Lung Transplantation in Idiopathic Pulmonary Fibrosis Patients in the European MultiPartner IPF Registry: Challenges for Health Equity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population and Collected Data
2.3. Subgroups of Eligible Patients with and Without LuTX
2.4. Assessment of LuTX Referral and Listing Practices Using a Questionnaire
2.5. Ethical Statement
2.6. Statistical Analysis
3. Results
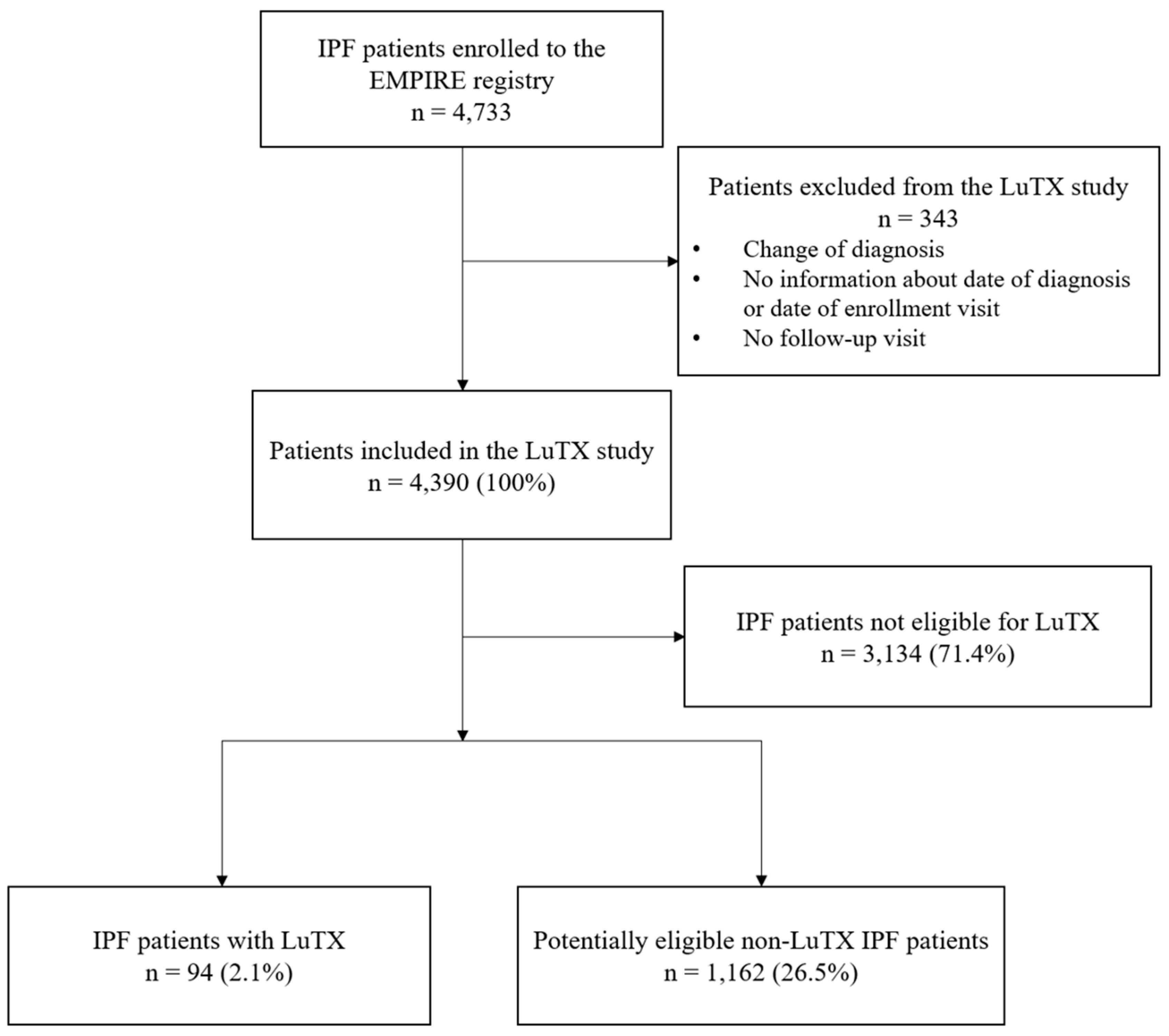
3.1. Lung Transplantation Rate and Exclusion Factors
3.2. Comparison of Clinical Characteristics Between Eligible Patients with and Without LuTX
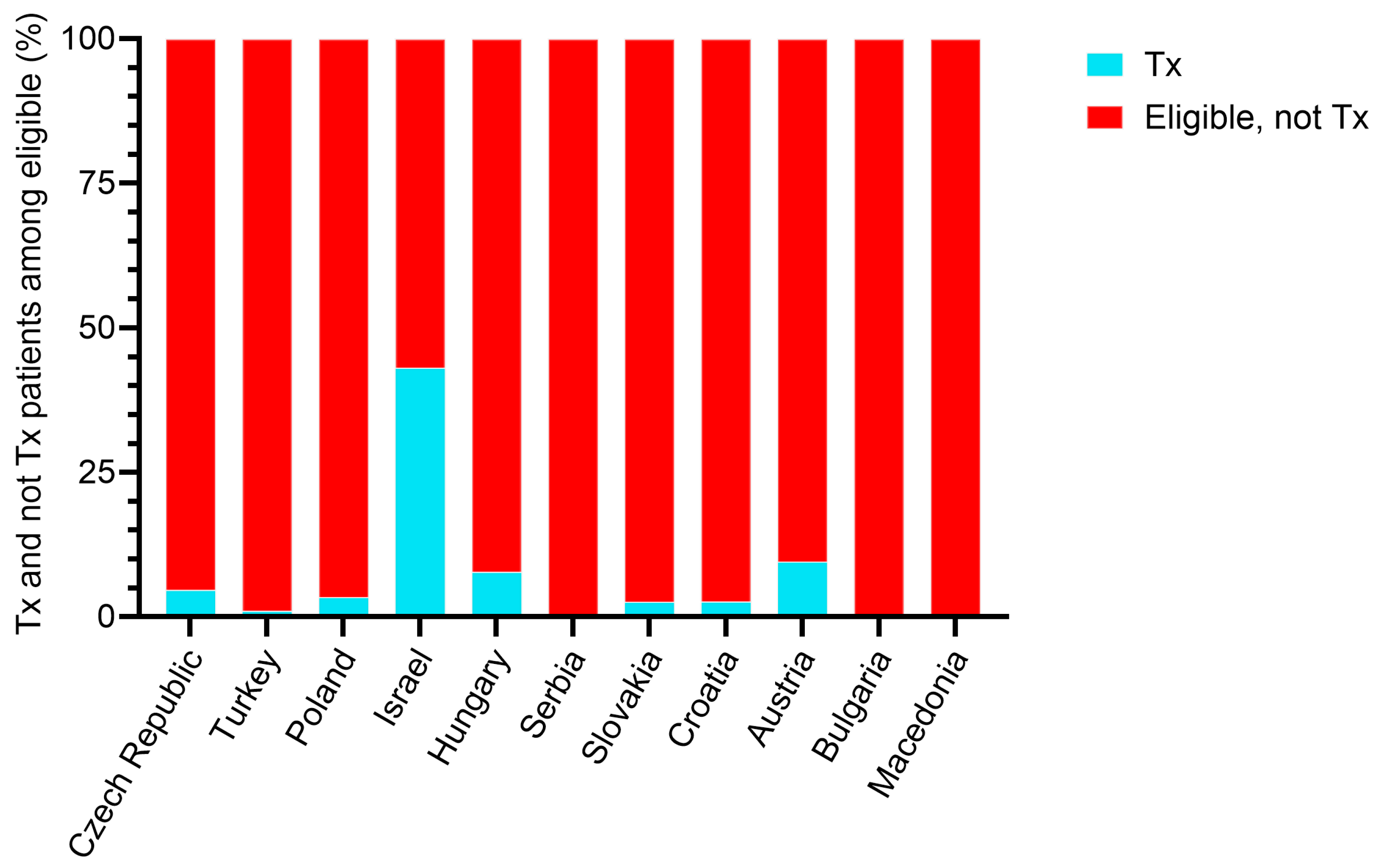
3.3. Patient Characteristics by Country
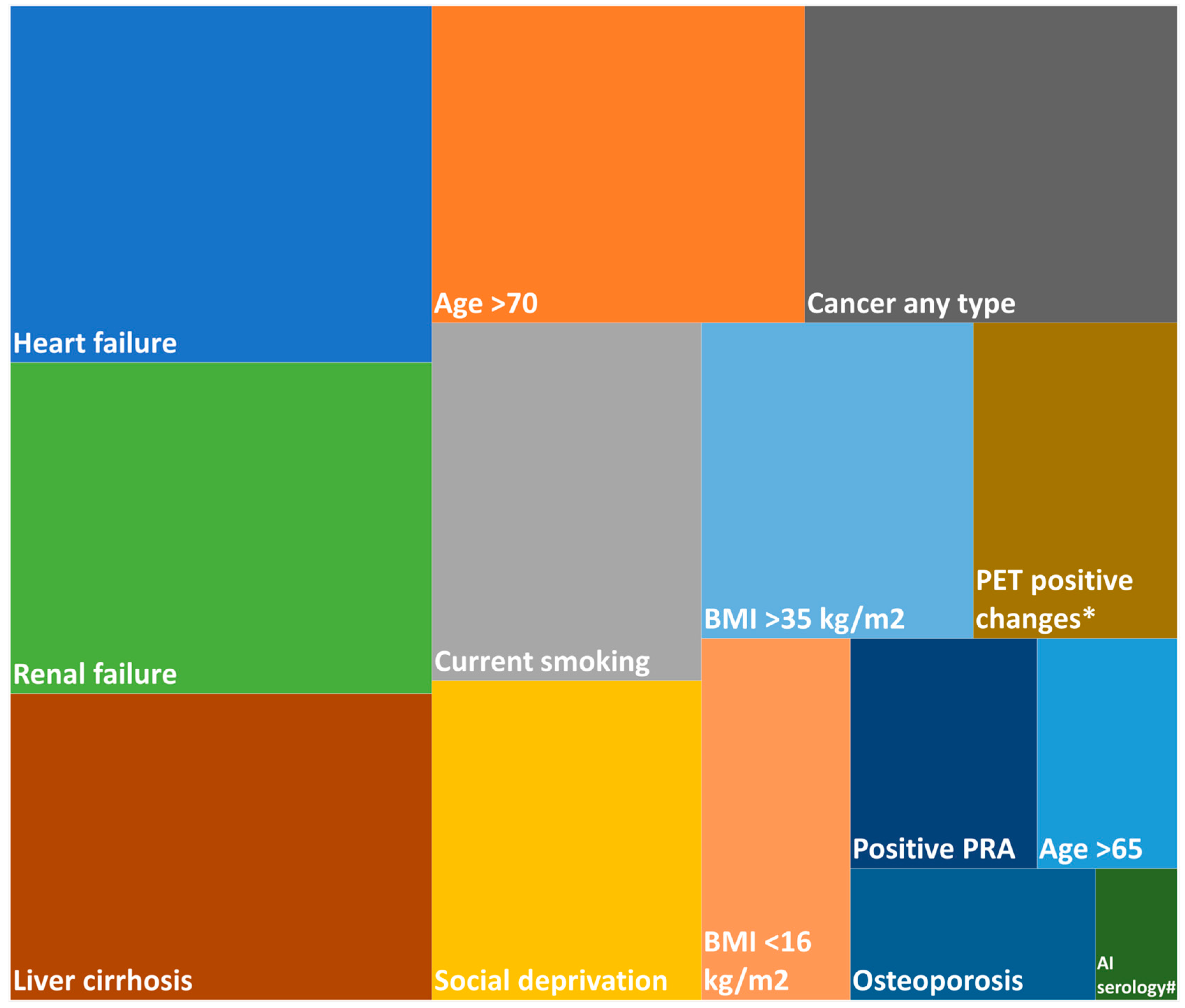
3.4. Clinical Factors Influencing Referral for LuTX by Experts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| CEE | Central and Eastern European |
| EMPIRE | European MultiPartner IPF Registry |
| FVC | forced vital capacity |
| GAP | Gender–Age–Physiology index |
| IPF | idiopathic pulmonary fibrosis |
| ISHLT | International Society of Heart and Lung Transplantation |
| LuTX | lung transplantation |
| TLCO | transfer factor of the lung for carbon monoxide |
| UIP | usual interstitial pneumonia |
References
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47. [Google Scholar] [CrossRef]
- Raghu, G.; Collard, H.R.; Egan, J.J.; Martinez, F.J.; Behr, J.; Brown, K.K.; Colby, T.V.; Cordier, J.F.; Flaherty, K.R.; Lasky, J.A.; et al. An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary fibrosis: Evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 2011, 183, 788–824. [Google Scholar] [CrossRef]
- Glass, D.S.; Grossfeld, D.; Renna, H.A.; Agarwala, P.; Spiegler, P.; Deleon, J.; Reiss, A.B. Idiopathic pulmonary fibrosis: Current and future treatment. Clin. Respir. J. 2022, 16, 84–96. [Google Scholar] [CrossRef]
- Leard, L.E.; Holm, A.M.; Valapour, M.; Glanville, A.R.; Attawar, S.; Aversa, M.; Campos, S.V.; Christon, L.M.; Cypel, M.; Dellgren, G.; et al. Consensus document for the selection of lung transplant candidates: An update from the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2021, 40, 1349–1379. [Google Scholar] [CrossRef]
- Kolonics-Farkas, A.M.; Sterclova, M.; Mogulkoc, N.; Lewandowska, K.; Muller, V.; Hajkova, M.; Kramer, M.; Jovanovic, D.; Tekavec-Trkanjec, J.; Studnicka, M.; et al. Differences in Baseline Characteristics and Access to Treatment of Newly Diagnosed Patients With IPF in the EMPIRE Countries. Front. Med. 2021, 8, 729203. [Google Scholar] [CrossRef]
- European MultiPartner IPF REgistry. Available online: https://empire.registry.cz/index-en.php (accessed on 8 December 2024).
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef]
- Ley, B.; Ryerson, C.J.; Vittinghoff, E.; Ryu, J.H.; Tomassetti, S.; Lee, J.S.; Poletti, V.; Buccioli, M.; Elicker, B.M.; Jones, K.D.; et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann. Intern. Med. 2012, 156, 684–691. [Google Scholar] [CrossRef]
- Perch, M.; Hayes, D., Jr.; Cherikh, W.S.; Zuckermann, A.; Harhay, M.O.; Hsich, E.; Potena, L.; Sadavarte, A.; Lindblad, K.; Singh, T.P.; et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-ninth adult lung transplantation report-2022; focus on lung transplant recipients with chronic obstructive pulmonary disease. J. Heart Lung Transplant. 2022, 41, 1335–1347. [Google Scholar] [CrossRef]
- Swaminathan, A.C.; Hellkamp, A.S.; Neely, M.L.; Bender, S.; Paoletti, L.; White, E.S.; Palmer, S.M.; Whelan, T.P.M.; Dilling, D.F.; Idiopathic Pulmonary Fibrosis Prospective Outcomes Registry Investigators. Disparities in Lung Transplant among Patients with Idiopathic Pulmonary Fibrosis: An Analysis of the IPF-PRO Registry. Ann. Am. Thorac. Soc. 2022, 19, 981–990. [Google Scholar] [CrossRef]
- Paoletti, L.; Palmer, S.; Yow, E.; Neely, M.L.; Gamerman, V.; Whelan, T. Underutilization of Lung Transplant Referral Among Patients with Newly Diagnosed Idiopathic Pulmonary Fibrosis (IPF). J. Heart Lung Transplant. 2017, 36, S115. [Google Scholar] [CrossRef]
- King, C.S.; White, E.; Aryal, S.; Shlobin, O.A.; Singhal, A.; Brown, W.; Thomas, C.; Khangoora, V.; Nyquist, A.; Flaherty, K.R.; et al. Factors associated with listing for lung transplantation in IPF patients: An analysis of the pulmonary fibrosis foundation registry. Heliyon 2023, 9, e18618. [Google Scholar] [CrossRef]
- Schaenman, J.M.; Diamond, J.M.; Greenland, J.R.; Gries, C.; Kennedy, C.C.; Parulekar, A.D.; Rozenberg, D.; Singer, J.P.; Singer, L.G.; Snyder, L.D.; et al. Frailty and aging-associated syndromes in lung transplant candidates and recipients. Am. J. Transplant. 2021, 21, 2018–2024. [Google Scholar] [CrossRef]
- Hayanga, A.J.; Aboagye, J.K.; Hayanga, H.E.; Morrell, M.; Huffman, L.; Shigemura, N.; Bhama, J.K.; D’Cunha, J.; Bermudez, C.A. Contemporary analysis of early outcomes after lung transplantation in the elderly using a national registry. J. Heart Lung Transplant. 2015, 34, 182–188. [Google Scholar] [CrossRef]
- Eurostat. Mortality and Life Expectancy Statistics. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Mortality_and_life_expectancy_statistics (accessed on 16 January 2024).
- Kistler, K.D.; Nalysnyk, L.; Rotella, P.; Esser, D. Lung transplantation in idiopathic pulmonary fibrosis: A systematic review of the literature. BMC Pulm. Med. 2014, 14, 139. [Google Scholar] [CrossRef]
- Riddell, P.; Kleinerova, J.; Eaton, D.; Healy, D.G.; Javadpour, H.; McCarthy, J.F.; Nolke, L.; Redmond, K.C.; Egan, J.J. Meaningful survival benefit for single lung transplantation in idiopathic pulmonary fibrosis patients over 65 years of age. Eur. Respir. J. 2020, 56, 1902413. [Google Scholar] [CrossRef]
- Li, D.; Liu, Y.; Wang, B. Single versus bilateral lung transplantation in idiopathic pulmonary fibrosis: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0233732. [Google Scholar] [CrossRef]
- Spratt, J.R.; Tomic, R.; Brown, R.Z.; Rudser, K.; Loor, G.; Hertz, M.; Shumway, S.; Kelly, R.F. Single Versus Bilateral Lung Transplantation for Idiopathic Pulmonary Fibrosis in the Lung Allocation Score Era. J. Surg. Res. 2019, 234, 84–95. [Google Scholar] [CrossRef]
- Villavicencio, M.A.; Axtell, A.L.; Osho, A.; Astor, T.; Roy, N.; Melnitchouk, S.; D’Alessandro, D.; Tolis, G.; Raz, Y.; Neuringer, I.; et al. Single- Versus Double-Lung Transplantation in Pulmonary Fibrosis: Impact of Age and Pulmonary Hypertension. Ann. Thorac. Surg. 2018, 106, 856–863. [Google Scholar] [CrossRef]
- Ranganath, N.K.; Malas, J.; Phillips, K.G.; Lesko, M.B.; Smith, D.E.; Angel, L.F.; Lonze, B.E.; Kon, Z.N. Single and Double Lung Transplantation Have Equivalent Survival for Idiopathic Pulmonary Fibrosis. Ann. Thorac. Surg. 2020, 109, 211–217. [Google Scholar] [CrossRef]
- Gulack, B.C.; Ganapathi, A.M.; Speicher, P.J.; Meza, J.M.; Hirji, S.A.; Snyder, L.D.; Davis, R.D.; Hartwig, M.G. What Is the Optimal Transplant for Older Patients With Idiopathic Pulmonary Fibrosis? Ann. Thorac. Surg. 2015, 100, 1826–1833. [Google Scholar] [CrossRef]
- Amor, M.S.; Rosengarten, D.; Shitenberg, D.; Pertzov, B.; Shostak, Y.; Kramer, M.R. Lung Transplantation in Idiopathic Pulmonary Fibrosis: Risk Factors and Outcome. Isr. Med. Assoc. J. 2020, 22, 741–746. [Google Scholar]
- Hanna, K.; Calvelli, H.; Kashem, M.A.; Zhao, H.; Cheng, K.; Leotta, E.; Yanagida, R.; Shigemura, N.; Toyoda, Y. Donor and Recipient Age in Interstitial Lung Disease: Types of Lung Transplant Survival Outcomes. J. Surg. Res. 2024, 293, 136–143. [Google Scholar] [CrossRef]
- Gershman, E.; Zer, A.; Pertzov, B.; Shtraichman, O.; Shitenberg, D.; Heching, M.; Rosengarten, D.; Kramer, M. Characteristics of lung cancer in idiopathic pulmonary fibrosis with single lung transplant versus non-transplanted patients: A retrospective observational study. BMJ Open Respir. Res. 2020, 7, e000566. [Google Scholar] [CrossRef]
- Invernizzi, R.; Barnett, J.; Rawal, B.; Nair, A.; Ghai, P.; Kingston, S.; Chua, F.; Wu, Z.; Wells, A.U.; Renzoni, E.R.; et al. Bacterial burden in the lower airways predicts disease progression in idiopathic pulmonary fibrosis and is independent of radiological disease extent. Eur. Respir. J. 2020, 55, 1901519. [Google Scholar] [CrossRef]
- Valenzi, E.; Yang, H.; Sembrat, J.C.; Yang, L.; Winters, S.; Nettles, R.; Kass, D.J.; Qin, S.; Wang, X.; Myerburg, M.M.; et al. Topographic heterogeneity of lung microbiota in end-stage idiopathic pulmonary fibrosis: The Microbiome in Lung Explants-2 (MiLEs-2) study. Thorax 2021, 76, 239–247. [Google Scholar] [CrossRef]
- Kirgou, P.; Sinis, S.I.; Dimeas, I.E.; Papanikolaou, I.C.; Tatsis, K.; Gogali, A.; Gourgoulianis, K.I.; Bogdanos, D.P.; Daniil, Z. Clinical relevance of circulating autoantibodies in idiopathic pulmonary fibrosis; A NAt hard to break. Front. Med. 2022, 9, 964722. [Google Scholar] [CrossRef]
- Kamiya, H.; Panlaqui, O.M. Systematic review and meta-analysis of clinical significance of autoantibodies for idiopathic pulmonary fibrosis. BMJ Open 2019, 9, e027849. [Google Scholar] [CrossRef]
- Alhamad, E.H.; Cal, J.G.; AlBoukai, A.A.; Shaik, S.A.; Omair, M.A. Autoimmune symptoms in idiopathic pulmonary fibrosis: Clinical significance. Clin. Respir. J. 2016, 10, 350–358. [Google Scholar] [CrossRef]
| Criteria | Total n = 4390 | LuTX n = 94 | No TX n = 4296 |
|---|---|---|---|
| Refused TX | 656 (14.9%) | 1 (1.1%) | 655 (15.2%) |
| Age > 70 years | 2163 (49.3%) | 7 (7.4%) | 2156 (50.2%) |
| Active tuberculosis | 93 (2.1%) | 0 (0.0%) | 93 (2.2%) |
| Liver cirrhosis | 14 (0.3%) | 0 (0.0%) | 14 (0.3%) |
| BMI > 35 kg/m2 OR < 16 kg/m2 | 348 (7.9%) | 8 (8.5%) | 340 (7.9%) |
| Current smoking | 213 (4.9%) | 5 (5.3%) | 208 (4.8%) |
| Malignancy | 575 (13.1%) | 8 (8.5%) | 567 (13.2%) |
| Atrial fibrillation | 234 (5.3%) | 0 (0.0%) | 234 (5.4%) |
| HIV infection | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Concurrent antiplatelet AND anticoagulant therapy | 205 (4.7%) | 5 (5.3%) | 200 (4.7%) |
| Any exclusion criterion | 3163 (72.1%) | 29 (30.9%) | 3134 (73.0%) |
| Potentially eligible for LuTX according to criteria OR transplanted | 1256 (28.6%) | 94 (100%) | 1162 (27.0%) |
| All EMPIRE Patients n = 4390 | All Patients Potentially Eligible for LuTX n = 1256 | Patients with LuTX n = 94 | Patients Without LuTX n = 1162 | |
|---|---|---|---|---|
| Considered for LuTX initially according to registry entry | ||||
| Yes | 1227 (27.9%) | 1227 (97.7%) | 65 (69.1%) | 1162 (100.0%) |
| No | 3163 (72.1%) | 29 (2.3%) | 29 (30.9%) | 0 (0.0%) |
| Reason if not considered | ||||
| Age | 2163 (68.4%) | 7 (24.1%) | 7 (24.1%) | - |
| Comorbidities | 347 (11.0%) | 7 (24.1%) | 7 (24.1%) | - |
| Refused | 370 (11.7%) | 1 (3.4%) | 1 (3.4%) | - |
| Other | 283 (8.9%) | 14 (48.3%) | 14 (48.3%) | - |
| Characteristics | All Patients Potentially Eligible for LuTX n = 1256 | Patients with LuTX n = 94 | Patients Without LuTX n = 1162 | p-Value * |
|---|---|---|---|---|
| Number of patients | ||||
| All countries | 1256 (100%) | 94 (100%) | 1162 (100%) | |
| Czech Republic | 431 (34.3%) | 20 (21.3%) | 411 (35.4%) | |
| Turkey | 192 (15.3%) | 2 (2.1%) | 190 (16.4%) | |
| Poland | 177 (14.1%) | 6 (6.4%) | 171 (14.7%) | |
| Israel | 123 (9.8%) | 53 (56.4%) | 70 (6.0%) | |
| Hungary | 103 (8.2%) | 8 (8.5%) | 95 (8.2%) | |
| Serbia | 79 (6.3%) | 0 (0.0%) | 79 (6.8%) | |
| Slovakia | 77 (6.1%) | 2 (2.1%) | 75 (6.5%) | |
| Croatia | 38 (3.0%) | 1 (1.1%) | 37 (3.2%) | |
| Austria | 21 (1.7%) | 2 (2.1%) | 19 (1.6%) | |
| Bulgaria | 10 (0.8%) | 0 (0.0%) | 10 (0.9%) | |
| Macedonia | 5 (0.4%) | 0 (0.0%) | 5 (0.4%) | |
| Men | 886 (70.5%) | 72 (76.6%) | 814 (70.1%) | 0.181 |
| Age at inclusion (years) (n = 1255) | 63.9 (59.4–67.3) | 60.7 (55.9–65.6) | 64.1 (59.7–67.3) | <0.001 |
| Age at diagnosis (years) (n = 1255) | 63 (58–67) | 59 (55–64) | 63 (59–67) | <0.001 |
| Duration of symptoms (months) (n = 1212) | 12 (6–24) | 24 (9–48) | 12 (6–24) | <0.001 |
| Length of follow-up from enrollment (months) | 19 (7–39) | 19 (11–36) | 19 (6–39) | 0.495 |
| Time from diagnosis to LuTX/last follow-up (months) | 29 (14–53) | 33 (20–51) | 29 (14–53) | 0.090 |
| BMI (kg/m2) (n = 1242) | 27.9 (25.4–30.5) | 29.4 (26.5–30.9) | 27.8 (25.3–30.5) | 0.005 |
| Smoking status | <0.001 | |||
| Never-smokers | 487 (39.0%) | 31 (33.3%) | 456 (39.4%) | |
| Ex-smokers | 758 (60.6%) | 57 (61.3%) | 701 (60.6%) | |
| Current smokers | 5 (0.4%) | 5 (5.4%) | 0 (0.0%) | |
| High-resolution CT pattern | 0.086 | |||
| UIP | 825 (65.7%) | 71 (75.5%) | 754 (64.9%) | |
| Probable/possible UIP | 330 (26.3%) | 21 (22.3%) | 309 (26.6%) | |
| Inconsistent with UIP/alternative diagnosis | 67 (5.3%) | 1 (1.1%) | 66 (5.7%) | |
| Unknown | 34 (2.7%) | 1 (1.1%) | 33 (2.8%) | |
| Number of comorbidities | 3.00 (1.00–5.00) | 4.00 (3.00–6.00) | 3.00 (1.00–5.00) | <0.001 |
| Heart and vascular | 830 (66.1%) | 68 (72.3%) | 762 (65.6%) | 0.183 |
| Pulmonary | 451 (35.9%) | 34 (36.2%) | 417 (35.9%) | 0.956 |
| Gastrointestinal | 701 (55.8%) | 73 (77.7%) | 628 (54.0%) | <0.001 |
| Urogenital | 133 (10.6%) | 13 (13.8%) | 120 (10.3%) | 0.288 |
| Hematologic and immune system | 79 (6.3%) | 13 (13.8%) | 66 (5.7%) | 0.002 |
| Arterial hypertension | 576 (45.9%) | 42 (44.7%) | 534 (46.0%) | 0.811 |
| Diabetes mellitus | 230 (18.3%) | 26 (27.7%) | 204 (17.6%) | 0.015 |
| Ischemic heart disease | 212 (16.9%) | 26 (27.7%) | 186 (16.0%) | 0.004 |
| Hyperlipidemia | 300 (23.9%) | 41 (43.6%) | 259 (22.3%) | <0.001 |
| Duodenal ulcer disease | 278 (22.1%) | 39 (41.5%) | 239 (20.6%) | <0.001 |
| At least one comedication | 991 (78.9%) | 73 (77.7%) | 918 (79.0%) | 0.759 |
| Number of comedications | 3.00 (1.00–4.00) | 3.00 (2.00–5.00) | 2.00 (1.00–4.00) | 0.003 |
| Beta-blockers | 285 (22.7%) | 20 (21.3%) | 265 (22.8%) | 0.734 |
| Angiotensin-converting enzyme inhibitor | 280 (22.3%) | 17 (18.1%) | 263 (22.6%) | 0.308 |
| Aspirin | 251 (20.0%) | 26 (27.7%) | 225 (19.4%) | 0.053 |
| Statins | 285 (22.7%) | 26 (27.7%) | 259 (22.3%) | 0.232 |
| Diuretics | 193 (15.4%) | 12 (12.8%) | 181 (15.6%) | 0.467 |
| Insulin | 209 (16.6%) | 21 (22.3%) | 188 (16.2%) | 0.123 |
| Pharmacological IPF treatment | 1046 (83.3%) | 90 (95.7%) | 956 (82.3%) | <0.001 |
| Antifibrotics | 861 (68.6%) | 83 (88.3%) | 778 (67.0%) | <0.001 |
| Rehabilitation | 319 (25.4%) | 42 (44.7%) | 277 (23.8%) | <0.001 |
| Long-term oxygen therapy | 465 (37.0%) | 77 (81.9%) | 388 (33.4%) | <0.001 |
| N-acetylcysteine | 221 (17.6%) | 17 (18.1%) | 204 (17.6%) | 0.897 |
| Proton pump inhibitors | 222 (17.7%) | 14 (14.9%) | 208 (17.9%) | 0.462 |
| Systemic corticosteroids | 276 (22.0%) | 33 (35.1%) | 243 (20.9%) | 0.001 |
| Azathioprine | 91 (7.2%) | 9 (9.6%) | 82 (7.1%) | 0.365 |
| Other cytostatic | 20 (1.6%) | 2 (2.1%) | 18 (1.5%) | 0.657 |
| First antifibrotic drug | <0.001 | |||
| Pirfenidone | 451 (35.9%) | 47 (50.0%) | 404 (34.8%) | |
| Nintedanib | 410 (32.6%) | 36 (38.3%) | 374 (32.2%) | |
| None | 395 (31.4%) | 11 (11.7%) | 384 (33.0%) | |
| First antifibrotic therapy duration (months) (n = 513) | 15 (7–29) | 23 (11–37) | 14 (6–28) | <0.001 |
| FVC (L) (n = 1106) | 2.58 (1.96–3.25) | 2.20 (1.75–2.82) | 2.62 (1.98–3.28) | <0.001 |
| FVC (%ref) (n = 1090) | 72 (58–87) | 60 (48–69) | 74 (60–87) | <0.001 |
| TLCO (mmol/kPa/min) (n = 1021) | 3.92 (2.92–5.09) | 3.45 (2.37–4.50) | 3.98 (2.92–5.14) | 0.015 |
| TLCO (%ref) (n = 949) | 47 (35–59) | 41 (32–51) | 47 (35–60) | <0.001 |
| 6 min walk test (m) (n = 546) | 416 (344–480) | 392 (334–469) | 420 (345–480) | 0.356 |
| GAP index | 0.014 | |||
| GAP I | 546 (54.2%) | 33 (39.3%) | 513 (55.6%) | |
| GAP II | 382 (37.9%) | 41 (48.8%) | 341 (36.9%) | |
| GAP III | 79 (7.8%) | 10 (11.9%) | 69 (7.5%) | |
| Dyspnea (New York Heart Association grade) | 0.140 | |||
| I | 54 (4.7%) | 1 (1.1%) | 53 (5.0%) | |
| II | 714 (61.7%) | 53 (57.6%) | 661 (62.1%) | |
| III | 368 (31.8%) | 37 (40.2%) | 331 (31.1%) | |
| IV | 21 (1.8%) | 1 (1.1%) | 20 (1.9%) | |
| Velcro-like crackles | 1166 (93.3%) | 90 (96.8%) | 1076 (93.0%) | 0.162 |
| Finger clubbing | 552 (44.2%) | 62 (66.7%) | 490 (42.4%) | <0.001 |
| Exertional dyspnea | 1157 (92.2%) | 92 (97.9%) | 1065 (91.7%) | 0.033 |
| Cough | 848 (67.5%) | 73 (77.7%) | 775 (66.7%) | 0.029 |
| Signs of connective tissue disease | 116 (9.2%) | 14 (14.9%) | 102 (8.8%) | 0.049 |
| Autoantibodies | 97 (7.7%) | 8 (8.5%) | 89 (7.7%) | 0.766 |
| Exposure to organic dusts | 169 (13.5%) | 12 (12.8%) | 157 (13.5%) | 0.839 |
| Exposure to inorganic dusts | 221 (17.6%) | 24 (25.5%) | 197 (17.0%) | 0.036 |
| Respiratory infections | 218 (17.4%) | 25 (26.9%) | 193 (16.7%) | 0.012 |
| Type of infection | 0.217 | |||
| Sporadic | 149 (69.3%) | 20 (80.0%) | 129 (67.9%) | |
| Frequent | 66 (30.7%) | 5 (20.0%) | 61 (32.1%) |
| All Patients Potentially Eligible for LuTX n = 1256 | Czech Republic n = 431 | Turkey n = 192 | Poland n = 177 | Israel n = 123 | Hungary n = 103 | Serbia n = 79 | Slovakia n = 77 | Croatia n = 38 | Austria n = 21 | Bulgaria n = 10 | Macedonia n = 5 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age at inclusion (years) | 63.9 (59.4–67.3) | 64.4 (60.0–67.4) | 63.1 (58.6–66.8) | 64.5 (61.6–67.4) | 64.4 (59.3–67.6) | 62.7 (59.1–67.2) | 61.1 (57.1–66.2) | 62.3 (55.9–67.2) | 63.6 (60.1–67.6) | 66.2 (63.1–68.2) | 67.5 (64.4–68.5) | 55.0 (53.9–65.3) |
| Men | 886 (70.5%) | 318 (73.8%) | 155 (80.7%) | 131 (74.0%) | 82 (66.7%) | 63 (61.2%) | 43 (54.4%) | 46 (59.7%) | 26 (68.4%) | 12 (57.1%) | 7 (70.0%) | 3 (60.0%) |
| Antifibrotic treatment | ||||||||||||
| Yes | 861 (68.6%) | 315 (73.1%) | 108 (56.3%) | 112 (63.3%) | 99 (80.5%) | 84 (81.6%) | 49 (62.0%) | 41 (53.2%) | 26 (68.4%) | 17 (81.0%) | 8 (80.0%) | 2 (40.0%) |
| No | 395 (31.4%) | 116 (26.9%) | 84 (43.8%) | 65 (36.7%) | 24 (19.5%) | 19 (18.4%) | 30 (38.0%) | 36 (46.8%) | 12 (31.6%) | 4 (19.0%) | 2 (20.0%) | 3 (60.0%) |
| Number of comorbidities | 3.00 (1.00–5.00) | 3.00 (2.00–5.00) | 3.00 (2.00–5.00) | 2.00 (1.00–4.00) | 5.00 (3.00–7.00) | 2.00 (1.00–3.00) | 1.00 (1.00–2.00) | 1.00 (1.00–3.00) | 4.00 (3.00–5.00) | 2.00 (1.00–3.00) | 1.50 (1.00–5.00) | 0 |
| Heart and vascular | 830 (66.1%) | 297 (68.9%) | 122 (63.5%) | 114 (64.4%) | 80 (65.0%) | 64 (62.1%) | 58 (73.4%) | 46 (59.7%) | 27 (71.1%) | 14 (66.7%) | 8 (80.0%) | 0 |
| Pulmonary | 451 (35.9%) | 168 (39.0%) | 150 (78.1%) | 35 (19.8%) | 32 (26.0%) | 22 (21.4%) | 10 (12.7%) | 11 (14.3%) | 19 (50.0%) | 3 (14.3%) | 1 (10.0%) | 0 |
| Gastrointestinal | 701 (55.8%) | 268 (62.2%) | 91 (47.4%) | 101 (57.1%) | 104 (84.6%) | 41 (39.8%) | 20 (25.3%) | 34 (44.2%) | 29 (76.3%) | 10 (47.6%) | 3 (30.0%) | 0 |
| Blood and immune system | 79 (6.3%) | 27 (6.3%) | 14 (7.3%) | 7 (4.0%) | 15 (12.2%) | 6 (5.8%) | 4 (5.1%) | 5 (6.5%) | 0 (0.0%) | 1 (4.8%) | 0 (0.0%) | 0 |
| Arterial hypertension | 576 (45.9%) | 212 (49.2%) | 67 (34.9%) | 87 (49.2%) | 50 (40.7%) | 46 (44.7%) | 38 (48.1%) | 38 (49.4%) | 19 (50.0%) | 13 (61.9%) | 6 (60.0%) | 0 |
| Diabetes mellitus | 230 (18.3%) | 80 (18.6%) | 37 (19.3%) | 35 (19.8%) | 34 (27.6%) | 13 (12.6%) | 10 (12.7%) | 11 (14.3%) | 7 (18.4%) | 1 (4.8%) | 2 (20.0%) | 0 |
| Ischemic heart disease | 212 (16.9%) | 76 (17.6%) | 32 (16.7%) | 31 (17.5%) | 34 (27.6%) | 12 (11.7%) | 3 (3.8%) | 9 (11.7%) | 10 (26.3%) | 1 (4.8%) | 4 (40.0%) | 0 |
| Hyperlipidemia | 300 (23.9%) | 133 (30.9%) | 9 (4.7%) | 48 (27.1%) | 80 (65.0%) | 5 (4.9%) | 0 (0.0%) | 11 (14.3%) | 6 (15.8%) | 8 (38.1%) | 0 (0.0%) | 0 |
| FVC (L) | 2.58 (1.96–3.25) | 2.74 (2.14–3.37) | 2.17 (1.71–2.71) | 2.99 (2.45–3.65) | 1.91 (1.44–2.49) | 2.46 (1.74–3.15) | 2.55 (2.03–3.07) | 2.82 (2.17–3.53) | 2.74 (2.35–3.78) | 2.74 (2.35–3.41) | 2.67 (2.40–3.51) | NA |
| FVC (%ref) | 72 (58–87) | 75 (62–86) | 63 (49–75) | 85 (71–102) | 60 (45–70) | 70 (56–81) | 73 (57–88) | 82 (67–96) | 82 (66–96) | 82 (66–91) | 80 (66–108) | NA |
| TLCO (mmol/kPa/min) | 3.92 (2.92–5.09) | 4.12 (3.08–5.22) | 3.22 (2.45–4.71) | 4.28 (3.14–5.50) | 3.35 (2.36–4.51) | 4.44 (3.55–6.06) | 3.09 (2.07–4.65) | 4.54 (3.05–5.06) | 3.83 (2.51–4.50) | 4.08 (3.00–5.14) | 2.84 (2.62–4.11) | NA |
| TLCO (%ref) | 47 (35–59) | 47 (36–59) | 40 (30–58) | 50 (37–63) | 42 (32–52) | 55 (44–73) | 35 (24–50) | 50 (36–60) | 43 (32–55) | 50 (35–59) | 40 (29–48) | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tóth, N.M.; Kramer, M.R.; Šterclová, M.; Müller, V.; Lewandowska, K.B.; Mogulkoc, N.; Hájková, M.; Studnicka, M.; Tekavec-Trkanjec, J.; Dimic-Janjic, S.; et al. Lung Transplantation in Idiopathic Pulmonary Fibrosis Patients in the European MultiPartner IPF Registry: Challenges for Health Equity. Biomedicines 2025, 13, 2684. https://doi.org/10.3390/biomedicines13112684
Tóth NM, Kramer MR, Šterclová M, Müller V, Lewandowska KB, Mogulkoc N, Hájková M, Studnicka M, Tekavec-Trkanjec J, Dimic-Janjic S, et al. Lung Transplantation in Idiopathic Pulmonary Fibrosis Patients in the European MultiPartner IPF Registry: Challenges for Health Equity. Biomedicines. 2025; 13(11):2684. https://doi.org/10.3390/biomedicines13112684
Chicago/Turabian StyleTóth, Nóra M., Mordechai R Kramer, Martina Šterclová, Veronika Müller, Katarzyna B. Lewandowska, Nesrin Mogulkoc, Marta Hájková, Michael Studnicka, Jasna Tekavec-Trkanjec, Sanja Dimic-Janjic, and et al. 2025. "Lung Transplantation in Idiopathic Pulmonary Fibrosis Patients in the European MultiPartner IPF Registry: Challenges for Health Equity" Biomedicines 13, no. 11: 2684. https://doi.org/10.3390/biomedicines13112684
APA StyleTóth, N. M., Kramer, M. R., Šterclová, M., Müller, V., Lewandowska, K. B., Mogulkoc, N., Hájková, M., Studnicka, M., Tekavec-Trkanjec, J., Dimic-Janjic, S., Penev, A., Arsovski, Z., Gregor, J., Ovesná, P., & Vašáková, M. K. (2025). Lung Transplantation in Idiopathic Pulmonary Fibrosis Patients in the European MultiPartner IPF Registry: Challenges for Health Equity. Biomedicines, 13(11), 2684. https://doi.org/10.3390/biomedicines13112684





