A Thymus-Independent Artificial Organoid System Supports Complete Thymopoiesis from Rhesus Macaque-Derived Hematopoietic Stem and Progenitor Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Construction of Recombinant Mouse Bone Marrow Stromal Cell (MS-5) Expressing Rhesus macaque (Macaca mulatta) Delta-Like Canonical Notch Ligand 1 (DLL1)
2.2. Characterization of Transduced MS-5 Expressing RhDLL1
2.3. Bioethical Approvals
2.4. Processing of Rhesus macaque Thymus Tissue
2.5. Processing of Rhesus macaque Peripheral Blood Mononuclear Cells
2.6. Processing of Human Mobilized Peripheral Mononuclear Cells
2.7. Processing of Rhesus macaque Bone Marrow-Derived Biospecimen
2.8. Enrichment and Isolation of CD3−CD34+ HSPCs
2.9. Establishment of a Thymus-Independent Artificial Organoid
2.10. RhATO Phenotypic Analysis by Flow Cytometry
2.11. T Cell Cytokine Assay
2.12. Antibodies
3. Results
3.1. Generation and Characterization of MS5 Stromal Cells Expressing Rhesus macaque (Macaca mulatta) DLL1
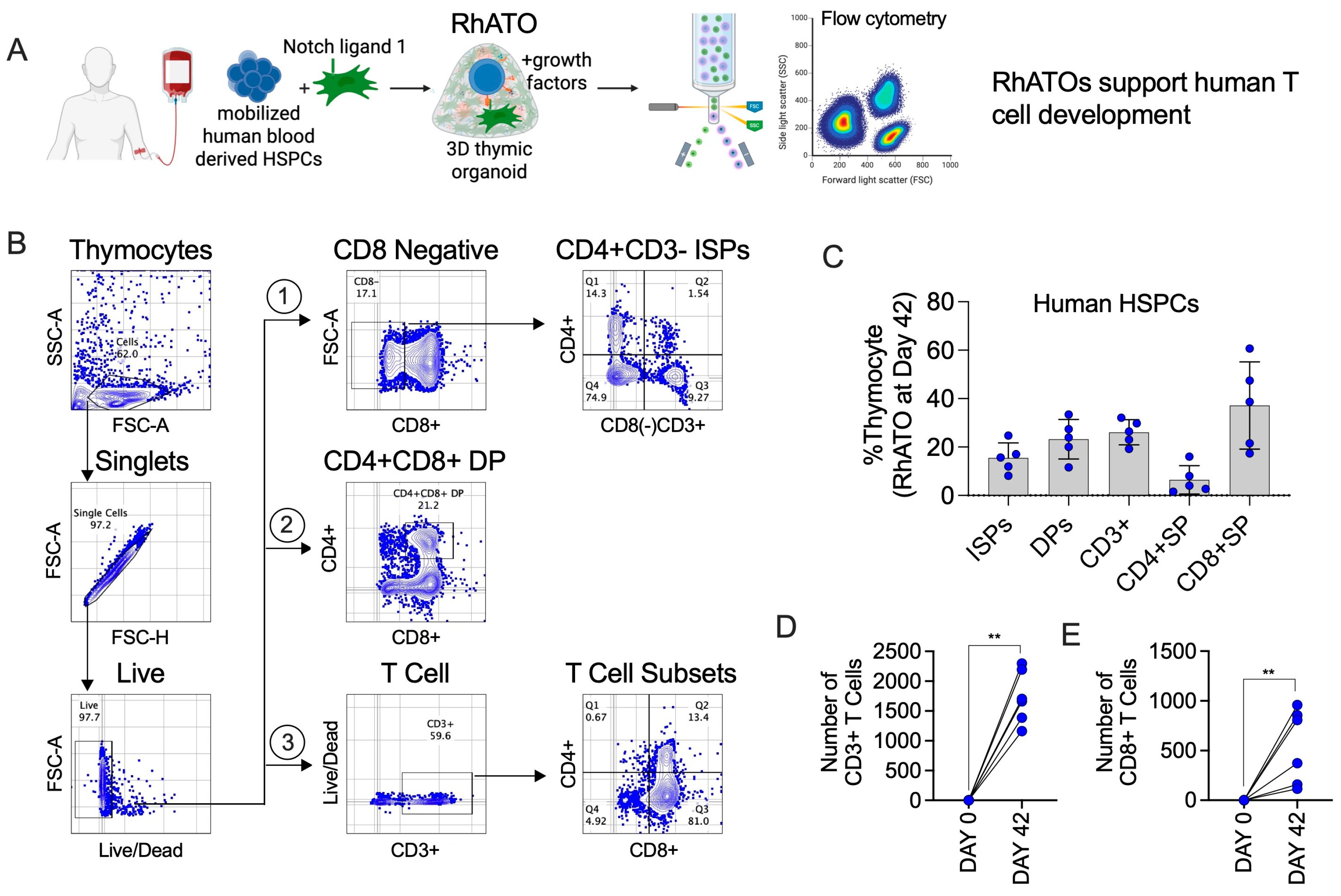
3.2. Three-Dimensional RhATO System Supported Robust T Cell Specification of Rhesus macaque HSPCs
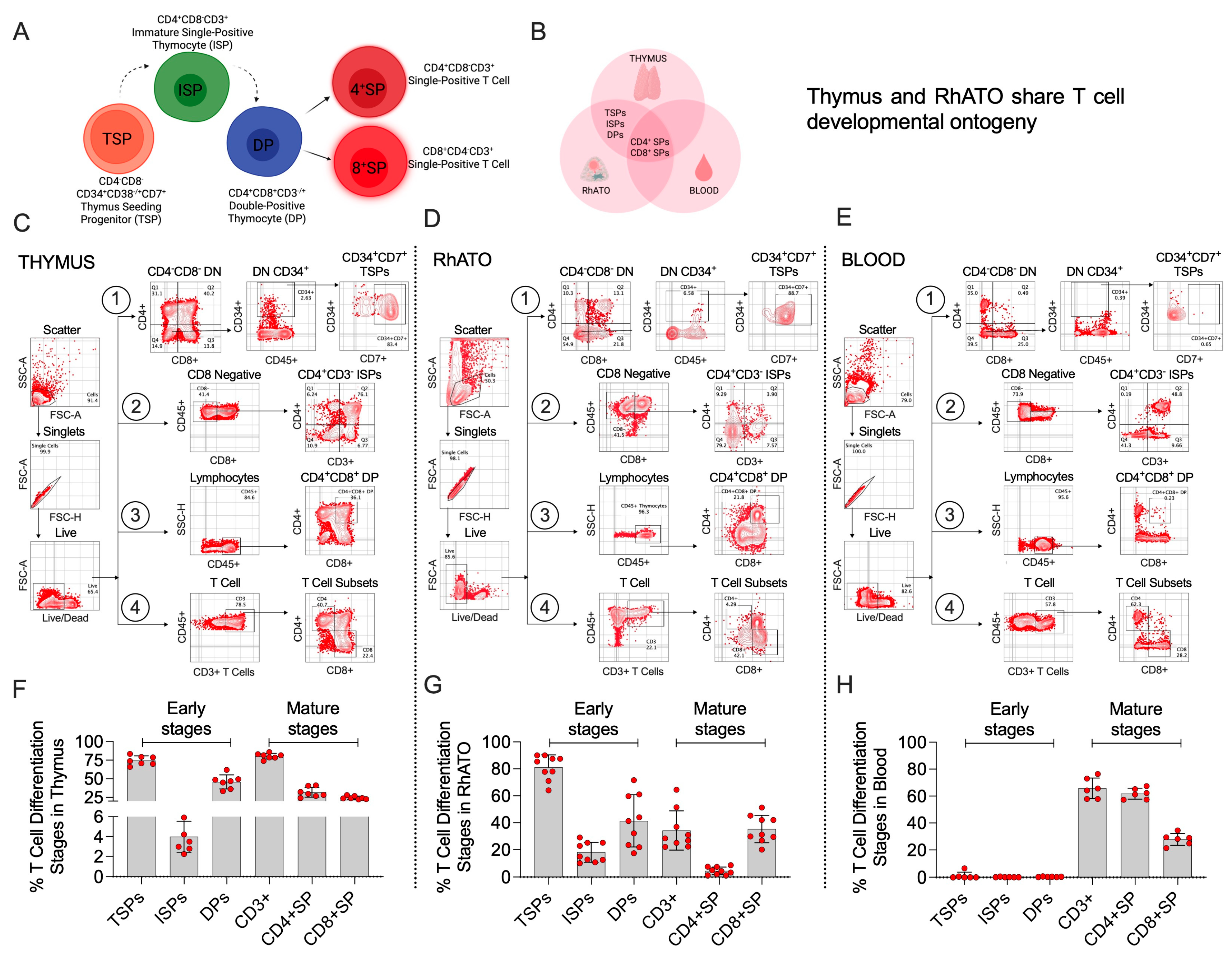
3.3. RhATO-Generated Intermediate Thymocyte Populations Mirroring Bonafide Thymus
3.4. The RhATO System Disfavored Non-T Cell-Biased Lineage Specification of HSPCs Akin to Thymus
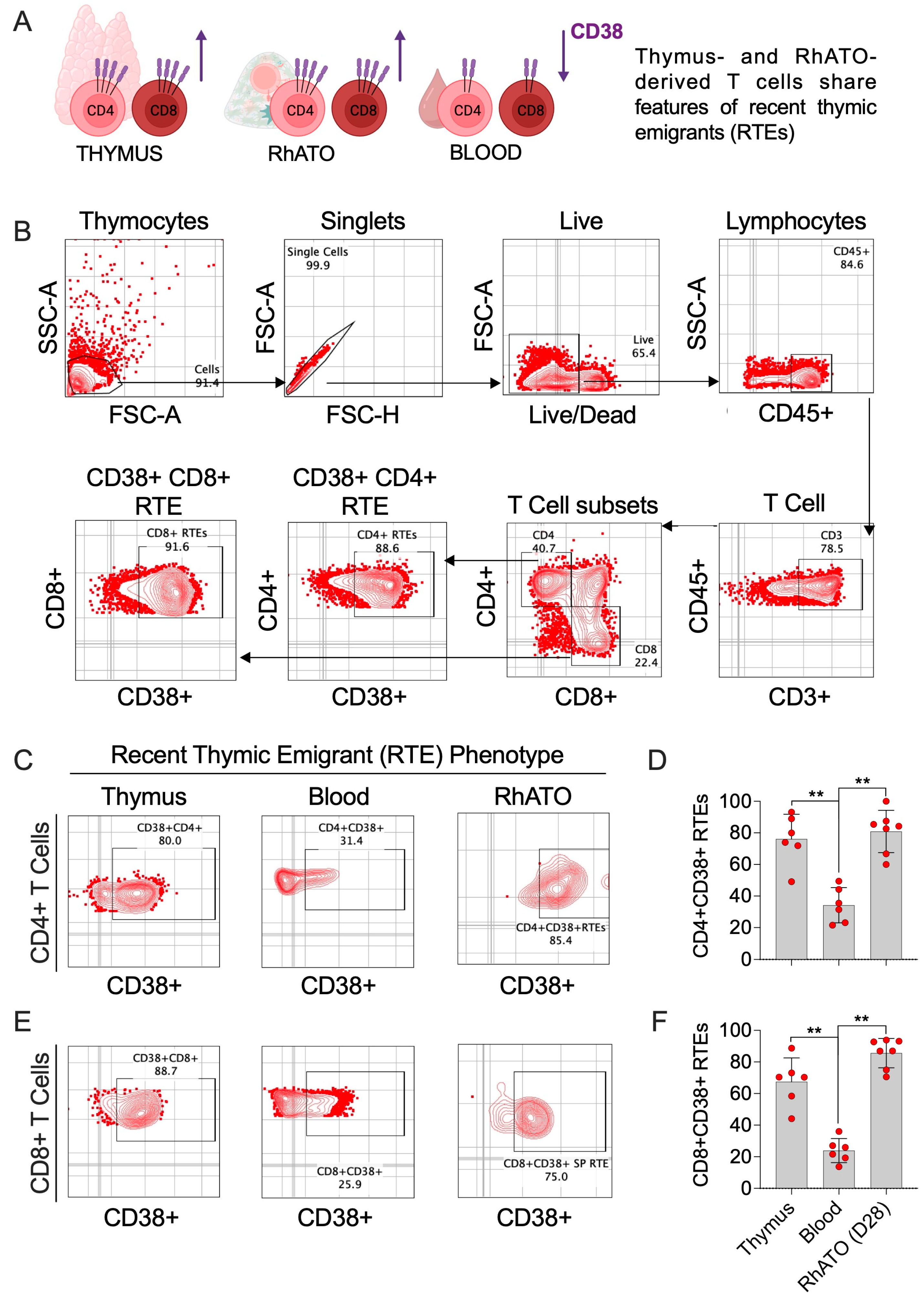
3.5. RhATO-Generated Mature T Cells Shared Features of Recent Thymic Emigrants
3.6. RhATO Supported Efficient TCR-Selected T Cell Development with Functional Potential
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cordes, M.; Cante-Barrett, K.; van den Akker, E.B.; Moretti, F.A.; Kielbasa, S.M.; Vloemans, S.A.; Garcia-Perez, L.; Teodosio, C.; van Dongen, J.J.M.; Pike-Overzet, K.; et al. Single-cell immune profiling reveals thymus-seeding populations, T cell commitment, and multilineage development in the human thymus. Sci. Immunol. 2022, 7, eade0182. [Google Scholar] [CrossRef]
- Hoebeke, I.; De Smedt, M.; Stolz, F.; Pike-Overzet, K.; Staal, F.J.; Plum, J.; Leclercq, G. T-, B- and NK-lymphoid, but not myeloid cells arise from human CD34(+)CD38(−)CD7(+) common lymphoid progenitors expressing lymphoid-specific genes. Leukemia 2007, 21, 311–319. [Google Scholar] [CrossRef]
- Six, E.M.; Bonhomme, D.; Monteiro, M.; Beldjord, K.; Jurkowska, M.; Cordier-Garcia, C.; Garrigue, A.; Dal Cortivo, L.; Rocha, B.; Fischer, A.; et al. A human postnatal lymphoid progenitor capable of circulating and seeding the thymus. J. Exp. Med. 2007, 204, 3085–3093. [Google Scholar] [CrossRef] [PubMed]
- Ashby, K.M.; Hogquist, K.A. Author Correction: A guide to thymic selection of T cells. Nat. Rev. Immunol. 2023, 23, 697. [Google Scholar] [CrossRef]
- Germain, R.N. T-cell development and the CD4-CD8 lineage decision. Nat. Rev. Immunol. 2002, 2, 309–322. [Google Scholar] [CrossRef] [PubMed]
- La Motte-Mohs, R.N.; Herer, E.; Zuniga-Pflucker, J.C. Induction of T-cell development from human cord blood hematopoietic stem cells by Delta-like 1 In Vitro. Blood 2005, 105, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Bohacova, P.; Terekhova, M.; Tsurinov, P.; Mullins, R.; Husarcikova, K.; Shchukina, I.; Antonova, A.U.; Echalar, B.; Kossl, J.; Saidu, A.; et al. Multidimensional profiling of human T cells reveals high CD38 expression, marking recent thymic emigrants and age-related naive T cell remodeling. Immunity 2024, 57, 2362–2379.e10. [Google Scholar] [CrossRef]
- Estes, J.D.; Wong, S.W.; Brenchley, J.M. Nonhuman primate models of human viral infections. Nat. Rev. Immunol. 2018, 18, 390–404. [Google Scholar] [CrossRef]
- Tarantal, A.F.; Noctor, S.C.; Hartigan-O’Connor, D.J. Nonhuman Primates in Translational Research. Annu. Rev. Anim. Biosci. 2022, 10, 441–468. [Google Scholar] [CrossRef]
- Rosenzweig, M.; Marks, D.F.; Hempel, D.; Johnson, R.P. In Vitro T lymphopoiesis: A model system for stem cell gene therapy for AIDS. J. Med. Primatol. 1996, 25, 192–200. [Google Scholar] [CrossRef]
- Rosenzweig, M.; Marks, D.F.; Zhu, H.; Hempel, D.; Mansfield, K.G.; Sehgal, P.K.; Kalams, S.; Scadden, D.T.; Johnson, R.P. In Vitro T lymphopoiesis of human and rhesus CD34+ progenitor cells. Blood 1996, 87, 4040–4048. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, M.; Yamada, K.; Harper, D.M.; Hempel, D.M.; Johnson, R.P. T-cell differentiation of human and non-human primate CD34+ hematopoietic progenitor cells using porcine thymic stroma. Xenotransplantation 2001, 8, 185–192. [Google Scholar] [CrossRef]
- Akkina, R.K.; Rosenblatt, J.D.; Campbell, A.G.; Chen, I.S.; Zack, J.A. Modeling human lymphoid precursor cell gene therapy in the SCID-hu mouse. Blood 1994, 84, 1393–1398. [Google Scholar] [CrossRef]
- Jenkinson, E.J.; Franchi, L.L.; Kingston, R.; Owen, J.J. Effect of deoxyguanosine on lymphopoiesis in the developing thymus rudiment In Vitro: Application in the production of chimeric thymus rudiments. Eur. J. Immunol. 1982, 12, 583–587. [Google Scholar] [CrossRef]
- Peault, B.; Weissman, I.L.; Baum, C.; McCune, J.M.; Tsukamoto, A. Lymphoid reconstitution of the human fetal thymus in SCID mice with CD34+ precursor cells. J. Exp. Med. 1991, 174, 1283–1286. [Google Scholar] [CrossRef] [PubMed]
- Yeoman, H.; Gress, R.E.; Bare, C.V.; Leary, A.G.; Boyse, E.A.; Bard, J.; Shultz, L.D.; Harris, D.T.; DeLuca, D. Human bone marrow and umbilical cord blood cells generate CD4+ and CD8+ single-positive T cells in murine fetal thymus organ culture. Proc. Natl. Acad. Sci. USA 1993, 90, 10778–10782. [Google Scholar] [CrossRef]
- Kutlesa, S.; Zayas, J.; Valle, A.; Levy, R.B.; Jurecic, R. T-cell differentiation of multipotent hematopoietic cell line EML in the OP9-DL1 coculture system. Exp. Hematol. 2009, 37, 909–923. [Google Scholar] [CrossRef][Green Version]
- Mohtashami, M.; Brauer, P.M.; Zuniga-Pflucker, J.C. Induction of Human T Cell Development In Vitro with OP9-DL4-7FS Cells Expressing Human Cytokines. Methods Mol. Biol. 2023, 2580, 249–260. [Google Scholar] [CrossRef]
- Mohtashami, M.; Shah, D.K.; Kianizad, K.; Awong, G.; Zuniga-Pflucker, J.C. Induction of T-cell development by Delta-like 4-expressing fibroblasts. Int. Immunol. 2013, 25, 601–611. [Google Scholar] [CrossRef]
- Schmitt, T.M.; Zuniga-Pflucker, J.C. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 In Vitro. Immunity 2002, 17, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Radtke, S.; Adair, J.E.; Giese, M.A.; Chan, Y.Y.; Norgaard, Z.K.; Enstrom, M.; Haworth, K.G.; Schefter, L.E.; Kiem, H.P. A distinct hematopoietic stem cell population for rapid multilineage engraftment in nonhuman primates. Sci. Transl. Med. 2017, 9, eaan1145. [Google Scholar] [CrossRef]
- Awong, G.; La Motte-Mohs, R.N.; Zuniga-Pflucker, J.C. In Vitro human T cell development directed by notch-ligand interactions. Methods Mol. Biol. 2008, 430, 135–142. [Google Scholar] [CrossRef]
- De Smedt, M.; Hoebeke, I.; Plum, J. Human bone marrow CD34+ progenitor cells mature to T cells on OP9-DL1 stromal cell line without thymus microenvironment. Blood Cells Mol. Dis. 2004, 33, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Awong, G.; Herer, E.; La Motte-Mohs, R.N.; Zuniga-Pflucker, J.C. Human CD8 T cells generated In Vitro from hematopoietic stem cells are functionally mature. BMC Immunol. 2011, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.; Montel-Hagen, A.; Ge, S.; Blumberg, G.; Kim, K.; Klein, S.; Zhu, Y.; Parekh, C.; Balamurugan, A.; Yang, O.O.; et al. Engineering the human thymic microenvironment to support thymopoiesis In Vivo. Stem Cells 2014, 32, 2386–2396. [Google Scholar] [CrossRef]
- Van Coppernolle, S.; Verstichel, G.; Timmermans, F.; Velghe, I.; Vermijlen, D.; De Smedt, M.; Leclercq, G.; Plum, J.; Taghon, T.; Vandekerckhove, B.; et al. Functionally mature CD4 and CD8 TCRalphabeta cells are generated in OP9-DL1 cultures from human CD34+ hematopoietic cells. J. Immunol. 2009, 183, 4859–4870. [Google Scholar] [CrossRef]
- Montel-Hagen, A.; Tsai, S.; Seet, C.S.; Crooks, G.M. Generation of Artificial Thymic Organoids from Human and Murine Hematopoietic Stem and Progenitor Cells. Curr. Protoc. 2022, 2, e403. [Google Scholar] [CrossRef]
- Seet, C.S.; He, C.; Bethune, M.T.; Li, S.; Chick, B.; Gschweng, E.H.; Zhu, Y.; Kim, K.; Kohn, D.B.; Baltimore, D.; et al. Generation of mature T cells from human hematopoietic stem and progenitor cells in artificial thymic organoids. Nat. Methods 2017, 14, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.A.; Yagnik, B.; Bally, A.P.; Morrow, K.N.; Wang, S.; Vanderford, T.H.; Freeman, G.J.; Ahmed, R.; Amara, R.R. PD-1 blockade and vaccination provide therapeutic benefit against SIV by inducing broad and functional CD8(+) T cells in lymphoid tissue. Sci. Immunol. 2021, 6, eabh3034. [Google Scholar] [CrossRef] [PubMed]
- de Pooter, R.; Zuniga-Pflucker, J.C. T-cell potential and development In Vitro: The OP9-DL1 approach. Curr. Opin. Immunol. 2007, 19, 163–168. [Google Scholar] [CrossRef]
- Poznansky, M.C.; Evans, R.H.; Foxall, R.B.; Olszak, I.T.; Piascik, A.H.; Hartman, K.E.; Brander, C.; Meyer, T.H.; Pykett, M.J.; Chabner, K.T.; et al. Efficient generation of human T cells from a tissue-engineered thymic organoid. Nat. Biotechnol. 2000, 18, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Sodora, D.L.; Milush, J.M.; Ware, F.; Wozniakowski, A.; Montgomery, L.; McClure, H.M.; Lackner, A.A.; Marthas, M.; Hirsch, V.; Johnson, R.P.; et al. Decreased levels of recent thymic emigrants in peripheral blood of simian immunodeficiency virus-infected macaques correlate with alterations within the thymus. J. Virol. 2002, 76, 9981–9990. [Google Scholar] [CrossRef] [PubMed]
- Gardner, J.P.; Rosenzweig, M.; Marks, D.F.; Harper, D.; Gaynor, K.; Fallon, R.J.; Wall, D.A.; Johnson, R.P.; Scadden, D.T. T-lymphopoietic capacity of cord blood-derived CD34+ progenitor cells. Exp. Hematol. 1998, 26, 991–999. [Google Scholar] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilde, C.; Anwar, S.; Yau, Y.-T.; Badve, S.; Gökmen-Polar, Y.; Roback, J.D.; Amara, R.R.; Johnson, R.P.; Rahman, S.A. A Thymus-Independent Artificial Organoid System Supports Complete Thymopoiesis from Rhesus Macaque-Derived Hematopoietic Stem and Progenitor Cells. Biomedicines 2025, 13, 2692. https://doi.org/10.3390/biomedicines13112692
Wilde C, Anwar S, Yau Y-T, Badve S, Gökmen-Polar Y, Roback JD, Amara RR, Johnson RP, Rahman SA. A Thymus-Independent Artificial Organoid System Supports Complete Thymopoiesis from Rhesus Macaque-Derived Hematopoietic Stem and Progenitor Cells. Biomedicines. 2025; 13(11):2692. https://doi.org/10.3390/biomedicines13112692
Chicago/Turabian StyleWilde, Callie, Saleem Anwar, Yu-Tim Yau, Sunil Badve, Yesim Gökmen-Polar, John D. Roback, Rama Rao Amara, R. Paul Johnson, and Sheikh Abdul Rahman. 2025. "A Thymus-Independent Artificial Organoid System Supports Complete Thymopoiesis from Rhesus Macaque-Derived Hematopoietic Stem and Progenitor Cells" Biomedicines 13, no. 11: 2692. https://doi.org/10.3390/biomedicines13112692
APA StyleWilde, C., Anwar, S., Yau, Y.-T., Badve, S., Gökmen-Polar, Y., Roback, J. D., Amara, R. R., Johnson, R. P., & Rahman, S. A. (2025). A Thymus-Independent Artificial Organoid System Supports Complete Thymopoiesis from Rhesus Macaque-Derived Hematopoietic Stem and Progenitor Cells. Biomedicines, 13(11), 2692. https://doi.org/10.3390/biomedicines13112692








