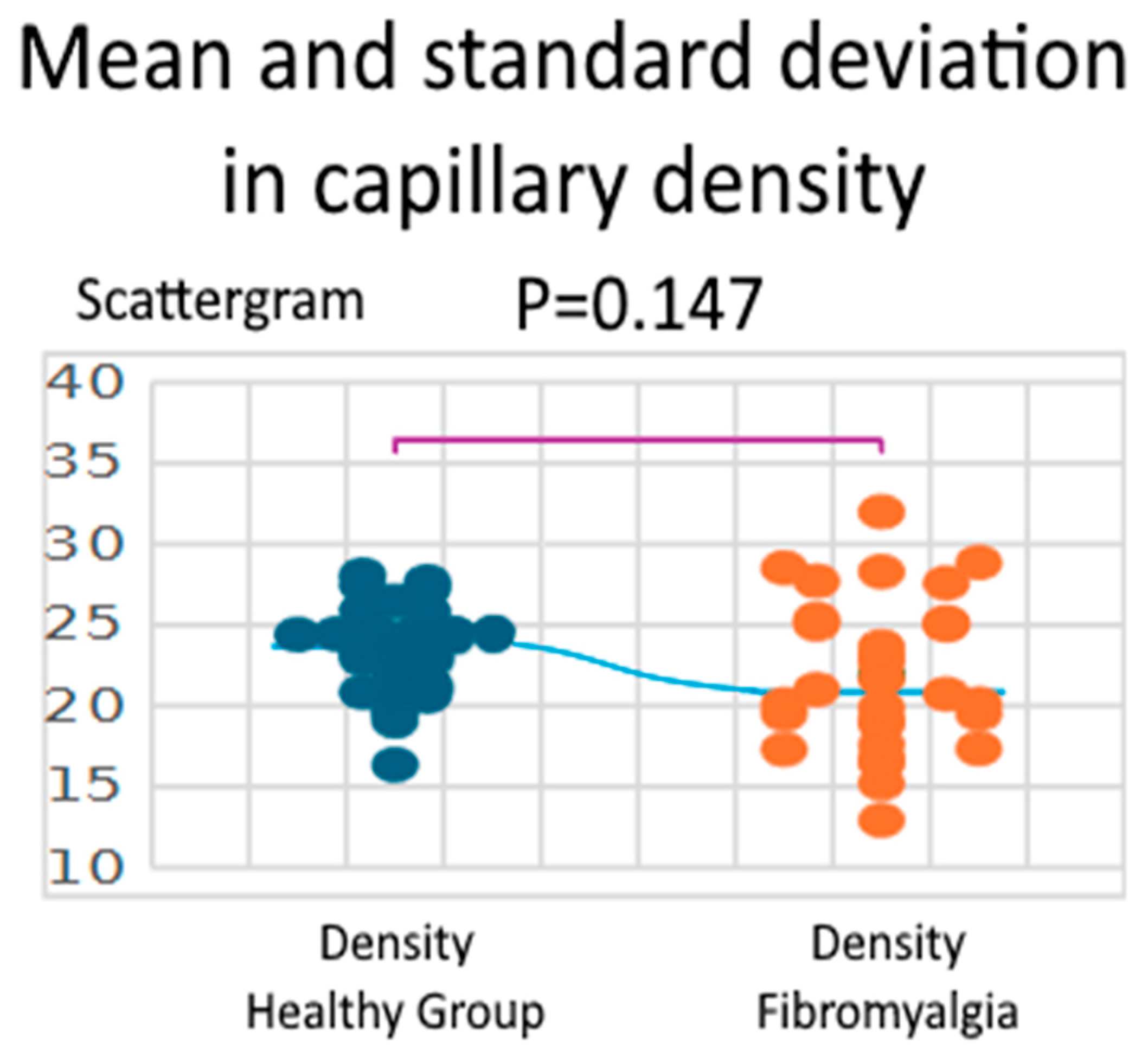
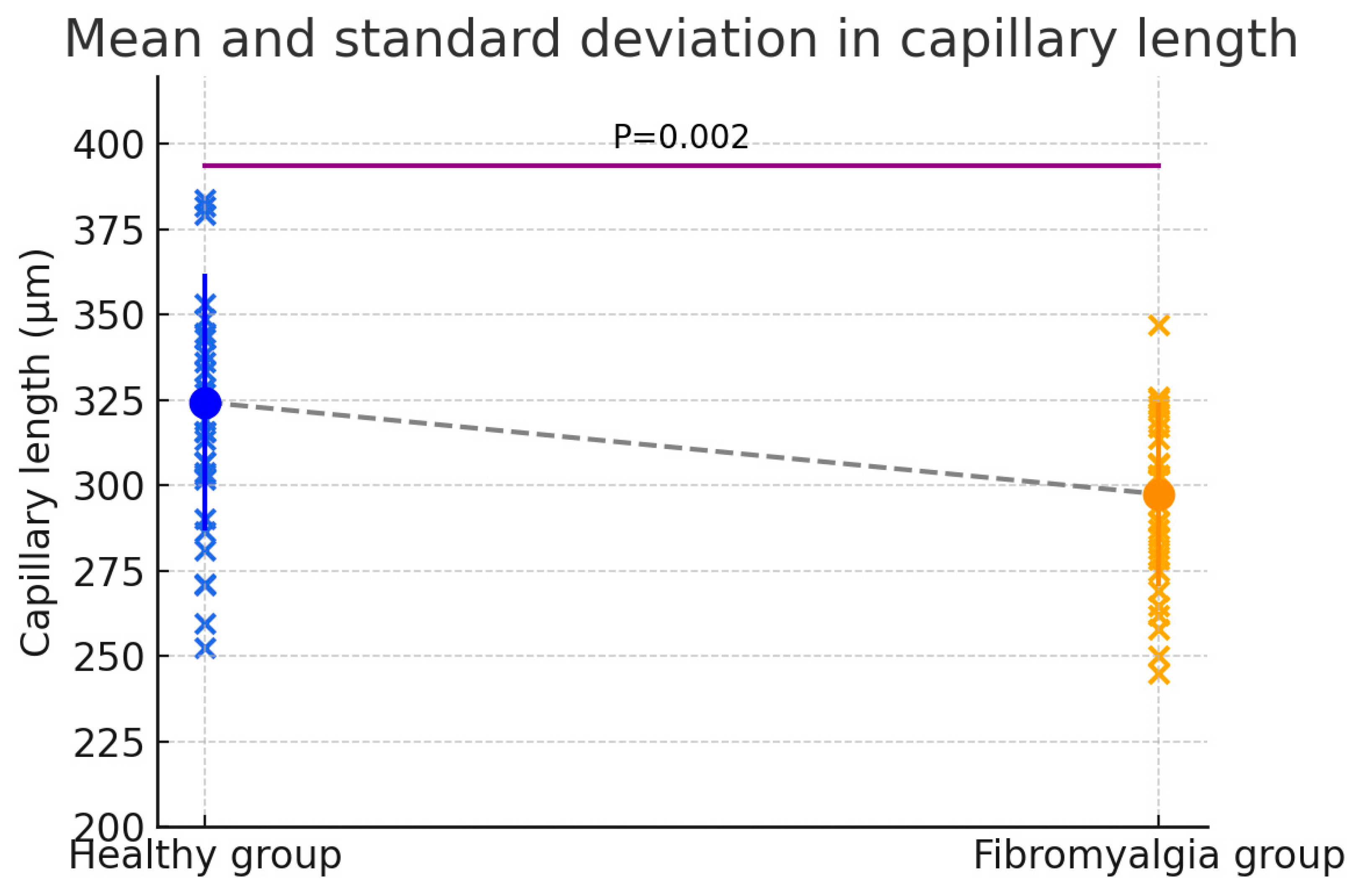
| Parameter | Healthy Group | Fibromyalgia | Difference (Fibro—Healthy) | 95% CI | t (df = 58) | p-Value | Hedges’ g |
|---|---|---|---|---|---|---|---|
| Caliber (µm) | 19.18 ± 0.74 (17.57–20.57) | 19.03 ± 0.83 (17.44–20.76) | 0.15 | −0.26–0.56 | 0.717 | 0.476 | 0.185 |
| Density (n/mm2) | 23.37 ± 2.81 (16.36–28.09) | 21.91 ± 4.66 (12.93–32.00) | 1.46 | −0.53–3.45 | 1.469 | 0.147 | 0.380 |
| Length (µm) | 324.43 ± 37.59 (277.53–417.56) | 297.49 ± 26.82 (218.63–374.92) | 26.94 | 10.06–43.81 | 3.195 | 0.002 * | 0.825 |
| Visibility—Healthy | Visibility—Fibromyalgia | |
|---|---|---|
| 1 Clearly | 113 | 110 |
| 2 Poorly | 5 | 6 |
| 3 Not visible | 2 | 4 |
| Orientation—Healthy | Orientation—Fibromyalgia | |
|---|---|---|
| A (Parallel) | 113 | 104 |
| B (Perpendicular) | 0 | 5 |
| AB (Mixed) | 5 | 7 |
| Microhemorrhages—Healthy | Microhemorrhages—Fibromyalgia | |
|---|---|---|
| Yes | 1 | 3 |
| No | 117 | 113 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nigliaccio, S.; Fontana, D.A.; Pusateri, F.; Di Vita, E.; Messina, P.; Cumbo, E.; Scardina, G.A. Evaluation of Oral Mucosa Capillaries in Fibromyalgia Patients. Biomedicines 2025, 13, 2701. https://doi.org/10.3390/biomedicines13112701
Nigliaccio S, Fontana DA, Pusateri F, Di Vita E, Messina P, Cumbo E, Scardina GA. Evaluation of Oral Mucosa Capillaries in Fibromyalgia Patients. Biomedicines. 2025; 13(11):2701. https://doi.org/10.3390/biomedicines13112701
Chicago/Turabian StyleNigliaccio, Salvatore, Davide Alessio Fontana, Francesca Pusateri, Emanuele Di Vita, Pietro Messina, Enzo Cumbo, and Giuseppe Alessandro Scardina. 2025. "Evaluation of Oral Mucosa Capillaries in Fibromyalgia Patients" Biomedicines 13, no. 11: 2701. https://doi.org/10.3390/biomedicines13112701
APA StyleNigliaccio, S., Fontana, D. A., Pusateri, F., Di Vita, E., Messina, P., Cumbo, E., & Scardina, G. A. (2025). Evaluation of Oral Mucosa Capillaries in Fibromyalgia Patients. Biomedicines, 13(11), 2701. https://doi.org/10.3390/biomedicines13112701








