Neuroplasticity Across the Autism–Schizophrenia Continuum
Abstract
1. Introduction
2. Methods
3. Molecular Pathways of Plasticity
4. The Case of Autism
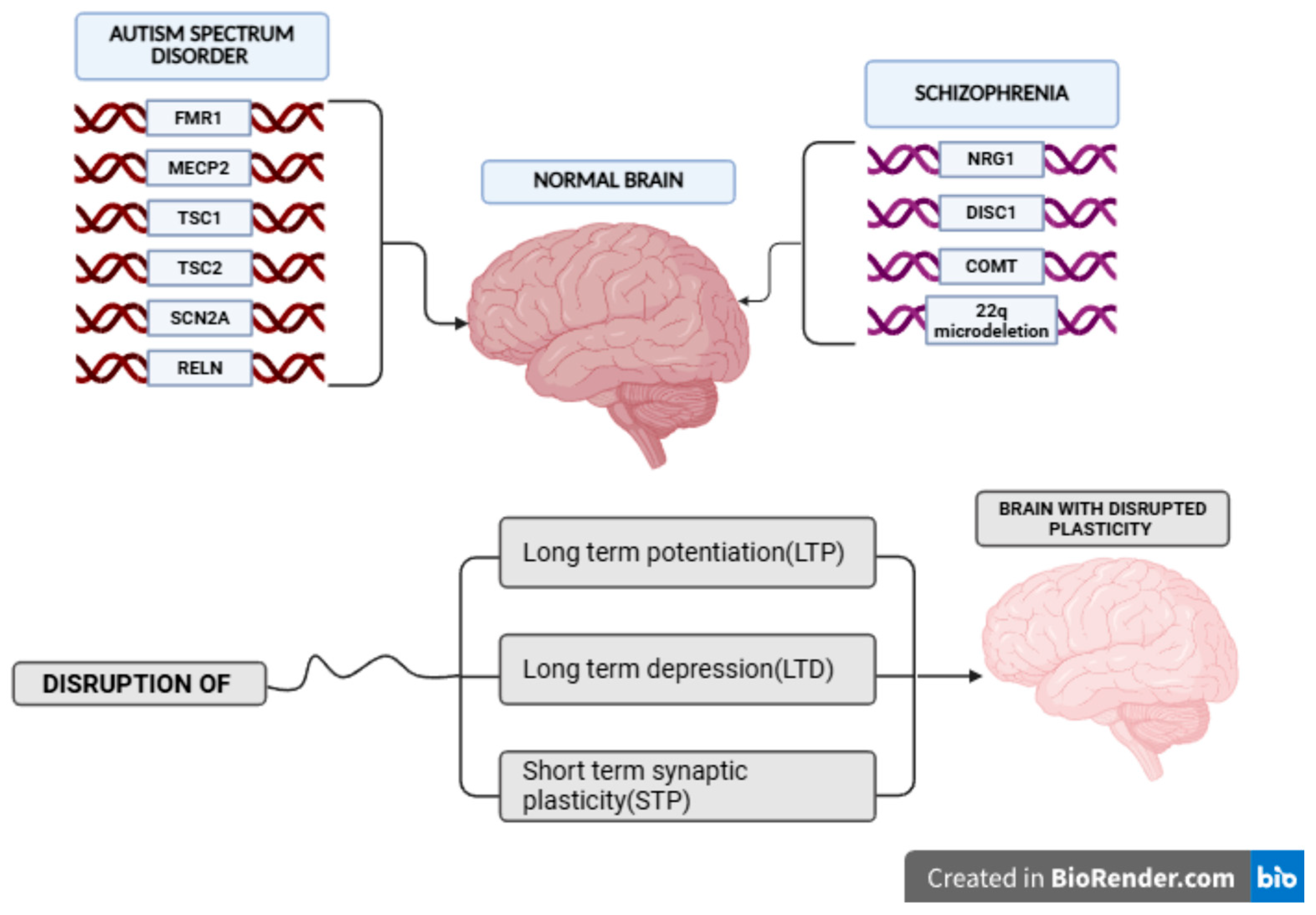
4.1. Definition, Epidemiology and Causes
4.2. Neuropathology, Neurogenetics and Neurochemistry Protagonists
4.3. Synaptic and Non-Synaptic Plasticity in Autism
5. The Case of Schizophrenia
5.1. Epidemiology and Stimuli
5.2. Synaptic and Non-Synaptic Plasticity in Schizophrenia
5.3. Genetic Predisposition
6. Rehabilitation
7. Limitations
8. Future Directions
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMPAR | α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid Receptor |
| ASD | Autism Spectrum Disorder |
| ASR | Age-Standardized Rate |
| DALYs | Disability-Adjusted Life Years |
| EEG | Electroencephalography |
| E/I | Excitation/Inhibition |
| EF | Executive Functions |
| FEP | Forehead Lobe Exercise Program |
| LTD | Long-Term Depression |
| LTP | Long-Term Potentiation |
| MRS | Magnetic Resonance Spectroscopy |
| NMDAR | N-Methyl-d-aspartate Receptor |
| rTMS | Repetitive Transcranial Magnetic Stimulation |
| SCZ | Schizophrenia |
| SLD | Specific Learning Difficulties |
| STP | Short-Term Plasticity |
| tDCS | Transcranial Direct Current Stimulation |
| VR | Virtual Reality |
References
- Mateos-Aparicio, P.; Rodríguez-Moreno, A. The Impact of Studying Brain Plasticity. Front. Cell. Neurosci. 2019, 13, 66. [Google Scholar] [CrossRef] [PubMed]
- Oberman, L.; Pascual-Leone, A. Changes in Plasticity Across the Lifespan. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2013; pp. 91–120. [Google Scholar] [CrossRef]
- Citri, A.; Malenka, R.C. Synaptic Plasticity: Multiple Forms, Functions, and Mechanisms. Neuropsychopharmacology 2008, 33, 18–41. [Google Scholar] [CrossRef]
- Katz, L.C.; Shatz, C.J. Synaptic Activity and the Construction of Cortical Circuits. Science 1996, 274, 1133–1138. [Google Scholar] [CrossRef]
- Monk, C.S.; Webb, S.J.; Nelson, C.A. Prenatal Neurobiological Development: Molecular Mechanisms and Anatomical Change. Dev. Neuropsychol. 2001, 19, 211–236. [Google Scholar] [CrossRef]
- Oppenheim, R.W. Cell Death During Development of the Nervous System. Annu. Rev. Neurosci. 1991, 14, 453–501. [Google Scholar] [CrossRef]
- Raff, M.C.; Barres, B.A.; Burne, J.F.; Coles, H.S.; Ishizaki, Y.; Jacobson, M.D. Programmed Cell Death and the Control of Cell Survival: Lessons from the Nervous System. Science 1993, 262, 695–700. [Google Scholar] [CrossRef]
- Smith, J. Neurulation: Coming to closure. Trends Neurosci. 1997, 20, 510–517. [Google Scholar] [CrossRef]
- Tessier-Lavigne, M.; Goodman, C.S. The Molecular Biology of Axon Guidance. Science 1996, 274, 1123–1133. [Google Scholar] [CrossRef]
- Cuestas Torres, D.M.; Cardenas, F.P. Synaptic plasticity in Alzheimer’s disease and healthy aging. Rev. Neurosci. 2020, 31, 245–268. [Google Scholar] [CrossRef] [PubMed]
- Engert, F.; Bonhoeffer, T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature 1999, 399, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, M.; Honkura, N.; Ellis-Davies, G.C.R.; Kasai, H. Structural basis of long-term potentiation in single dendritic spines. Nature 2004, 429, 761–766. [Google Scholar] [CrossRef]
- Xu, T.; Yu, X.; Perlik, A.J.; Tobin, W.F.; Zweig, J.A.; Tennant, K.; Jones, T.; Zuo, Y. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature 2009, 462, 915–919. [Google Scholar] [CrossRef]
- Lai, C.S.W.; Franke, T.F.; Gan, W.-B. Opposite effects of fear conditioning and extinction on dendritic spine remodelling. Nature 2012, 483, 87–91. [Google Scholar] [CrossRef]
- Ziemann, U.; Iliać, T.V.; Pauli, C.; Meintzschel, F.; Ruge, D. Learning Modifies Subsequent Induction of Long-Term Potentiation-Like and Long-Term Depression-Like Plasticity in Human Motor Cortex. J. Neurosci. 2004, 24, 1666–1672. [Google Scholar] [CrossRef]
- Lynch, M.A. Long-Term Potentiation and Memory. Physiol. Rev. 2004, 84, 87–136. [Google Scholar] [CrossRef]
- Kauer, J.A.; Malenka, R.C. Synaptic plasticity and addiction. Nat. Rev. Neurosci. 2007, 8, 844–858. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.G.; Zukin, R.S. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat. Rev. Neurosci. 2007, 8, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Malenka, R.C.; Bear, M.F. LTP and LTD. Neuron 2004, 44, 5–21. [Google Scholar] [CrossRef]
- Lisman, J.; Schulman, H.; Cline, H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat. Rev. Neurosci. 2002, 3, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, R.J.; Govindarajan, A.; Jung, H.-Y.; Kang, H.; Tonegawa, S. Translational Control by MAPK Signaling in Long-Term Synaptic Plasticity and Memory. Cell 2004, 116, 467–479. [Google Scholar] [CrossRef]
- Sweatt, J.D. The neuronal MAP kinase cascade: A biochemical signal integration system subserving synaptic plasticity and memory. J. Neurochem. 2001, 76, 1–10. [Google Scholar] [CrossRef]
- Zhou, Q.; Homma, K.J.; Poo, M. Shrinkage of Dendritic Spines Associated with Long-Term Depression of Hippocampal Synapses. Neuron 2004, 44, 749–757. [Google Scholar] [CrossRef]
- Wilson, R.I.; Nicoll, R.A. Endocannabinoid Signaling in the Brain. Science 2002, 296, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Olusanya, B.O.; Davis, A.C.; Wertlieb, D.; Boo, N.-Y.; Nair, M.K.C.; Halpern, R.; Kuper, H.; Breinbauer, C.; De Vries, P.J.; Gladstone, M.; et al. Developmental disabilities among children younger than 5 years in 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Glob. Health 2018, 6, e1100–e1121, Erratum in Lancet Glob. Health 2018, 6, e1287. https://doi.org/10.1016/S2214-109X(18)30443-1. [Google Scholar] [CrossRef]
- Sauer, A.K.; Stanton, J.E.; Hans, S.; Grabrucker, A.M. Autism Spectrum Disorders: Etiology and Pathology. In Autism Spectrum Disorders [Internet]; Grabrucker, A.M., Ed.; Exon Publications: Brisbane City, Australia, 2021; pp. 1–16. [Google Scholar] [CrossRef]
- Hodges, H.; Fealko, C.; Soares, N. Autism spectrum disorder: Definition, epidemiology, causes, and clinical evaluation. Transl. Pediatr. 2020, 9, S55–S65. [Google Scholar] [CrossRef] [PubMed]
- Vahia, V. Diagnostic and statistical manual of mental disorders 5: A quick glance. Indian J. Psychiatry 2013, 55, 220. [Google Scholar] [CrossRef]
- Habela, C.W.; Song, H.; Ming, G. Modeling synaptogenesis in schizophrenia and autism using human iPSC derived neurons. Mol. Cell. Neurosci. 2016, 73, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-A.; Chen, Z.-J.; Li, X.-D.; Gu, M.-H.; Xia, N.; Gong, C.; Zhou, Z.-W.; Yasin, G.; Xie, H.-Y.; Wei, X.-P.; et al. Epidemiology of autism spectrum disorders: Global burden of disease 2019 and bibliometric analysis of risk factors. Front. Pediatr. 2022, 10, 972809. [Google Scholar] [CrossRef]
- Masi, A.; DeMayo, M.M.; Glozier, N.; Guastella, A.J. An Overview of Autism Spectrum Disorder, Heterogeneity and Treatment Options. Neurosci. Bull. 2017, 33, 183–193. [Google Scholar] [CrossRef]
- Cohen, S.; Conduit, R.; Lockley, S.W.; Rajaratnam, S.M.; Cornish, K.M. The relationship between sleep and behavior in autism spectrum disorder (ASD): A review. J. Neurodev. Disord. 2014, 6, 44. [Google Scholar] [CrossRef]
- Yoon, S.; Choi, J.; Lee, W.; Do, J. Genetic and Epigenetic Etiology Underlying Autism Spectrum Disorder. J. Clin. Med. 2020, 9, 966. [Google Scholar] [CrossRef] [PubMed]
- Sacco, R.; Gabriele, S.; Persico, A.M. Head circumference and brain size in autism spectrum disorder: A systematic review and meta-analysis. Psychiatry Res. Neuroimaging 2015, 234, 239–251. [Google Scholar] [CrossRef]
- Cheffer, A.; Flitsch, L.J.; Krutenko, T.; Röderer, P.; Sokhranyaeva, L.; Iefremova, V.; Hajo, M.; Peitz, M.; Schwarz, M.K.; Brüstle, O. Human stem cell-based models for studying autism spectrum disorder-related neuronal dysfunction. Mol. Autism 2020, 11, 99. [Google Scholar] [CrossRef] [PubMed]
- Carroll, L.; Braeutigam, S.; Dawes, J.M.; Krsnik, Z.; Kostovic, I.; Coutinho, E.; Dewing, J.M.; Horton, C.A.; Gomez-Nicola, D.; Menassa, D.A. Autism Spectrum Disorders: Multiple Routes to, and Multiple Consequences of, Abnormal Synaptic Function and Connectivity. Neuroscientist 2021, 27, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Huttenlocher, J. Language Input and Language Growth. Prev. Med. 1998, 27, 195–199. [Google Scholar] [CrossRef]
- KostoviÄ, I.; KostoviÄ SrzentiÄ, M.; Benjak, V.; Jovanov-MiloÅeviÄ, N.; RadoÅ, M. Developmental Dynamics of Radial Vulnerability in the Cerebral Compartments in Preterm Infants and Neonates. Front. Neurol. 2014, 5, 139. [Google Scholar] [CrossRef]
- Gilmore, J.H.; Knickmeyer, R.C.; Gao, W. Imaging structural and functional brain development in early childhood. Nat. Rev. Neurosci. 2018, 19, 123–137. [Google Scholar] [CrossRef]
- Salami, M.; Itami, C.; Tsumoto, T.; Kimura, F. Change of conduction velocity by regional myelination yields constant latency irrespective of distance between thalamus and cortex. Proc. Natl. Acad. Sci. USA 2003, 100, 6174–6179. [Google Scholar] [CrossRef]
- Ciarrusta, J.; O’Muircheartaigh, J.; Dimitrova, R.; Batalle, D.; Cordero-Grande, L.; Price, A.; Hughes, E.; Steinweg, J.K.; Kangas, J.; Perry, E.; et al. Social Brain Functional Maturation in Newborn Infants with and Without a Family History of Autism Spectrum Disorder. JAMA Netw. Open 2019, 2, e191868. [Google Scholar] [CrossRef]
- Just, M.A.; Keller, T.A.; Malave, V.L.; Kana, R.K.; Varma, S. Autism as a neural systems disorder: A theory of frontal-posterior underconnectivity. Neurosci. Biobehav. Rev. 2012, 36, 1292–1313. [Google Scholar] [CrossRef]
- Koshino, H.; Kana, R.K.; Keller, T.A.; Cherkassky, V.L.; Minshew, N.J.; Just, M.A. fMRI Investigation of Working Memory for Faces in Autism: Visual Coding and Underconnectivity with Frontal Areas. Cereb. Cortex 2008, 18, 289–300. [Google Scholar] [CrossRef]
- Rubenstein, J.L.R.; Merzenich, M.M. Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003, 2, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Suprunowicz, M.; Bogucka, J.; Szczerbińska, N.; Modzelewski, S.; Oracz, A.J.; Konarzewska, B.; Waszkiewicz, N. Neuroplasticity-Based Approaches to Sensory Processing Alterations in Autism Spectrum Disorder. Int. J. Mol. Sci. 2025, 26, 7102. [Google Scholar] [CrossRef] [PubMed]
- Uzunova, G.; Pallanti, S.; Hollander, E. Excitatory/inhibitory imbalance in autism spectrum disorders: Implications for interventions and therapeutics. World J. Biol. Psychiatry 2016, 17, 174–186. [Google Scholar] [CrossRef]
- Shaw, P.; Kabani, N.J.; Lerch, J.P.; Eckstrand, K.; Lenroot, R.; Gogtay, N.; Greenstein, D.; Clasen, L.; Evans, A.; Rapoport, J.L.; et al. Neurodevelopmental Trajectories of the Human Cerebral Cortex. J. Neurosci. 2008, 28, 3586–3594. [Google Scholar] [CrossRef]
- Kéïta, L.; Mottron, L.; Bertone, A. Far visual acuity is unremarkable in autism: Do we need to focus on crowding? Autism Res. 2010, 3, 333–341. [Google Scholar] [CrossRef]
- Tran The, J.; Magistretti, P.J.; Ansermet, F. The critical periods of cerebral plasticity: A key aspect in a dialog between psychoanalysis and neuroscience centered on the psychopathology of schizophrenia. Front. Mol. Neurosci. 2022, 15, 1057539. [Google Scholar] [CrossRef]
- Marder, S.R.; Cannon, T.D. Schizophrenia. N. Engl. J. Med. 2019, 381, 1753–1761. [Google Scholar] [CrossRef]
- Hoseth, E.Z.; Ueland, T.; Dieset, I.; Birnbaum, R.; Shin, J.H.; Kleinman, J.E.; Hyde, T.M.; Mørch, R.H.; Hope, S.; Lekva, T.; et al. A Study of TNF Pathway Activation in Schizophrenia and Bipolar Disorder in Plasma and Brain Tissue. Schizophr. Bull. 2017, 43, sbw183. [Google Scholar] [CrossRef]
- Tandon, R.; Keshavan, M.S.; Nasrallah, H.A. Schizophrenia, “Just the Facts”: What we know in 2008. Schizophr. Res. 2008, 100, 4–19. [Google Scholar] [CrossRef] [PubMed]
- Stephan, K.E.; Baldeweg, T.; Friston, K.J. Synaptic Plasticity and Dysconnection in Schizophrenia. Biol. Psychiatry 2006, 59, 929–939. [Google Scholar] [CrossRef]
- Keshavan, M.S.; Anderson, S.; Pettergrew, J.W. Is Schizophrenia due to excessive synaptic pruning in the prefrontal cortex? The Feinberg hypothesis revisited. J. Psychiatr. Res. 1994, 28, 239–265. [Google Scholar] [CrossRef]
- Murray, R.M.; Lewis, S.W. Is schizophrenia a neurodevelopmental disorder? BMJ 1988, 296, 63. [Google Scholar] [CrossRef]
- Feinberg, I. Schizophrenia: Caused by a fault in programmed synaptic elimination during adolescence? J. Psychiatr. Res. 1982, 17, 319–334. [Google Scholar] [CrossRef]
- Harrison, P.J.; Owen, M.J. Genes for schizophrenia? Recent findings and their pathophysiological implications. Lancet 2003, 361, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Barr, M.S.; Farzan, F.; Rajji, T.K.; Voineskos, A.N.; Blumberger, D.M.; Arenovich, T.; Fitzgerald, P.B.; Daskalakis, Z.J. Can Repetitive Magnetic Stimulation Improve Cognition in Schizophrenia? Pilot Data from a Randomized Controlled Trial. Biol. Psychiatry 2013, 73, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.M.; Moran, J.L.; Fromer, M.; Ruderfer, D.; Solovieff, N.; Roussos, P.; O’Dushlaine, C.; Chambert, K.; Bergen, S.E.; Kähler, A.; et al. A polygenic burden of rare disruptive mutations in schizophrenia. Nature 2014, 506, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Doorduin, J.; De Vries, E.F.J.; Willemsen, A.T.M.; De Groot, J.C.; Dierckx, R.A.; Klein, H.C. Neuroinflammation in Schizophrenia-Related Psychosis: A PET Study. J. Nucl. Med. 2009, 50, 1801–1807. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Chiba, K. Role of Microglial M1/M2 Polarization in Relapse and Remission of Psychiatric Disorders and Diseases. Pharmaceuticals 2014, 7, 1028–1048. [Google Scholar] [CrossRef]
- Kukreja, I.; Bhagwath, A.; Shankaran, A. Synaptic Pruning in Schizophrenia: Manifestations and Antecedents. Int. J. Multidiscip. Res. 2025, 7, 44004. [Google Scholar] [CrossRef]
- Hashemi, E.; Ariza, J.; Rogers, H.; Noctor, S.C.; Martínez-Cerdeño, V. The Number of Parvalbumin-Expressing Interneurons Is Decreased in the Prefrontal Cortex in Autism. Cereb. Cortex 2017, 27, 1931–1943, Erratum in Cereb. Cortex 2018, 28, 690. https://doi.org/10.1093/cercor/bhx063. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, A.; Voineskos, D.; Daskalakis, Z.J.; Rajji, T.K.; Blumberger, D.M. A Review of Impaired Neuroplasticity in Schizophrenia Investigated with Non-invasive Brain Stimulation. Front. Psychiatry 2016, 7, 45. [Google Scholar] [CrossRef]
- Kaneko, N.; Wada, M.; Nakajima, S.; Takano, M.; Taniguchi, K.; Honda, S.; Mimura, M.; Noda, Y. Neuroplasticity of the left dorsolateral prefrontal cortex in patients with treatment-resistant depression as indexed with paired associative stimulation: A TMS–EEG study. Cereb. Cortex 2024, 34, bhad515. [Google Scholar] [CrossRef] [PubMed]
- Figurov, A.; Pozzo-Miller, L.D.; Olafsson, P.; Wang, T.; Lu, B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature 1996, 381, 706–709. [Google Scholar] [CrossRef]
- Pillai, A.; Terry, A.V.; Mahadik, S.P. Differential effects of long-term treatment with typical and atypical antipsychotics on NGF and BDNF levels in rat striatum and hippocampus. Schizophr. Res. 2006, 82, 95–106. [Google Scholar] [CrossRef]
- Frantseva, M.V.; Fitzgerald, P.B.; Chen, R.; Moller, B.; Daigle, M.; Daskalakis, Z.J. Evidence for Impaired Long-Term Potentiation in Schizophrenia and Its Relationship to Motor Skill Leaning. Cereb. Cortex 2008, 18, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Wamsley, E.J.; Tucker, M.A.; Shinn, A.K.; Ono, K.E.; McKinley, S.K.; Ely, A.V.; Goff, D.C.; Stickgold, R.; Manoach, D.S. Reduced Sleep Spindles and Spindle Coherence in Schizophrenia: Mechanisms of Impaired Memory Consolidation? Biol. Psychiatry 2012, 71, 154–161. [Google Scholar] [CrossRef]
- Balu, D.T.; Coyle, J.T. Neuroplasticity signaling pathways linked to the pathophysiology of schizophrenia. Neurosci. Biobehav. Rev. 2011, 35, 848–870. [Google Scholar] [CrossRef]
- Liu, J.; Hao, Y.; Du, M.; Wang, X.; Zhang, J.; Manor, B.; Jiang, X.; Fang, W.; Wang, D. Quantitative cerebral blood flow mapping and functional connectivity of postherpetic neuralgia pain: A perfusion fMRI study. Pain 2013, 154, 110–118. [Google Scholar] [CrossRef]
- Bjarnadottir, M.; Misner, D.L.; Haverfield-Gross, S.; Bruun, S.; Helgason, V.G.; Stefansson, H.; Sigmundsson, A.; Firth, D.R.; Nielsen, B.; Stefansdottir, R.; et al. Neuregulin1 (NRG1) Signaling through Fyn Modulates NMDA Receptor Phosphorylation: Differential Synaptic Function in NRG1+/− Knock-Outs Compared with Wild-Type Mice. J. Neurosci. 2007, 27, 4519–4529. [Google Scholar] [CrossRef]
- Millar, J.K.; Christie, S.; Anderson, S.; Lawson, D.; Loh, D.H.-W.; Devon, R.S.; Arveiler, B.; Muir, W.J.; Blackwood, D.H.R.; Porteous, D.J. Genomic structure and localisation within a linkage hotspot of Disrupted In Schizophrenia 1, a gene disrupted by a translocation segregating with schizophrenia. Mol. Psychiatry 2001, 6, 173–178. [Google Scholar] [CrossRef]
- Xie, G.; Clapcote, S.J.; Nieman, B.J.; Tallerico, T.; Huang, Y.; Vukobradovic, I.; Cordes, S.P.; Osborne, L.R.; Rossant, J.; Sled, J.G.; et al. Forward genetic screen of mouse reveals dominant missense mutation in the P/Q-type voltage-dependent calcium channel, CACNA1A. Genes Brain Behav. 2007, 6, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Ibi, D.; Nagai, T.; Koike, H.; Kitahara, Y.; Mizoguchi, H.; Niwa, M.; Jaaro-Peled, H.; Nitta, A.; Yoneda, Y.; Nabeshima, T. Combined effect of neonatal immune activation and mutant DISC1 on phenotypic changes in adulthood. Behav. Brain Res. 2010, 206, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Arguello, P.A.; Markx, S.; Gogos, J.A.; Karayiorgou, M. Development of animal models for schizophrenia. Dis. Model. Mech. 2010, 3, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Trubetskoy, V.; Pardiñas, A.F.; Qi, T.; Panagiotaropoulou, G.; Awasthi, S.; Bigdeli, T.B.; Bryois, J.; Chen, C.-Y.; Dennison, C.A.; Hall, L.S.; et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature 2022, 604, 502–508. [Google Scholar] [CrossRef]
- Eack, S.M.; Greeno, C.G.; Pogue-Geile, M.F.; Newhill, C.E.; Hogarty, G.E.; Keshavan, M.S. Assessing Social-Cognitive Deficits in Schizophrenia with the Mayer-Salovey-Caruso Emotional Intelligence Test. Schizophr. Bull. 2010, 36, 370–380. [Google Scholar] [CrossRef]
- Penadés, R.; Pujol, N.; Catalán, R.; Massana, G.; Rametti, G.; García-Rizo, C.; Bargalló, N.; Gastó, C.; Bernardo, M.; Junqué, C. Brain Effects of Cognitive Remediation Therapy in Schizophrenia: A Structural and Functional Neuroimaging Study. Biol. Psychiatry 2013, 73, 1015–1023. [Google Scholar] [CrossRef]
- Fisher, M.; Holland, C.; Subramaniam, K.; Vinogradov, S. Neuroplasticity-Based Cognitive Training in Schizophrenia: An Interim Report on the Effects 6 Months Later. Schizophr. Bull. 2010, 36, 869–879. [Google Scholar] [CrossRef]
- Sommer, I.E.C.; Slotema, C.W.; Daskalakis, Z.J.; Derks, E.M.; Blom, J.D.; Van Der Gaag, M. The Treatment of Hallucinations in Schizophrenia Spectrum Disorders. Schizophr. Bull. 2012, 38, 704–714. [Google Scholar] [CrossRef]
- Molteni, R.; Wu, A.; Vaynman, S.; Ying, Z.; Barnard, R.J.; Gómez-Pinilla, F. Exercise reverses the harmful effects of consumption of a high-fat diet on synaptic and behavioral plasticity associated to the action of brain-derived neurotrophic factor. Neuroscience 2004, 123, 429–440. [Google Scholar] [CrossRef]
- Di Giusto, V.; Purpura, G.; Zorzi, C.F.; Blonda, R.; Brazzoli, E.; Meriggi, P.; Reina, T.; Rezzonico, S.; Sala, R.; Olivieri, I.; et al. Virtual reality rehabilitation program on executive functions of children with specific learning disorders: A pilot study. Front. Psychol. 2023, 14, 1241860. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.O.; Tamplain, P.; Duarte, N.A.C.; Comodo, A.C.M.; Ferreira, G.O.A.; Queiróga, A.; Oliveira, C.S.; Collange-Grecco, L.A. Transcranial direct current stimulation to facilitate neurofunctional rehabilitation in children with autism spectrum disorder: A protocol for a randomized, sham-controlled, double-blind clinical trial. Front. Neurol. 2023, 14, 1196585. [Google Scholar] [CrossRef]
- Atigh, A.; Alizadeh Zarei, M. The effect of cognitive rehabilitation therapy (CRT) on the executive functions of children with autism spectrum disorder (ASD). Chronic Dis. J. 2019, 7, 137–147. [Google Scholar] [CrossRef]
- Tieri, G.; Morone, G.; Paolucci, S.; Iosa, M. Virtual reality in cognitive and motor rehabilitation: Facts, fiction and fallacies. Expert Rev. Med. Devices 2018, 15, 107–117. [Google Scholar] [CrossRef] [PubMed]

| Dimension | ASD (Typical Direction/Timing) | SCZ (Typical Direction/Timing) | Key Molecular Nodes | Phys/Imaging Markers | Therapeutic Implications |
|---|---|---|---|---|---|
| Synaptic plasticity | Early hyperplasticity; excess spine formation in early childhood | Hypoplasticity/overpruning in adolescence | FMRP, mTOR/PI3K, SHANK3 (ASD); NRG1, DISC1, C4/C3 (SCZ) | Spine density, dendritic morphology (postmortem/iPSC), MRI cortical thickness | Early cognitive training, EF interventions (ASD); rTMS, cognitive remediation (SCZ) |
| Connectivity | Local hyperconnectivity, long-range underconnectivity; early critical period | Reduced long-range connectivity; delayed circuit refinement | NRXN/NLGN/SHANK, GABA/glutamate receptors | DTI, fMRI resting-state networks | Circuit-targeted VR, behavioral therapies (ASD); network-guided rTMS (SCZ) |
| Critical periods | Early sensory/social circuits (0–3 years) | Adolescent refinement (12–25 years) | GABAergic maturation, parvalbumin interneurons | MRS GABA/Glu, EEG/ERP | Developmentally timed interventions; experience-dependent plasticity harnessing |
| Excitation/inhibition balance | E/I shift favoring excitation → hyper-responsivity | E/I shift favoring reduced inhibition → network instability | GABA, Glutamate, mTOR, SHANK, NMDAR | MRS Glu/GABA, TMS plasticity metrics | Pharmacological modulation, activity-dependent interventions |
| Microglia/pruning | Impaired pruning → excess early connections | Excess pruning → synapse loss, network dysregulation | Complement (C3/C4), microglia activation | TSPO-PET, postmortem histology | Anti-inflammatory approaches; precision-timed interventions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kesidou, E.; Mitsoudis, N.; Damianidou, O.; Taloumtzis, C.; Tsakiridou, M.; Polyzoidou, E.; Grigoriadou, E.; Bakirtzis, C.; Spandou, E.; Simeonidou, C. Neuroplasticity Across the Autism–Schizophrenia Continuum. Biomedicines 2025, 13, 2695. https://doi.org/10.3390/biomedicines13112695
Kesidou E, Mitsoudis N, Damianidou O, Taloumtzis C, Tsakiridou M, Polyzoidou E, Grigoriadou E, Bakirtzis C, Spandou E, Simeonidou C. Neuroplasticity Across the Autism–Schizophrenia Continuum. Biomedicines. 2025; 13(11):2695. https://doi.org/10.3390/biomedicines13112695
Chicago/Turabian StyleKesidou, Evangelia, Nikolaos Mitsoudis, Olympia Damianidou, Charilaos Taloumtzis, Marianna Tsakiridou, Eleni Polyzoidou, Eleni Grigoriadou, Christos Bakirtzis, Evangelia Spandou, and Constantina Simeonidou. 2025. "Neuroplasticity Across the Autism–Schizophrenia Continuum" Biomedicines 13, no. 11: 2695. https://doi.org/10.3390/biomedicines13112695
APA StyleKesidou, E., Mitsoudis, N., Damianidou, O., Taloumtzis, C., Tsakiridou, M., Polyzoidou, E., Grigoriadou, E., Bakirtzis, C., Spandou, E., & Simeonidou, C. (2025). Neuroplasticity Across the Autism–Schizophrenia Continuum. Biomedicines, 13(11), 2695. https://doi.org/10.3390/biomedicines13112695









