Intracellular Calcium Dysregulation: The Hidden Culprit in the Diabetes–Gout Nexus
Abstract
1. Introduction
2. The Pathogenesis of Type 2 Diabetes Coexisting with Gout
3. Intracellular Calcium Homeostasis and Type 2 Diabetes
3.1. Intracellular Calcium Homeostasis and β-Cell Injury
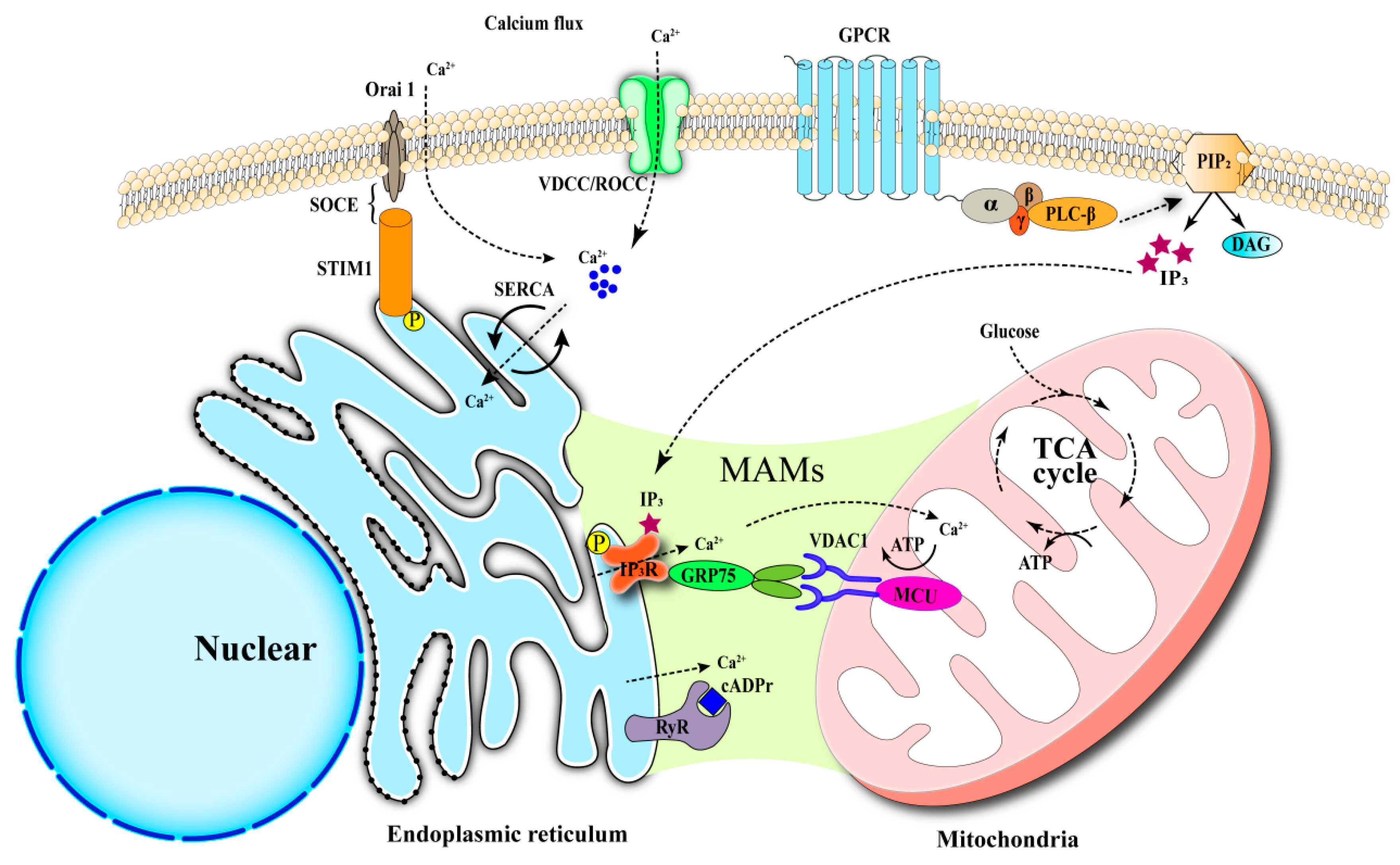
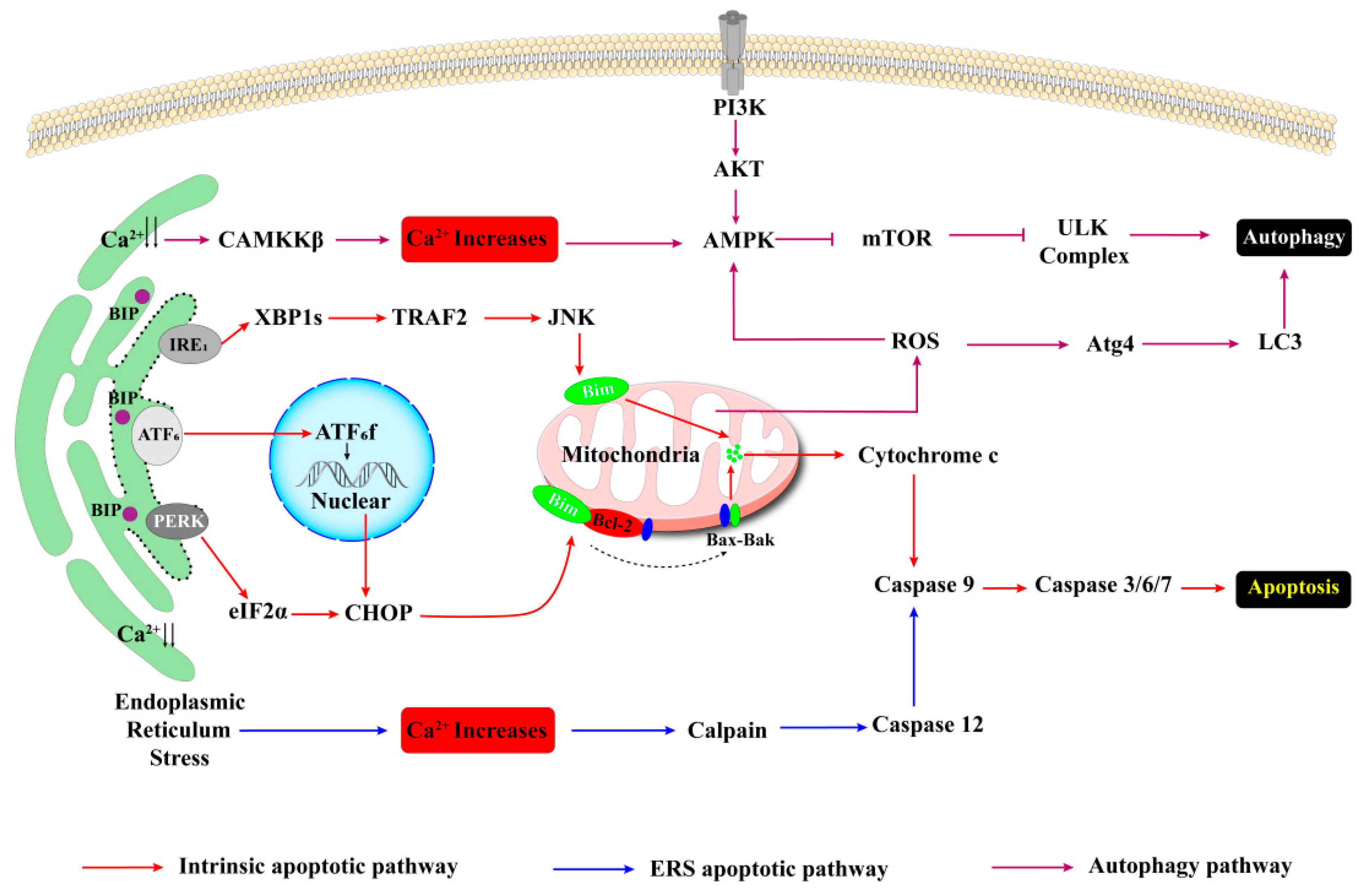
3.1.1. Apoptosis in Pancreatic β-Cells
Endoplasmic Reticulum-Related Apoptosis
Mitochondria-Associated Apoptosis
3.1.2. Autophagy in Pancreatic β-Cells
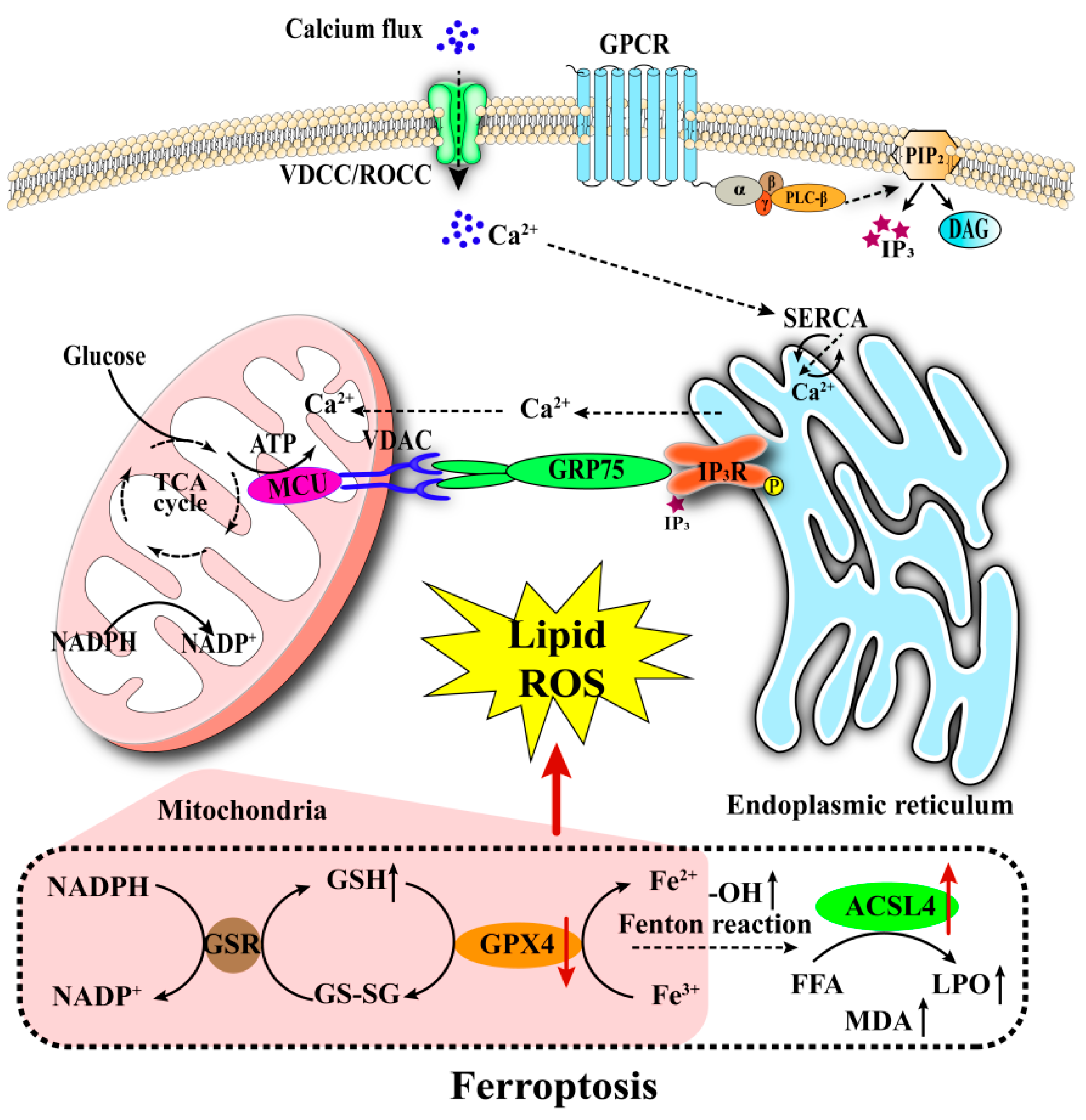
3.1.3. Ferroptosis in Pancreatic β-Cells
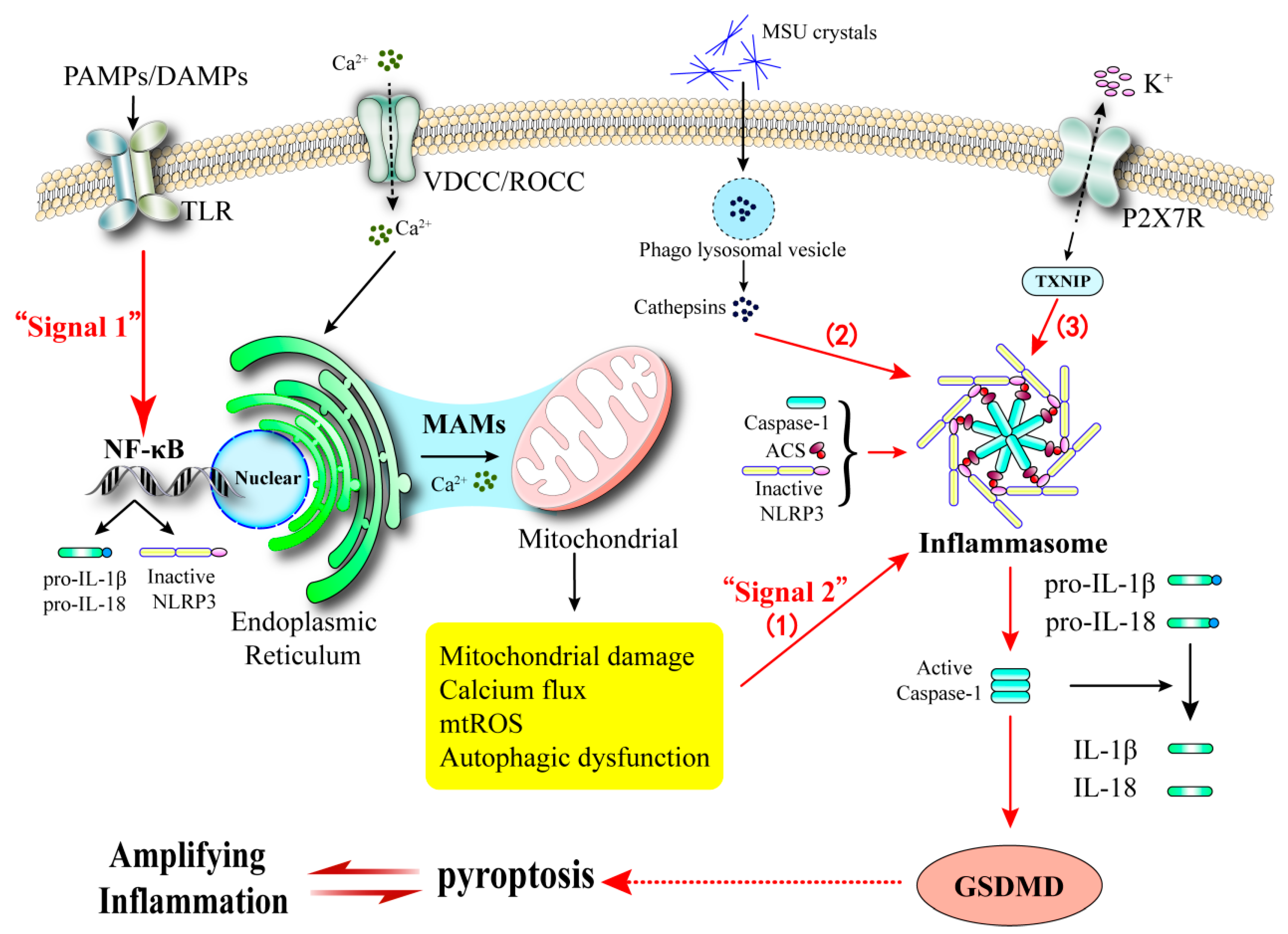
3.1.4. Pyroptosis in Pancreatic β-Cells
3.2. Calcium Homeostasis and Insulin Resistance in Diabetes
3.2.1. The Process of Insulin Signaling Transduction
3.2.2. Insulin Resistance in Target Organs
4. Calcium Homeostasis and Gout
4.1. Abnormal Calcium Signaling in Immune Cells
4.1.1. T Cells and Calcium Homeostasis
4.1.2. Macrophages and Calcium Homeostasis
4.2. Insulin Resistance and Imbalance of Calcium Homeostasis in Gout
5. The Association Between Intracellular Calcium Homeostasis and Comorbid Gout in Type 2 Diabetes
5.1. Linking Effects of Insulin Resistance
5.2. The Amplification of Inflammatory Signals
5.3. Linking β-Cell Death Networks to Gouty Inflammation via Calcium Signaling
6. Therapeutic Perspectives and Challenges
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chan, J.C.N.; Lim, L.-L.; Wareham, N.J.; Shaw, J.E.; Orchard, T.J.; Zhang, P.; Lau, E.S.H.; Eliasson, B.; Kong, A.P.S.; Ezzati, M.; et al. The Lancet Commission on diabetes: Using data to transform diabetes care and patient lives. Lancet 2020, 396, 2019–2082. [Google Scholar] [CrossRef]
- Dieleman, J.L.; Cao, J.; Chapin, A.; Chen, C.; Li, Z.; Liu, A.; Horst, C.; Kaldjian, A.; Matyasz, T.; Scott, K.W.; et al. US Health Care Spending by Payer and Health Condition, 1996–2016. JAMA 2020, 323, 863–884. [Google Scholar] [CrossRef]
- Maria, J.L.; Anand, T.N.; Dona, B.; Prinu, J.; Prabhakaran, D.; Jeemon, P. Task-sharing interventions for improving control of diabetes in low-income and middle-income countries: A systematic review and meta-analysis. Lancet Glob. Health 2021, 9, e170–e180. [Google Scholar] [CrossRef]
- International Diabetes Federation (IDF). IDF Diabetes Atlas, 11th ed.; International Diabetes Federation: Brussels, Belgium, 2025. [Google Scholar]
- Jiang, J.; Zhang, T.; Liu, Y.; Chang, Q.; Zhao, Y.; Guo, C.; Xia, Y. Prevalence of Diabetes in Patients with Hyperuricemia and Gout: A Systematic Review and Meta-analysis. Curr. Diabetes Rep. 2023, 23, 103–117. [Google Scholar] [CrossRef]
- Wijnands, J.M.A.; van Durme, C.M.P.G.; Driessen, J.H.M.; Boonen, A.; Klop, C.; Leufkens, B.; Cooper, C.; Stehouwer, C.D.A.; de Vries, F. Individuals with Type 2 Diabetes Mellitus are at an Increased Risk of Gout but this is not Due to Diabetes. Medicine 2015, 94, e1358. [Google Scholar] [CrossRef]
- Matsuura, F.; Yamashita, S.; Nakamura, T.; Nishida, M.; Nozaki, S.; Funahashi, T.; Matsuzawa, Y. Effect of visceral fat accumulation on uric acid metabolism in male obese subjects: Visceral fat obesity is linked more closely to overproduction of uric acid than subcutaneous fat obesity. Metabolism 1998, 47, 929–933. [Google Scholar] [CrossRef]
- Toyoki, D.; Shibata, S.; Kuribayashi-Okuma, E.; Xu, N.; Ishizawa, K.; Hosoyamada, M.; Uchida, S. Insulin stimulates uric acid reabsorption via regulating urate transporter 1 and ATP-binding cassette subfamily G member 2. Am. J. Physiol. Ren. Physiol. 2017, 313, F826–F834. [Google Scholar] [CrossRef]
- Maaten, J.T.; Voorburg, A.; Heine, R.J.; Wee, P.T.; Donker, A.J.M.; Gans, R.O.B. Renal handling of urate and sodium during acute physiological hyperinsulinaemia in healthy subjects. Clin. Sci. 1997, 92, 51–58. [Google Scholar] [CrossRef]
- Henning, C.; Liehr, K.; Girndt, M.; Ulrich, C.; Glomb, M.A. Extending the Spectrum of α-Dicarbonyl Compounds in Vivo. J. Biol. Chem. 2014, 289, 28676–28688. [Google Scholar] [CrossRef]
- Srivastava, A.; Kaze, A.D.; McMullan, C.J.; Isakova, T.; Waikar, S.S. Uric Acid and the Risks of Kidney Failure and Death in Individuals With CKD. Am. J. Kidney Dis. 2018, 71, 362–370. [Google Scholar] [CrossRef]
- Sanchez-Lozada, L.G.; Rodriguez-Iturbe, B.; Kelley, E.E.; Nakagawa, T.; Madero, M.; Feig, D.I.; Borghi, C.; Piani, F.; Cara-Fuentes, G.; Bjornstad, P.; et al. Uric Acid and Hypertension: An Update with Recommendations. Am. J. Hypertens. 2020, 33, 583–594. [Google Scholar] [CrossRef]
- Bertrand, L.; Zhi, L.; Yuzhang, Z.; Tianliang, H.; Hisatome, I.; Yamamoto, T.; Jidong, C. High Uric Acid Induces Insulin Resistance in Cardiomyocytes In Vitro and In Vivo. PLoS ONE 2016, 11, e0147737. [Google Scholar]
- Baldwin, W.; McRae, S.; Marek, G.; Wymer, D.; Pannu, V.; Baylis, C.; Johnson, R.J.; Sautin, Y.Y. Hyperuricemia as a mediator of the proinflammatory endocrine imbalance in the adipose tissue in a murine model of the metabolic syndrome. Diabetes 2011, 60, 1258–1269. [Google Scholar] [CrossRef]
- Tassone, E.J.; Cimellaro, A.; Perticone, M.; Hribal, M.L.; Sciacqua, A.; Andreozzi, F.; Sesti, G.; Perticone, F. Uric Acid Impairs Insulin Signaling by Promoting Enpp1 Binding to Insulin Receptor in Human Umbilical Vein Endothelial Cells. Front. Endocrinol. 2018, 9, 98. [Google Scholar] [CrossRef]
- Raffaello, A.; Mammucari, C.; Gherardi, G.; Rizzuto, R. Calcium at the Center of Cell Signaling: Interplay between Endoplasmic Reticulum, Mitochondria, and Lysosomes. Trends Biochem. Sci. 2016, 41, 1035–1049. [Google Scholar] [CrossRef]
- Puzianowska-Kuznicka, M.; Kuznicki, J. The ER and ageing II: Calcium homeostasis. Ageing Res. Rev. 2009, 8, 160–172. [Google Scholar] [CrossRef]
- Petersen, O.H.; Petersen, C.C.; Kasai, H. Calcium and hormone action. Annu. Rev. Physiol. 1994, 56, 297–319. [Google Scholar] [CrossRef]
- Guerrero-Hernandez, A.V.A. Calcium signalling in diabetes. Cell Calcium 2014, 56, 297–301. [Google Scholar] [CrossRef]
- Hodeify, R.; Nandakumar, M.; Own, M.; Courjaret, R.J.; Graumann, J.; Hubrack, S.Z.; Machaca, K. The CCT chaperonin is a novel regulator of Ca2+ signaling through modulation of Orai1 trafficking. Sci. Adv. 2018, 4, eaau1935. [Google Scholar] [CrossRef]
- Vultur, A.; Gibhardt, C.S.; Stanisz, H.; Bogeski, I. The role of the mitochondrial calcium uniporter (MCU) complex in cancer. Pflügers Arch. Eur. J. Physiol. 2018, 470, 1149–1163. [Google Scholar] [CrossRef]
- Bernhard, W.; Rouiller, C. Close topographical relationship between mitochondria and ergastoplasm of liver cells in a definite phase of cellular activity. J. Biophys. Biochem. Cytol. 1956, 2, 73–78. [Google Scholar] [CrossRef]
- Peng, W.; Wong, Y.C.; Krainc, D. Mitochondria-lysosome contacts regulate mitochondrial Ca2+ dynamics via lysosomal TRPML1. Proc. Natl. Acad. Sci. USA 2020, 117, 19266–19275. [Google Scholar] [CrossRef]
- Yang, W.; Li, Y.; Feng, R.; Liang, P.; Tian, K.; Hu, L.; Wang, K.; Qiu, T.; Zhang, J.; Sun, X.; et al. PFOS causes lysosomes-regulated mitochondrial fission through TRPML1-VDAC1 and oligomerization of MCU/ATP5J2. J. Hazard. Mater. 2025, 489, 137685. [Google Scholar] [CrossRef]
- Song, S.-E.; Shin, S.-K.; Ju, H.Y.; Im, S.-S.; Song, D.-K. Role of cytosolic and endoplasmic reticulum Ca2+ in pancreatic beta-cells: Pros and cons. Pflügers Arch. Eur. J. Physiol. 2023, 476, 151–161. [Google Scholar] [CrossRef]
- Mu-u-min, R.B.A.; Diane, A.; Allouch, A.; Al-Siddiqi, H.H. Ca2+-Mediated Signaling Pathways: A Promising Target for the Successful Generation of Mature and Functional Stem Cell-Derived Pancreatic Beta Cells In Vitro. Biomedicines 2023, 11, 1577. [Google Scholar] [CrossRef]
- Idevall-Hagren, O.; Tengholm, A. Metabolic regulation of calcium signaling in beta cells. Semin. Cell Dev. Biol. 2020, 103, 20–30. [Google Scholar] [CrossRef]
- Kim, M.J.; Min, S.H.; Shin, S.Y.; Kim, M.N.; Lee, H.; Jang, J.Y.; Kim, S.-W.; Park, K.S.; Jung, H.S. Attenuation of PERK enhances glucose-stimulated insulin secretion in islets. J. Endocrinol. 2018, 236, 125–136. [Google Scholar] [CrossRef]
- Voelker, D.R. Bridging gaps in phospholipid transport. Trends Biochem. Sci. 2005, 30, 396–404. [Google Scholar] [CrossRef]
- Rizzuto, R.; Duchen, M.R.; Pozzan, T. Flirting in Little Space: The ER/Mitochondria Ca2+ Liaison. Sci. STKE 2004, 2004, re1. [Google Scholar] [CrossRef]
- Hajnóczky, G.; Davies, E.; Madesh, M. Calcium signaling and apoptosis. Biochem. Biophys. Res. Commun. 2003, 304, 445–454. [Google Scholar] [CrossRef]
- Carnevale, V.; Nieddu, L.; Scillitani, A.; Tinti, M.G.; Eller-Vainicher, C.; Cosso, R.; Rendina, D.; Falchetti, A. Calcium-phosphate homeostasis and insulin resistance in men. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 353–359. [Google Scholar] [CrossRef]
- Missiroli, S.; Patergnani, S.; Caroccia, N.; Pedriali, G.; Perrone, M.; Previati, M.; Wieckowski, M.R.; Giorgi, C. Mitochondria-associated membranes (MAMs) and inflammation. Cell Death Dis. 2018, 9, 329. [Google Scholar] [CrossRef]
- Lee, J.W.; Gu, H.-O.; Jung, Y.; Jung, Y.; Seo, S.-Y.; Hong, J.-H.; Hong, I.-S.; Lee, D.H.; Kim, O.-H.; Oh, B.-C. Candesartan, an angiotensin-II receptor blocker, ameliorates insulin resistance and hepatosteatosis by reducing intracellular calcium overload and lipid accumulation. Exp. Mol. Med. 2023, 55, 910–925. [Google Scholar] [CrossRef]
- Arruda, A.P.; Hotamisligil, G.S. Calcium Homeostasis and Organelle Function in the Pathogenesis of Obesity and Diabetes. Cell Metab. 2015, 22, 381–397. [Google Scholar] [CrossRef]
- Fu, S.; Yang, L.; Li, P.; Hofmann, O.; Dicker, L.; Hide, W.; Lin, X.; Watkins, S.M.; Ivanov, A.R.; Hotamisligil, G.S. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature 2011, 473, 528–531. [Google Scholar] [CrossRef]
- Mohan, A.A.; Talwar, P. MAM kinases: Physiological roles, related diseases, and therapeutic perspectives—A systematic review. Cell. Mol. Biol. Lett. 2025, 30, 35. [Google Scholar] [CrossRef]
- Hong, Q.; Qi, K.; Feng, Z.; Huang, Z.; Cui, S.; Wang, L.; Fu, B.; Ding, R.; Yang, J.; Chen, X.; et al. Hyperuricemia induces endothelial dysfunction via mitochondrial Na+/Ca2+ exchanger-mediated mitochondrial calcium overload. Cell Calcium 2012, 51, 402–410. [Google Scholar] [CrossRef]
- Hernández-Alvarez, M.I.; Sebastián, D.; Vives, S.; Ivanova, S.; Bartoccioni, P.; Kakimoto, P.; Plana, N.; Veiga, S.R.; Hernández, V.; Vasconcelos, N.; et al. Deficient Endoplasmic Reticulum-Mitochondrial Phosphatidylserine Transfer Causes Liver Disease. Cell 2019, 177, 881–895.e17. [Google Scholar] [CrossRef]
- Tong, X.K.T.; Anderson-Baucum, E.K.; Yamamoto, W.; Gilon, P.; Lebeche, D.; Day, R.N.; Shull, G.E.; Evans-Molina, C. SERCA2 Deficiency Impairs Pancreatic β-Cell Function in Response to Diet-Induced Obesity. Diabetes 2016, 65, 3039–3052. [Google Scholar] [CrossRef]
- Kang, S.; Dahl, R.; Hsieh, W.; Shin, A.; Zsebo, K.M.; Buettner, C.; Hajjar, J.R. Small Molecular Allosteric Activator of the Sarco/Endoplasmic Reticulum Ca2+-ATPase (SERCA) Attenuates Diabetes and Metabolic Disorders. J. Biol. Chem. 2016, 291, 5185–5198. [Google Scholar] [CrossRef]
- Ali, S.I.; Elkhalifa, A.M.E.; Nabi, S.U.; Hayyat, F.S.; Nazar, M.; Taifa, S.; Rakhshan, R.; Shah, I.H.; Shaheen, M.; Wani, I.A.; et al. Aged garlic extract preserves beta-cell functioning via modulation of nuclear factor kappa-B (NF-κB)/Toll-like receptor (TLR)-4 and sarco endoplasmic reticulum calcium ATPase (SERCA)/Ca2+ in diabetes mellitus. Diabetol. Metab. Syndr. 2024, 16, 110. [Google Scholar] [CrossRef]
- Luciani, D.S.; Gwiazda, K.S.; Yang, T.-L.B.; Kalynyak, T.B.; Bychkivska, Y.; Frey, M.H.Z.; Jeffrey, K.D.; Sampaio, A.V.; Underhill, T.M.; Johnson, J.D. Roles of IP3R and RyR Ca2+ Channels in Endoplasmic Reticulum Stress and β-Cell Death. Diabetes 2009, 58, 422–432. [Google Scholar] [CrossRef]
- Yamamoto, W.R.; Bone, R.N.; Sohn, P.; Syed, F.; Reissaus, C.A.; Mosley, A.L.; Wijeratne, A.B.; True, J.D.; Tong, X.; Kono, T.; et al. Endoplasmic reticulum stress alters ryanodine receptor function in the murine pancreatic β cell. J. Biol. Chem. 2019, 294, 168–181. [Google Scholar] [CrossRef]
- Song, S.-E.; Shin, S.-K.; Kim, Y.-W.; Do, Y.R.; Lim, A.K.; Bae, J.-H.; Jeong, G.-S.; Im, S.-S.; Song, D.-K. Lupenone attenuates thapsigargin-induced endoplasmic reticulum stress and apoptosis in pancreatic beta cells possibly through inhibition of protein tyrosine kinase 2 activity. Life Sci. 2023, 332, 122107. [Google Scholar] [CrossRef]
- Meng, M.; Jiang, Y.; Wang, Y.; Huo, R.; Ma, N.; Shen, X.; Chang, G. β-carotene targets IP3R/GRP75/VDAC1-MCU axis to renovate LPS-induced mitochondrial oxidative damage by regulating STIM1. Free Radic. Biol. Med. 2023, 205, 25–46. [Google Scholar] [CrossRef]
- Li, A.; Yi, B.; Han, H.; Yang, S.; Hu, Z.; Zheng, L.; Wang, J.; Liao, Q.; Zhang, H. Vitamin D-VDR (vitamin D receptor) regulates defective autophagy in renal tubular epithelial cell in streptozotocin-induced diabetic mice via the AMPK pathway. Autophagy 2021, 18, 877–890. [Google Scholar] [CrossRef]
- Ren, S.; Liang, P.; Feng, R.; Yang, W.; Qiu, T.; Zhang, J.; Li, Q.; Yang, G.; Sun, X.; Yao, X. The phosphorylation of Smad3 by CaMKIIγ leads to the hepatocyte pyroptosis under perfluorooctane sulfonate exposure. Ecotoxicol. Environ. Saf. 2024, 284, 116924. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, X.; Ishikura, S.; Zhang, D.; Gao, J.; Sun, Y.; Contreras-Ferrat, A.; Foley, K.P.; Lavandero, S.; Yao, Z.; et al. Ca2+ signals promote GLUT4 exocytosis and reduce its endocytosis in muscle cells. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E209–E224. [Google Scholar] [CrossRef]
- Li, Z.; Ran, Q.; Qu, C.; Hu, S.; Cui, S.; Zhou, Y.; Shen, B.; Yang, B. Sigma-1 receptor activation attenuates DOX-induced cardiotoxicity by alleviating endoplasmic reticulum stress and mitochondrial calcium overload via PERK and IP3R-VDAC1-MCU signaling pathways. Biol. Direct 2025, 20, 23. [Google Scholar] [CrossRef]
- Park, K.; Lim, H.; Kim, J.; Hwang, Y.; Lee, Y.S.; Bae, S.H.; Kim, H.; Kim, H.; Kang, S.-W.; Kim, J.Y.; et al. Lysosomal Ca2+-mediated TFEB activation modulates mitophagy and functional adaptation of pancreatic β-cells to metabolic stress. Nat. Commun. 2022, 13, 1300. [Google Scholar] [CrossRef]
- Zhang, I.X.; Ren, J.; Vadrevu, S.; Raghavan, M.; Satin, L.S. ER stress increases store-operated Ca2+ entry (SOCE) and augments basal insulin secretion in pancreatic beta cells. J. Biol. Chem. 2020, 295, 5685–5700. [Google Scholar] [CrossRef]
- Heger, V.; Benesova, B.; Viskupicova, J.; Majekova, M.; Zoofishan, Z.; Hunyadi, A.; Horakova, L. Phenolic Compounds from Morus nigra Regulate Viability and Apoptosis of Pancreatic β-Cells Possibly via SERCA Activity. ACS Med. Chem. Lett. 2020, 11, 1006–1013. [Google Scholar] [CrossRef]
- PrayGod, G.; Filteau, S.; Range, N.; Kitilya, B.; Kavishe, B.B.; Ramaiya, K.; Jeremiah, K.; Rehman, A.M.; Changalucha, J.; Olsen, M.F.; et al. β-cell dysfunction and insulin resistance in relation to pre-diabetes and diabetes among adults in north-western Tanzania: A cross-sectional study. Trop. Med. Int. Health 2021, 26, 435–443. [Google Scholar] [CrossRef]
- Wang, G.; Zheng, S.; Xu, H.; Zhou, H.; Ren, X.; Han, T.; Chen, Y.; Qiu, H.; Wu, P.; Zheng, J.; et al. Associations of lipid profiles with insulin resistance and β cell function in adults with normal glucose tolerance and different categories of impaired glucose regulation. PLoS ONE 2017, 12, e0172221. [Google Scholar]
- Ha, K.H.; Park, C.Y.; Jeong, I.K.; Kim, H.J.; Kim, S.-Y.; Kim, W.J.; Yoon, J.S.; Kim, I.J.; Kim, D.J.; Kim, S. Clinical Characteristics of People with Newly Diagnosed Type 2 Diabetes between 2015 and 2016: Difference by Age and Body Mass Index. Diabetes Metab. J. 2018, 42, 137–146. [Google Scholar] [CrossRef]
- Zhou, Y.; Chung, A.C.K.; Fan, R.; Lee, H.M.; Xu, G.; Tomlinson, B.; Chan, J.C.N.; Kong, A.P.S. Sirt3 Deficiency Increased the Vulnerability of Pancreatic Beta Cells to Oxidative Stress-Induced Dysfunction. Antioxid. Redox Signal. 2017, 27, 962–976. [Google Scholar] [CrossRef]
- Cras-Méneur, C.; Uhlemeyer, C.; Müller, N.; Grieß, K.; Wessel, C.; Schlegel, C.; Kuboth, J.; Belgardt, B.-F. ATM and P53 differentially regulate pancreatic beta cell survival in Ins1E cells. PLoS ONE 2020, 15, e0237669. [Google Scholar]
- Nguyen, H.T.; Noriega Polo, C.; Wiederkehr, A.; Wollheim, C.B.; Park, K.S. CDN1163, an activator of sarco/endoplasmic reticulum Ca2+ ATPase, up-regulates mitochondrial functions and protects against lipotoxicity in pancreatic β-cells. Br. J. Pharmacol. 2023, 180, 2762–2776. [Google Scholar] [CrossRef]
- Wang, R.; Hu, W. Asprosin promotes β-cell apoptosis by inhibiting the autophagy of β-cell via AMPK-mTOR pathway. J. Cell. Physiol. 2020, 236, 215–221. [Google Scholar] [CrossRef]
- Li, X.; Bai, C.; Wang, H.; Wan, T.; Li, Y. LncRNA MEG3 regulates autophagy and pyroptosis via FOXO1 in pancreatic β-cells. Cell. Signal. 2022, 92, 110247. [Google Scholar] [CrossRef]
- Gwiazda, K.S.; Yang, T.-L.B.; Lin, Y.; Johnson, J.D. Effects of palmitate on ER and cytosolic Ca2+ homeostasis in β-cells. Am. J. Physiol. -Endocrinol. Metab. 2009, 296, E690–E701. [Google Scholar] [CrossRef]
- Liu, H.; Tang, D.; Zhou, X.; Yang, X.; Chen, A.F. PhospholipaseCγ1/calcium-dependent membranous localization of Gsdmd-N drives endothelial pyroptosis, contributing to lipopolysaccharide-induced fatal outcome. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H1482–H1495. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, J.; Xu, F.; Shang, L.; Liu, Q.; Shen, C. TAK-242 alleviates diabetic cardiomyopathy via inhibiting pyroptosis and TLR4/CaMKII/NLRP3 pathway. Open Life Sci. 2024, 19, 20220957. [Google Scholar] [CrossRef]
- Huo, M.; Guo, W.; Ding, L. Benidipine Hydrochloride Inhibits NLRP3 Inflammasome Activation by Inhibiting LPS-Induced NF-κB Signaling in THP-1 Macrophages. J. Inflamm. Res. 2024, 17, 6307–6316. [Google Scholar] [CrossRef]
- Pathak, S.; Pham, T.T.; Jeong, J.-H.; Byun, Y. Immunoisolation of pancreatic islets via thin-layer surface modification. J. Control. Release 2019, 305, 176–193. [Google Scholar] [CrossRef]
- Kim, K.; Chung, M.H.; Park, S.; Cha, J.; Baek, J.H.; Lee, S.-Y.; Choi, S.-Y. ER stress attenuation by Aloe-derived polysaccharides in the protection of pancreatic β-cells from free fatty acid-induced lipotoxicity. Biochem. Biophys. Res. Commun. 2018, 500, 797–803. [Google Scholar] [CrossRef]
- Zhao, S.; Yuan, C.; Tuo, X.; Zhou, C.; Zhao, Q.; Shen, T. MCLR induces dysregulation of calcium homeostasis and endoplasmic reticulum stress resulting in apoptosis in Sertoli cells. Chemosphere 2021, 263, 127868. [Google Scholar] [CrossRef]
- Huang, K.-J.; Feng, L.; Wu, P.; Liu, Y.; Zhang, L.; Mi, H.-F.; Zhou, X.-Q.; Jiang, W.-D. Hypoxia leads to gill endoplasmic reticulum stress and disruption of mitochondrial homeostasis in grass carp (Ctenopharyngodon idella): Mitigation effect of thiamine. J. Hazard. Mater. 2024, 469, 134005. [Google Scholar] [CrossRef]
- Gerber, P.A.; Rutter, G.A. The Role of Oxidative Stress and Hypoxia in Pancreatic Beta-Cell Dysfunction in Diabetes Mellitus. Antioxid. Redox Signal. 2017, 26, 501–518. [Google Scholar] [CrossRef]
- Deng, J.; Zheng, C.; Hua, Z.; Ci, H.; Wang, G.; Chen, L. Diosmin mitigates high glucose-induced endoplasmic reticulum stress through PI3K/AKT pathway in HK-2 cells. BMC Complement. Med. Ther. 2022, 22, 116. [Google Scholar] [CrossRef]
- Ozcan, L.; Tabas, I. Calcium signalling and ER stress in insulin resistance and atherosclerosis. J. Intern. Med. 2016, 280, 457–464. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Li, B. Chlorogenic acid and β-glucan from highland barley grain ameliorate β-cell dysfunction via inhibiting apoptosis and improving cell proliferation. Food Funct. 2021, 12, 10040–10052. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Z.; Zhang, Y.; Zhang, M.; Zhu, X.; Fan, Y.; Liu, Z. Rhein protects pancreatic β-cells from dynamin-related protein-1-mediated mitochondrial fission and cell apoptosis under hyperglycemia. Diabetes 2013, 62, 3927–3935. [Google Scholar] [CrossRef]
- Karunakaran, U.; Lee, J.E.; Elumalai, S.; Moon, J.S.; Won, K.C. Myricetin prevents thapsigargin-induced CDK5-P66Shc signalosome mediated pancreatic β-cell dysfunction. Free Radic. Biol. Med. 2019, 141, 59–66. [Google Scholar] [CrossRef]
- Park, S.; Lim, W.; Song, G. Delphinidin induces antiproliferation and apoptosis of endometrial cells by regulating cytosolic calcium levels and mitochondrial membrane potential depolarization. J. Cell. Biochem. 2018, 120, 5072–5084. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Lim, W.; Ham, J.; Kim, J.; You, S.; Song, G. Ivermectin induces apoptosis of porcine trophectoderm and uterine luminal epithelial cells through loss of mitochondrial membrane potential, mitochondrial calcium ion overload, and reactive oxygen species generation. Pestic. Biochem. Physiol. 2019, 159, 144–153. [Google Scholar] [CrossRef]
- Magnuson, M.A.; Osipovich, A.B. Ca2+ signaling and metabolic stress-induced pancreatic β-cell failure. Front. Endocrinol. 2024, 15, 1412411. [Google Scholar] [CrossRef]
- He, Q.; Qu, M.; Shen, T.; Su, J.; Xu, Y.; Xu, C.; Barkat, M.Q.; Cai, J.; Zhu, H.; Zeng, L.-H.; et al. Control of mitochondria-associated endoplasmic reticulum membranes by protein S-palmitoylation: Novel therapeutic targets for neurodegenerative diseases. Ageing Res. Rev. 2023, 87, 101920. [Google Scholar] [CrossRef]
- Velmurugan, S.; Liu, T.; Chen, K.C.; Despa, F.; O’Rourke, B.; Despa, S. Distinct Effects of Mitochondrial Na+/Ca2+ Exchanger Inhibition and Ca2+ Uniporter Activation on Ca2+ Sparks and Arrhythmogenesis in Diabetic Rats. J. Am. Heart Assoc. 2023, 12, e029997. [Google Scholar] [CrossRef]
- Emdad, L.; Bhoopathi, P.; Talukdar, S.; Pradhan, A.K.; Sarkar, D.; Wang, X.-Y.; Das, S.K.; Fisher, P.B. Recent insights into apoptosis and toxic autophagy: The roles of MDA-7/IL-24, a multidimensional anti-cancer therapeutic. Semin. Cancer Biol. 2020, 66, 140–154. [Google Scholar] [CrossRef]
- Gupta, R.; Ambasta, R.K.; Pravir, K. Autophagy and apoptosis cascade: Which is more prominent in neuronal death? Cell. Mol. Life Sci. 2021, 78, 8001–8047. [Google Scholar] [CrossRef]
- Gao, W.F.; Xu, Y.Y.; Ge, J.F.; Chen, F.H. Inhibition of acid-sensing ion channel 1a attenuates acid-induced activation of autophagy via a calcium signaling pathway in articular chondrocytes. Int. J. Mol. Med. 2019, 43, 1778–1788. [Google Scholar] [CrossRef]
- Zheng, X.; Pang, Y.; Hasenbilige; Yang, Y.; Li, Q.; Liu, Y.; Cao, J. ATF4-mediated different mode of interaction between autophagy and mTOR determines cell fate dependent on the level of ER stress induced by Cr(VI). Ecotoxicol. Environ. Saf. 2024, 281, 116639. [Google Scholar] [CrossRef]
- Qi, M.; Jiang, Q.; Yang, S.; Zhang, C.; Liu, J.; Liu, W.; Lin, P.; Chen, H.; Zhou, D.; Tang, K.; et al. The endoplasmic reticulum stress-mediated unfolded protein response protects against infection of goat endometrial epithelial cells by Trueperella pyogenesvia autophagy. Virulence 2021, 13, 122–136. [Google Scholar] [CrossRef]
- Dalle Pezze, P.; Ruf, S.; Sonntag, A.G.; Langelaar-Makkinje, M.; Hall, P.; Heberle, A.M.; Razquin Navas, P.; van Eunen, K.; Tölle, R.C.; Schwarz, J.J.; et al. A systems study reveals concurrent activation of AMPK and mTOR by amino acids. Nat. Commun. 2016, 7, 13254. [Google Scholar] [CrossRef]
- Aoyagi, K.; Yamashita, S.-I.; Akimoto, Y.; Nishiwaki, C.; Nakamichi, Y.; Udagawa, H.; Abe, M.; Sakimura, K.; Kanki, T.; Ohara-Imaizumi, M. A new beta cell-specific mitophagy reporter mouse shows that metabolic stress leads to accumulation of dysfunctional mitochondria despite increased mitophagy. Diabetologia 2022, 66, 147–162. [Google Scholar] [CrossRef]
- Dai Ly, L.; Da Ly, D.; Nguyen, N.T.; Kim, J.H.; Yoo, H.; Chung, J.; Lee, M.-S.; Cha, S.-K.; Park, K.-S. Mitochondrial Ca2+ Uptake Relieves Palmitate-Induced Cytosolic Ca2+ Overload in MIN6 Cells. Mol. Cells 2020, 43, 66–75. [Google Scholar]
- Zummo, F.P.; Cullen, K.S.; Honkanen-Scott, M.; Shaw, J.A.; Lovat, P.E.; Arden, C. Glucagon-Like Peptide 1 Protects Pancreatic β-Cells from Death by Increasing Autophagic Flux and Restoring Lysosomal Function. Diabetes 2017, 66, 1272–1285. [Google Scholar] [CrossRef]
- Israeli, T.; Riahi, Y.; Garzon, P.; Louzada, R.A.; Werneck-de-Castro, J.P.; Blandino-Rosano, M.; Yeroslaviz-Stolper, R.; Kadosh, L.; Tornovsky-Babeay, S.; Hacker, G.; et al. Nutrient Sensor mTORC1 Regulates Insulin Secretion by Modulating β-Cell Autophagy. Diabetes 2022, 71, 453–469. [Google Scholar] [CrossRef]
- Aoyama, S.; Nishida, Y.; Uzawa, H.; Himuro, M.; Kanai, A.; Ueki, K.; Ito, M.; Iida, H.; Tanida, I.; Miyatsuka, T.; et al. Monitoring autophagic flux in vivo revealed its physiological response and significance of heterogeneity in pancreatic beta cells. Cell Chem. Biol. 2023, 30, 658–671.e4. [Google Scholar] [CrossRef]
- Ru, Q.; Li, Y.; Chen, L.; Wu, Y.; Min, J.; Wang, F. Iron homeostasis and ferroptosis in human diseases: Mechanisms and therapeutic prospects. Signal Transduct. Target. Ther. 2024, 9, 271. [Google Scholar] [CrossRef]
- Gleitze, S.; Paula-Lima, A.; Núñez, M.T.; Hidalgo, C. The calcium–iron connection in ferroptosis-mediated neuronal death. Free Radic. Biol. Med. 2021, 175, 28–41. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Yu, J.; Wang, W.; Du, Z.; Gao, S.; Ma, Y.; Tang, R.; Liu, T.; Ma, S.; et al. Saikosaponin B2 ameliorates depression-induced microglia activation by inhibiting ferroptosis-mediated neuroinflammation and ER stress. J. Ethnopharmacol. 2023, 316, 116729. [Google Scholar] [CrossRef]
- Sun, Y.; Bai, Y.-P.; Wang, D.-G.; Xing, Y.-J.; Zhang, T.; Wang, W.; Zhou, S.-M.; Cheng, J.-H.; Chang, W.-W.; Kong, X.; et al. Protective effects of metformin on pancreatic β-cell ferroptosis in type 2 diabetes in vivo. Biomed. Pharmacother. 2023, 168, 116729. [Google Scholar] [CrossRef]
- Miao, R.; Fang, X.; Zhang, Y.; Wei, J.; Zhang, Y.; Tian, J. Iron metabolism and ferroptosis in type 2 diabetes mellitus and complications: Mechanisms and therapeutic opportunities. Cell Death Dis. 2023, 14, 186. [Google Scholar] [CrossRef]
- Shu, T.; Lv, Z.; Xie, Y.; Tang, J.; Mao, X. Hepcidin as a key iron regulator mediates glucotoxicity-induced pancreatic β-cell dysfunction. Endocr. Connect. 2019, 8, 150–161. [Google Scholar] [CrossRef]
- Huang, J.; Meng, P.; Liang, Y.; Li, X.; Zhou, S.; Li, J.; Wang, X.; Miao, J.; Shen, W.; Zhou, L. Tubular CD44 plays a key role in aggravating AKI through NF-κB p65-mediated mitochondrial dysfunction. Cell Death Dis. 2025, 16, 119. [Google Scholar] [CrossRef]
- Hong, H.; Lin, X.; Xu, Y.; Tong, T.; Zhang, J.; He, H.; Yang, L.; Lu, Y.; Zhou, Z. Cadmium induces ferroptosis mediated inflammation by activating Gpx4/Ager/p65 axis in pancreatic β-cells. Sci. Total Environ. 2022, 849, 157819. [Google Scholar] [CrossRef]
- Wei, S.; Qiu, T.; Yao, X.; Wang, N.; Jiang, L.; Jia, X.; Tao, Y.; Wang, Z.; Pei, P.; Zhang, J.; et al. Arsenic induces pancreatic dysfunction and ferroptosis via mitochondrial ROS-autophagy-lysosomal pathway. J. Hazard. Mater. 2020, 384, 121390. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, L.; Chen, H.; Wei, S.; Yao, K.; Sun, X.; Yang, G.; Jiang, L.; Zhang, C.; Wang, N.; et al. Resveratrol protected acrolein-induced ferroptosis and insulin secretion dysfunction via ER-stress- related PERK pathway in MIN6 cells. Toxicology 2022, 465, 153048. [Google Scholar] [CrossRef]
- Nemecz, M.; Constantin, A.; Dumitrescu, M.; Alexandru, N.; Filippi, A.; Tanko, G.; Georgescu, A. The Distinct Effects of Palmitic and Oleic Acid on Pancreatic Beta Cell Function: The Elucidation of Associated Mechanisms and Effector Molecules. Front. Pharmacol. 2019, 9, 1554. [Google Scholar] [CrossRef]
- Lee, H.J.; Jung, Y.H.; Choi, G.E.; Kim, J.S.; Chae, C.W.; Lim, J.R.; Kim, S.Y.; Yoon, J.H.; Cho, J.H.; Lee, S.-J.; et al. Urolithin A suppresses high glucose-induced neuronal amyloidogenesis by modulating TGM2-dependent ER-mitochondria contacts and calcium homeostasis. Cell Death Differ. 2020, 28, 184–202. [Google Scholar] [CrossRef]
- Hsu, C.C.; Fidler, T.P.; Kanter, J.E.; Kothari, V.; Kramer, F.; Tang, J.; Tall, A.R.; Bornfeldt, K.E. Hematopoietic NLRP3 and AIM2 Inflammasomes Promote Diabetes-Accelerated Atherosclerosis, but Increased Necrosis Is Independent of Pyroptosis. Diabetes 2023, 72, 999–1011. [Google Scholar] [CrossRef]
- Fu, F.; Luo, H.; Du, Y.; Chen, Y.; Tian, K.; Pan, J.; Li, J.; Wang, N.; Bao, R.; Jin, H.; et al. AR/PCC herb pair inhibits osteoblast pyroptosis to alleviate diabetes-related osteoporosis by activating Nrf2/Keap1 pathway. J. Cell. Mol. Med. 2023, 27, 3601–3613. [Google Scholar] [CrossRef]
- Wu, Q.; Guan, Y.-B.; Zhang, K.-J.; Li, L.; Zhou, Y. Tanshinone IIA mediates protection from diabetes kidney disease by inhibiting oxidative stress induced pyroptosis. J. Ethnopharmacol. 2023, 316, 3601–3613. [Google Scholar] [CrossRef]
- Wei, H.; Sun, M.; Wang, R.; Zeng, H.; Zhao, B.; Jin, S. Puerarin mitigated LPS-ATP or HG-primed endothelial cells damage and diabetes-associated cardiovascular disease via ROS-NLRP3 signalling. J. Cell. Mol. Med. 2024, 28, e18239. [Google Scholar] [CrossRef]
- Han, J.-J.; Li, J.; Huang, D.-H. Mesenchymal Stem Cell-Derived Extracellular Vesicles Carrying Circ-Tulp4 Attenuate Diabetes Mellitus with Nonalcoholic Fatty Liver Disease by Inhibiting Cell Pyroptosis through the HNRNPC/ABHD6 Axis. Tissue Eng. Regen. Med. 2024, 22, 23–41. [Google Scholar] [CrossRef]
- Du, G.; Healy, L.B.; David, L.; Walker, C.; El-Baba, T.J.; Lutomski, C.A.; Goh, B.; Gu, B.; Pi, X.; Devant, P.; et al. ROS-dependent S-palmitoylation activates cleaved and intact gasdermin D. Nature 2024, 630, 437–446. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, X.; Zhu, Z.; Luo, Z.; Hao, Y.; Yang, X.; Feng, J.; Zhang, Z.; Hu, J.; Jian, Y.; et al. STING contributes to lipopolysaccharide-induced tubular cell inflammation and pyroptosis by activating endoplasmic reticulum stress in acute kidney injury. Cell Death Dis. 2024, 15, 217. [Google Scholar] [CrossRef]
- Ma, L.; Han, Z.; Yin, H.; Tian, J.; Zhang, J.; Li, N.; Ding, C.; Zhang, L. Characterization of Cathepsin B in Mediating Silica Nanoparticle-Induced Macrophage Pyroptosis via an NLRP3-Dependent Manner. J. Inflamm. Res. 2022, 15, 4537–4545. [Google Scholar] [CrossRef]
- Shi, J.; Gao, W.; Shao, F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem. Sci. 2017, 42, 245–254. [Google Scholar] [CrossRef]
- Kesavardhana, S.; Malireddi, R.K.S.; Kanneganti, T.-D. Caspases in Cell Death, Inflammation, and Pyroptosis. Annu. Rev. Immunol. 2020, 38, 567–595. [Google Scholar] [CrossRef]
- Devant, P.; Kagan, J.C. Molecular mechanisms of gasdermin D pore-forming activity. Nat. Immunol. 2023, 24, 1064–1075. [Google Scholar] [CrossRef]
- Miao, R.; Jiang, C.; Chang, W.Y.; Zhang, H.; An, J.; Ho, F.; Chen, P.; Zhang, H.; Junqueira, C.; Amgalan, D.; et al. Gasdermin D permeabilization of mitochondrial inner and outer membranes accelerates and enhances pyroptosis. Immunity 2023, 56, 2523–2541.e8. [Google Scholar] [CrossRef]
- Li, X.; Xiao, G.-Y.; Guo, T.; Song, Y.-J.; Li, Q.-M. Potential therapeutic role of pyroptosis mediated by the NLRP3 inflammasome in type 2 diabetes and its complications. Front. Endocrinol. 2022, 13, 986565. [Google Scholar] [CrossRef]
- Al Mamun, A.; Wu, Y.; Nasrin, F.; Akter, A.; Taniya, M.A.; Munir, F.; Jia, C.; Xiao, J. Role of Pyroptosis in Diabetes and Its Therapeutic Implications. J. Inflamm. Res. 2021, 14, 2187–2206. [Google Scholar] [CrossRef]
- Lee, K.-H.; Kang, T.-B. The Molecular Links between Cell Death and Inflammasome. Cells 2019, 8, 1057. [Google Scholar] [CrossRef]
- Cao, Z.; Huang, D.; Tang, C.; Lu, Y.; Huang, S.; Peng, C.; Hu, X. Pyroptosis in diabetes and diabetic nephropathy. Clin. Chim. Acta 2022, 531, 188–196. [Google Scholar] [CrossRef]
- Hocevar, S.E.; Kamendulis, L.M.; Hocevar, B.A. Perfluorooctanoic acid activates the unfolded protein response in pancreatic acinar cells. J. Biochem. Mol. Toxicol. 2020, 34, e22561. [Google Scholar] [CrossRef]
- Zhang, L.; Duan, X.; Sun, W.; Sun, H. Perfluorooctane sulfonate acute exposure stimulates insulin secretion via GPR40 pathway. Sci. Total Environ. 2020, 726, 138498. [Google Scholar] [CrossRef]
- Jeyarajan, S.; Zhang, I.X.; Arvan, P.; Lentz, S.I.; Satin, L.S. Simultaneous Measurement of Changes in Mitochondrial and Endoplasmic Reticulum Free Calcium in Pancreatic Beta Cells. Biosensors 2023, 13, 382. [Google Scholar] [CrossRef]
- Wu, Q.-R.; Yang, H.; Zhang, H.-D.; Cai, Y.-J.; Zheng, Y.-X.; Fang, H.; Wang, Z.-F.; Kuang, S.-J.; Rao, F.; Huang, H.-L.; et al. IP3R2-mediated Ca2+ release promotes LPS-induced cardiomyocyte pyroptosis via the activation of NLRP3/Caspase-1/GSDMD pathway. Cell Death Discov. 2024, 10, 91. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, H.; Zhao, B.; Fei, H. IL-1β caused pancreatic β-cells apoptosis is mediated in part by endoplasmic reticulum stress via the induction of endoplasmic reticulum Ca2+ release through the c-Jun N-terminal kinase pathway. Mol. Cell. Biochem. 2008, 324, 183–190. [Google Scholar] [CrossRef]
- Eriksson, J.; Franssila-Kallunki, A.; Ekstrand, A.; Saloranta, C.; Widén, E.; Schalin, C.; Groop, L. Early metabolic defects in persons at increased risk for non-insulin-dependent diabetes mellitus. N. Engl. J. Med. 1989, 321, 337–343. [Google Scholar] [CrossRef]
- Gunaid, A.A.; Al-Kebsi, M.M.; Bamashmus, M.A.; Al-Akily, S.A.; Al-Radaei, A.N. Clinical phenotyping of newly diagnosed type 2 diabetes in Yemen. BMJ Open Diabetes Res. Care 2018, 6, e000587. [Google Scholar] [CrossRef]
- Kashyap, B.; Saikia, K.; Samanta, S.K.; Thakur, D.; Banerjee, S.K.; Borah, J.C.; Talukdar, N.C. Kaempferol 3-O-rutinoside from Antidesma acidum Retz. Stimulates glucose uptake through SIRT1 induction followed by GLUT4 translocation in skeletal muscle L6 cells. J. Ethnopharmacol. 2023, 301, 115788. [Google Scholar] [CrossRef]
- Kang, J.K.; Kim, O.-H.; Hur, J.; Yu, S.H.; Lamichhane, S.; Lee, J.W.; Ojha, U.; Hong, J.H.; Lee, C.S.; Cha, J.-Y.; et al. Increased intracellular Ca2+ concentrations prevent membrane localization of PH domains through the formation of Ca2+ -phosphoinositides. Proc. Natl. Acad. Sci. USA 2017, 114, 11926–11931. [Google Scholar] [CrossRef]
- Draznin, B. Cytosolic Calcium and Insulin Resistance. Am. J. Kidney Dis. 1993, 21, S32–S38. [Google Scholar] [CrossRef]
- Dai, W.; Choubey, M.; Patel, S.; Singer, H.A.; Ozcan, L. Adipocyte CAMK2 deficiency improves obesity-associated glucose intolerance. Mol. Metab. 2021, 53, 101300. [Google Scholar] [CrossRef]
- Saini, V. Molecular mechanisms of insulin resistance in type 2 diabetes mellitus. World J. Diabetes 2010, 1, 68–75. [Google Scholar] [CrossRef]
- Zhao, X.; An, X.; Yang, C.; Sun, W.; Ji, H.; Lian, F. The crucial role and mechanism of insulin resistance in metabolic disease. Front. Endocrinol. 2023, 14, 1149239. [Google Scholar] [CrossRef]
- Taddeo, E.P.; Laker, R.C.; Breen, D.S.; Akhtar, Y.N.; Kenwood, B.M.; Liao, J.A.; Zhang, M.; Fazakerley, D.J.; Tomsig, J.L.; Harris, T.E.; et al. Opening of the mitochondrial permeability transition pore links mitochondrial dysfunction to insulin resistance in skeletal muscle. Mol. Metab. 2014, 3, 124–134. [Google Scholar] [CrossRef]
- Song, Q.; Sergeev, I.N. Calcium and vitamin D in obesity. Nutr. Res. Rev. 2012, 25, 130–141. [Google Scholar] [CrossRef]
- Tamas, I.; Major, E.; Horvath, D.; Keller, I.; Ungvari, A.; Haystead, T.A.; MacDonald, J.A.; Lontay, B. Mechanisms by which smoothelin-like protein 1 reverses insulin resistance in myotubules and mice. Mol. Cell. Endocrinol. 2022, 551, 111663. [Google Scholar] [CrossRef]
- Lee, S.-H.; Park, S.-Y.; Choi, C.S. Insulin Resistance: From Mechanisms to Therapeutic Strategies. Diabetes Metab. J. 2022, 46, 15–37. [Google Scholar] [CrossRef]
- Schubert, K.M.; Scheid, M.P.; Duronio, V. Ceramide inhibits protein kinase B/Akt by promoting dephosphorylation of serine 473. J. Biol. Chem. 2000, 275, 13330–13335. [Google Scholar] [CrossRef]
- Kitessa, S.; Abeywardena, M. Lipid-Induced Insulin Resistance in Skeletal Muscle: The Chase for the Culprit Goes from Total Intramuscular Fat to Lipid Intermediates, and Finally to Species of Lipid Intermediates. Nutrients 2016, 8, 466. [Google Scholar] [CrossRef]
- Morris, A.D.; Donnelly, R.; Connell, J.M.C.; Reid, J.L. Effects of the calcium antagonist lacidipine on insulin sensitivity in essential hypertension. A placebo-controlled study. Horm. Metab. Res. 1994, 26, 257–259. [Google Scholar] [CrossRef]
- Di Giovine, F.S.; Malawista, S.E.; Thornton, E.; Duff, G.W. Urate crystals stimulate production of tumor necrosis factor alpha from human blood monocytes and synovial cells. Cytokine mRNA and protein kinetics, and cellular distribution. J. Clin. Investig. 1991, 87, 1375–1381. [Google Scholar] [CrossRef]
- Trebak, M.; Kinet, J.-P. Calcium signalling in T cells. Nat. Rev. Immunol. 2019, 19, 154–169. [Google Scholar] [CrossRef]
- Zi, X.; Su, R.; Su, R.; Wang, H.; Li, B.; Gao, C.; Li, X.; Wang, C. Elevated serum IL-2 and Th17/Treg imbalance are associated with gout. Clin. Exp. Med. 2024, 24, 9. [Google Scholar] [CrossRef]
- Giri, P.S.; Bharti, A.H.; Begum, R.; Dwivedi, M. Calcium controlled NFATc1 activation enhances suppressive capacity of regulatory T cells isolated from generalized vitiligo patients. Immunology 2022, 167, 314–327. [Google Scholar] [CrossRef]
- Häusler, D.; Torke, S.; Peelen, E.; Bertsch, T.; Djukic, M.; Nau, R.; Larochelle, C.; Zamvil, S.S.; Brück, W.; Weber, M.S. High dose vitamin D exacerbates central nervous system autoimmunity by raising T-cell excitatory calcium. Brain 2019, 142, 2737–2755. [Google Scholar] [CrossRef]
- Hainberger, D.; Stolz, V.; Zhu, C.; Schuster, M.; Müller, L.; Hamminger, P.; Rica, R.; Waltenberger, D.; Alteneder, M.; Krausgruber, T.; et al. NCOR1 Orchestrates Transcriptional Landscapes and Effector Functions of CD4+ T Cells. Front. Immunol. 2020, 11, 579. [Google Scholar] [CrossRef]
- So, A.K.; Martinon, F. Inflammation in gout: Mechanisms and therapeutic targets. Nat. Rev. Rheumatol. 2017, 13, 639–647. [Google Scholar] [CrossRef]
- Du, L.; Zong, Y.; Li, H.; Wang, Q.; Xie, L.; Yang, B.; Pang, Y.; Zhang, C.; Zhong, Z.; Gao, J. Hyperuricemia and its related diseases: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 212. [Google Scholar] [CrossRef]
- Chang, W.-C.; Jan Wu, Y.-J.; Chung, W.-H.; Lee, Y.-S.; Chin, S.-W.; Chen, T.-J.; Chang, Y.-S.; Chen, D.-Y.; Hung, S.-I. Genetic variants of PPAR-gamma coactivator 1B augment NLRP3-mediated inflammation in gouty arthritis. Rheumatology 2016, 56, 457–466. [Google Scholar] [CrossRef]
- Choi, N.; Yang, G.; Jang, J.H.; Kang, H.C.; Cho, Y.-Y.; Lee, H.S.; Lee, J.Y. Loganin Alleviates Gout Inflammation by Suppressing NLRP3 Inflammasome Activation and Mitochondrial Damage. Molecules 2021, 26, 1071. [Google Scholar] [CrossRef]
- Kowaltowski, A.J.; Menezes-Filho, S.L.; Assali, E.A.; Gonçalves, I.G.; Cabral-Costa, J.V.; Abreu, P.; Miller, N.; Nolasco, P.; Laurindo, F.R.M.; Bruni-Cardoso, A.; et al. Mitochondrial morphology regulates organellar Ca2+ uptake and changes cellular Ca2+ homeostasis. FASEB J. 2019, 33, 13176–13188. [Google Scholar] [CrossRef]
- Li, X.; Gao, J.; Tao, J. Purinergic Signaling in the Regulation of Gout Flare and Resolution. Front. Immunol. 2021, 12, 785425. [Google Scholar] [CrossRef]
- Huang, Z.; Xie, N.; Illes, P.; Di Virgilio, F.; Ulrich, H.; Semyanov, A.; Verkhratsky, A.; Sperlagh, B.; Yu, S.-G.; Huang, C.; et al. From purines to purinergic signalling: Molecular functions and human diseases. Signal Transduct. Target. Ther. 2021, 6, 162. [Google Scholar] [CrossRef]
- Akbal, A.; Dernst, A.; Lovotti, M.; Mangan, M.S.J.; McManus, R.M.; Latz, E. How location and cellular signaling combine to activate the NLRP3 inflammasome. Cell. Mol. Immunol. 2022, 19, 1201–1214. [Google Scholar] [CrossRef]
- Hamilton, C.; Anand, P.K. Right place, right time: Localisation and assembly of the NLRP3 inflammasome. F1000Research 2019, 8, F1000 Faculty Rev-676. [Google Scholar] [CrossRef]
- Nawaz, S.; Kulyar, M.F.; Mo, Q.; Zhang, Z.; Quan, C.; Iqbal, M.; Imad, E.F.; Li, J. Thiram-induced ER stress promotes mitochondrial calcium signaling and NLRP3 inflammasome activation in a tissue specific manner. Ecotoxicol. Environ. Saf. 2025, 293, 118026. [Google Scholar] [CrossRef]
- Smith, A.N.; Altara, R.; Amin, G.; Habeichi, N.J.; Thomas, D.G.; Jun, S.; Kaplan, A.; Booz, G.W.; Zouein, F.A. Genomic, Proteomic, and Metabolic Comparisons of Small Animal Models of Heart Failure with Preserved Ejection Fraction: A Tale of Mice, Rats, and Cats. J. Am. Heart Assoc. 2022, 11, e026071. [Google Scholar] [CrossRef]
- Shandilya, S.; Kesari, K.K.; Ruokolainen, J. Vitamin K2 Modulates Organelle Damage and Tauopathy Induced by Streptozotocin and Menadione in SH-SY5Y Cells. Antioxidants 2021, 10, 983. [Google Scholar] [CrossRef]
- Shishkova, D.; Lobov, A.; Repkin, E.; Markova, V.; Markova, Y.; Sinitskaya, A.; Sinitsky, M.; Kondratiev, E.; Torgunakova, E.; Kutikhin, A. Calciprotein Particles Induce Cellular Compartment-Specific Proteome Alterations in Human Arterial Endothelial Cells. J. Cardiovasc. Dev. Dis. 2023, 11, 5. [Google Scholar] [CrossRef]
- Lee, H.E.; Yang, G.; Park, Y.B.; Kang, H.C.; Cho, Y.-Y.; Lee, H.S.; Lee, J.Y. Epigallocatechin-3-Gallate Prevents Acute Gout by Suppressing NLRP3 Inflammasome Activation and Mitochondrial DNA Synthesis. Molecules 2019, 24, 2138. [Google Scholar] [CrossRef]
- McCormick, N.; O’Connor, M.J.; Yokose, C.; Merriman, T.R.; Mount, D.B.; Leong, A.; Choi, H.K. Assessing the Causal Relationships Between Insulin Resistance and Hyperuricemia and Gout Using Bidirectional Mendelian Randomization. Arthritis Rheumatol. 2021, 73, 2096–2104. [Google Scholar] [CrossRef]
- Gheita, T.A.; El-Fishawy, H.S.; Nasrallah, M.M.; Hussein, H. Insulin resistance and metabolic syndrome in primary gout: Relation to punched-out erosions. Int. J. Rheum. Dis. 2012, 15, 521–525. [Google Scholar] [CrossRef]
- Ahn, C.; Kang, H.-S.; Lee, J.-H.; Hong, E.-J.; Jung, E.-M.; Yoo, Y.-M.; Jeung, E.-B. Bisphenol A and octylphenol exacerbate type 1 diabetes mellitus by disrupting calcium homeostasis in mouse pancreas. Toxicol. Lett. 2018, 295, 162–172. [Google Scholar] [CrossRef]
- Mandal, A.K.; Leask, M.P.; Estiverne, C.; Choi, H.K.; Merriman, T.R.; Mount, D.B. Genetic and Physiological Effects of Insulin on Human Urate Homeostasis. Front. Physiol. 2021, 12, 713710. [Google Scholar] [CrossRef]
- Renaudin, F.; Orliaguet, L.; Castelli, F.; Fenaille, F.; Prignon, A.; Alzaid, F.; Combes, C.; Delvaux, A.; Adimy, Y.; Cohen-Solal, M.; et al. Gout and pseudo-gout-related crystals promote GLUT1-mediated glycolysis that governs NLRP3 and interleukin-1β activation on macrophages. Ann. Rheum. Dis. 2020, 79, 1506–1514. [Google Scholar] [CrossRef]
- Zhu, P.; Hu, S.; Jin, Q.; Li, D.; Tian, F.; Toan, S.; Li, Y.; Zhou, H.; Chen, Y. Ripk3 promotes ER stress-induced necroptosis in cardiac IR injury: A mechanism involving calcium overload/XO/ROS/mPTP pathway. Redox Biol. 2018, 16, 157–168. [Google Scholar] [CrossRef]
- Collier, A.; Stirling, A.; Cameron, L.; Hair, M.; Crosbie, D. Gout and diabetes: A common combination. Postgrad. Med. J. 2016, 92, 372–378. [Google Scholar] [CrossRef]
- Tung, Y.-C.; Lee, S.-S.; Tsai, W.-C.; Lin, G.-T.; Chang, H.-W.; Tu, H.-P. Association Between Gout and Incident Type 2 Diabetes Mellitus: A Retrospective Cohort Study. Am. J. Med. 2016, 129, 1219.e17–1219.e25. [Google Scholar] [CrossRef]
- Zheliabina, O.V.; Eliseev, M.S.; Glukhova, S.I.; Nasonov, E.L. Contributing Factors of Diabetes Mellitus among Patients with Gout (Results of the Long-Term Prospective Study). Dokl. Biochem. Biophys. 2023, 511, 195–202. [Google Scholar] [CrossRef]
- Zhang, W.; Dun, Y.; You, B.; Qiu, L.; Ripley-Gonzalez, J.W.; Cheng, J.; Fu, S.; Li, C.; Liu, S. Trimetazidine and exercise offer analogous improvements to the skeletal muscle insulin resistance of mice through Nrf2 signaling. BMJ Open Diabetes Res. Care 2022, 10, e002699. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, H.; Lu, J.; Xie, D.; Wang, Q.; Huang, T.; Xin, H.; Hisatome, I.; Yamamoto, T.; Wang, W.; et al. High uric acid promotes dysfunction in pancreatic β cells by blocking IRS2/AKT signalling. Mol. Cell. Endocrinol. 2021, 520, 111070. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, X.-R.; Ye, M.-Y.; Xu, X.-Q.; Zhang, Y.-W.; Liu, H.; Huang, X.-Z. RBP4 Is Associated with Insulin Resistance in Hyperuricemia-Induced Rats and Patients with Hyperuricemia. Front. Endocrinol. 2021, 12, 653819. [Google Scholar] [CrossRef]
- Ouyang, R.; Zhao, X.; Zhang, R.; Yang, J.; Li, S.; Deng, D. FGF21 attenuates high uric acid-induced endoplasmic reticulum stress, inflammation and vascular endothelial cell dysfunction by activating Sirt1. Mol. Med. Rep. 2022, 25, 35. [Google Scholar] [CrossRef]
- Minamino, T.; Zhou, Y.; Sun, P.; Wang, T.; Chen, K.; Zhu, W.; Wang, H. Inhibition of Calcium Influx Reduces Dysfunction and Apoptosis in Lipotoxic Pancreatic β-Cells via Regulation of Endoplasmic Reticulum Stress. PLoS ONE 2015, 10, e0132411. [Google Scholar]
- Jiang, H.; Chen, F.; Song, D.; Zhou, X.; Ren, L.; Zeng, M.; Pagliaro, P. Dynamin-Related Protein 1 Is Involved in Mitochondrial Damage, Defective Mitophagy, and NLRP3 Inflammasome Activation Induced by MSU Crystals. Oxidative Med. Cell. Longev. 2022, 2022, 1–22. [Google Scholar] [CrossRef]
- Cao, X.; Li, Y.; Luo, Y.; Chu, T.; Yang, H.; Wen, J.; Liu, Y.; Zhao, Y.; Herrmann, M. Transient receptor potential melastatin 2 regulates neutrophil extracellular traps formation and delays resolution of neutrophil-driven sterile inflammation. J. Inflamm. 2023, 20, 7. [Google Scholar] [CrossRef]
- Ji, S.Y.; Lee, H.; Hwangbo, H.; Hong, S.-H.; Cha, H.-J.; Park, C.; Kim, D.-H.; Kim, G.-Y.; Kim, S.; Kim, H.-S.; et al. A Novel Peptide Oligomer of Bacitracin Induces M1 Macrophage Polarization by Facilitating Ca2+ Influx. Nutrients 2020, 12, 1603. [Google Scholar] [CrossRef]
- Dror, E.; Dalmas, E.; Meier, D.T.; Wueest, S.; Thévenet, J.; Thienel, C.; Timper, K.; Nordmann, T.M.; Traub, S.; Schulze, F.; et al. Postprandial macrophage-derived IL-1β stimulates insulin, and both synergistically promote glucose disposal and inflammation. Nat. Immunol. 2017, 18, 283–292. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- Dickerson, M.T.; Bogart, A.M.; Altman, M.K.; Milian, S.C.; Jordan, K.L.; Dadi, P.K.; Jacobson, D.A. Cytokine-mediated changes in K+ channel activity promotes an adaptive Ca2+ response that sustains β-cell insulin secretion during inflammation. Sci. Rep. 2018, 8, 1158. [Google Scholar] [CrossRef]
- Zha, J.; Chi, X.W.; Yu, X.L.; Liu, X.M.; Liu, D.Q.; Zhu, J.; Ji, H.; Liu, R.T. Interleukin-1β-Targeted Vaccine Improves Glucose Control and β-Cell Function in a Diabetic KK-Ay Mouse Model. PLoS ONE 2016, 11, e0154298. [Google Scholar] [CrossRef]
- Delgadillo-Silva, L.F.; Tsakmaki, A.; Akhtar, N.; Franklin, Z.J.; Konantz, J.; Bewick, G.A.; Ninov, N. Modelling pancreatic β-cell inflammation in zebrafish identifies the natural product wedelolactone for human islet protection. Dis. Models Mech. 2019, 12, dmm036004. [Google Scholar] [CrossRef]
- Clark, A.L.; Kanekura, K.; Lavagnino, Z.; Spears, L.D.; Abreu, D.; Mahadevan, J.; Yagi, T.; Semenkovich, C.F.; Piston, D.W.; Urano, F. Targeting Cellular Calcium Homeostasis to Prevent Cytokine-Mediated Beta Cell Death. Sci. Rep. 2017, 7, 5611. [Google Scholar] [CrossRef]
- Wang, T.Y.; Liu, X.J.; Xie, J.Y.; Yuan, Q.Z.; Wang, Y. Cask methylation involved in the injury of insulin secretion function caused by interleukin1-β. J. Cell. Mol. Med. 2020, 24, 14247–14256. [Google Scholar] [CrossRef]
- Coope, A.; Torsoni, A.S.; Velloso, L.A. Metabolic and inflammatory pathways on the pathogenesis of type 2 diabetes. Eur. J. Endocrinol. 2016, 174, R175–R187. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef]
- Kurajoh, M.; Fukumoto, S.; Akari, S.; Murase, T.; Nakamura, T.; Takahashi, K.; Yoshida, H.; Nakatani, S.; Tsuda, A.; Morioka, T.; et al. Possible role of insulin resistance in activation of plasma xanthine oxidoreductase in health check-up examinees. Sci. Rep. 2022, 12, 10281. [Google Scholar] [CrossRef]
- Martínez-Sánchez, F.D.; Vargas-Abonce, V.P.; Guerrero-Castillo, A.P.; Santos-Villavicencio, M.D.l.; Eseiza-Acevedo, J.; Meza-Arana, C.E.; Gulias-Herrero, A.; Gómez-Sámano, M.Á. Serum Uric Acid concentration is associated with insulin resistance and impaired insulin secretion in adults at risk for Type 2 Diabetes. Prim. Care Diabetes 2021, 15, 293–299. [Google Scholar] [CrossRef]
| Name/Location | Function | Main Regulators | References |
|---|---|---|---|
| SERCA (ER membrane) | Ca2+ uptake from cytosol to ER lumen | Thapsigargin (inhibitor); CDN1163 (activators); Aged garlic extract (activators); | [40,41,42,43] |
| IP3R (ER membrane) | Ca2+ release from ER to cytosol | Xestospongin C (inhibitor); ryanodine (inhibitor); 2-APB (inhibitor); carbachol (activators); IP3 (activators); | [43,44] |
| RyR (ER membrane) | Ca2+ release from ER to cytosol | Dantrolene (inhibitor); High Ca2+ (inhibitor); Low Ca2+ (activator); Caffeine (activator); | [43,44] |
| STIM (ER membrane) | Ca2+ entry from extracellular space to cytosol | Ca2+ depletion (activator); Low 2APB (activator); SKF96365 (inhibitor); Lupenone (inhibitor); BTP2 (inhibitor); | [20,45,46] |
| Orai (Plasma Membrane) | Coupled with STIM, Ca2+ entry from extracellular milieu to cytosol | A chaperone complex (Regulating agent); BTP2 (inhibitor); | [20,46] |
| CaMKII (Cytoplasm) | Regulated by the interaction between Ca2+ and calmodulin | Ionomycin (activator); | [45,47,48,49] |
| VDAC (Mitochondrial outer membrane) | Forms a channel in the outer mitochondrial membrane and coupled to MCU to allow Ca2+ diffusion | BD1047 (Regulating agent); | [24,46,50] |
| MCU (Mitochondrial inner membrane) | Ca2+ uptake into the mitochondria | Ruthenium Red (inhibitor) BAPTA-AM (Regulating agent); | [24,46,50] |
| TRPML1 (Lysosome) | Mediating lysosomal Ca2+ efflux and promoting autophagy | PI (3,5) P2 (activator) | [51] |
| Forms of Injury | Calcium Channels (Activation/Inhibition) | Mechanisms | References |
|---|---|---|---|
| Apoptosis | SERCA (inhibition); | Improving the calcium storage in the endoplasmic reticulum and alleviating endoplasmic reticulum stress. | [40,42,43,53] |
| IP3R, RyR (activation); | Resulting in depletion of calcium ions in the endoplasmic reticulum, triggering endoplasmic reticulum stress. | [43,44,62] | |
| SOCE (activation) | Increasing the intracellular calcium ion concentration, causing endoplasmic reticulum stress. | [45] | |
| Autophagy | TRPML1 (activation); | Mediating the release of calcium ions from lysosomes, activating Calcineurin, and inducing autophagy. | [51] |
| CRAC (activation); | Mediating the inward flow of extracellular calcium ions and promoting autophagy. | [51] | |
| Pyroptosis | IP3R (activation) | PLCγ1 indirectly activating IP3R to release endoplasmic reticulum calcium to induce pyroptosis. | [63] |
| CaMKII (activation) | Promoting the assembly of the NLRP3 inflammasome and activating pyroptosis. | [64] | |
| L-type calcium channel (inhibition) | Reducing the intracellular calcium ion concentration and inhibiting the activation of the NLRP3 inflammasome. | [65] |
| Calcium Channels | Animals/Cells | Intervention Reagents | Treatment Time | Disease Type | Mechanisms | References |
|---|---|---|---|---|---|---|
| SERCA (inhibitor) | ob/ob mice | CDN1163 | 5 days | Insulin resistance and prediabetes | Activating Ca2+-ATPase activity and improving endoplasmic reticulum stress | [41] |
| SOCE (activator) | Male C57BL/6 mice HepG2 cells | Candesartan Azilsartan, candesartan, | 21 days 16 h | Obesity; Insulin resistance | Mediated calcium influx, inhibition of AKT phosphorylation | [34] |
| CaMKII (inhibitor) | L6-GLUT4myc cells | Lonomycin | 48 h | Muscle Insulin resistance | Activating AMPK-PKC phosphorylation pathway to enhance GLUT4 exocytosis and endocytosis | [49] |
| CAMK2 (activator) | Ai-CAMK2 KO mice; OP9 cells | Tamoxifen KN93 | 5 days 1 h | Obesity; Insulin resistance | CAMK2 activation decreases the number of insulin receptors (INSR) | [130] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, H.; Shan, Y.; Qian, K.; Zhao, R.; Li, H. Intracellular Calcium Dysregulation: The Hidden Culprit in the Diabetes–Gout Nexus. Biomedicines 2025, 13, 2694. https://doi.org/10.3390/biomedicines13112694
Shi H, Shan Y, Qian K, Zhao R, Li H. Intracellular Calcium Dysregulation: The Hidden Culprit in the Diabetes–Gout Nexus. Biomedicines. 2025; 13(11):2694. https://doi.org/10.3390/biomedicines13112694
Chicago/Turabian StyleShi, Hongbin, Yisi Shan, Kewei Qian, Ruofei Zhao, and Hong Li. 2025. "Intracellular Calcium Dysregulation: The Hidden Culprit in the Diabetes–Gout Nexus" Biomedicines 13, no. 11: 2694. https://doi.org/10.3390/biomedicines13112694
APA StyleShi, H., Shan, Y., Qian, K., Zhao, R., & Li, H. (2025). Intracellular Calcium Dysregulation: The Hidden Culprit in the Diabetes–Gout Nexus. Biomedicines, 13(11), 2694. https://doi.org/10.3390/biomedicines13112694






