Do Cancer Patients Present a Different Phenotype of Atrial Fibrillation or a Different Arrhythmia Management?
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Statistical Analysis
3. Results
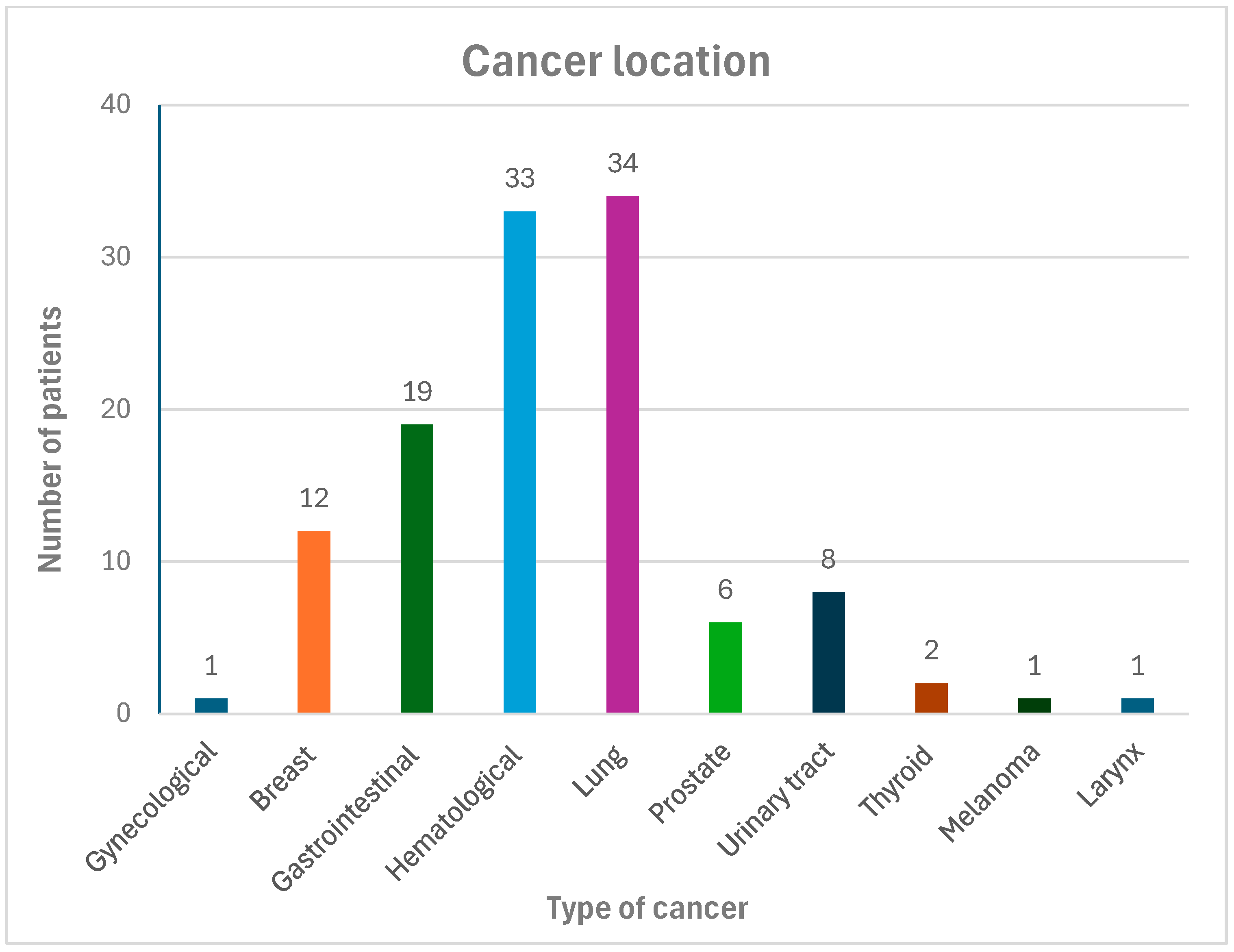
3.1. Clinical Features
3.2. Echocardiographic Parameters
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AF | atrial fibrillation |
| LV | left ventricular |
| CVRF | cardiovascular risk factors |
| LDL | low-density lipoprotein |
| VKA | vitamin K antagonists |
| LMWH | low-molecular-weight heparin |
| CRNMB | clinically relevant non-major bleeding |
| CCI | Charlson Comorbidity Index |
| DOAC | direct oral anticoagulant |
| LAA | left atrial appendage |
| COPD | chronic obstructive pulmonary disease |
| NTpro-BNP | N-terminal pro-B-type natriuretic peptide |
| BNP | B-type natriuretic peptide |
| BMI | body mass index |
| LA | left atrium |
| LVEF | left ventricular ejection |
| LV GLS | left global longitudinal strain |
| CA | catheter ablation |
| ICD | implantable cardioverter defibrillation |
References
- Norbiato, C.; Marengo, S.; Parrini, I. Cancer and arrhythmias. e-J. Cardiol. Pract. 2021, 19, 21. [Google Scholar]
- Hawryszko, M.; Sławiński, G.; Tomasik, B.; Lewicka, E. Cardiac Arrhythmias in Patients Treated for Lung Cancer: A Review. Cancers 2023, 15, 5723. [Google Scholar] [CrossRef]
- Pérez-Callejo, D.; Torrente, M.; Brenes, M.A.; Núñez, B.; Provencio, M. Lung cancer as a cardiotoxic state: A review. Med. Oncol. 2017, 34, 159. [Google Scholar] [CrossRef]
- Tamargo, J.; Caballero, R.; Delpón, E. Cancer chemotherapy and cardiac arrhythmias: A review. Drug Saf. 2015, 38, 129–152. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Miqdad, M.A.; Alatta, L.; Mohamed, D.S.; Syed, N.; Ali, M.; Elomeiri, L.; Alamin, A.; Zubair, H.; Abdalla, Y.; Abdelrahman, N. The Mysterious Association Between Atrial Fibrillation and Cancer: A Literature Review. Cureus 2023, 15, e47278. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Buza, V.; Rajagopalan, B.; Curtis, A.B. Cancer Treatment-Induced Arrhythmias: Focus on Chemotherapy and Targeted Therapies. Circ. Arrhythm. Electrophysiol. 2017, 10, e005443. [Google Scholar] [CrossRef] [PubMed]
- Font, J.; Milliez, P.; Ouazar, A.B.; Klok, F.A.; Alexandre, J. Atrial fibrillation, cancer and anticancer drugs. Arch. Cardiovasc. Dis. 2023, 116, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Imperatori, A.; Mariscalco, G.; Riganti, G.; Rotolo, N.; Conti, V.; Dominioni, L. Atrial fibrillation after pulmonary lobectomy for lung cancer affects long-term survival in a prospective single-center study. J. Cardiothorac. Surg. 2012, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Undas, A.; Drabik, L.; Potpara, T. Bleeding in anticoagulated patients with atrial fibrillation: Practical considerations. Pol. Arch. Intern. Med. 2020, 130, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Kaatz, S.; Ahmad, D.; Spyropoulos, A.C.; Schulman, S.; Subcommittee on Control of Anticoagulation. Subcommittee on Control of Anticoagulation. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2015, 13, 2119–2126. [Google Scholar] [CrossRef]
- Vizzardi, E.; Curnis, A.; Latini, M.G.; Salghetti, F.; Rocco, E.; Lupi, L.; Rovetta, R.; Quinzani, F.; Bonadei, I.; Bontempi, L.; et al. Risk factors for atrial fibrillation recurrence: A literature review. J. Cardiovasc. Med. 2014, 15, 235–253. [Google Scholar] [CrossRef]
- Bizhanov, K.A.; Abzaliyev, K.B.; Baimbetov, A.K.; Sarsenbayeva, A.B.; Lyan, E. Atrial fibrillation: Epidemiology, pathophysiology, and clinical complications. J. Cardiovasc. Electrophysiol. 2023, 34, 153–165. [Google Scholar] [CrossRef]
- Jung, M.; Yang, P.S.; Kim, D.; Sung, J.-H.; Jang, E.; Yu, H.T.; Kim, T.-H.; Uhm, J.-S.; Pak, H.-N.; Lee, M.-H.; et al. Multimorbidity in atrial fibrillation for clinical implications using the Charlson Comorbidity Index. Int. J. Cardiol. 2024, 398, 131605. [Google Scholar] [CrossRef]
- Menichelli, D.; Sciacqua, A.; Cangemi, R.; Andreozzi, P.; Del Sole, F.; Violi, F.; Pignatelli, P.; Pastori, D.; The ATHERO-AF Study Group; Saliola, M.; et al. Atrial fibrillation pattern, left atrial diameter and risk of cardiovascular events and mortality. A prospective multicenter cohort study. Int. J. Clin. Pract. 2021, 75, e13771. [Google Scholar] [CrossRef]
- Pierucci, N.; Mariani, M.V.; Iannetti, G.; Maffei, L.; Coluccio, A.; Laviola, D.; Palombi, M.; Trivigno, S.; Spadafora, L.; Chourda, E.; et al. Atrial cardiomyopathy: New pathophysiological and clinical aspects. Minerva Cardiol Angiol. 2025. [Google Scholar] [CrossRef]
- Maciorowska, M.; Uziębło-Życzkowska, B.; Gorczyca-Głowacka, I.; Wożakowska-Kapłon, B.; Jelonek, O.; Wójcik, M.; Błaszczyk, R.; Kapłon-Cieślicka, A.; Gawałko, M.; Tokarek, T.; et al. Oral anticoagulation therapy in atrial fibrillation patients at high risk of bleeding: Clinical characteristics and treatment strategies based on data from the Polish multicenter register of atrial fibrillation (POL-AF). Kardiol. Pol. 2024, 82, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Menezes Fernandes, R.; Mota, T.F.; Costa, H.A.; Santo, M.E.; Bento, D.; Candeias, R.; Mimoso, J.; Jesus, I. Cancer and atrial fibrillation/flutter: An analysis of patients referred to electrical cardioversion. Eur. Heart J. 2021, 42, 1. [Google Scholar] [CrossRef]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef]
- Giustozzi, M.; Ali, H.; Reboldi, G.; Balla, C.; Foresti, S.; de Ambroggi, G.; Lupo, P.P.; Agnelli, G.; Cappato, R. Safety of catheter ablation of atrial fibrillation in cancer survivors. J. Interv. Card. Electrophysiol. 2021, 60, 419–426. [Google Scholar] [CrossRef]
- Ganatra, S.; Abraham, S.; Kumar, A.; Parikh, R.; Patel, R.; Khadke, S.; Kumar, A.; Liu, V.; Diaz, A.N.R.; Neilan, T.G.; et al. Efficacy and safety of catheter ablation for atrial fibrillation in patients with history of cancer. Cardiooncology 2023, 9, 19. [Google Scholar] [CrossRef]
- Farkowski, M.M.; Ciszewski, J.; Maciąg, A.; Jasek, S.; Zastawna, I.; Jędrychowski, T.; Kapała, A.; Gil, R.J.; Pytkowski, M. A pilot study of high-density mapping of left atrial scarring in patients with a history of cancer treatment undergoing ablation of arrhythmia: The Pilot OncoLA Study. Kardiol. Pol. 2024, 82, 537–539. [Google Scholar] [CrossRef]
- Uemura, K.; Nishimori, M.; Nagai, S.; Takeuchi, M.; Nishihara, Y.; Todo, S.; Oota, E.; Odajima, S.; Takeuchi, K.; Ichikawa, Y.; et al. Identification of factors associated with progression of left atrial enlargement in patients with atrial fibrillation. Echocardiography 2023, 40, 976–982. [Google Scholar] [CrossRef]
| Variable | All Patients n = 217 | Cancer Patients n = 110 | Control Group n = 107 | p Value |
|---|---|---|---|---|
| Age (years) | 70 (10) | 70 (9) | 70 (10) | 0.855 |
| Males, n (%) | 124 (57.4) | 63 (57.3) | 61 (57) | 0.964 |
| BMI (kg/m2) | 28 (25–31) | 27 (25–30) | 29 (26–32) | 0.028 |
| Systolic blood pressure (mmHg) | 130 (19) | 131 (21) | 128 (16) | 0.274 |
| Diastolic blood pressure (mmHg) | 77 (11) | 78 (12) | 76 (10) | 0.228 |
| Paroxysmal atrial fibrillation, n (%) | 135 (62.2) | 70 (63.6) | 65 (60.7) | 0.765 |
| Persistent atrial fibrillation, n (%) | 27 (12.5) | 10 (9.1) | 17 (15.9) | 0.134 |
| Permanent atrial fibrillation, n (%) | 55 (25.3) | 30 (27.3) | 25 (23.4) | 0.53 |
| Coronary artery disease, n (%) | 65 (30) | 28 (25.5) | 37 (34.6) | 0.13 |
| Hypertension, n (%) | 128 (59) | 87 (79.1) | 81 (75.7) | 0.549 |
| Diabetes, n (%) | 53 (24.4) | 29 (26.4) | 24 (22.4) | 0.493 |
| Chronic heart failure, n (%) | 75 (34.6) | 35 (32.8) | 40 (37.4) | 0.478 |
| Stroke/TIA history, n (%) | 24 (11.1) | 8 (7.3) | 16 (14.9) | 0.071 |
| CHA2DS2-VA Score, median and range | 3 (2–4) | 3 (2–4) | 3 (2–4) | 0.897 |
| HAS-BLED Score, median and range | 2 (1–2 | 2 (2–2) | 2 (1–2) | 0.702 |
| COPD, n (%) | 24 (11,1) | 16 (14,5) | 6 (5,6) | 0.029 |
| Active or former smoking, n (%) | 91 (41.9) | 55 (50) | 36 (33.6) | 0.014 |
| ≥2 cardiovascular risk factors, n (%) | 172 (79.3) | 90 (81.8) | 82 (76.6) | 0.35 |
| ≥3 cardiovascular risk factors, n (%) | 101 (46.5) | 52 (47.3) | 49 (45.8) | 0.83 |
| Charlson Comorbidity Index, median and range | 3 (1–5) | 4 (3–8) | 2 (0–3) | <0.001 |
| Creatinine (mg/dL) | 1.00 (0.84–1.24) | 1.00 (0.83–1.25) | 1.00 (0.85–1.21) | 0.963 |
| NT-proBNP (pg/mL) | 1135 (244–3171) | 703 (210–2222) | 1549 (850–4084) | 0.010 |
| BNP (pg/mL) | 209 (90–434) | 129 (66–451) | 220 (91–432) | 0.463 |
| LAA thrombi, n (%) | 6 (2.8) | 1 (0.9) | 5 (4.7) | 0.089 |
| Major bleeding, n (%) | 13 (6) | 9 (8.2) | 4 (3.7) | 0.168 |
| Pulmonary vein isolation, n (%) | 25 (11.5) | 2 (1,8) | 23 (21.5) | <0.001 |
| Electrical cardioversion, n (%) | 55 (25.3) | 18 (16.4) | 37 (34.6) | 0.002 |
| Antiarrhythmic drugs #, n (%) | 63 (29) | 16 (14.5) | 47 (44) | <0.001 |
| VKA, n (%) | 20 (9.3) | 10 (9) | 10 (9.3) | 0.965 |
| LMWH, n (%) | 18 (8.3) | 17 (15.9) | 1 (0.9) | <0.001 |
| DOAC, n (%) | 143 (65.9) | 68 (61.8) | 75 (70.1) | 0.759 |
| Variable | All Patients n = 217 | Cancer Patients n = 110 | Control Group n = 107 | p Value |
|---|---|---|---|---|
| LVEDV (mL) | 119 (55) | 107 (41) | 132 (64) | 0.059 |
| LVESV (mL) | 60 (44) | 51 (33) | 70 (52) | 0.032 |
| LVIDd (mm) | 50 (8) | 49 (7) | 52 (9) | 0.018 |
| LVIDs (mm) | 35 (9) | 33 (8) | 36 (10) | 0.302 |
| IVS (mm) | 11 (2) | 12 (2) | 11 (2) | 0.629 |
| PW (mm) | 11 (2) | 11 (2) | 10 (2) | 0.151 |
| LA diameter (mm) | 45 (7) | 43 (6) | 47 (7) | 0.015 |
| LA diameter > 40 mm (n, %) | 111 (51%) | 48 (44%) | 63 (59%) | 0.020 |
| LAA (cm2) | 30 (10) | 29 (9) | 30 (11) | 0.701 |
| LAA > 20 cm2 (n, %) | 58 (27%) | 32 (29%) | 26 (25%) | 0.446 |
| LA volume (mL) | 102 (54) | 90 (31) | 114 (66) | 0.043 |
| LAVI (mL/m2) | 53 (26) | 49 (19) | 58 (31) | 0.087 |
| LAVI > 34 mL/m2 (n, %) | 93 (43%) | 45 (41%) | 48 (45%) | 0.514 |
| RAA (cm2) | 25 (8) | 24 (7) | 25 (9) | 0.704 |
| RAA > 18 cm2 (n, %) | 49 (23%) | 16 (15%) | 33 (31%) | 0.004 |
| LVEF (%) | 53 (11) | 54 (9) | 51 (12) | 0.025 |
| LV GLS (%) | −15 (4) | −16 (4) | −13 (3) | 0.016 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hawryszko, M.; Sławiński, G.; Pusz-Bulas, W.; Młyński, M.; Wiśniewska, K.; Macuk, P.; Liżewska-Springer, A.; Daniłowicz-Szymanowicz, L.; Lewicka, E. Do Cancer Patients Present a Different Phenotype of Atrial Fibrillation or a Different Arrhythmia Management? Biomedicines 2025, 13, 2700. https://doi.org/10.3390/biomedicines13112700
Hawryszko M, Sławiński G, Pusz-Bulas W, Młyński M, Wiśniewska K, Macuk P, Liżewska-Springer A, Daniłowicz-Szymanowicz L, Lewicka E. Do Cancer Patients Present a Different Phenotype of Atrial Fibrillation or a Different Arrhythmia Management? Biomedicines. 2025; 13(11):2700. https://doi.org/10.3390/biomedicines13112700
Chicago/Turabian StyleHawryszko, Maja, Grzegorz Sławiński, Weronika Pusz-Bulas, Mikołaj Młyński, Kalina Wiśniewska, Patryk Macuk, Aleksandra Liżewska-Springer, Ludmiła Daniłowicz-Szymanowicz, and Ewa Lewicka. 2025. "Do Cancer Patients Present a Different Phenotype of Atrial Fibrillation or a Different Arrhythmia Management?" Biomedicines 13, no. 11: 2700. https://doi.org/10.3390/biomedicines13112700
APA StyleHawryszko, M., Sławiński, G., Pusz-Bulas, W., Młyński, M., Wiśniewska, K., Macuk, P., Liżewska-Springer, A., Daniłowicz-Szymanowicz, L., & Lewicka, E. (2025). Do Cancer Patients Present a Different Phenotype of Atrial Fibrillation or a Different Arrhythmia Management? Biomedicines, 13(11), 2700. https://doi.org/10.3390/biomedicines13112700









