Real-Life Efficacy of Single-Inhaler Triple Therapy with Budesonide/Glycopyrronium/Formoterol Fumarate in Persistent COPD Users: A Retrospective Database Study
Abstract
1. Introduction
2. Materials and Methods
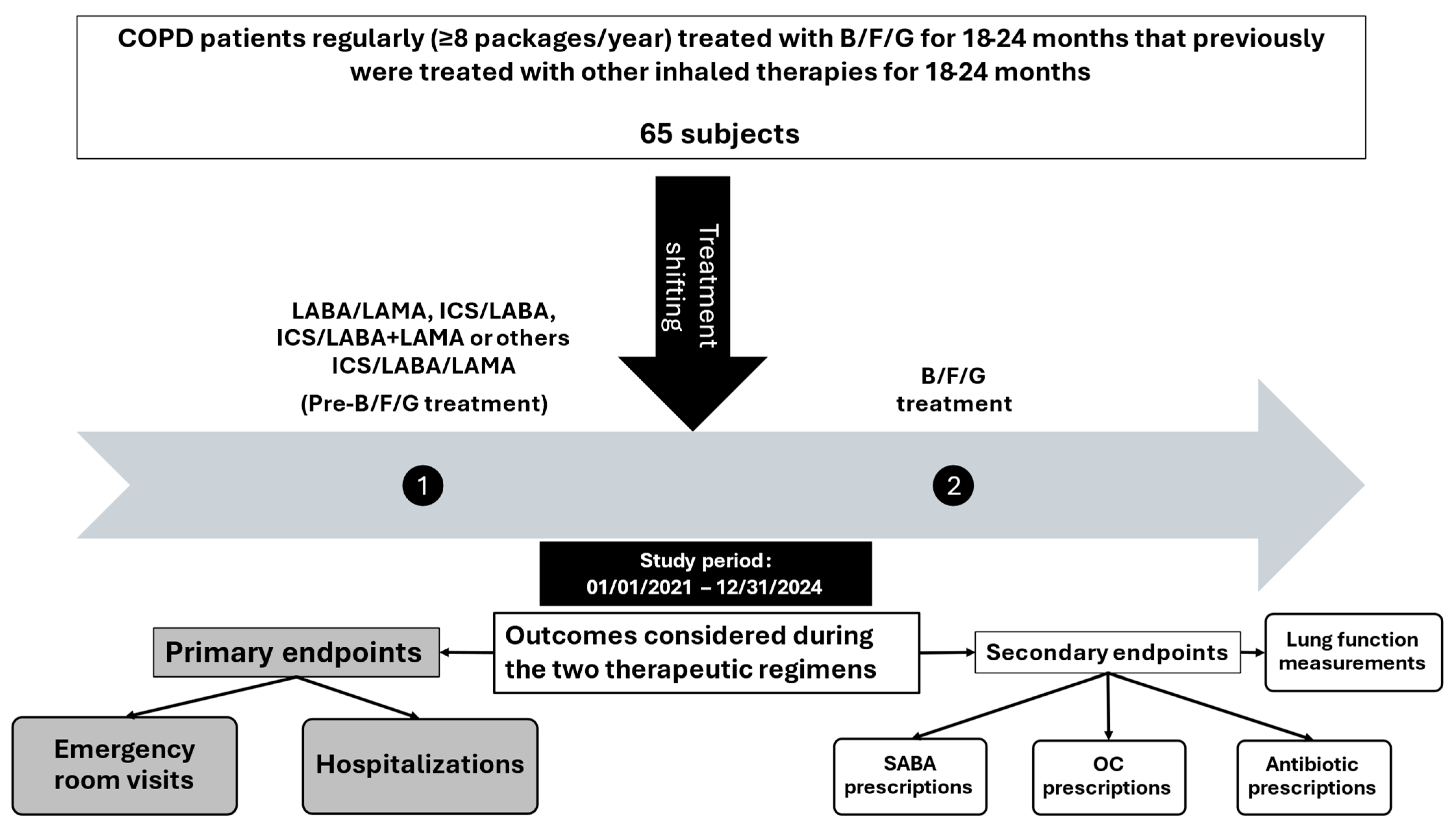
2.1. Study Design
2.2. Setting and Participants
2.3. Variables and Measurements
2.4. Statistical Analysis
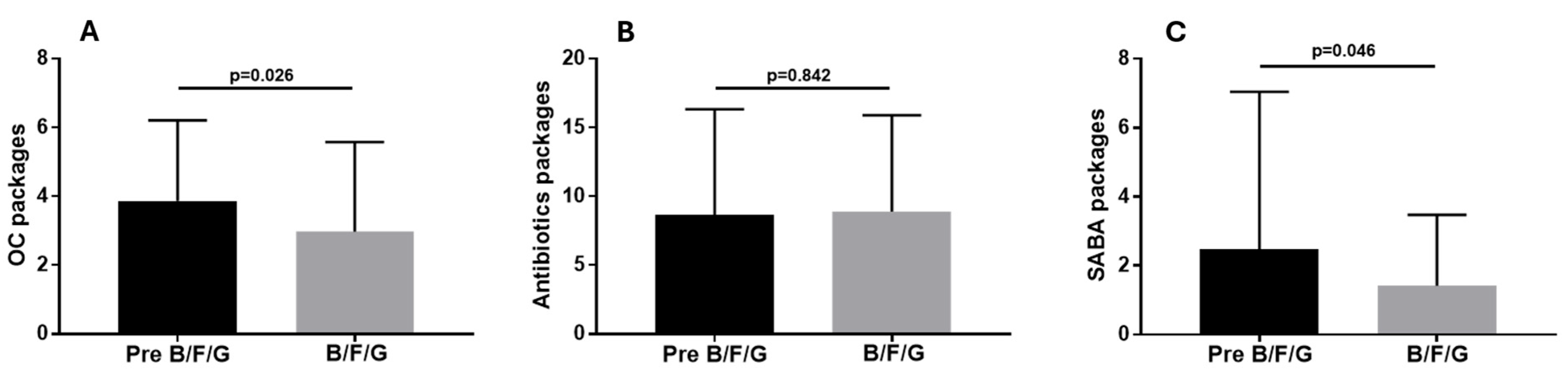
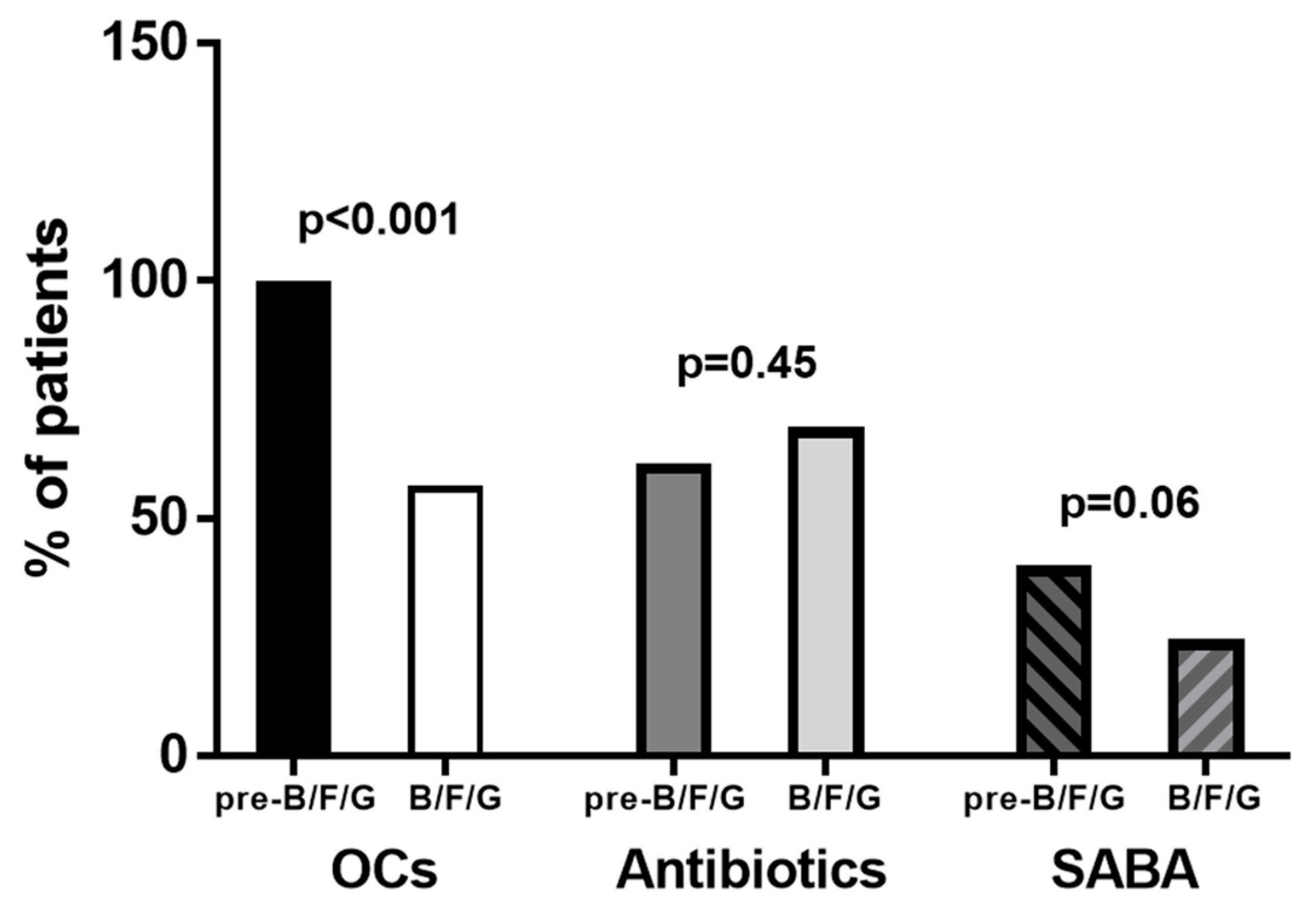
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Strategy for Prevention, Diagnosis and Management of Copd: 2025 Report. Available online: https://goldcopd.org/2025-gold-report/ (accessed on 7 July 2025).
- Ferguson, G.T.; Rabe, K.F.; Martinez, F.J.; Fabbri, L.M.; Wang, C.; Ichinose, M.; Bourne, E.; Ballal, S.; Darken, P.; DeAngelis, K.; et al. Triple therapy with budesonide/glycopyrrolate/formoterol fumarate with co-suspension delivery technology versus dual therapies in chronic obstructive pulmonary disease (KRONOS): A double-blind, parallel-group, multicentre, phase 3 randomised controlled trial. Lancet Respir. Med. 2018, 6, 747–758, Erratum in Lancet Respir. Med. 2018, 6, E55. [Google Scholar]
- Rabe, K.F.; Martinez, F.J.; Ferguson, G.T.; Wang, C.; Singh, D.; Wedzicha, J.A.; Trivedi, R.; St Rose, E.; Ballal, S.; McLaren, J.; et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N. Engl. J. Med. 2020, 383, 35–48. [Google Scholar] [CrossRef]
- Martinez, F.J.; Rabe, K.F.; Ferguson, G.T.; Wedzicha, J.A.; Trivedi, R.; Jenkins, M.; Darken, P.; Aurivillius, M.; Dorinsky, P. Benefits of budesonide/glycopyrrolate/formoterol fumarate (BGF) on symptoms and quality of life in patients with COPD in the ETHOS trial. Respir. Med. 2021, 185, 106509. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Montero, A.; de Miguel Diez, J.; de Simón Gutiérrez, R.; Campos Téllez, S.; Chacón Moreno, A.D.; Alonso Avilés, R.; González Alonso, N.; Montero Solís, A.; Escribano Pardo, D.; APyC Group. Triple inhaled therapy of formoterol/glycopyrrolate/budesonide reduces the use of oral corticosteroids and antibiotics during COPD exacerbations in real-world conditions. Semergen 2025, 51, 102418. [Google Scholar] [CrossRef]
- Ferguson, G.T.; Darken, P.; Ballal, S.; Siddiqui, M.K.; Singh, B.; Attri, S.; Holmgren, U.; de Nigris, E. Efficacy of budesonide/glycopyrronium/formoterol fumarate metered dose inhaler (BGF MDI) versus other inhaled corticosteroid/long-acting muscarinic antagonist/long-acting β2-agonist (ICS/LAMA/LABA) triple combinations in COPD: A systematic literature review and network meta-analysis. Adv. Ther. 2020, 37, 2956–2975. [Google Scholar] [PubMed]
- Bourdin, A.; Molinari, N.; Ferguson, G.T.; Singh, B.; Siddiqui, M.K.; Holmgren, U.; Ouwens, M.; Jenkins, M.; De Nigris, E. Efficacy and safety of budesonide/glycopyrronium/formoterol fumarate versus other triple combinations in COPD: A systematic literature review and network meta-analysis. Adv. Ther. 2021, 38, 3089–3112. [Google Scholar]
- Müllerová, H.; Chan, J.S.K.; Heatley, H.; Carter, V.; Townend, J.; Skinner, D.; Franzén, S.; Marshall, J.; Price, D. Budesonide/glycopyrrolate/formoterol for the management of COPD in a UK primary care population: Real-world use and early medication success. Int. J. Chron. Obstruct. Pulmon. Dis. 2024, 19, 1153–1166. [Google Scholar] [CrossRef] [PubMed]
- Dondi, L.; Ronconi, G.; Calabria, S.; Dell’Anno, I.; Piccinni, C.; Brignoli, O.; Canonica, G.W.; Carone, M.; Di Marco, F.; Micheletto, C.; et al. Clinical characteristics, use and switch of drugs for obstructive airway diseases among patients with COPD experiencing an exacerbation: A retrospective analysis of Italian administrative healthcare data. BMC Pulm. Med. 2024, 24, 525, Erratum in BMC Pulm. Med. 2024, 24, 606. [Google Scholar]
- Calle Rubio, M.; Trigueros Carrero, J.A.; Chaparro Briones, P.; Escudero Herrera, L.; Pollack, M.; Hernández Subirá, I.; Sánchez-Covisa, J. Characteristics of patients receiving budesonide/glycopyrronium/formoterol for chronic obstructive pulmonary disease in Spain: The AURA study. Int. J. Chron. Obstruct. Pulmon. Dis. 2025, 20, 999–1008. [Google Scholar] [CrossRef]
- Suissa, S.; Dell’Aniello, S.; Ernst, P. Long-term natural history of chronic obstructive pulmonary disease: Severe exacerbations and mortality. Thorax 2012, 67, 957–963. [Google Scholar] [CrossRef]
- Halpin, D.M.; Decramer, M.; Celli, B.; Kesten, S.; Liu, D.; Tashkin, D.P. Exacerbation frequency and course of COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2012, 7, 653–661. [Google Scholar]
- Rhodes, K.; Jenkins, M.; de Nigris, E.; Aurivillius, M.; Ouwens, M. Relationship between risk, cumulative burden of exacerbations and mortality in patients with COPD: Modelling analysis using data from the ETHOS study. BMC Med. Res. Methodol. 2022, 22, 150. [Google Scholar]
- Martinez, F.J.; Rabe, K.F.; Ferguson, G.T.; Wedzicha, J.A.; Singh, D.; Wang, C.; Rossman, K.; St Rose, E.; Trivedi, R.; Ballal, S.; et al. Reduced all-cause mortality in the ETHOS trial of budesonide/glycopyrrolate/formoterol for chronic obstructive pulmonary disease. A randomized, double-blind, multicenter, parallel-group study. Am. J. Respir. Crit. Care Med. 2021, 203, 553–564. [Google Scholar] [CrossRef]
- Löfdahl, C.G.; Postma, D.S.; Pride, N.B.; Boe, J.; Thorén, A. Possible protection by inhaled budesonide against ischaemic cardiac events in mild COPD. Eur. Respir. J. 2007, 29, 1115–1119. [Google Scholar] [CrossRef]
- Shin, J.; Yoon, H.Y.; Lee, Y.M.; Ha, E.; Lee, J.H. Inhaled corticosteroids in COPD and the risk for coronary heart disease: A nationwide cohort study. Sci. Rep. 2020, 10, 18973. [Google Scholar] [CrossRef]
- Chen, H.; Deng, Z.X.; Sun, J.; Huang, Q.; Huang, L.; He, Y.H.; Ma, C.; Wang, K. Association of inhaled corticosteroids with all-cause mortality risk in patients with COPD: A meta-analysis of 60 randomized controlled trials. Chest 2023, 163, 100–114. [Google Scholar] [PubMed]
- Fan, V.S.; Gylys-Colwell, I.; Locke, E.; Sumino, K.; Nguyen, H.Q.; Thomas, R.M.; Magzamen, S. Overuse of short-acting beta-agonist bronchodilators in COPD during periods of clinical stability. Respir. Med. 2016, 116, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, K.; Harada, N.; Horiuchi, A.; Umemoto, S.; Kurabatashi, R.; Yui, A.; Yamamura, H.; Shinka, Y.; Miyao, N. Therapeutic response to single-inhaler triple therapies in moderate-to-severe COPD. Respir. Care 2023, 68, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Rabe, K.F.; Martinez, F.J.; Singh, D.; Trivedi, R.; Jenkins, M.; Darken, P.; Aurivillius, M.; Dorinsky, P. Improvements in lung function with budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler versus dual therapies in patients with COPD: A sub-study of the ETHOS trial. Ther. Adv. Respir. Dis. 2021, 15, 17534666211034329. [Google Scholar]
- Requena, G.; Camidge, L.J.; Ford, A.; Yarita, M.; Hashimoto, K.; Jennison, T.; Massey, O.S.; Noorduyn, S.G.; Mizukami, A. Effectiveness of switching from multiple-inhaler to once-daily single-inhaler triple therapy in patients with COPD in a real-world setting in Japan. Int. J. Chron. Obstruct. Pulmon. Dis. 2025, 20, 565–580. [Google Scholar] [CrossRef]
- Sposato, B.; Ricci, A.; Lacerenza, L.G.; Petrucci, E.; Cresti, A.; Baratta, P.; Perrella, A.; Serafini, A.; Scalese, M. Triple therapy in COPD in real life: Is it better to use single or multiple inhalers? J. Clin. Med. 2024, 13, 6191. [Google Scholar] [CrossRef]
- Jokšaitė, S.; Wood, R.; Ismaila, A.; Camidge, L.; Mizukami, A.; Czira, A.; Massey, O.; Yarita, M.; Compton, C.; Siddiqui, R.; et al. Comparative adherence and persistence of single-inhaler and multiple-inhaler triple therapies among patients with chronic obstructive pulmonary disease in Japan: A retrospective cohort study. BMJ Open 2024, 14, e080864. [Google Scholar]
- Vogelmeier, C.F.; Beeh, K.M.; Schultze, M.; Kossack, N.; Richter, L.M.; Claussen, J.; Compton, C.; Noorduyn, S.G.; Ismaila, A.S.; Requena, G. Evaluation of adherence and persistence to triple therapy in patients with COPD: A German claims data study. Int. J. Chron. Obstruct. Pulmon. Dis. 2024, 19, 1835–1848. [Google Scholar] [CrossRef] [PubMed]
- Deslee, G.; Fabry-Vendrand, C.; Poccardi, N.; Thabut, G.; Eteve Pitsaer, C.; Coriat, A.; Renaudat, C.; Maguire, A.; Pinto, T. Use and persistence of single and multiple inhaler triple therapy prescribed for patients with COPD in France: A retrospective study on THIN database (OPTI study). BMJ Open Respir. Res. 2023, 10, e001585. [Google Scholar] [CrossRef]
- Lin, L.; Liu, C.; Cheng, W.; Song, Q.; Zeng, Y.; Li, X.; Deng, D.; Liu, D.; Chen, Y.; Cai, S.; et al. Comparison of treatment persistence, adherence, and risk of exacerbation in patients with COPD treated with single-inhaler versus multiple-inhaler triple therapy: A prospective observational study in China. Front. Pharmacol. 2023, 14, 1147985. [Google Scholar]
- Turégano-Yedro, M.; Trillo-Calvo, E.; Navarro i Ros, F.; Maya-Viejo, J.D.; González Villaescusa, C.; Echave Sustaeta, J.M.; Doña, E.; Alcázar Navarrete, B. Inhaler adherence in COPD: A crucial step towards the correct treatment. Int. J. Chron. Obstruct. Pulmon. Dis. 2023, 18, 2887–2893. [Google Scholar]
- Toy, E.L.; Beaulieu, N.U.; McHale, J.M.; Welland, T.R.; Plauschinat, C.A.; Swensen, A.; Duh, M.S. Treatment of COPD: Relationships between daily dosing frequency, adherence, resource use, and costs. Respir. Med. 2011, 105, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Ismaila, A.; Corriveau, D.; Vaillancourt, J.; Parsons, D.; Dalal, A.; Su, Z.; Sampalis, J.S. Impact of adherence to treatment with tiotropium and fluticasone propionate/salmeterol in chronic obstructive pulmonary diseases patients. Curr. Med. Res. Opin. 2014, 30, 1427–1436. [Google Scholar] [PubMed]
- Vestbo, J.; Anderson, J.A.; Calverley, P.M.; Celli, B.; Ferguson, G.T.; Jenkins, C.; Knobil, K.; Willits, L.R.; Yates, J.C.; Jones, P.W. Adherence to inhaled therapy, mortality and hospital admission in COPD. Thorax 2009, 64, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Gaduzo, S.; McGovern, V.; Roberts, J.; Scullion, J.E.; Singh, D. When to use single-inhaler triple therapy in COPD: A practical approach for primary care health care professionals. Int. J. Chron. Obstruct. Pulmon. Dis. 2019, 14, 391–401. [Google Scholar] [CrossRef]
- Calzetta, L.; Matera, M.G.; Cazzola, M. Pharmacological mechanisms leading to synergy in fixed-dose dual bronchodilator therapy. Curr. Opin. Pharmacol. 2018, 40, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Calzetta, L.; Matera, M.G.; Cazzola, M.; Rogliani, P. Optimizing the development strategy of combination therapy in respiratory medicine: From isolated airways to patients. Adv. Ther. 2019, 36, 3291–3298. [Google Scholar] [CrossRef]
- Sposato, B.; Petrucci, E.; Serafini, A.; Lena, F.; Lacerenza, L.G.; Montagnani, A.; Alessandri, M.; Cresti, A.; Scala, R.; Rogliani, P.; et al. Which LABA/LAMA should be chosen in COPD patients in real life? Pulm. Pharmacol. Ther. 2021, 71, 102076. [Google Scholar] [CrossRef] [PubMed]
- Rogliani, P.; Matera, M.G.; Facciolo, F.; Page, C.; Cazzola, M.; Calzetta, L. Beclomethasone dipropionate, formoterol fumarate and glycopyrronium bromide: Synergy of triple combination therapy on human airway smooth muscle ex vivo. Br. J. Pharmacol. 2020, 177, 1150–1163. [Google Scholar] [PubMed]





| Age | 72.5 ± 9.04 |
| Sex (M/F) | 38/27 |
| Ex smokers | 63 (96.9%) |
| Smokers | 2 (3.1%) |
| GOLD stage II | 15 (23.1%) |
| GOLD stage III | 31 (52.3%) |
| GOLD stage IV | 16 (24.6%) |
| Blood eosinophils count ≥ 300/µL (evaluated on 58 patients) | 7 (12%) |
| Blood eosinophils count between 200 and 299/µL (evaluated on 58 patients) | 13 (22.4%) |
| Blood eosinophils count between 100 and 199/µL (evaluated on 58 patients) | 38 (65.6%) |
| Cardiovascular diseases | 52 (80%) |
| GER/DY | 52 (80%) |
| Hypertension | 51 (78.4%) |
| Dyslipidemia | 24 (36.9%) |
| Cancer | 22 (33.8%) |
| Psychiatric disorders | 20 (30.7%) |
| Hyperuricemia | 18 (27.7%) |
| Diabetes | 15 (23.1%) |
| Prostatic hyperplasia | 14 (21.5%) |
| Anemia | 12 (18.4%) |
| Others | 32 (49.2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sposato, B.; Lacerenza, L.G.; Croce, S.; Petrucci, E.; Fabbrini, V.; Giannini, L.; Baratta, P.; Cresti, A.; Ricci, A.; Micheletto, C.; et al. Real-Life Efficacy of Single-Inhaler Triple Therapy with Budesonide/Glycopyrronium/Formoterol Fumarate in Persistent COPD Users: A Retrospective Database Study. Biomedicines 2025, 13, 2681. https://doi.org/10.3390/biomedicines13112681
Sposato B, Lacerenza LG, Croce S, Petrucci E, Fabbrini V, Giannini L, Baratta P, Cresti A, Ricci A, Micheletto C, et al. Real-Life Efficacy of Single-Inhaler Triple Therapy with Budesonide/Glycopyrronium/Formoterol Fumarate in Persistent COPD Users: A Retrospective Database Study. Biomedicines. 2025; 13(11):2681. https://doi.org/10.3390/biomedicines13112681
Chicago/Turabian StyleSposato, Bruno, Leonardo Gianluca Lacerenza, Sara Croce, Elisa Petrucci, Valentina Fabbrini, Laura Giannini, Pasquale Baratta, Alberto Cresti, Alberto Ricci, Claudio Micheletto, and et al. 2025. "Real-Life Efficacy of Single-Inhaler Triple Therapy with Budesonide/Glycopyrronium/Formoterol Fumarate in Persistent COPD Users: A Retrospective Database Study" Biomedicines 13, no. 11: 2681. https://doi.org/10.3390/biomedicines13112681
APA StyleSposato, B., Lacerenza, L. G., Croce, S., Petrucci, E., Fabbrini, V., Giannini, L., Baratta, P., Cresti, A., Ricci, A., Micheletto, C., Perrella, A., Alonzi, V., Serafini, A., & Scalese, M. (2025). Real-Life Efficacy of Single-Inhaler Triple Therapy with Budesonide/Glycopyrronium/Formoterol Fumarate in Persistent COPD Users: A Retrospective Database Study. Biomedicines, 13(11), 2681. https://doi.org/10.3390/biomedicines13112681









