The Long-Term Outcomes of Corticosteroid Use in COVID-19 Patients with Cardiovascular Disease: A Propensity-Matched Analysis from the Multi-Center International Prospective Registry (HOPE-2)
Abstract
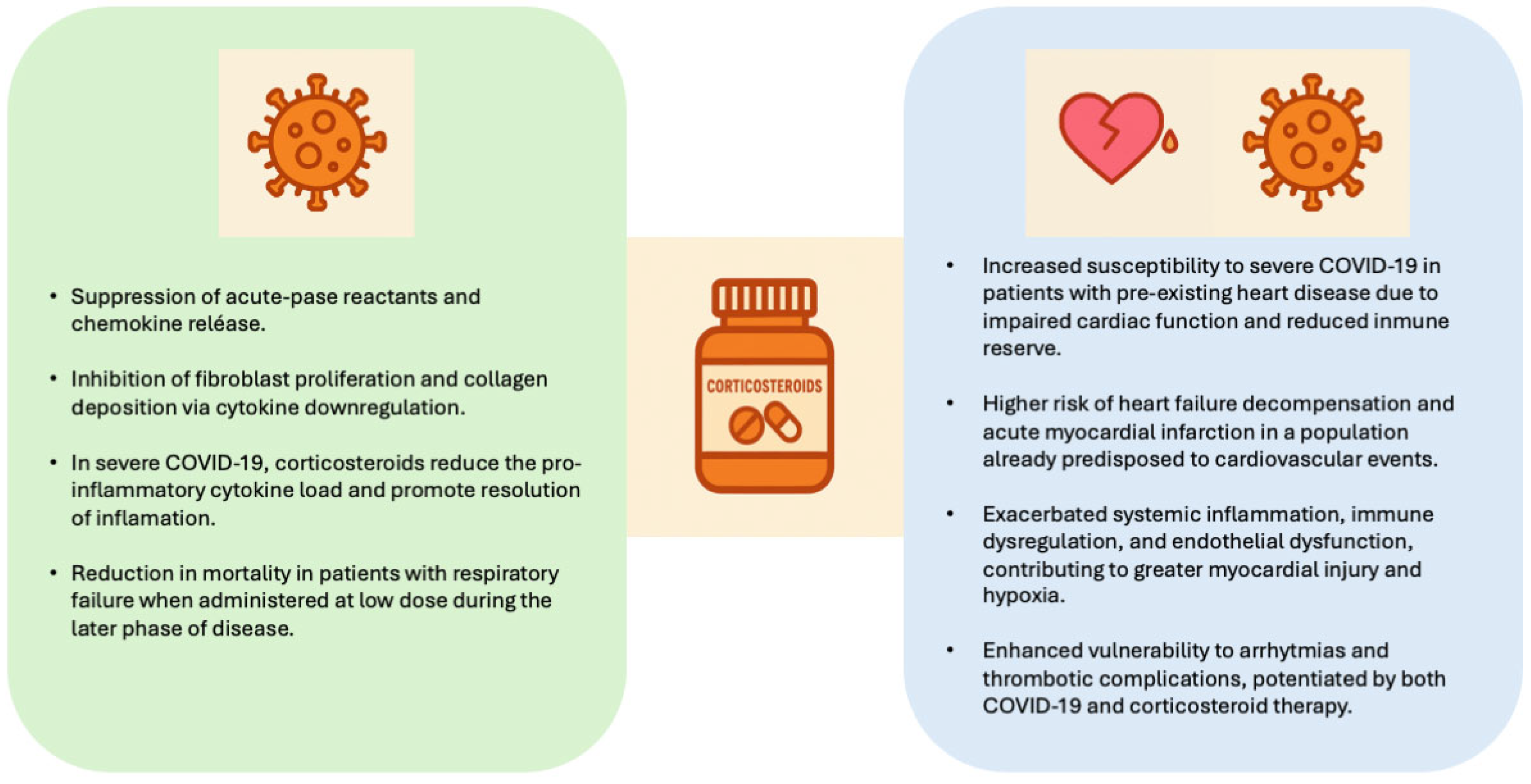
1. Introduction
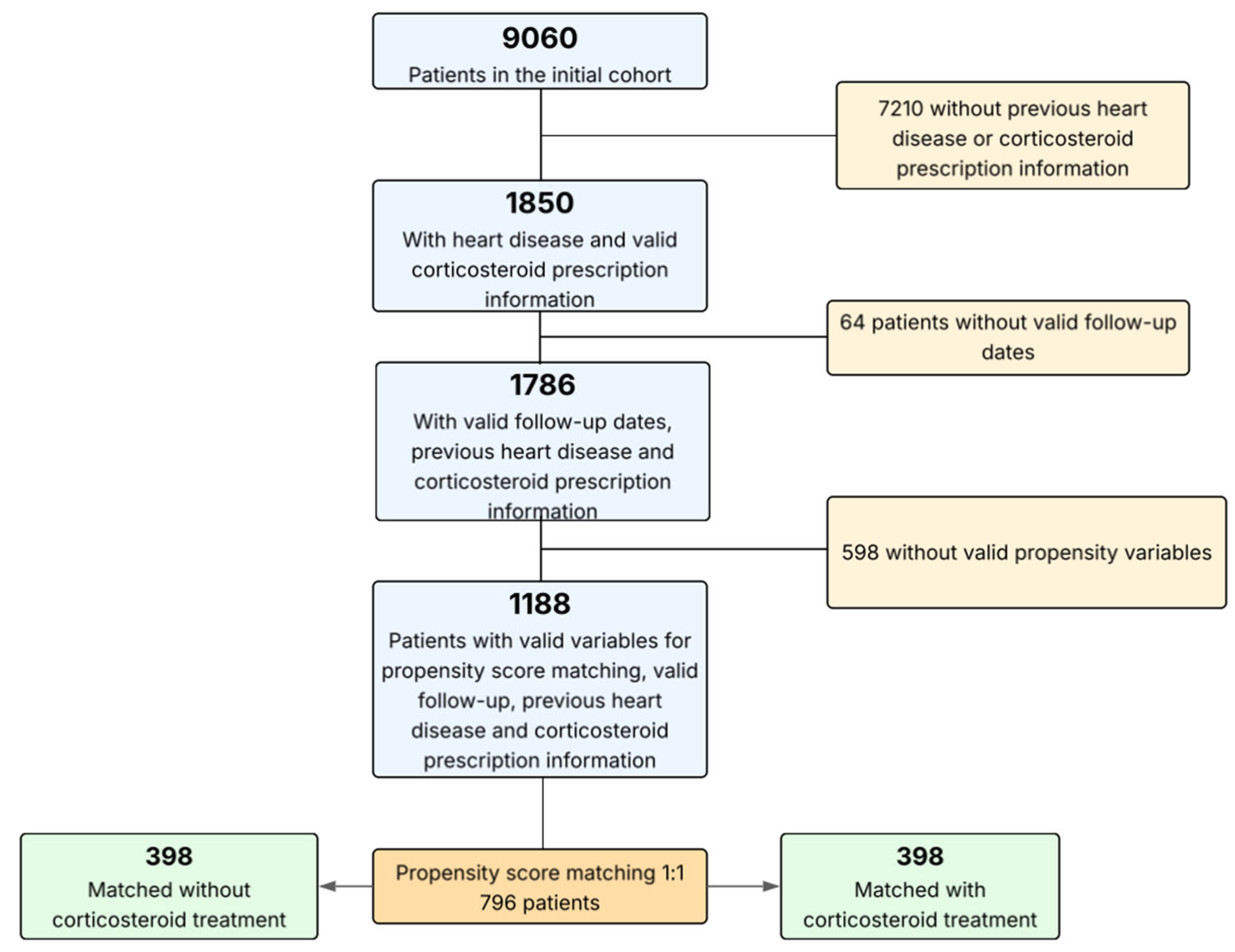
2. Materials and Methods
2.1. Study Design and Participation Criteria
2.2. Data Acquisition and Study Definitions
2.3. Study Follow-Up and Outcomes
2.4. Statistical Analysis
3. Results
4. Discussion
Clinical Implications
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- RECOVERY Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in hospitalized patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar]
- Núñez-Gil, I.J.; Fernández-Ortiz, A.; Maroud Eid, C.; Huang, J.; Romero, R.; Becerra-Muñoz, V.M.; Uribarri, A.; Feltes, G.; Trabatoni, D.; Fernandez-Rozas, I.; et al. Underlying heart diseases and acute COVID-19 outcomes. Cardiol. J. 2021, 28, 202–221. [Google Scholar] [CrossRef]
- Matthay, M.A.; Wick, K.D. Corticosteroids, COVID-19 pneumonia, and acute respiratory distress syndrome. J. Clin. Investig. 2020, 130, 6218–6221. [Google Scholar] [CrossRef] [PubMed]
- Pérez de Llano, L.A.; Golpe, R.; Pérez-Ortiz, D.; Menéndez, R.; España Yandiola, P.P.; Artaraz, A.; Zalacain, R.; Cilloniz, C.; Torres, A. Early initiation of corticosteroids might be harmful in patients hospitalized with COVID-19 pneumonia: A multicenter propensity score analysis. Arch. De Bronconeumol. 2021, 58, 281. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.-T.; Bani-Sadr, F.; Robineau, O.; Perpoint, T.; Perrodeau, E.; Gallay, L.; Ravaud, P.; Goehringer, F.; Lescure, F.-X.; Ismaël, S.; et al. Corticosteroids in patients hospitalized for COVID-19 pneumonia who require oxygen: Observational comparative study using routine care data. Clin. Microbiol. Infect. 2021, 27, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Vazirani, R.; Feltes, G.; Hoyo, R.S.-D.; Viana-Llamas, M.C.; Raposeiras-Roubín, S.; Romero, R.; Alfonso-Rodríguez, E.; Uribarri, A.; Santoro, F.; Becerra-Muñoz, V.; et al. Elevated troponins after COVID-19 hospitalization and long-term COVID-19 symptoms: Incidence, prognosis, and clinical outcomes–results from a multi-center international prospective registry (HOPE-2). J. Clin. Med. 2024, 13, 2596. [Google Scholar] [CrossRef]
- Choi, K.Y.; Lee, H.J.; Lee, H.W.; Park, T.Y.; Heo, E.Y.; Kim, D.K.; Lee, J.-K. Systemic corticosteroid use and cardiovascular risk in patients hospitalized for pneumonia. Steroids 2023, 191, 109161. [Google Scholar] [CrossRef]
- Núñez-Gil, I.J.; Feltes, G.; Viana-Llamas, M.C.; Raposeiras-Roubin, S.; Romero, R.; Alfonso-Rodríguez, E.; Uribarri, A.; Santoro, F.; Becerra-Muñoz, V.; Pepe, M.; et al. Post-COVID-19 symptoms and heart disease: Incidence, prognostic factors, outcomes and vaccination: Results from a multi-center international prospective registry (HOPE 2). J. Clin. Med. 2023, 12, 706. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, R.; Zhang, C.; Guo, Y.; Lv, M.; Zhang, C.; Bian, C.; Jiang, R.; Zhou, W.; Guo, L. Effects of the pre-existing coronary heart disease on the prognosis of COVID-19 patients: A systematic review and meta-analysis. PLoS ONE. 2023, 18, e0292021. [Google Scholar] [CrossRef]
- World Health Organization. Therapeutics and COVID-19: Living Guideline; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Varas-Lorenzo, C.; Rodriguez, L.A.; Maguire, A.; Castellsague, J.; Perez-Gutthann, S. Use of oral corticosteroids and the risk of acute myocardial infarction. Atherosclerosis 2007, 192, 376–383. [Google Scholar] [CrossRef]
- Chen, L.; Hao, G. The role of angiotensin-converting enzyme 2 in coronaviruses/influenza viruses and cardiovascular disease. Cardiovasc. Res. 2020, 116, 1932–1936. [Google Scholar] [CrossRef] [PubMed]
- Burrell, L.M.; Risvanis, J.; Kubota, E.; Dean, R.G.; MacDonald, P.S.; Lu, S.; Tikellis, C.; Grant, S.L.; Lew, R.A.; Smith, A.I.; et al. Myocardial infarction increases ACE2 expression in rat and humans. Eur. Heart J. 2005, 26, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Soro-Paavonen, A.; Gordin, D.; Forsblom, C.; Rosengard-Barlund, M.; Waden, J.; Thorn, L.; Sandholm, N.; Thomas, M.C.; Groop, P.-H. Circulating ACE2 activity is increased in patients with type 1 diabetes and vascular complications. J. Hypertens. 2012, 30, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Aparisi, Á.; Amat-Santos, I.J.; López Otero, D.; Marcos-Mangas, M.; González-Juanatey, J.R.; San Román, J.A. Impact of statins in patients with COVID-19. Rev. Esp. Cardiol. 2021, 74, 637–640. [Google Scholar] [CrossRef]
- van der Hooft, C.S.; Heeringa, J.; Brusselle, G.G.; Hofman, A.; Witteman, J.C.; Kingma, J.H.; Sturkenboom, M.C.; Stricker, B.H. Corticosteroids and the risk of atrial fibrillation. Arch. Intern. Med. 2006, 166, 1016–1020. [Google Scholar] [CrossRef]
- Fardet, L.; Fève, B. Systemic glucocorticoid therapy: A review of its metabolic and cardiovascular adverse events. Drugs 2014, 74, 1731–1745. [Google Scholar] [CrossRef]
- Goodwin, J.E.; Zhang, J.; Geller, D.S. A critical role for vascular smooth muscle in acute glucocorticoid-induced hypertension. J. Am. Soc. Nephrol. 2008, 19, 1291–1299. [Google Scholar] [CrossRef]
- Pujades-Rodriguez, M.; Morgan, A.W.; Cubbon, R.M.; Wu, J. Dose-dependent oral glucocorticoid cardiovascular risks in people with immune-mediated inflammatory diseases: A population-based cohort study. PLoS Med. 2020, 17, e1003432. [Google Scholar] [CrossRef]
- Zhang, Y.; Coats, A.J.S.; Zheng, Z.; Adamo, M.; Ambrosio, G.; Anker, S.D.; Butler, J.; Xu, D.; Mao, J.; Khan, M.S.; et al. Management of heart failure patients with COVID-19: A joint position paper of the Chinese Heart Failure Association and National Heart Failure Committee and the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 941–956. [Google Scholar] [CrossRef]
- Souverein, P.C.; Berard, A.; Van Staa, T.P.; Cooper, C.; Egberts, A.C.G.; Leufkens, H.G.M.; Walker, B.R. Use of oral glucocorticoids and risk of cardiovascular and cerebrovascular disease in a population-based case-control study. Heart 2004, 90, 859–865. [Google Scholar] [CrossRef]
- Wei, L.; MacDonald, T.M.; Walker, B.R. Taking glucocorticoids by prescription is associated with subsequent cardiovascular disease. Ann. Intern. Med. 2004, 141, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Narum, S.; Westergren, T.; Klemp, M. Corticosteroids and risk of gastrointestinal bleeding: A systematic review and meta-analysis. BMJ Open 2014, 4, e004587. [Google Scholar] [CrossRef] [PubMed]
- Rentsch, C.T.; Beckman, J.A.; Tomlinson, L.; Gellad, W.F.; Alcorn, C.; Kidwai-Khan, F.; Skanderson, M.; Brittain, E.; King, J.T.; Ho, Y.-L.; et al. Early initiation of prophylactic anticoagulation for prevention of coronavirus disease 2019 mortality in patients admitted to hospital in the United States: Cohort study. BMJ 2021, 372, n311. [Google Scholar] [CrossRef] [PubMed]
- Santoro, F.; Núñez-Gil, I.J.; Vitale, E.; Viana-Llamas, M.C.; Romero, R.; Eid, C.M.; Guzman, G.F.; Becerra-Muñoz, V.M.; Rozas, I.F.; Uribarri, A.; et al. Aspirin therapy on prophylactic anticoagulation for patients hospitalized with COVID-19: A propensity score-matched cohort analysis of the HOPE-COVID-19 registry. J. Am. Heart Assoc. 2022, 11, e024530. [Google Scholar] [CrossRef]
- Yao, T.C.; Huang, Y.W.; Chang, S.M.; Tsai, S.Y.; Wu, A.C.; Tsai, H.J. Association between oral corticosteroid bursts and severe adverse events: A nationwide population-based cohort study. Ann. Intern. Med. 2020, 173, 325–330. [Google Scholar] [CrossRef]
- Zamarrón, E.; Carpio, C.; Villamañán, E.; Álvarez-Sala, R.; Borobia, A.M.; Gómez-Carrera, L.; Buño, A.; Prados, C.; COVID@HULP Working Group; POSTCOVID@HULP Working Group. Impact of systemic corticosteroids on hospital length of stay among patients with COVID-19. Farm. Hosp. 2023, 47, 55–63. [Google Scholar] [CrossRef]
- Ibrahim, S.; Siemieniuk, R.A.C.; Oliveros, M.J.; Islam, N.; Martinez, J.P.D.; Izcovich, A.; Qasim, A.; Zhao, Y.; Zaror, C.; Yao, L.; et al. Drug treatments for mild or moderate COVID-19: Systematic review and network meta-analysis. BMJ 2025, 389, e081165. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- PHOSP-COVIDStudy Collaborative Group; Lawson, C.A.; Moss, A.J.; Arnold, J.R.; Bagot, C.; Banerjee, A.; Berry, C.; Greenwood, J.; Hughes, A.D.; Khunti, K.; et al. Long COVID and cardiovascular disease: A prospective cohort study. Open Heart 2024, 11, e002662. [Google Scholar] [CrossRef]
- Freund, O.; Breslavsky, A.; Fried, S.; Givoli-Vilensky, R.; Cohen-Rubin, S.; Zacks, N.; Kleinhendler, E.; Unterman, A.; Frydman, S.; Wand, O.; et al. Interactions and clinical implications of serological and respiratory variables 3 months after acute COVID-19. Clin. Exp. Med. 2023, 23, 6331–6340. [Google Scholar] [CrossRef]
- Davelaar, J.; Jessurun, N.; Schaap, G.; Bode, C.; Vonkeman, H. The effect of corticosteroids, antibiotics, and anticoagulants on the development of post-COVID-19 syndrome in COVID-19 hospitalized patients 6 months after discharge: A retrospective follow-up study. Clin. Exp. Med. 2023, 23, 4881–4888. [Google Scholar] [CrossRef]

| Matched Population | Matched Population (796) | Corticosteroid Treatment (398) | Non-Corticosteroid Treatment (398) | p-Value |
|---|---|---|---|---|
| Age, years | 76.4 ± 12.5 | 76.4 ± 12.7 | 76.3 ± 12.3 | 0.964 |
| Male | 533 (67.0%) | 266 (66.8%) | 267 (67.1%) | 0.940 |
| Hypertension | 646 (81.2%) | 323 (81.2%) | 323 (81.2%) | 1.000 |
| Obesity | 197 (24.7%) | 95 (23.9%) | 102 (25.6%) | 0.565 |
| Type 2 diabetes mellitus | 268 (33.7%) | 136 (34.2%) | 132 (33.2%) | 0.764 |
| Dyslipidemia | 420 (52.8%) | 207 (52.0%) | 213 (53.5%) | 0.670 |
| Smoking | 53 (6.7%) | 28 (7.0%) | 25 (6.3%) | 0.670 |
| Chronic kidney disease | 151 (19.0%) | 74 (18.6%) | 77 (19.3%) | 0.786 |
| Lung disease | 344 (43.2%) | 174 (43.7%) | 170 (42.7%) | 0.775 |
| Cerebrovascular disease | 139 (17.5%) | 72 (18.1%) | 67 (16.8%) | 0.641 |
| Liver disease | 50 (6.3%) | 25 (6.3%) | 25 (6.3%) | 1.000 |
| History of cancer | 154 (19.3%) | 75 (18.8%) | 79 (19.8%) | 0.720 |
| Immunosuppression | 79 (9.9%) | 41 (10.3%) | 38 (9.5%) | 0.722 |
| Previous vaccination | 122 (47.7%) | 54 (41.9%) | 68 (53.5%) | 0.061 |
| Anticoagulation therapy | 314 (39.4%) | 164 (41.2%) | 150 (37.7%) | 0.310 |
| Aspirin | 74 (30.2%) | 33 (27.0%) | 41 (33.3%) | 0.284 |
| ACEI/ARB | 457 (57.4%) | 233 (58.0%) | 224 (56.3%) | 0.519 |
| Statins | 91 (37.9%) | 37 (30.3%) | 54 (43.9%) | 0.028 |
| Respiratory failure during admission | 629 (79.0%) | 312 (78.4%) | 317 (79.6%) | 0.663 |
| Heart failure during admission | 165 (20.7%) | 85 (21.4%) | 80 (20.1%) | 0.662 |
| Kidney failure during admission | 280 (35.2%) | 144 (36.2%) | 136 (34.2%) | 0.553 |
| Upper respiratory tract infection | 133 (16.7%) | 69 (17.3%) | 64 (16.1%) | 0.635 |
| Pneumonia | 744 (93.5%) | 371 (93.2%) | 373 (93.7%) | 0.774 |
| Sepsis | 139 (17.5%) | 72 (18.1%) | 67 (16.8%) | 0.641 |
| SIRS | 244 (30.7%) | 133 (33.4%) | 111 (27.9%) | 0.910 |
| ECMO or any circulatory support | 68 (8.5%) | 35 (8.8%) | 33 (8.3%) | 0.800 |
| Non-invasive mechanical ventilation | 144 (18.1%) | 80 (20.1%) | 64 (16.1%) | 0.141 |
| Invasive mechanical ventilation | 85 (10.7%) | 52 (13.1%) | 33 (8.3%) | 0.029 |
| High-flow nasal cannula | 223 (28.3%) | 113 (28.4%) | 112 (28.1%) | 0.937 |
| In-hospital relevant bleeding | 53 (6.7%) | 35 (8.8%) | 18 (4.5%) | 0.016 |
| Hospital stay (days) | 9.5 {5–16} | 8.0 {4–14} | 11 {7–18} | <0.001 |
| In-hospital mortality | 309 (38.8%) | 164 (41.2%) | 145 (36.4%) | 0.167 |
| All-cause mortality | 333 (41.8%) | 175 (44.0%) | 158 (39.7%) | 0.222 |
| Follow-up time, days | 16 [7.0–50.7] | 17 [8–42.7] | 16 [6–56.2] | 0.450 |
| Main Heart Disease | Matched Population (796) | Corticosteroid Treatment (398) | Non-Corticosteroid Treatment (398) |
|---|---|---|---|
| Arrhythmias | 211 (26.5%) | 111 (27.9%) | 100 (25.1%) |
| Coronary | 231 (29.0%) | 109 (27.4%) | 122 (30.7%) |
| Heart failure | 81 (10.2%) | 43 (10.8%) | 38 (9.5%) |
| Valvular disease | 67 (8.4%) | 31 (7.8%) | 36 (9.0%) |
| Combined | 180 (22.6%) | 90 (22.6%) | 90 (22.6%) |
| Not recorded | 26 (3.3%) | 14 (3.5%) | 12 (3.0%) |
| Long-Term COVID-19 Symptoms | Matched Population (487) | Corticosteroid Treatment (234) | Non-Corticosteroid Treatment (253) | p-Value |
|---|---|---|---|---|
| Long-Term COVID-19 Cardiovascular Traits | ||||
| Fatigue | 107/245 (43.7%) | 52/122 (42.6%) | 55/123 (44.7%) | 0.777 |
| Dizziness | 31/245 (12.7%) | 20/122 (16.4%) | 11/123 (8.9%) | 0.079 |
| Chest pain | 24/245 (9.8%) | 8/122 (6.6%) | 16/123 (13.0%) | 0.089 |
| Acute coronary syndrome | 8/245 (3.3%) | 5/122 (4.1%) | 3/123 (2.4%) | 0.500 |
| Palpitations | 38/245 (15.5%) | 23/122 (18.9%) | 15/123 (12.2%) | 0.150 |
| Resting heart rate increase | 18/245 (7.3%) | 12/122 (9.8%) | 6/123 (4.9%) | 0.150 |
| Syncope | 7/245 (2.9%) | 4/122 (3.3%) | 3/123 (2.4%) | 0.722 |
| Any arrhythmia | 40/245 (16.3%) | 22/122 (18%) | 18/123 (14.6%) | 0.472 |
| Atrial fibrillation | 29/245 (11.8%) | 15/122 (18%) | 14/123 (11.4%) | 0.825 |
| Perimyocarditis | 2/245 (0.8%) | 2/122 (1.6%) | 0/123 (0.0%) | 0.247 |
| Edema | 18/245 (7.3%) | 9/122 (7.4%) | 9 (7.3%) /123 (0.0%) | 0.986 |
| Incident hypertension | 5/245 (2.0%) | 3/122 (2.5%) | 2/123 (1.6%) | 0.684 |
| Left ventricular dysfunction | 12/245 (4.9%) | 8/122 (6.6%) | 4/123 (3.3%) | 0.254 |
| Relevant bleeding | 5/245 (2.0%) | 5/122 (4.1%) | 0/123 (0.0%) | 0.029 |
| Long-Term COVID-19 Neuro-Psychological Traits | ||||
| Headache | 11/245 (4.5%) | 6/122 (4.9%) | 5/123 (4.1%) | 0.747 |
| Migraine | 8/245 (3.3%) | 5/122 (4.1%) | 3/123 (2.4%) | 0.500 |
| Ageusia | 18/245 (7.3%) | 7/122 (5.7%) | 11/123 (8.9%) | 0.336 |
| Cognitive disorder | 19/245 (7.8%) | 8/122 (6.6%) | 11/123 (8.9%) | 0.485 |
| Anxiety | 28/245 (11.4%) | 18/122 (14.8%) | 10/123 (8.1%) | 0.103 |
| Depression | 20/245 (8.2%) | 11/122 (9%) | 9/123 (7.3%) | 0.627 |
| Tinnitus or hearing loss | 14/245 (5.7%) | 6/122 (4.9%) | 8/123 (6.5%) | 0.593 |
| Sleep disorder | 26/245 (10.6%) | 13/122 (10.7%) | 13/123 (10.6%) | 0.982 |
| Mood disorder | 21/245 (8.6%) | 13/122 (10.7%) | 8/123 (6.5%) | 0.246 |
| Paranoia | 17/245 (6.9%) | 11/122 (9.0%) | 6/123 (4.9%) | 0.202 |
| Other Long-Term COVID-19 Symptoms | ||||
| Cough | 37/245 (15.1%) | 20/122 (16.4%) | 17/123 (13.8%) | 0.574 |
| Polypnea | 16/245 (6.5%) | 10/122 (8.2%) | 6/123 (4.9%) | 0.293 |
| Sleep apnea | 15/245 (6.1%) | 9/122 (7.4%) | 6/123 (4.9%) | 0.415 |
| Tongue involvement | 5/245 (2.0%) | 2/122 (1.6%) | 3/123 (2.4%) | 1.000 |
| Digestive disorders | 13/245 (5.3%) | 5/122 (4.1%) | 8/123 (6.5%) | 0.401 |
| Nausea and vomiting | 7/245 (2.9%) | 5/122 (4.1%) | 2/123 (1.6%) | 0.281 |
| Intermittent fever | 5/245 (2.0%) | 4/122 (3.3%) | 1/123 (0.8%) | 0.213 |
| Chills | 6/245 (2.4%) | 5/122 (4.1%) | 1/123 (0.8%) | 0.120 |
| Hair loss | 9/245 (3.7%) | 5/122 (4.1%) | 4/123 (3.3%) | 0.749 |
| Joint pain | 14/245 (5.7%) | 6/122 (4.9%) | 8/123 (6.5%) | 0.593 |
| Myalgias | 17/245 (6.9%) | 3/122 (2%) | 14/123 (11.4%) | 0.06 |
| Sweats | 5/245 (2.0%) | 2/122 (1.6%) | 3/123 (2.4%) | 1.000 |
| Weight loss | 23/245 (9.4%) | 9/122 (7.4%) | 14/123 (11.4%) | 0.381 |
| Anosmia | 13/245 (5.3%) | 6/122 (4.9%) | 7/123 (5.7%) | 0.787 |
| Attention disorder | 19/245 (7.8%) | 10/122 (8.2%) | 9/123 (7.3%) | 0.797 |
| Memory loss | 24/245 (9.8%) | 12/122 (9.8%) | 12/123 (9.8%) | 0.983 |
| Follow-up time, days | 35 [15–361] | 31.5 [15–311] | 36 [14–378] | 0.450 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Onrubia, J.; Vazirani, R.; Feltes, G.; Sánchez-Del Hoyo, R.; Viana-Llamas, M.C.; Raposeiras-Roubín, S.; Romero, R.; Alfonso-Rodríguez, E.; Uribarri, A.; Santoro, F.; et al. The Long-Term Outcomes of Corticosteroid Use in COVID-19 Patients with Cardiovascular Disease: A Propensity-Matched Analysis from the Multi-Center International Prospective Registry (HOPE-2). Biomedicines 2025, 13, 2665. https://doi.org/10.3390/biomedicines13112665
García-Onrubia J, Vazirani R, Feltes G, Sánchez-Del Hoyo R, Viana-Llamas MC, Raposeiras-Roubín S, Romero R, Alfonso-Rodríguez E, Uribarri A, Santoro F, et al. The Long-Term Outcomes of Corticosteroid Use in COVID-19 Patients with Cardiovascular Disease: A Propensity-Matched Analysis from the Multi-Center International Prospective Registry (HOPE-2). Biomedicines. 2025; 13(11):2665. https://doi.org/10.3390/biomedicines13112665
Chicago/Turabian StyleGarcía-Onrubia, Jorge, Ravi Vazirani, Gisela Feltes, Rafael Sánchez-Del Hoyo, María C. Viana-Llamas, Sergio Raposeiras-Roubín, Rodolfo Romero, Emilio Alfonso-Rodríguez, Aitor Uribarri, Francesco Santoro, and et al. 2025. "The Long-Term Outcomes of Corticosteroid Use in COVID-19 Patients with Cardiovascular Disease: A Propensity-Matched Analysis from the Multi-Center International Prospective Registry (HOPE-2)" Biomedicines 13, no. 11: 2665. https://doi.org/10.3390/biomedicines13112665
APA StyleGarcía-Onrubia, J., Vazirani, R., Feltes, G., Sánchez-Del Hoyo, R., Viana-Llamas, M. C., Raposeiras-Roubín, S., Romero, R., Alfonso-Rodríguez, E., Uribarri, A., Santoro, F., Becerra-Muñoz, V., Pepe, M., Castro-Mejía, A. F., Signes-Costa, J., Gonzalez, A., Marín, F., Lopez-País, J., Cerrato, E., Vázquez-Cancela, O., ... Nuñez-Gil, I. J. (2025). The Long-Term Outcomes of Corticosteroid Use in COVID-19 Patients with Cardiovascular Disease: A Propensity-Matched Analysis from the Multi-Center International Prospective Registry (HOPE-2). Biomedicines, 13(11), 2665. https://doi.org/10.3390/biomedicines13112665










