Mulberrin Alleviates Renal Ischemia–Reperfusion by Inhibiting Ferroptosis and Oxidative Stress Through Sirt3 Activation
Abstract
1. Introduction
2. Methods
2.1. Experimental Animals
2.2. Cell Culture and Transfection
2.3. Renal Function Tests
2.4. Immunohistochemistry (IHC)
2.5. Hematoxylin and Eosin (H&E) Staining
2.6. TdT-Mediated dUTP Nick-End Labeling (TUNEL)
2.7. ROS Assay
2.8. Western Blot
2.9. Mitochondrial Membrane Potential (MMP) Assay
2.10. Malondialdehyde (MDA), Glutathione (GSH) ASSAY, Fe2+ Levels
2.11. Immunofluorescence Staining
2.12. Statistical Analysis
3. Results
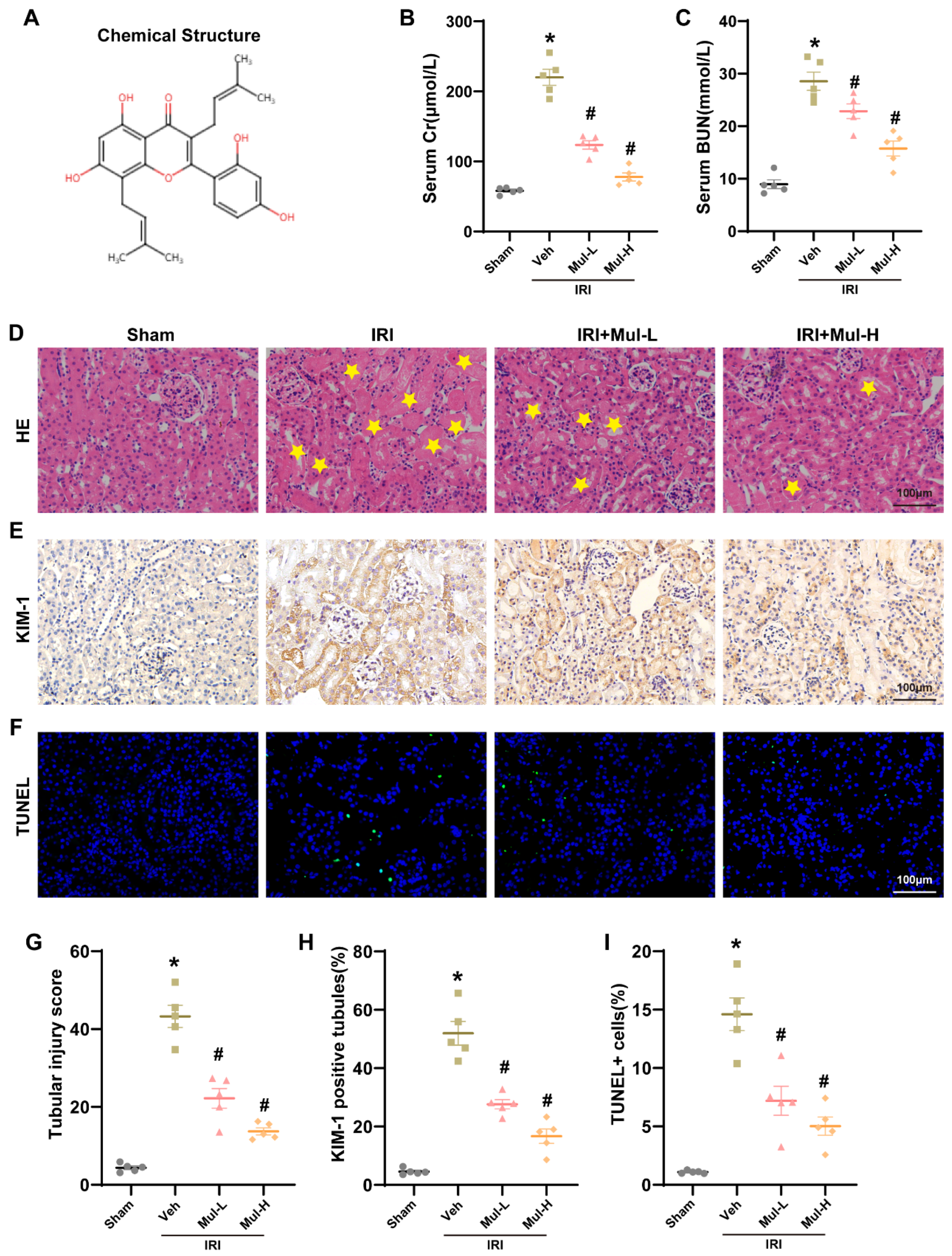
3.1. Mul Played a Protective Role in Renal IRI in Mice
3.2. Mul Alleviated Oxidative Stress and Ferroptosis Induced by Renal IRI
3.3. Mul Attenuated Oxidative Stress to Protect HK-2 Cells from H/R Injury
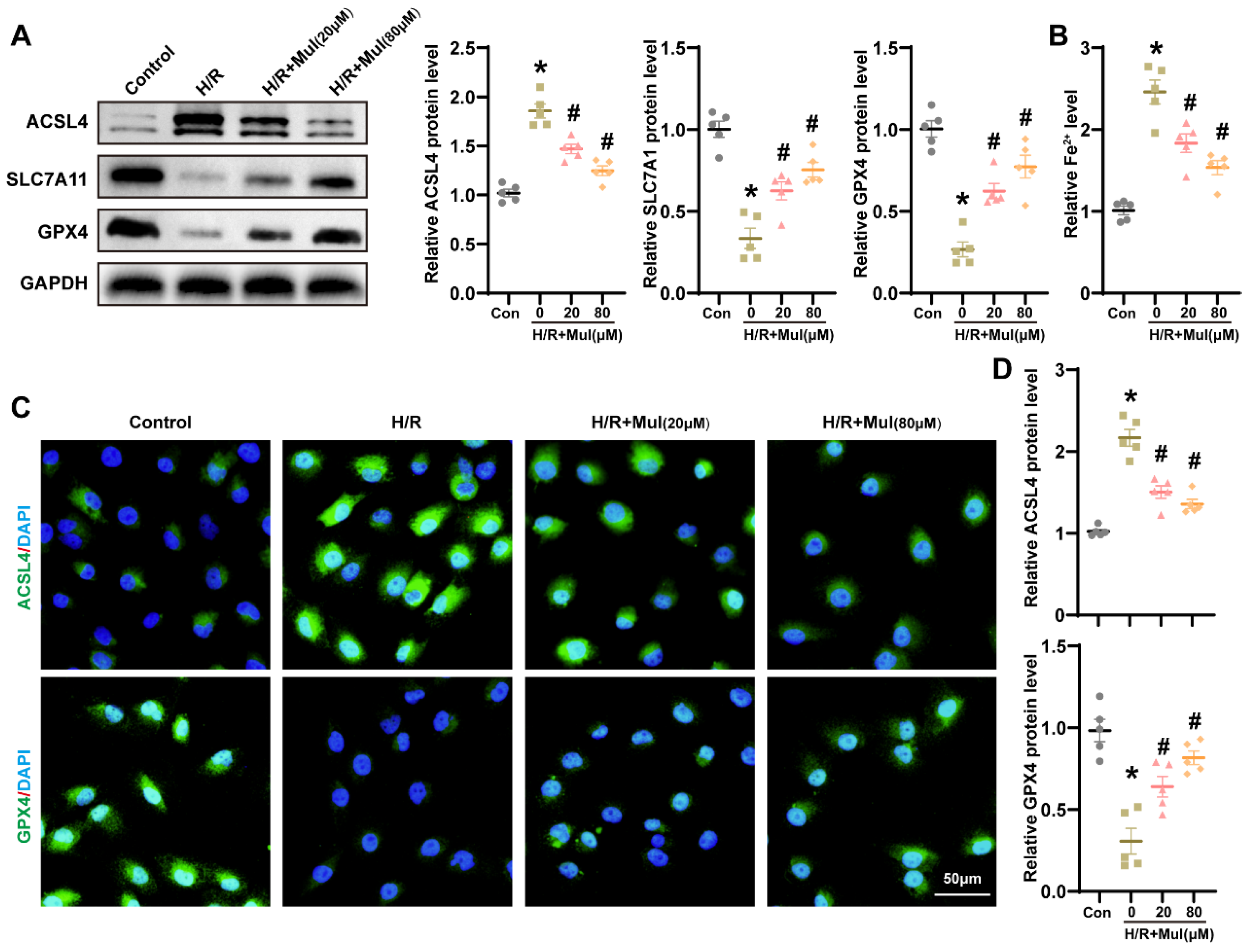
3.4. Mul Suppressed H/R-Induced Ferroptosis in HK-2 Cells
3.5. Mul Enhances Sirt3 Expression Following IRI and H/R
3.6. The Reduction of SIRT3 Hindered Mul’s Ability to Combat Oxidative Stress Triggered by H/R
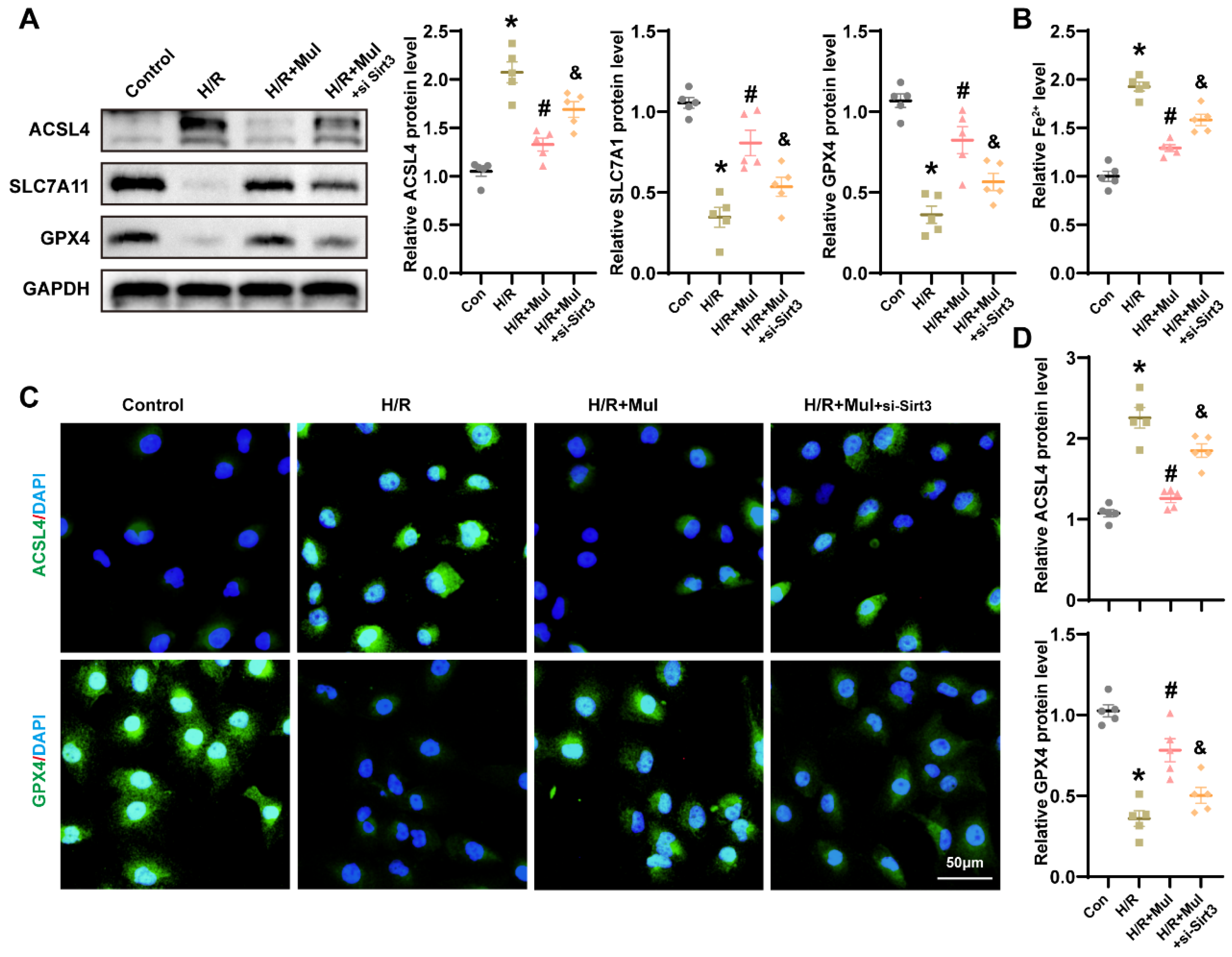
3.7. Loss of Sirt3 Blocked Mul’s Anti-Ferroptotic Action During H/R Challenge
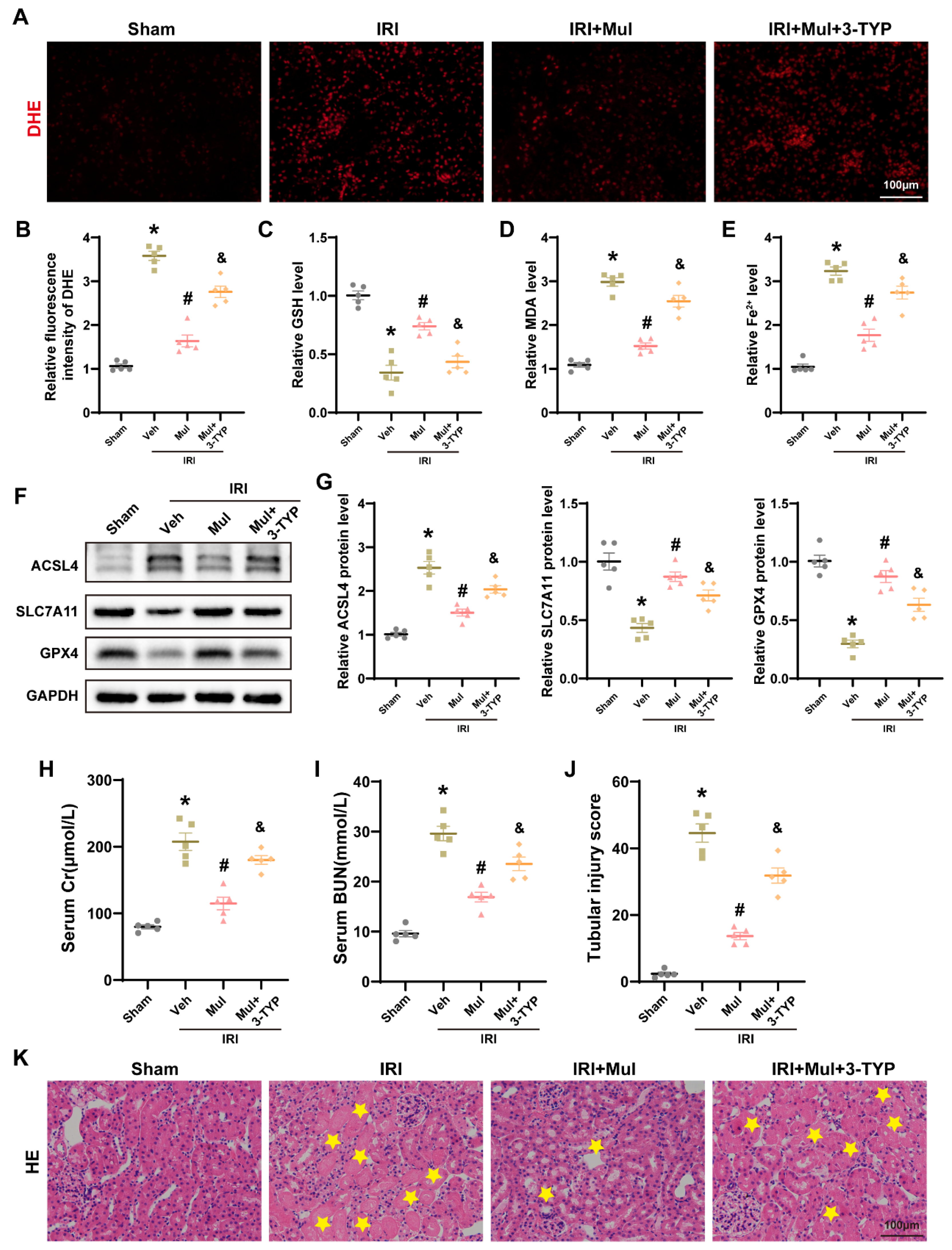
3.8. Sirt3 Depletion Negated Mul’s Protection from IRI-Driven Oxidative Injury and Ferroptosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ostermann, M.; Lumlertgul, N.; Jeong, R.; See, E.; Joannidis, M.; James, M. Acute kidney injury. Lancet 2025, 405, 241–256. [Google Scholar] [CrossRef]
- Matsuura, R.; Doi, K.; Rabb, H. Acute kidney injury and distant organ dysfunction-network system analysis. Kidney Int. 2023, 103, 1041–1055. [Google Scholar] [CrossRef]
- Funk, J.A.; Schnellmann, R.G. Persistent disruption of mitochondrial homeostasis after acute kidney injury. Am. J. Physiol. Renal Physiol. 2012, 302, F853–F864. [Google Scholar] [CrossRef]
- Bhargava, P.; Schnellmann, R.G. Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 2017, 13, 629–646. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Jin, F.; Liu, D.; Shu, G.; Wang, X.; Qi, J.; Sun, M.; Yang, P.; Jiang, S.; Ying, X.; et al. ROS-responsive nano-drug delivery system combining mitochondria-targeting ceria nanoparticles with atorvastatin for acute kidney injury. Theranostics 2020, 10, 2342–2357. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Han, S.; Guo, J.; Qiu, T.; Zhou, J.; Shen, L. Ferroptosis-A New Dawn in the Treatment of Organ Ischemia-Reperfusion Injury. Cells 2022, 11, 3653. [Google Scholar] [CrossRef]
- Shi, L.; Song, Z.; Li, Y.; Huang, J.; Zhao, F.; Luo, Y.; Wang, J.; Deng, F.; Shadekejiang, H.; Zhang, M.; et al. MiR-20a-5p alleviates kidney ischemia/reperfusion injury by targeting ACSL4-dependent ferroptosis. Am. J. Transplant. 2023, 23, 11–25. [Google Scholar] [CrossRef]
- Deng, Y.; Zeng, L.; Liu, H.; Zuo, A.; Zhou, J.; Yang, Y.; You, Y.; Zhou, X.; Peng, B.; Lu, H.; et al. Silibinin attenuates ferroptosis in acute kidney injury by targeting FTH1. Redox Biol. 2024, 77, 103360. [Google Scholar] [CrossRef]
- Hosohata, K.; Harnsirikarn, T.; Chokesuwattanaskul, S. Ferroptosis: A Potential Therapeutic Target in Acute Kidney Injury. Int. J. Mol. Sci. 2022, 23, 6583. [Google Scholar] [CrossRef]
- Shi, Z.; Du, Y.; Zheng, J.; Tang, W.; Liang, Q.; Zheng, Z.; Liu, B.; Sun, H.; Wang, K.; Shao, C. Liproxstatin-1 Alleviated Ischemia/Reperfusion-Induced Acute Kidney Injury via Inhibiting Ferroptosis. Antioxidants 2024, 13, 182. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, D.; Fedorova, J.; He, Z.; Li, J. SIRT1/SIRT3 Modulates Redox Homeostasis during Ischemia/Reperfusion in the Aging Heart. Antioxidants 2020, 9, 858. [Google Scholar] [CrossRef] [PubMed]
- Klimova, N.; Fearnow, A.; Long, A.; Kristian, T. NAD(+) precursor modulates post-ischemic mitochondrial fragmentation and reactive oxygen species generation via SIRT3 dependent mechanisms. Exp. Neurol. 2020, 325, 113144. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Xiong, R.; Li, G.; Wang, B.; Geng, Q. PM2.5 contributed to pulmonary epithelial senescence and ferroptosis by regulating USP3-SIRT3-P53 axis. Free Radic. Biol. Med. 2023, 205, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Hu, P.; Wang, X.; Ding, X.; Wang, Q.; Luo, L.; Zhang, Y.; Li, M.; Zhao, Y.; Li, S.; et al. Sirtuin-3 activation by honokiol attenuated anesthesia/surgery-induced cognitive impairment and neuronal ferroptosis via inhibiting mitochondrial GPX4 acetylation. J. Nanobiotechnol. 2025, 23, 414. [Google Scholar] [CrossRef]
- Ma, D.; Fang, W.; Cai, L.; Li, W.; Su, H. Norepinephrine exacerbates LPS-induced cardiomyopathy via SIRT3/HO-1 axis-mediated ferroptosis. Crit. Care 2025, 29, 354. [Google Scholar] [CrossRef]
- Xia, K.; Jin, Z.; Qiu, Q.; Zhou, Y.; Lu, Y.; Qiu, T.; Zhou, J.; Chen, Z. Ligustilide alleviates oxidative stress during renal ischemia-reperfusion injury through maintaining Sirt3-dependent mitochondrial homeostasis. Phytomedicine 2024, 134, 155975. [Google Scholar] [CrossRef]
- Liu, D.; Tang, S.; Gan, L.; Cui, W. Renal-Protective Effects and Potential Mechanisms of Traditional Chinese Medicine after Ischemia-Reperfusion Injury. Evid. Based Complement. Altern. Med. 2021, 2021, 5579327. [Google Scholar] [CrossRef]
- Li, Y.; Dong, M.; Qin, H.; An, G.; Cen, L.; Deng, L.; Cui, H. Mulberrin suppresses gastric cancer progression and enhances chemosensitivity to oxaliplatin through HSP90AA1/PI3K/AKT axis. Phytomedicine 2025, 139, 156441. [Google Scholar] [CrossRef]
- Morante-Carriel, J.; Živković, S.; Nájera, H.; Sellés-Marchart, S.; Martínez-Márquez, A.; Martínez-Esteso, M.J.; Obrebska, A.; Samper-Herrero, A.; Bru-Martínez, R. Prenylated Flavonoids of the Moraceae Family: A Comprehensive Review of Their Biological Activities. Plants 2024, 13, 1211. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kwon, E.B.; Kim, B.; Chung, H.S.; Choi, G.; Kim, Y.H.; Choi, J.G. Mulberry Component Kuwanon C Exerts Potent Therapeutic Efficacy In Vitro against COVID-19 by Blocking the SARS-CoV-2 Spike S1 RBD:ACE2 Receptor Interaction. Int. J. Mol. Sci. 2022, 23, 12516. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Gao, X.; Duan, L.; Zhang, W.; Sun, Y.F. Mulberrin (Mul) reduces spinal cord injury (SCI)-induced apoptosis, inflammation and oxidative stress in rats via miroRNA-337 by targeting Nrf-2. Biomed. Pharmacother. 2018, 107, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Tan, J.; Lou, D.; Zhu, L.; Zhong, Z.; Dai, X.; Sun, Y.; Kuang, Q.; Zhao, J.; Wang, L.; et al. Mulberrin confers protection against hepatic fibrosis by Trim31/Nrf2 signaling. Redox Biol. 2022, 51, 102274. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, L.; Weng, X.; Chen, H.; Du, Y.; Diao, C.; Chen, Z.; Liu, X. Inhibition of Brd4 alleviates renal ischemia/reperfusion injury-induced apoptosis and endoplasmic reticulum stress by blocking FoxO4-mediated oxidative stress. Redox Biol. 2019, 24, 101195. [Google Scholar] [CrossRef]
- Han, S.; Guo, J.; Kong, C.; Li, J.; Lin, F.; Zhu, J.; Wang, T.; Chen, Q.; Liu, Y.; Hu, H.; et al. ANKRD1 aggravates renal ischaemia-reperfusion injury via promoting TRIM25-mediated ubiquitination of ACSL3. Clin. Transl. Med. 2024, 14, e70024. [Google Scholar] [CrossRef]
- Xia, K.; Guo, J.; Yu, B.; Wang, T.; Qiu, Q.; Chen, Q.; Qiu, T.; Zhou, J.; Zheng, S. Sentrin-specific protease 1 maintains mitochondrial homeostasis through targeting the deSUMOylation of sirtuin-3 to alleviate oxidative damage induced by hepatic ischemia/reperfusion. Free Radic. Biol. Med. 2024, 210, 378–389. [Google Scholar] [CrossRef]
- Su, Z.; Li, P.; Ding, W.; Gao, Y. Urolithin A improves myocardial ischemia-reperfusion injury by attenuating oxidative stress and ferroptosis through Nrf2 pathway. Int. Immunopharmacol. 2024, 143 Pt 2, 113394. [Google Scholar] [CrossRef]
- Xu, C.; Wang, H.; Wang, H.; Man, J.; Deng, Y.; Li, Y.; Cheng, K.; Niu, J.; Gui, H.; Fu, S.; et al. Schisandrin B regulates mitochondrial dynamics via AKT1 activation and mitochondrial targeting to ameliorate renal ischemia-reperfusion injury. Phytomedicine 2025, 141, 156672. [Google Scholar] [CrossRef]
- Cao, W.; Dong, Y.; Zhao, W.; Lu, X.; Sun, L. Mulberrin attenuates 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine (MPTP)-induced Parkinson’s disease by promoting Wnt/β-catenin signaling pathway. J. Chem. Neuroanat. 2019, 98, 63–70. [Google Scholar] [CrossRef]
- Ma, Y.; Fei, S.; Chen, X.; Gui, Y.; Zhou, B.; Xiang, T.; Liu, J.; Yue, K.; Li, Q.; Jiang, W.; et al. Chemerin attenuates acute kidney injury by inhibiting ferroptosis via the AMPK/NRF2/SLC7A11 axis. Commun. Biol. 2024, 7, 1679. [Google Scholar] [CrossRef]
- Gao, G.; Xia, H.; Shi, J.; Zheng, P.; Wu, W.; Wu, S.; Qi, T.; Song, H.; Gu, Y.; Li, J.; et al. Carbon Dot Nanozymes with Ferrous Ion-Chelating and Antioxidative Activity Inhibiting Ferroptosis to Alleviate Renal Ischemia-Reperfusion Injury. Small 2025, 21, e2407372. [Google Scholar] [CrossRef]
- Tao, W.H.; Shan, X.S.; Zhang, J.X.; Liu, H.Y.; Wang, B.Y.; Wei, X.; Zhang, M.; Peng, K.; Ding, J.; Xu, S.X.; et al. Dexmedetomidine Attenuates Ferroptosis-Mediated Renal Ischemia/Reperfusion Injury and Inflammation by Inhibiting ACSL4 via α2-AR. Front. Pharmacol. 2022, 13, 782466. [Google Scholar] [CrossRef]
- Zhao, W.; Nikolic-Paterson, D.J.; Li, K.; Li, Y.; Wang, Y.; Chen, X.; Duan, Z.; Zhang, Y.; Liu, P.; Lu, S.; et al. Selenium binding protein 1 protects renal tubular epithelial cells from ferroptosis by upregulating glutathione peroxidase 4. Chem. Biol. Interact. 2024, 393, 110944. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Hue, M.; García-Caballero, C.; Palomino-Antolín, A.; Rubio-Navarro, A.; Vázquez-Carballo, C.; Herencia, C.; Martín-Sanchez, D.; Farré-Alins, V.; Egea, J.; Cannata, P.; et al. Curcumin reduces renal damage associated with rhabdomyolysis by decreasing ferroptosis-mediated cell death. FASEB J. 2019, 33, 8961–8975. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Quan, F.; Cao, Q.; Lin, Y.; Yue, C.; Bi, R.; Cui, X.; Yang, H.; Yang, Y.; Birnbaumer, L.; et al. Quercetin alleviates acute kidney injury by inhibiting ferroptosis. J. Adv. Res. 2021, 28, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.W.; Wang, Q.L.; Luo, M.; Zhu, M.D.; Liang, H.M.; Li, W.J.; Cai, H.; Zhou, Z.B.; Wang, H.; Tong, S.Q.; et al. Phytochemistry and pharmacology of natural prenylated flavonoids. Arch. Pharm. Res. 2023, 46, 207–272. [Google Scholar] [CrossRef]
- Deng, Z.; He, M.; Hu, H.; Zhang, W.; Zhang, Y.; Ge, Y.; Ma, T.; Wu, J.; Li, L.; Sun, M.; et al. Melatonin attenuates sepsis-induced acute kidney injury by promoting mitophagy through SIRT3-mediated TFAM deacetylation. Autophagy 2024, 20, 151–165. [Google Scholar] [CrossRef]
- Yuan, Y.; Yuan, L.; Yang, J.; Liu, F.; Liu, S.; Li, L.; Liao, G.; Tang, X.; Cheng, J.; Liu, J.; et al. Autophagy-deficient macrophages exacerbate cisplatin-induced mitochondrial dysfunction and kidney injury via miR-195a-5p-SIRT3 axis. Nat. Commun. 2024, 15, 4383. [Google Scholar] [CrossRef]
- Li, J.; Jia, Y.C.; Ding, Y.X.; Bai, J.; Cao, F.; Li, F. The crosstalk between ferroptosis and mitochondrial dynamic regulatory networks. Int. J. Biol. Sci. 2023, 19, 2756–2771. [Google Scholar] [CrossRef]
- She, H.; Tan, L.; Du, Y.; Zhou, Y.; Guo, N.; Zhang, J.; Du, Y.; Wang, Y.; Wu, Z.; Ma, C.; et al. VDAC2 malonylation participates in sepsis-induced myocardial dysfunction via mitochondrial-related ferroptosis. Int. J. Biol. Sci. 2023, 19, 3143–3158. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, X.; Wang, J.; Shi, Y.; Hu, Q.; Cui, W.; Bai, H.; Zhou, J.; Du, Y.; Han, L.; et al. Adiponectin/AdiopR1 signaling prevents mitochondrial dysfunction and oxidative injury after traumatic brain injury in a SIRT3 dependent manner. Redox Biol. 2022, 54, 102390. [Google Scholar] [CrossRef]
- Liu, X.; Li, D.; Pi, W.; Wang, B.; Xu, S.; Yu, L.; Yao, L.; Sun, Z.; Jiang, J.; Mi, Y. LCZ696 protects against doxorubicin-induced cardiotoxicity by inhibiting ferroptosis via AKT/SIRT3/SOD2 signaling pathway activation. Int. Immunopharmacol. 2022, 113 Pt A, 109379. [Google Scholar] [CrossRef]
- Li, Q.; Liao, J.; Chen, W.; Zhang, K.; Li, H.; Ma, F.; Zhang, H.; Han, Q.; Guo, J.; Li, Y.; et al. NAC alleviative ferroptosis in diabetic nephropathy via maintaining mitochondrial redox homeostasis through activating SIRT3-SOD2/Gpx4 pathway. Free Radic. Biol. Med. 2022, 187, 158–170. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, Z.; Wang, H.; Jiang, M.; Lu, H.; Wei, Y.; Hu, Y.; Mo, L.; Liu, Y.; Zhou, C.; et al. Isovitexin targets SIRT3 to prevent steroid-induced osteonecrosis of the femoral head by modulating mitophagy-mediated ferroptosis. Bone Res. 2025, 13, 18. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Q.; Chen, Z.; Yang, W.; Zhou, Y.; Jiang, N.; Jiang, J.; He, D.; Lu, Y.; Yu, B.; Qiu, T.; et al. Mulberrin Alleviates Renal Ischemia–Reperfusion by Inhibiting Ferroptosis and Oxidative Stress Through Sirt3 Activation. Biomedicines 2025, 13, 2687. https://doi.org/10.3390/biomedicines13112687
Qiu Q, Chen Z, Yang W, Zhou Y, Jiang N, Jiang J, He D, Lu Y, Yu B, Qiu T, et al. Mulberrin Alleviates Renal Ischemia–Reperfusion by Inhibiting Ferroptosis and Oxidative Stress Through Sirt3 Activation. Biomedicines. 2025; 13(11):2687. https://doi.org/10.3390/biomedicines13112687
Chicago/Turabian StyleQiu, Qiangmin, Zhan Chen, Wenbin Yang, Yujie Zhou, Nan Jiang, Jiahao Jiang, Dalin He, Yifan Lu, Bo Yu, Tao Qiu, and et al. 2025. "Mulberrin Alleviates Renal Ischemia–Reperfusion by Inhibiting Ferroptosis and Oxidative Stress Through Sirt3 Activation" Biomedicines 13, no. 11: 2687. https://doi.org/10.3390/biomedicines13112687
APA StyleQiu, Q., Chen, Z., Yang, W., Zhou, Y., Jiang, N., Jiang, J., He, D., Lu, Y., Yu, B., Qiu, T., & Zhou, J. (2025). Mulberrin Alleviates Renal Ischemia–Reperfusion by Inhibiting Ferroptosis and Oxidative Stress Through Sirt3 Activation. Biomedicines, 13(11), 2687. https://doi.org/10.3390/biomedicines13112687






