Deep Brain Stimulation: Mechanisms, Cost-Effectiveness, and Precision Applications Across Neurology and Psychiatry
Abstract
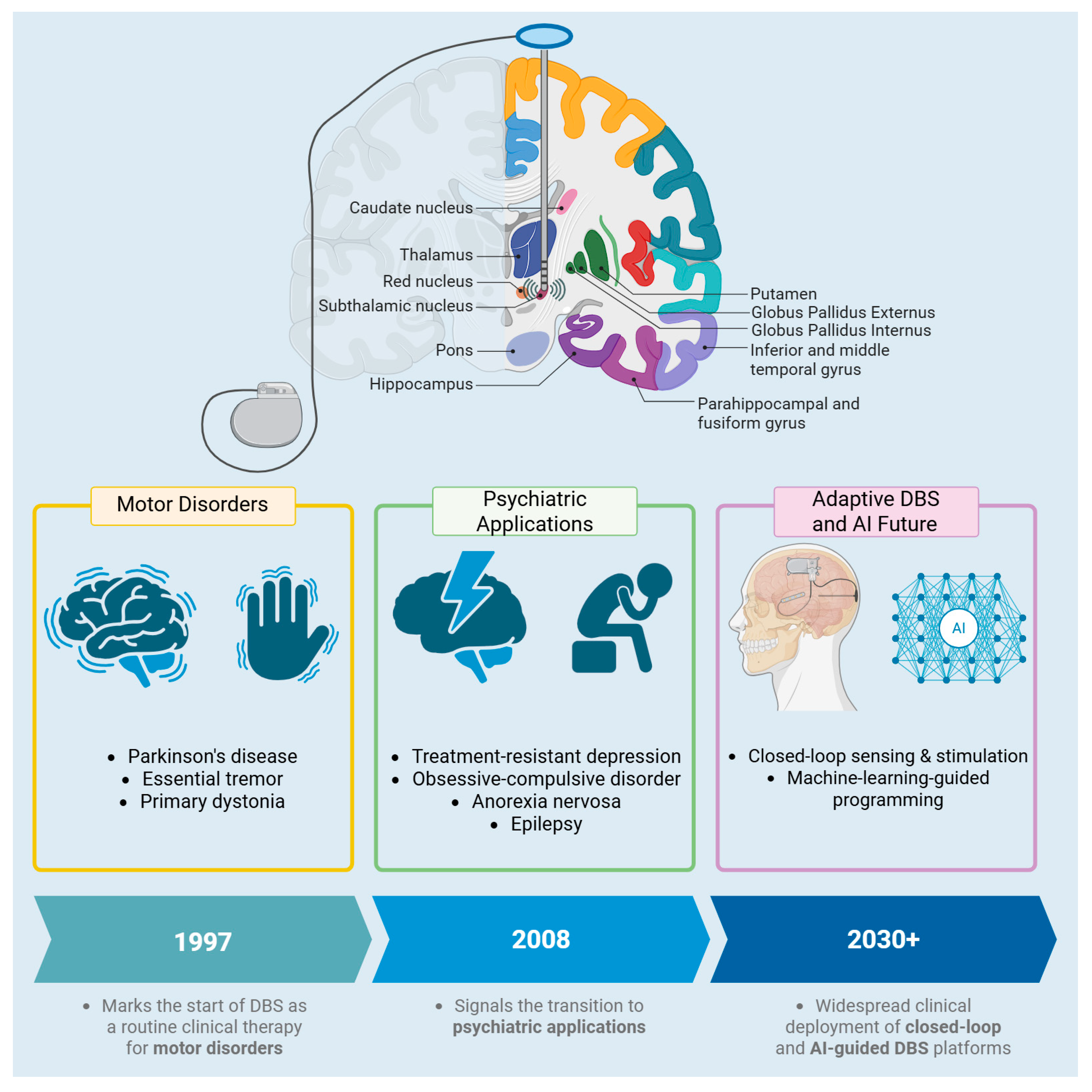
1. Introduction
2. Targets
3. Mechanism
4. DBS Stimulation in Other Pathologies (Psychiatric Pathologies)
5. Complications and Adverse Effects of the DBS
| # | Study (First Author, Year) | Design/Scope | Patients (n) | DBS Location(s) | Principal Pathology/Indications | Salient Complication Findings |
|---|---|---|---|---|---|---|
| 1 | Rasiah et al. (2023) [89] | Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) meta-analysis of 262 studies | 21,261 | STN, GPi, ViM | Parkinson’s disease | Revision 4.9%; infection 4.2%; lead malposition 3.3%; hemorrhage 2.4% (risk rises with >1 MER track) |
| 2 | Tian et al. (2022) [90] | Meta-analysis of 10 studies | 1354 | STN, GPi, ViM, PSA | Predominantly PD/ET | Pooled PLE incidence 35.8%; symptomatic PLE 3.1% |
| 3 | Bullard et al. (2020) [91] | Systematic review of 240 studies | 34,089 | Mixed targets | Broad neurology and psychiatry indications | Infection 4.57%; IPG malfunction 3.25%; hemorrhage 2.86%; lead fracture 2.56% |
| 4 | Radziunas et al. (2018) [92] | Prospective single-center cohort | 22 | STN | Parkinson’s disease | 31.8% developed early neuro-psychiatric events (psychosis, delirium) after STN-DBS |
| 5 | Jitkritsadakul et al. (2017) [79] | Systematic review of 96 studies | 8983 | Mixed (STN, GPi, ViM, etc.) | PD, dystonia, Tourette, epilepsy, cluster HA, OCD | Hardware complications overall 11.75%; infections 5.1%; lead migration 1.6% |
6. DBS and Connectomics
7. DBS and AI
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Emamzadeh, F.N.; Surguchov, A. Parkinson’s Disease: Biomarkers, Treatment, and Risk Factors. Front. Neurosci. 2018, 12, 612. [Google Scholar] [CrossRef] [PubMed]
- Balestrino, R.; Schapira, A.H.V. Parkinson disease. Eur. J. Neurol. 2020, 27, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Tanner, C.M.; Ostrem, J.L. Parkinson’s Disease. N. Engl. J. Med. 2024, 391, 442–452. [Google Scholar] [CrossRef]
- Lozano, A.M.; Lipsman, N.; Bergman, H.; Brown, P.; Chabardes, S.; Chang, J.W.; Matthews, K.; McIntyre, C.C.; Schlaepfer, T.E.; Schulder, M.; et al. Deep brain stimulation: Current challenges and future directions. Nat. Rev. Neurol. 2019, 15, 148–160. [Google Scholar] [CrossRef]
- Mahlknecht, P.; Foltynie, T.; Limousin, P.; Poewe, W. How Does Deep Brain Stimulation Change the Course of Parkinson’s Disease? Mov. Disord. Off. J. Mov. Disord. Soc. 2022, 37, 1581–1592. [Google Scholar] [CrossRef]
- Hartmann, C.J.; Fliegen, S.; Groiss, S.J.; Wojtecki, L.; Schnitzler, A. An update on best practice of deep brain stimulation in Parkinson’s disease. Ther. Adv. Neurol. Disord. 2019, 12, 1756286419838096. [Google Scholar] [CrossRef]
- Mahajan, A.; Butala, A.; Okun, M.S.; Mari, Z.; Mills, K.A. Global Variability in Deep Brain Stimulation Practices for Parkinson’s Disease. Front. Hum. Neurosci. 2021, 15, 667035. [Google Scholar] [CrossRef] [PubMed]
- Bove, F.; Mulas, D.; Cavallieri, F.; Castrioto, A.; Chabardès, S.; Meoni, S.; Schmitt, E.; Bichon, A.; Di Stasio, E.; Kistner, A.; et al. Long-term Outcomes (15 Years) After Subthalamic Nucleus Deep Brain Stimulation in Patients with Parkinson Disease. Neurology 2021, 97, e254–e262. [Google Scholar] [CrossRef]
- Gessani, A.; Cavallieri, F.; Fioravanti, V.; Campanini, I.; Merlo, A.; Di Rauso, G.; Damiano, B.; Scaltriti, S.; Bardi, E.; Corni, M.G.; et al. Long-term effects of subthalamic nucleus deep brain stimulation on speech in Parkinson’s disease. Sci. Rep. 2023, 13, 11462. [Google Scholar] [CrossRef]
- Defer, G.L.; Widner, H.; Marié, R.M.; Rémy, P.; Levivier, M. Core assessment program for surgical interventional therapies in Parkinson’s disease (CAPSIT-PD). Mov. Disord. Off. J. Mov. Disord. Soc. 1999, 14, 572–584. [Google Scholar] [CrossRef]
- Morgante, L.; Morgante, F.; Moro, E.; Epifanio, A.; Girlanda, P.; Ragonese, P.; Antonini, A.; Barone, P.; Bonuccelli, U.; Contarino, M.F.; et al. How many parkinsonian patients are suitable candidates for deep brain stimulation of subthalamic nucleus? Results of a questionnaire. Park. Relat. Disord. 2007, 13, 528–531. [Google Scholar] [CrossRef]
- Lannon, M.; Duda, T.; Mastrolonardo, A.; Huang, E.; Martyniuk, A.; Farrokhyar, F.; Xie, F.; Bhandari, M.; Kalia, S.K.; Sharma, S. Economic Evaluations Comparing Deep Brain Stimulation to Best Medical Therapy for Movement Disorders: A Meta-Analysis. PharmacoEconomics 2024, 42, 41–68. [Google Scholar] [CrossRef]
- Nyholm, D.; Eggington, S.; Holm, A. Therapies for Advanced Parkinson’s Disease in Sweden: A Cost-Effectiveness Analysis Using Real-World Data. Neurol. Ther. 2025, 14, 801–812. [Google Scholar] [CrossRef]
- Kabotyanski, K.E.; Najera, R.A.; Banks, G.P.; Sharma, H.; Provenza, N.R.; Hayden, B.Y.; Mathew, S.J.; Sheth, S.A. Cost-effectiveness and threshold analysis of deep brain stimulation vs. treatment-as-usual for treatment-resistant depression. Transl. Psychiatry 2024, 14, 243. [Google Scholar] [CrossRef]
- Najera, R.A.; Kabotyanski, K.E.; McLaughlin, N.C.; Gregory, S.T.; Anand, A.; Shofty, B.; Provenza, N.R.; Storch, E.A.; Goodman, W.K.; Sheth, S.A. Cost-effectiveness analysis of deep brain stimulation versus treatment as usual for treatment-resistant obsessive-compulsive disorder. J. Neurosurg. 2025, 1, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.Y.; Wijnen, B.F.M.; Majoie, M.H.J.M.; Evers, S.M.A.A.; Hiligsmann, M. Economic evaluation of deep brain stimulation compared with vagus nerve stimulation and usual care for patients with refractory epilepsy: A lifetime decision analytic model. Epilepsia 2022, 63, 641–651. [Google Scholar] [CrossRef]
- Deep-Brain Stimulation of the Subthalamic Nucleus or the Pars Interna of the Globus Pallidus in Parkinson’s Disease. N. Engl. J. Med. 2001, 345, 956–963. [CrossRef] [PubMed]
- Stefani, A.; Lozano, A.M.; Peppe, A.; Stanzione, P.; Galati, S.; Tropepi, D.; Pierantozzi, M.; Brusa, L.; Scarnati, E.; Mazzone, P. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson’s disease. Brain 2007, 130, 1596–1607. [Google Scholar] [CrossRef]
- Vitek, J.L.; Hashimoto, T.; Peoples, J.; DeLong, M.R.; Bakay, R.A.E. Acute stimulation in the external segment of the globus pallidus improves parkinsonian motor signs. Mov. Disord. 2004, 19, 907–915. [Google Scholar] [CrossRef]
- Drouot, X.; Oshino, S.; Jarraya, B.; Besret, L.; Kishima, H.; Remy, P.; Dauguet, J.; Lefaucheur, J.P.; Dollé, F.; Condé, F.; et al. Functional Recovery in a Primate Model of Parkinson’s Disease following Motor Cortex Stimulation. Neuron 2004, 44, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Andrews, L.; Ali, A.M.S.; Elmolla, M.; Keller, S.S.; Bhojak, M.; Osman-Farah, J.; Macerollo, A. Directional deep brain stimulation for cervical dystonia: Outcomes, challenges and future directions. Deep Brain Stimul. 2024, 7, 7–13. [Google Scholar] [CrossRef]
- Guehl, D.; Guillaud, E.; Langbour, N.; Doat, E.; Auzou, N.; Courtin, E.; Branchard, O.; Engelhardt, J.; Benazzouz, A.; Eusebio, A.; et al. Usefulness of thalamic beta activity for closed-loop therapy in essential tremor. Sci. Rep. 2023, 13, 22332. [Google Scholar] [CrossRef] [PubMed]
- Stover, N.P.; Okun, M.S.; Evatt, M.L.; Raju, D.V.; Bakay, R.A.E.; Vitek, J.L. Stimulation of the Subthalamic Nucleus in a Patient with Parkinson Disease and Essential Tremor. Arch. Neurol. 2005, 62, 141. [Google Scholar] [CrossRef]
- Diamond, A.; Shahed, J.; Jankovic, J. The effects of subthalamic nucleus deep brain stimulation on parkinsonian tremor. J. Neurol. Sci. 2007, 260, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Lachenmayer, M.L.; Mürset, M.; Antih, N.; Debove, I.; Muellner, J.; Bompart, M.; Schlaeppi, J.-A.; Nowacki, A.; You, H.; Michelis, J.P.; et al. Subthalamic and pallidal deep brain stimulation for Parkinson’s disease—Meta-analysis of outcomes. Npj Park. Dis. 2021, 7, 77. [Google Scholar] [CrossRef]
- Fan, S.; Liu, D.; Shi, L.; Meng, F.; Fang, H.; Liu, H.; Zhang, H.; Yang, A.; Zhang, J. Differential Effects of Subthalamic Nucleus and Globus Pallidus Internus Deep Brain Stimulation on Motor Subtypes in Parkinson’s Disease. World Neurosurg. 2022, 164, e245–e255. [Google Scholar] [CrossRef] [PubMed]
- Huhn, M.; Prewett, M.; Rossignol, J.; Dunbar, G.L. Comparison of the Long-Term Efficacy of Targeting the Subthalamic Nucleus Versus the Globus Pallidus Interna for Deep Brain Stimulation Treatment of Motor Dysfunction in Patients with Parkinson’s Disease: A Meta-Analysis Study. Park. Dis. 2024, 2024, 5157873. [Google Scholar] [CrossRef]
- Rajamani, N.; Friedrich, H.; Butenko, K.; Dembek, T.; Lange, F.; Navrátil, P.; Zvarova, P.; Hollunder, B.; De Bie, R.M.A.; Odekerken, V.J.J.; et al. Deep brain stimulation of symptom-specific networks in Parkinson’s disease. Nat. Commun. 2024, 15, 4662. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Chen, F.; Liu, X.; Jiang, C.; Hu, X.; Ma, L.; Xu, Z. STN versus GPi deep brain stimulation for dyskinesia improvement in advanced Parkinson’s disease: A meta-analysis of randomized controlled trials. Clin. Neurol. Neurosurg. 2021, 201, 106450. [Google Scholar] [CrossRef]
- Schmidt, S.L.; Chowdhury, A.H.; Mitchell, K.T.; Peters, J.J.; Gao, Q.; Lee, H.-J.; Genty, K.; Chow, S.-C.; Grill, W.M.; Pajic, M.; et al. At home adaptive dual target deep brain stimulation in Parkinson’s disease with proportional control. Brain 2024, 147, 911–922. [Google Scholar] [CrossRef]
- Deuschl, G.; Schade-Brittinger, C.; Krack, P.; Volkmann, J.; Schäfer, H.; Bötzel, K.; Daniels, C.; Deutschländer, A.; Dillmann, U.; Eisner, W.; et al. A Randomized Trial of Deep-Brain Stimulation for Parkinson’s Disease. N. Engl. J. Med. 2006, 355, 896–908. [Google Scholar] [CrossRef] [PubMed]
- Weaver, F.M. Bilateral Deep Brain Stimulation vs Best Medical Therapy for Patients with Advanced Parkinson Disease A Randomized Controlled Trial. JAMA 2009, 301, 63. [Google Scholar] [CrossRef] [PubMed]
- Fenoy, A.J.; Schiess, M.C. Deep Brain Stimulation of the Dentato-Rubro-Thalamic Tract: Outcomes of Direct Targeting for Tremor. Neuromodul. Technol. Neural Interface 2017, 20, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Chazen, J.L.; Sarva, H.; Stieg, P.E.; Min, R.J.; Ballon, D.J.; Pryor, K.O.; Riegelhaupt, P.M.; Kaplitt, M.G. Clinical improvement associated with targeted interruption of the cerebellothalamic tract following MR-guided focused ultrasound for essential tremor. J. Neurosurg. 2018, 129, 315–323. [Google Scholar] [CrossRef]
- Dembek, T.A.; Petry-Schmelzer, J.N.; Reker, P.; Wirths, J.; Hamacher, S.; Steffen, J.; Dafsari, H.S.; Hövels, M.; Fink, G.R.; Visser-Vandewalle, V.; et al. PSA and VIM DBS efficiency in essential tremor depends on distance to the dentatorubrothalamic tract. NeuroImage Clin. 2020, 26, 102235. [Google Scholar] [CrossRef]
- Krauss, J.K.; Lipsman, N.; Aziz, T.; Boutet, A.; Brown, P.; Chang, J.W.; Davidson, B.; Grill, W.M.; Hariz, M.I.; Horn, A.; et al. Technology of deep brain stimulation: Current status and future directions. Nat. Rev. Neurol. 2021, 17, 75–87. [Google Scholar] [CrossRef]
- Alvarez, L.; Macias, R.; Pavón, N.; López, G.; Rodríguez-Oroz, M.C.; Rodríguez, R.; Alvarez, M.; Pedroso, I.; Teijeiro, J.; Fernández, R.; et al. Therapeutic efficacy of unilateral subthalamotomy in Parkinson’s disease: Results in 89 patients followed for up to 36 months. J. Neurol. Neurosurg. Psychiatry 2009, 80, 979–985. [Google Scholar] [CrossRef]
- Rodriguez-Rojas, R.; Pineda-Pardo, J.A.; Martinez-Fernandez, R.; Kogan, R.V.; Sanchez-Catasus, C.A.; Del Alamo, M.; Hernández, F.; García-Cañamaque, L.; Leenders, K.L.; Obeso, J.A. Functional impact of subthalamotomy by magnetic resonance-guided focused ultrasound in Parkinson’s disease: A hybrid PET/MR study of resting-state brain metabolism. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 425–436. [Google Scholar] [CrossRef]
- Martínez-Fernández, R.; Máñez-Miró, J.U.; Rodríguez-Rojas, R.; Del Álamo, M.; Shah, B.B.; Hernández-Fernández, F.; Pineda-Pardo, J.A.; Monje, M.H.G.; Fernández-Rodríguez, B.; Sperling, S.A.; et al. Randomized Trial of Focused Ultrasound Subthalamotomy for Parkinson’s Disease. N. Engl. J. Med. 2020, 383, 2501–2513. [Google Scholar] [CrossRef]
- Benazzouz, A.; Hallett, M. Mechanism of action of deep brain stimulation. Neurology 2000, 55, S13–S16. [Google Scholar]
- Davidson, B.; Milosevic, L.; Kondrataviciute, L.; Kalia, L.V.; Kalia, S.K. Neuroscience fundamentals relevant to neuromodulation: Neurobiology of deep brain stimulation in Parkinson’s disease. Neurotherapeutics 2024, 21, e00348. [Google Scholar] [CrossRef]
- Anderson, R.W.; Farokhniaee, A.; Gunalan, K.; Howell, B.; McIntyre, C.C. Action potential initiation, propagation, and cortical invasion in the hyperdirect pathway during subthalamic deep brain stimulation. Brain Stimulat. 2018, 11, 1140–1150. [Google Scholar] [CrossRef]
- Bourne, S.K.; Eckhardt, C.A.; Sheth, S.A.; Eskandar, E.N. Mechanisms of deep brain stimulation for obsessive compulsive disorder: Effects upon cells and circuits. Front. Integr. Neurosci. 2012, 6, 29. [Google Scholar] [CrossRef]
- Grill, W.M.; Snyder, A.N.; Miocinovic, S. Deep brain stimulation creates an informational lesion of the stimulated nucleus. Neuroreport 2004, 15, 1137–1140. [Google Scholar] [CrossRef]
- Lowet, E.; Kondabolu, K.; Zhou, S.; Mount, R.A.; Wang, Y.; Ravasio, C.R.; Han, X. Deep brain stimulation creates informational lesion through membrane depolarization in mouse hippocampus. Nat. Commun. 2022, 13, 7709. [Google Scholar] [CrossRef]
- Chen, Z.; Tsytsarev, V.; Finfrock, Y.Z.; Antipova, O.A.; Cai, Z.; Arakawa, H.; Lischka, F.W.; Hooks, B.M.; Wilton, R.; Wang, D.; et al. Wireless Optogenetic Modulation of Cortical Neurons Enabled by Radioluminescent Nanoparticles. ACS Nano 2021, 15, 5201–5208. [Google Scholar] [CrossRef]
- Matsubara, T.; Yanagida, T.; Kawaguchi, N.; Nakano, T.; Yoshimoto, J.; Sezaki, M.; Takizawa, H.; Tsunoda, S.P.; Horigane, S.; Ueda, S.; et al. Remote control of neural function by X-ray-induced scintillation. Nat. Commun. 2021, 12, 4478. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, M.; Koshimizu, M.; Asada, Y.; Fukumitsu, K.; Ohkuma, M.; Sang, N.; Nakano, T.; Kunikata, T.; Okazaki, K.; Kawaguchi, N.; et al. Comparative Validation of Scintillator Materials for X-Ray-Mediated Neuronal Control in the Deep Brain. Int. J. Mol. Sci. 2024, 25, 11365. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ke, Y.; Chan, D.C.W.; Qian, Z.-M.; Yung, K.K.L.; Ko, H.; Arbuthnott, G.W.; Yung, W.-H. Therapeutic deep brain stimulation in Parkinsonian rats directly influences motor cortex. Neuron 2012, 76, 1030–1041. [Google Scholar] [CrossRef]
- Sanders, T.H.; Jaeger, D. Optogenetic stimulation of cortico-subthalamic projections is sufficient to ameliorate bradykinesia in 6-ohda lesioned mice. Neurobiol. Dis. 2016, 95, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; de Hemptinne, C.; Miller, A.M.; Leibbrand, M.; Little, S.J.; Lim, D.A.; Larson, P.S.; Starr, P.A. Prefrontal-Subthalamic Hyperdirect Pathway Modulates Movement Inhibition in Humans. Neuron 2020, 106, 579–588.e3. [Google Scholar] [CrossRef]
- Jorge, A.; Lipski, W.J.; Wang, D.; Crammond, D.J.; Turner, R.S.; Richardson, R.M. Hyperdirect connectivity of opercular speech network to the subthalamic nucleus. Cell Rep. 2022, 38, 110477. [Google Scholar] [CrossRef]
- Johnson, L.A.; Wang, J.; Nebeck, S.D.; Zhang, J.; Johnson, M.D.; Vitek, J.L. Direct Activation of Primary Motor Cortex during Subthalamic But Not Pallidal Deep Brain Stimulation. J. Neurosci. Off. J. Soc. Neurosci. 2020, 40, 2166–2177. [Google Scholar] [CrossRef]
- Miocinovic, S.; de Hemptinne, C.; Chen, W.; Isbaine, F.; Willie, J.T.; Ostrem, J.L.; Starr, P.A. Cortical Potentials Evoked by Subthalamic Stimulation Demonstrate a Short Latency Hyperdirect Pathway in Humans. J. Neurosci. Off. J. Soc. Neurosci. 2018, 38, 9129–9141. [Google Scholar] [CrossRef] [PubMed]
- Holtzheimer, P.E.; Husain, M.M.; Lisanby, S.H.; Taylor, S.F.; Whitworth, L.A.; McClintock, S.; Slavin, K.V.; Berman, J.; McKhann, G.M.; Patil, P.G.; et al. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: A multisite, randomised, sham-controlled trial. Lancet Psychiatry 2017, 4, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Raymaekers, S.; Luyten, L.; Bervoets, C.; Gabriëls, L.; Nuttin, B. Deep brain stimulation for treatment-resistant major depressive disorder: A comparison of two targets and long-term follow-up. Transl. Psychiatry 2017, 7, e1251. [Google Scholar] [CrossRef] [PubMed]
- Pycroft, L.; Stein, J.; Aziz, T. Deep brain stimulation: An overview of history, methods, and future developments. Brain Neurosci. Adv. 2018, 2, 2398212818816017. [Google Scholar] [CrossRef]
- Alagapan, S.; Choi, K.S.; Heisig, S.; Riva-Posse, P.; Crowell, A.; Tiruvadi, V.; Obatusin, M.; Veerakumar, A.; Waters, A.C.; Gross, R.E.; et al. Cingulate dynamics track depression recovery with deep brain stimulation. Nature 2023, 622, 130–138. [Google Scholar] [CrossRef]
- Widge, A.S. Closing the loop in psychiatric deep brain stimulation: Physiology, psychometrics, and plasticity. Neuropsychopharmacology 2024, 49, 138–149. [Google Scholar] [CrossRef]
- Vahia, V.N. Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Association: Washington, WA, USA, 2013. [Google Scholar]
- Strom, N.I.; Soda, T.; Mathews, C.A.; Davis, L.K. A dimensional perspective on the genetics of obsessive-compulsive disorder. Transl. Psychiatry 2021, 11, 401. [Google Scholar] [CrossRef]
- Koran, L.M.; Hanna, G.L.; Hollander, E.; Nestadt, G.; Simpson, H.B. American Psychiatric Association Practice guideline for the treatment of patients with obsessive-compulsive disorder. Am. J. Psychiatry 2007, 164, 5–53. [Google Scholar]
- Romanelli, R.J.; Wu, F.M.; Gamba, R.; Mojtabai, R.; Segal, J.B. Behavioral therapy and serotonin reuptake inhibitor pharmacotherapy in the treatment of obsessive-compulsive disorder: A systematic review and meta-analysis of head-to-head randomized controlled trials: Review: Behavioral Therapy and SRIs in the Treatment of OCD. Depress. Anxiety 2014, 31, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Fineberg, N.A.; Brown, A.; Reghunandanan, S.; Pampaloni, I. Evidence-based pharmacotherapy of obsessive-compulsive disorder. Int. J. Neuropsychopharmacol. 2012, 15, 1173–1191. [Google Scholar] [CrossRef]
- Carmi, L.; Tendler, A.; Bystritsky, A.; Hollander, E.; Blumberger, D.M.; Daskalakis, J.; Ward, H.; Lapidus, K.; Goodman, W.; Casuto, L.; et al. Efficacy and Safety of Deep Transcranial Magnetic Stimulation for Obsessive-Compulsive Disorder: A Prospective Multicenter Randomized Double-Blind Placebo-Controlled Trial. Am. J. Psychiatry 2019, 176, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Goodman, W.K.; Storch, E.A.; Cohn, J.F.; Sheth, S.A. Deep Brain Stimulation for Intractable Obsessive-Compulsive Disorder: Progress and Opportunities. Am. J. Psychiatry 2020, 177, 200–203. [Google Scholar] [CrossRef]
- Hageman, S.B.; Van Rooijen, G.; Bergfeld, I.O.; Schirmbeck, F.; De Koning, P.; Schuurman, P.R.; Denys, D. Deep brain stimulation versus ablative surgery for treatment-refractory obsessive-compulsive disorder: A meta-analysis. Acta Psychiatr. Scand. 2021, 143, 307–318. [Google Scholar] [CrossRef]
- Allam, A.K.; Giridharan, N.; Hasen, M.; Banks, G.P.; Reyes, G.; Dang, H.; Kabotyanski, K.E.; Hertz, A.G.; Heilbronner, S.R.; Provenza, N.; et al. Effective deep brain stimulation for obsessive-compulsive disorder after failed anterior capsulotomy: Illustrative cases. J. Neurosurg. Case Lessons 2024, 8, CASE24289. [Google Scholar] [CrossRef]
- Solmi, M.; Seitidis, G.; Mavridis, D.; Correll, C.U.; Dragioti, E.; Guimond, S.; Tuominen, L.; Dargél, A.; Carvalho, A.F.; Fornaro, M.; et al. Incidence, prevalence, and global burden of schizophrenia—Data, with critical appraisal, from the Global Burden of Disease (GBD) 2019. Mol. Psychiatry 2023, 28, 5319–5327. [Google Scholar] [CrossRef]
- Marzouk, T.; Winkelbeiner, S.; Azizi, H.; Malhotra, A.K.; Homan, P. Transcranial Magnetic Stimulation for Positive Symptoms in Schizophrenia: A Systematic Review. Neuropsychobiology 2020, 79, 384–396. [Google Scholar] [CrossRef]
- Brunelin, J.; Mondino, M.; Gassab, L.; Haesebaert, F.; Gaha, L.; Suaud-Chagny, M.-F.; Saoud, M.; Mechri, A.; Poulet, E. Examining Transcranial Direct-Current Stimulation (tDCS) as a Treatment for Hallucinations in Schizophrenia. Am. J. Psychiatry 2012, 169, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Dokucu, M.E. Neuromodulation Treatments for Schizophrenia. Curr. Treat. Options Psychiatry 2015, 2, 339–348. [Google Scholar] [CrossRef]
- Plewnia, C.; Schober, F.; Rilk, A.; Buchkremer, G.; Reimold, M.; Wächter, T.; Breit, S.; Weiss, D.; Krüger, R.; Freudenstein, D. Sustained improvement of obsessive-compulsive disorder by deep brain stimulation in a woman with residual schizophrenia. Int. J. Neuropsychopharmacol. 2008, 11, 1181–1183. [Google Scholar] [CrossRef]
- Sheth, S.A.; Bijanki, K.R.; Metzger, B.; Allawala, A.; Pirtle, V.; Adkinson, J.A.; Myers, J.; Mathura, R.K.; Oswalt, D.; Tsolaki, E.; et al. Deep Brain Stimulation for Depression Informed by Intracranial Recordings. Biol. Psychiatry 2022, 92, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Lipsman, N.; Lam, E.; Volpini, M.; Sutandar, K.; Twose, R.; Giacobbe, P.; Sodums, D.J.; Smith, G.S.; Woodside, D.B.; Lozano, A.M. Deep brain stimulation of the subcallosal cingulate for treatment-refractory anorexia nervosa: 1 year follow-up of an open-label trial. Lancet Psychiatry 2017, 4, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-W.; Li, D.-Y.; Zhao, J.; Guan, Y.-H.; Sun, B.-M.; Zuo, C.-T. Metabolic imaging of deep brain stimulation in anorexia nervosa: A 18F-FDG PET/CT study. Clin. Nucl. Med. 2013, 38, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Lapa, J.D.S.; Duarte, J.F.S.; Campos, A.C.P.; Davidson, B.; Nestor, S.M.; Rabin, J.S.; Giacobbe, P.; Lipsman, N.; Hamani, C. Adverse Effects of Deep Brain Stimulation for Treatment-Resistant Depression: A Scoping Review. Neurosurgery 2024, 95, 509–516. [Google Scholar] [CrossRef]
- Tabaja, H.; Yuen, J.; Tai, D.B.G.; Campioli, C.C.; Chesdachai, S.; DeSimone, D.C.; Hassan, A.; Klassen, B.T.; Miller, K.J.; Lee, K.H.; et al. Deep Brain Stimulator Device Infection: The Mayo Clinic Rochester Experience. Open Forum Infect. Dis. 2023, 10, ofac631. [Google Scholar] [CrossRef] [PubMed]
- Jitkritsadakul, O.; Bhidayasiri, R.; Kalia, S.K.; Hodaie, M.; Lozano, A.M.; Fasano, A. Systematic review of hardware-related complications of Deep Brain Stimulation: Do new indications pose an increased risk? Brain Stimulat. 2017, 10, 967–976. [Google Scholar] [CrossRef]
- Hamani, C.; Lozano, A.M. Hardware-Related Complications of Deep Brain Stimulation: A Review of the Published Literature. Stereotact. Funct. Neurosurg. 2006, 84, 248–251. [Google Scholar] [CrossRef]
- Fernández, F.S.; Alvarez Vega, M.A.; Antuña Ramos, A.; Fernández González, F.; Lozano Aragoneses, B. Lead Fractures in Deep Brain Stimulation during Long-Term Follow-Up. Park. Dis. 2010, 2010, 409356. [Google Scholar] [CrossRef]
- Jiang, C.; Mo, X.; Dong, Y.; Meng, F.; Hao, H.; Zhang, J.; Feng, X.; Li, L. An Experimental Study of Deep Brain Stimulation Lead Fracture: Possible Fatigue Mechanisms and Prevention Approach. Neuromodul. Technol. Neural Interface 2015, 18, 243–248. [Google Scholar] [CrossRef]
- Mackel, C.E.; Papavassiliou, E.; Alterman, R.L. Risk Factors for Wire Fracture or Tethering in Deep Brain Stimulation: A 15-Year Experience. Oper. Neurosurg. Hagerstown Md 2020, 19, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Pajarín, G.; Sesar, A.; Ares, B.; Relova, J.L.; Arán, E.; Gelabert-González, M.; Castro, A. Delayed complications of deep brain stimulation: 16-year experience in 249 patients. Acta Neurochir. 2017, 159, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- Ineichen, C.; Shepherd, N.R.; Sürücü, O. Understanding the Effects and Adverse Reactions of Deep Brain Stimulation: Is It Time for a Paradigm Shift Toward a Focus on Heterogenous Biophysical Tissue Properties Instead of Electrode Design Only? Front. Hum. Neurosci. 2018, 12, 468. [Google Scholar] [CrossRef] [PubMed]
- Morrison, M.A.; Lee, A.T.; Martin, A.J.; Dietiker, C.; Brown, E.G.; Wang, D.D. DBS targeting for essential tremor using intersectional dentato-rubro-thalamic tractography and direct proton density visualization of the VIM: Technical note on 2 cases. J. Neurosurg. 2021, 135, 806–814. [Google Scholar] [CrossRef]
- Rahimpour, S.; Kiyani, M.; Hodges, S.E.; Turner, D.A. Deep brain stimulation and electromagnetic interference. Clin. Neurol. Neurosurg. 2021, 203, 106577. [Google Scholar] [CrossRef]
- Bishay, A.E.; Lyons, A.T.; Koester, S.W.; Paulo, D.L.; Liles, C.; Dambrino, R.J.; Feldman, M.J.; Ball, T.J.; Bick, S.K.; Englot, D.J.; et al. Global Economic Evaluation of the Reported Costs of Deep Brain Stimulation. Stereotact. Funct. Neurosurg. 2024, 102, 257–274. [Google Scholar] [CrossRef]
- Rasiah, N.P.; Maheshwary, R.; Kwon, C.-S.; Bloomstein, J.D.; Girgis, F. Complications of Deep Brain Stimulation for Parkinson Disease and Relationship between Micro-electrode tracks and hemorrhage: Systematic Review and Meta-Analysis. World Neurosurg. 2023, 171, e8–e23. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, J.; Jiang, L.; Feng, Z.; Shi, X.; Hao, Y. The need to be alert to complications of peri-lead cerebral edema caused by deep brain stimulation implantation: A systematic literature review and meta-analysis study. CNS Neurosci. Ther. 2022, 28, 332–342. [Google Scholar] [CrossRef]
- Bullard, A.J.; Hutchison, B.C.; Lee, J.; Chestek, C.A.; Patil, P.G. Estimating Risk for Future Intracranial, Fully Implanted, Modular Neuroprosthetic Systems: A Systematic Review of Hardware Complications in Clinical Deep Brain Stimulation and Experimental Human Intracortical Arrays. Neuromodul. Technol. Neural Interface 2020, 23, 411–426. [Google Scholar] [CrossRef]
- Radziunas, A.; Deltuva, V.P.; Tamasauskas, A.; Gleizniene, R.; Pranckeviciene, A.; Surkiene, D.; Bunevicius, A. Neuropsychiatric complications and neuroimaging characteristics after deep brain stimulation surgery for Parkinson’s disease. Brain Imaging Behav. 2020, 14, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Kühn, A.A.; Volkmann, J. Innovations in deep brain stimulation methodology. Mov. Disord. 2017, 32, 11–19. [Google Scholar] [CrossRef]
- Kehnemouyi, Y.M.; Wilkins, K.B.; Anidi, C.M.; Anderson, R.W.; Afzal, M.F.; Bronte-Stewart, H.M. Modulation of beta bursts in subthalamic sensorimotor circuits predicts improvement in bradykinesia. Brain J. Neurol. 2021, 144, 473–486. [Google Scholar] [CrossRef]
- Gilron, R.; Little, S.; Perrone, R.; Wilt, R.; De Hemptinne, C.; Yaroshinsky, M.S.; Racine, C.A.; Wang, S.S.; Ostrem, J.L.; Larson, P.S.; et al. Long-term wireless streaming of neural recordings for circuit discovery and adaptive stimulation in individuals with Parkinson’s disease. Nat. Biotechnol. 2021, 39, 1078–1085. [Google Scholar] [CrossRef]
- Swann, N.C.; De Hemptinne, C.; Miocinovic, S.; Qasim, S.; Ostrem, J.L.; Galifianakis, N.B.; Luciano, M.S.; Wang, S.S.; Ziman, N.; Taylor, R.; et al. Chronic multisite brain recordings from a totally implantable bidirectional neural interface: Experience in 5 patients with Parkinson’s disease. J. Neurosurg. 2018, 128, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Horn, A.; Reich, M.; Vorwerk, J.; Li, N.; Wenzel, G.; Fang, Q.; Schmitz-Hübsch, T.; Nickl, R.; Kupsch, A.; Volkmann, J.; et al. Connectivity Predicts deep brain stimulation outcome in P arkinson disease. Ann. Neurol. 2017, 82, 67–78. [Google Scholar] [CrossRef]
- Sobesky, L.; Goede, L.; Odekerken, V.J.J.; Wang, Q.; Li, N.; Neudorfer, C.; Rajamani, N.; Al-Fatly, B.; Reich, M.; Volkmann, J.; et al. Subthalamic and pallidal deep brain stimulation: Are we modulating the same network? Brain 2022, 145, 251–262. [Google Scholar] [CrossRef]
- Al-Fatly, B.; Ewert, S.; Kübler, D.; Kroneberg, D.; Horn, A.; Kühn, A.A. Connectivity profile of thalamic deep brain stimulation to effectively treat essential tremor. Brain 2019, 142, 3086–3098. [Google Scholar] [CrossRef]
- Okromelidze, L.; Tsuboi, T.; Eisinger, R.S.; Burns, M.R.; Charbel, M.; Rana, M.; Grewal, S.S.; Lu, C.-Q.; Almeida, L.; Foote, K.D.; et al. Functional and Structural Connectivity Patterns Associated with Clinical Outcomes in Deep Brain Stimulation of the Globus Pallidus Internus for Generalized Dystonia. Am. J. Neuroradiol. 2020, 41, 508–514. [Google Scholar] [CrossRef]
- Milosevic, L.; Kalia, S.K.; Hodaie, M.; Lozano, A.; Popovic, M.R.; Hutchison, W. Subthalamic suppression defines therapeutic threshold of deep brain stimulation in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1105–1108. [Google Scholar] [CrossRef] [PubMed]
- Milosevic, L.; Kalia, S.K.; Hodaie, M.; Lozano, A.M.; Fasano, A.; Popovic, M.R.; Hutchison, W.D. Neuronal inhibition and synaptic plasticity of basal ganglia neurons in Parkinson’s disease. Brain J. Neurol. 2018, 141, 177–190. [Google Scholar] [CrossRef]
- Milosevic, L.; Kalia, S.K.; Hodaie, M.; Lozano, A.M.; Popovic, M.R.; Hutchison, W.D.; Lankarany, M. A theoretical framework for the site-specific and frequency-dependent neuronal effects of deep brain stimulation. Brain Stimulat. 2021, 14, 807–821. [Google Scholar] [CrossRef]
- Milosevic, L.; Kalia, S.K.; Hodaie, M.; Lozano, A.M.; Popovic, M.R.; Hutchison, W.D. Physiological mechanisms of thalamic ventral intermediate nucleus stimulation for tremor suppression. Brain J. Neurol. 2018, 141, 2142–2155. [Google Scholar] [CrossRef]
- Bower, K.L.; McIntyre, C.C. Deep brain stimulation of terminating axons. Brain Stimulat. 2020, 13, 1863–1870. [Google Scholar] [CrossRef]
- McIntyre, C.C.; Grill, W.M.; Sherman, D.L.; Thakor, N.V. Cellular effects of deep brain stimulation: Model-based analysis of activation and inhibition. J. Neurophysiol. 2004, 91, 1457–1469. [Google Scholar] [CrossRef]
- Fahle, S.; Prinz, C.; Kuhlenkötter, B. Systematic review on machine learning (ML) methods for manufacturing processes—Identifying artificial intelligence (AI) methods for field application. Procedia CIRP 2020, 93, 413–418. [Google Scholar] [CrossRef]
- Lavallin, A.; Downs, J.A. Machine learning in geography–Past, present, and future. Geogr. Compass 2021, 15, e12563. [Google Scholar] [CrossRef]
- Awad, M.; Khanna, R. Efficient Learning Machines: Theories, Concepts, and Applications for Engineers and System Designers; Springer Nature: Berlin, Germany, 2015; ISBN 978-1-4302-5989-3. [Google Scholar]
- Makridakis, S.; Spiliotis, E.; Assimakopoulos, V. Statistical and Machine Learning forecasting methods: Concerns and ways forward. PLoS ONE 2018, 13, e0194889. [Google Scholar] [CrossRef] [PubMed]
- Celtikci, E. A Systematic Review on Machine Learning in Neurosurgery: The Future of Decision-Making in Patient Care. Turk. Neurosurg. 2018, 28, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Buchlak, Q.D.; Esmaili, N.; Leveque, J.-C.; Farrokhi, F.; Bennett, C.; Piccardi, M.; Sethi, R.K. Machine learning applications to clinical decision support in neurosurgery: An artificial intelligence augmented systematic review. Neurosurg. Rev. 2020, 43, 1235–1253. [Google Scholar] [CrossRef]
- Senders, J.T.; Arnaout, O.; Karhade, A.V.; Dasenbrock, H.H.; Gormley, W.B.; Broekman, M.L.; Smith, T.R. Natural and Artificial Intelligence in Neurosurgery: A Systematic Review. Neurosurgery 2018, 83, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Senders, J.T.; Zaki, M.M.; Karhade, A.V.; Chang, B.; Gormley, W.B.; Broekman, M.L.; Smith, T.R.; Arnaout, O. An introduction and overview of machine learning in neurosurgical care. Acta Neurochir. 2018, 160, 29–38. [Google Scholar] [CrossRef]
- Stanslaski, S.; Summers, R.L.S.; Tonder, L.; Tan, Y.; Case, M.; Raike, R.S.; Morelli, N.; Herrington, T.M.; Beudel, M.; Ostrem, J.L.; et al. Sensing data and methodology from the Adaptive DBS Algorithm for Personalized Therapy in Parkinson’s Disease (ADAPT-PD) clinical trial. Npj Park. Dis. 2024, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Allen, B. Discovering Themes in Deep Brain Stimulation Research Using Explainable Artificial Intelligence. Biomedicines 2023, 11, 771. [Google Scholar] [CrossRef]
- Jovanov, I.; Nauman, M.; Kumaravelu, K.; Lesi, V.; Zutshi, A.; Grill, W.M.; Pajic, M. Learning-Based Control Design for Deep Brain Stimulation. In Proceedings of the 2018 ACM/IEEE 9th International Conference on Cyber-Physical Systems (ICCPS), Porto, Portugal, 11–13 April 2018; IEEE: Porto, Portugal, 2018; pp. 349–350. [Google Scholar]
- Zhu, B.; Farivar, M.; Shoaran, M. ResOT: Resource-Efficient Oblique Trees for Neural Signal Classification. IEEE Trans. Biomed. Circuits Syst. 2020, 14, 692–704. [Google Scholar] [CrossRef] [PubMed]
- De La Pava, I.; Mejía, J.; Álvarez-Meza, A.; Álvarez, M.; Orozco, A.; Henao, O. A Hierarchical K-Nearest Neighbor Approach for Volume of Tissue Activated Estimation. In Progress in Pattern Recognition, Image Analysis, Computer Vision, and Applications; Beltrán-Castañón, C., Nyström, I., Famili, F., Eds.; Lecture Notes in Computer Science; Springer International Publishing: Cham, Germany, 2017; Volume 10125, pp. 125–133. [Google Scholar]
- Bermudez, C.; Dawant, B.M.; Landman, B.A.; Hainline, A.E.; Huo, Y.; Rodriguez, W.J.; Li, R.; Schults, R.; D’Haese, P.D.; Konrad, P.E. Towards machine learning prediction of deep brain stimulation (DBS) intra-operative efficacy maps. In Medical Imaging 2019: Image Processing; Angelini, E.D., Landman, B.A., Eds.; SPIE: San Diego, CA, USA, 2019; p. 72. [Google Scholar]
- Park, S.-C.; Cha, J.H.; Lee, S.; Jang, W.; Lee, C.S.; Lee, J.K. Deep Learning-Based Deep Brain Stimulation Targeting and Clinical Applications. Front. Neurosci. 2019, 13, 1128. [Google Scholar] [CrossRef]
- Ligaard, J.; Sannæs, J.; Pihlstrøm, L. Deep brain stimulation and genetic variability in Parkinson’s disease: A review of the literature. Npj Park. Dis. 2019, 5, 18. [Google Scholar] [CrossRef]
- De Oliveira, L.M.; Barbosa, E.R.; Aquino, C.C.; Munhoz, R.P.; Fasano, A.; Cury, R.G. Deep Brain Stimulation in Patients with Mutations in Parkinson’s Disease–Related Genes: A Systematic Review. Mov. Disord. Clin. Pract. 2019, 6, 359–368. [Google Scholar] [CrossRef]
- Ferrea, E.; Negahbani, F.; Cebi, I.; Weiss, D.; Gharabaghi, A. Machine learning explains response variability of deep brain stimulation on Parkinson’s disease quality of life. Npj Digit. Med. 2024, 7, 269. [Google Scholar] [CrossRef]
- Halász, L.; Sajonz, B.E.A.; Miklós, G.; Van Elswijk, G.; Hagh Gooie, S.; Várkuti, B.; Tamás, G.; Coenen, V.A.; Erōss, L. Predictive modeling of sensory responses in deep brain stimulation. Front. Neurol. 2024, 15, 1467307. [Google Scholar] [CrossRef]
| Criteria | Requirement |
|---|---|
| Diagnosis | Confirmed Idiopathic Parkinson’s Disease (IPD) with at least 5 years of disease duration. |
| Dopaminergic Responsiveness | A pharmacologic test with L-dopa or apomorphine should show at least a 33% decrease in the Unified Parkinson’s Disease Rating Scale (UPDRS) Part III score. |
| Motor Symptoms | Severe motor fluctuations, dyskinesias, or medication-resistant tremors significantly impair quality of life. |
| Cognitive and Behavioral Status | No severe cognitive impairment or dementia (Mattis Dementia Rating Scale cut-off: 130 or 120). |
| Psychiatric Exclusion Criteria | No severe psychiatric disorders, particularly major depression (Montgomery-Asberg Depression Rating Scale (MADRS): 7–19 cut-off) or active psychosis. |
| Medication Stability | Stable antiparkinsonian medication for at least 3 months preoperatively. |
| Age Consideration | No strict age limit, but younger patients tend to respond better; caution in elderly patients with cognitive risk. |
| Imaging Exclusion | No structural brain abnormalities suggestive of atypical parkinsonism (MRI recommended). |
| Functional Impairment | Significant functional disability despite optimal medical therapy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costin, H.P.; Brehar, F.-M.; Corlatescu, A.-D.; Pruna, V.M. Deep Brain Stimulation: Mechanisms, Cost-Effectiveness, and Precision Applications Across Neurology and Psychiatry. Biomedicines 2025, 13, 2691. https://doi.org/10.3390/biomedicines13112691
Costin HP, Brehar F-M, Corlatescu A-D, Pruna VM. Deep Brain Stimulation: Mechanisms, Cost-Effectiveness, and Precision Applications Across Neurology and Psychiatry. Biomedicines. 2025; 13(11):2691. https://doi.org/10.3390/biomedicines13112691
Chicago/Turabian StyleCostin, Horia Petre, Felix-Mircea Brehar, Antonio-Daniel Corlatescu, and Viorel Mihai Pruna. 2025. "Deep Brain Stimulation: Mechanisms, Cost-Effectiveness, and Precision Applications Across Neurology and Psychiatry" Biomedicines 13, no. 11: 2691. https://doi.org/10.3390/biomedicines13112691
APA StyleCostin, H. P., Brehar, F.-M., Corlatescu, A.-D., & Pruna, V. M. (2025). Deep Brain Stimulation: Mechanisms, Cost-Effectiveness, and Precision Applications Across Neurology and Psychiatry. Biomedicines, 13(11), 2691. https://doi.org/10.3390/biomedicines13112691






