Bidirectional Mendelian Randomization Analysis of the Causal Relationship Between Uterine Fibroids and Breast Cancer in East Asian Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Sources
2.3. Selection of the Genetic Instrumental Variables
2.4. Mendelian Randomization
2.5. Statistical Analysis
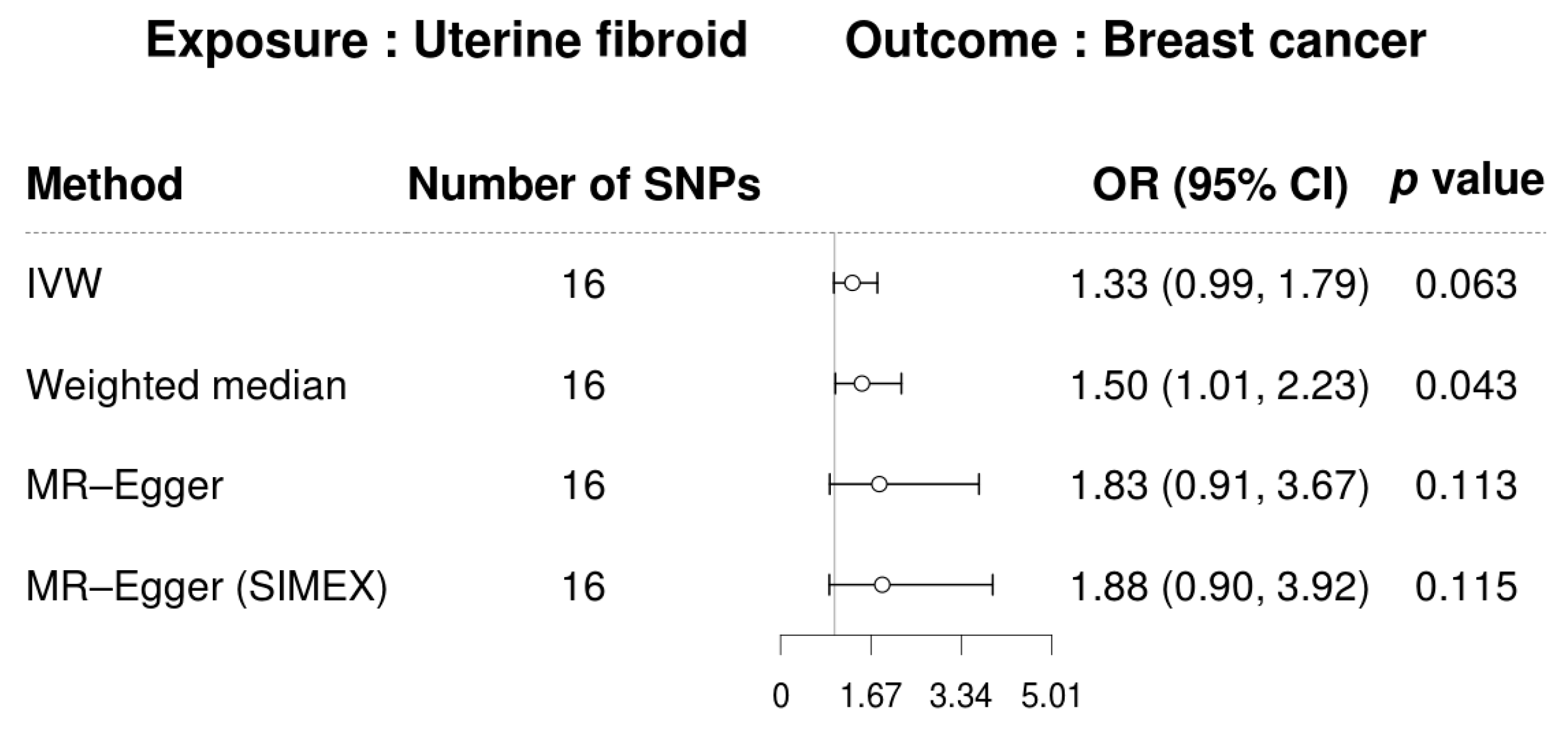
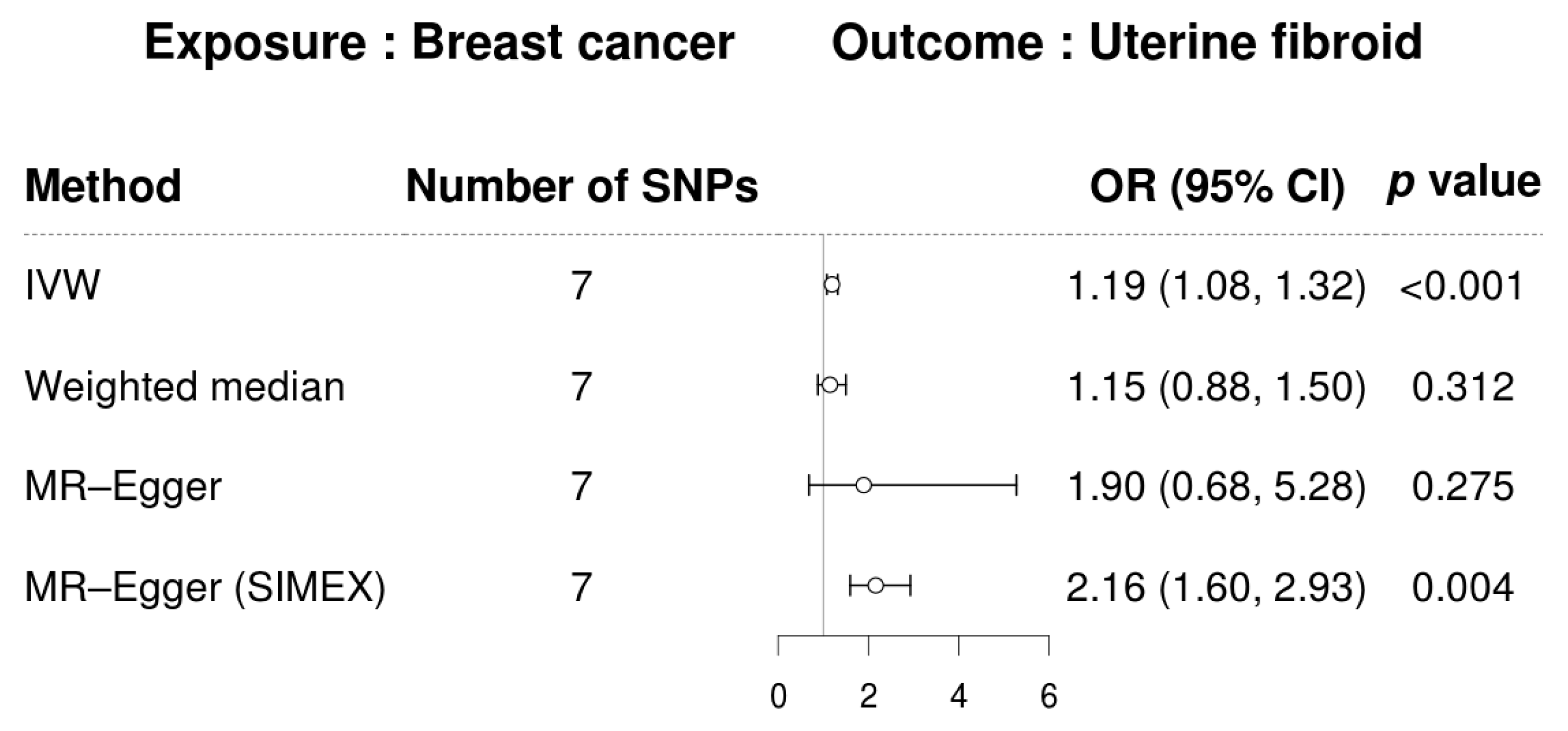
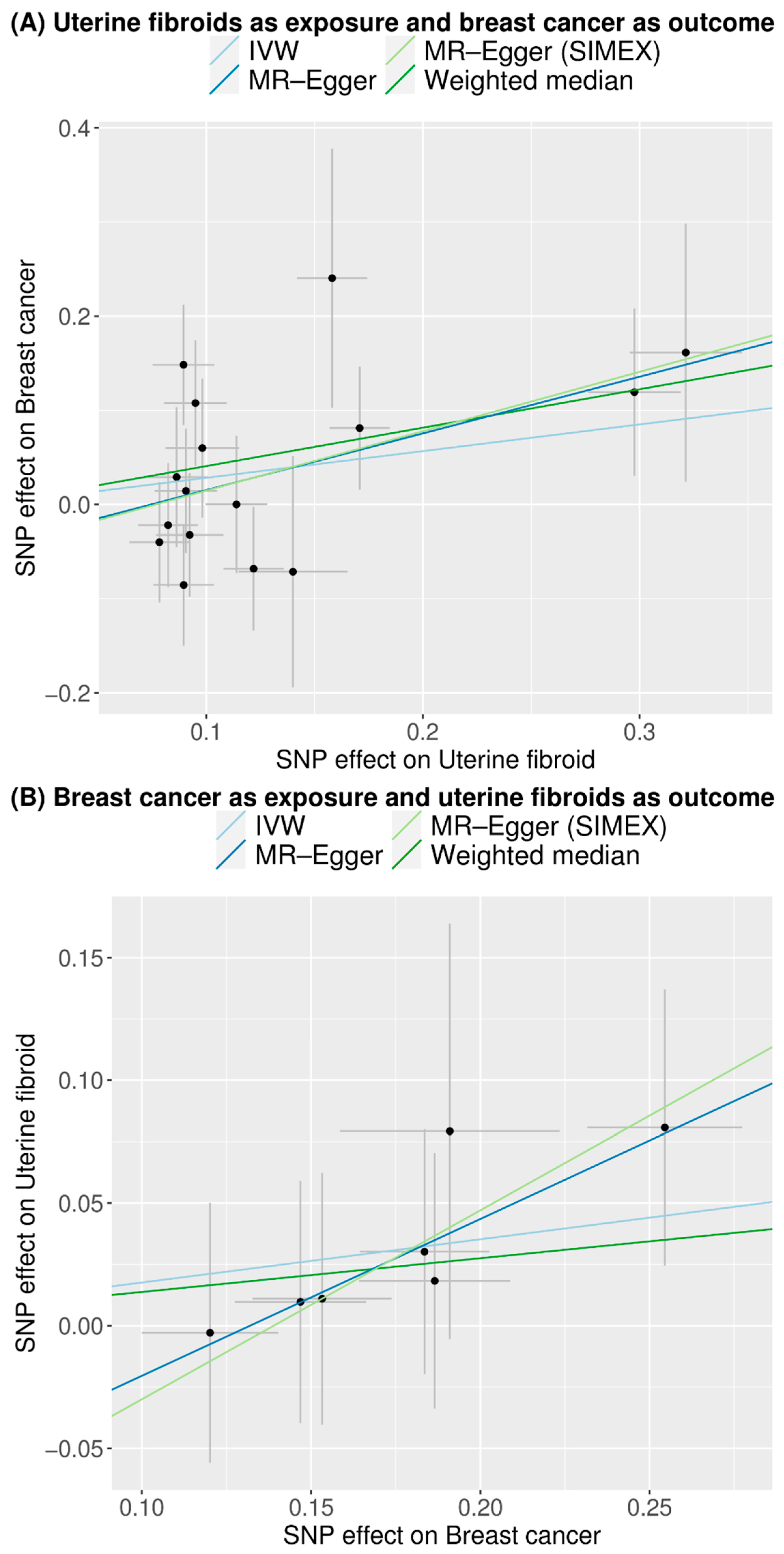
3. Results
3.1. Selection of Instrumental Variables
3.2. Heterogeneity and Horizontal Pleiotropy of the Instrumental Variables
3.3. Mendelian Randomization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BBJ | Biobank Japan |
| BC | Breast cancer |
| CI | Confidence interval |
| CKB | China Kadoorie Biobank |
| F | Mean F-statistic |
| GWAS | Genome-wide association study |
| HR | Hazard ratio |
| IV | Instrumental variable |
| IVW | Inverse-variance-weighted |
| KoGES | Korean Genome and Epidemiology Study |
| LD | Linkage disequilibrium |
| MR | Mendelian randomization |
| N | Number of instruments |
| NOME | No measurement error |
| OR | Odds ratio |
| PRESSO | Pleiotropy residual sum and outlier |
| SE | Standard error |
| SIMEX | Simulation extrapolation |
| SNP | Single-nucleotide polymorphism |
| UF | Uterine fibroids |
| β | Beta coefficient |
References
- Kim, J.; Harper, A.; McCormack, V.; Sung, H.; Houssami, N.; Morgan, E.; Mutebi, M.; Garvey, G.; Soerjomataram, I.; Fidler-Benaoudia, M.M. Global patterns and trends in breast cancer incidence and mortality across 185 countries. Nat. Med. 2025, 31, 1154–1162. [Google Scholar] [CrossRef]
- Dai, Y.; Chen, H.; Yu, J.; Cai, J.; Lu, B.; Dai, M.; Zhu, L. Global and regional trends in the incidence and prevalence of uterine fibroids and attributable risk factors at the national level from 2010 to 2019: A worldwide database study. Chin. Med. J. 2024, 137, 2583–2589. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Kumar, V.; Brahmachari, S.; Pandya, B. A Study on Clinico-Pathological Profile of Breast Cancer Patients and Their Correlation with Uterine Fibroids Using Hormone Level and Receptor Status Assessment. Breast Cancer 2022, 16, 11782234221090197. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Ciebiera, M.; Vafaei, S.; Alkhrait, S.; Chen, H.Y.; Chiang, Y.F.; Huang, K.C.; Feduniw, S.; Hsia, S.M.; Al-Hendy, A. Progesterone Signaling and Uterine Fibroid Pathogenesis; Molecular Mechanisms and Potential Therapeutics. Cells 2023, 12, 1117. [Google Scholar] [CrossRef]
- Yuk, J.S.; Yang, S.W.; Yoon, S.H.; Kim, M.H.; Seo, Y.S.; Lee, Y.; Joo, Y.; Kim, J.; Yoon, S.Y.; Cho, H.; et al. Association between breast diseases and symptomatic uterine fibroids by using South Korean National Health Insurance database. Sci. Rep. 2023, 13, 16772. [Google Scholar] [CrossRef]
- Marsh, E.E.; Bulun, S.E. Steroid hormones and leiomyomas. Obstet. Gynecol. Clin. N. Am. 2006, 33, 59–67. [Google Scholar] [CrossRef]
- Barbarisi, A.; Petillo, O.; Di Lieto, A.; Melone, M.A.; Margarucci, S.; Cannas, M.; Peluso, G. 17-beta estradiol elicits an autocrine leiomyoma cell proliferation: Evidence for a stimulation of protein kinase-dependent pathway. J. Cell Physiol. 2001, 186, 414–424. [Google Scholar] [CrossRef]
- Ishikawa, H.; Ishi, K.; Serna, V.A.; Kakazu, R.; Bulun, S.E.; Kurita, T. Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology 2010, 151, 2433–2442. [Google Scholar] [CrossRef]
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; LaCroix, A.Z.; Kooperberg, C.; Stefanick, M.L.; Jackson, R.D.; Beresford, S.A.; Howard, B.V.; Johnson, K.C.; et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2002, 288, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Teng, M.; Rosa, M.; Wang, X. Unique ER PR expression pattern in breast cancers with CHEK2 mutation: A hormone receptor and HER2 analysis based on germline cancer predisposition genes. Breast Cancer Res. 2022, 24, 11. [Google Scholar] [CrossRef]
- Collaborative Group on Hormonal Factors in Breast, C. Menarche, menopause, and breast cancer risk: Individual participant meta-analysis, including 118,964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012, 13, 1141–1151. [Google Scholar] [CrossRef]
- Travis, R.C.; Key, T.J. Oestrogen exposure and breast cancer risk. Breast Cancer Res. 2003, 5, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.W.; Oh, J.H.; Wang, E.K.; Bao, Y.; Kim, Y.B.; Lee, M.H.; Lee, Y.J.; Jo, Y.S.; Ku, C.R.; Lee, E.J. Excess endocrine growth hormone in acromegaly promotes the aggressiveness and metastasis of triple-negative breast cancer. iScience 2024, 27, 110137. [Google Scholar] [CrossRef] [PubMed]
- Tseng, J.J.; Chen, Y.H.; Chiang, H.Y.; Lin, C.H. Increased risk of breast cancer in women with uterine myoma: A nationwide, population-based, case-control study. J. Gynecol. Oncol. 2017, 28, e35. [Google Scholar] [CrossRef] [PubMed]
- Zeldin, J.; Sandler, D.P.; Ogunsina, K.; O’Brien, K.M. Association of Fibroids, Endometriosis, and Gynecologic Surgeries with Breast Cancer Incidence and Hormone Receptor Subtypes. Cancer Epidemiol. Biomark. Prev. 2024, 33, 576–585. [Google Scholar] [CrossRef]
- Lawlor, D.A.; Harbord, R.M.; Sterne, J.A.; Timpson, N.; Davey Smith, G. Mendelian randomization: Using genes as instruments for making causal inferences in epidemiology. Stat. Med. 2008, 27, 1133–1163. [Google Scholar] [CrossRef]
- Seo, J.H.; Lee, Y. Possible Causal Association between Type 2 Diabetes and Glycaemic Traits in Primary Open-Angle Glaucoma: A Two-Sample Mendelian Randomisation Study. Biomedicines 2024, 12, 866. [Google Scholar] [CrossRef]
- Jin, H.; Seo, J.H.; Lee, Y.; Won, S. Genetic risk factors associated with ocular perfusion pressure in primary open-angle glaucoma. Hum. Genom. 2025, 19, 31. [Google Scholar] [CrossRef]
- Zhao, C.; Shang, A.; Wu, H.; Li, Q.; Peng, L.; Yue, C. Causal relationship between genetically predicted uterine leiomyoma and cancer risk: A two-sample Mendelian randomization. Front. Endocrinol. 2024, 15, 1429165. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, M.; Wang, T.; Li, F.; Zhu, Y. A cause-effect relationship between uterine diseases and breast cancer: A bidirectional Mendelian randomization study. Heliyon 2024, 10, e38130. [Google Scholar] [CrossRef]
- Marshall, L.M.; Spiegelman, D.; Barbieri, R.L.; Goldman, M.B.; Manson, J.E.; Colditz, G.A.; Willett, W.C.; Hunter, D.J. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet. Gynecol. 1997, 90, 967–973. [Google Scholar] [CrossRef]
- Kjerulff, K.H.; Langenberg, P.; Seidman, J.D.; Stolley, P.D.; Guzinski, G.M. Uterine leiomyomas. Racial differences in severity, symptoms and age at diagnosis. J. Reprod. Med. 1996, 41, 483–490. [Google Scholar]
- Mitro, S.D.; Dyer, W.; Lee, C.; Bindra, A.; Wang, L.; Ritterman Weintraub, M.; Hedderson, M.M.; Zaritsky, E. Uterine Fibroid Diagnosis by Race and Ethnicity in an Integrated Health Care System. JAMA Netw. Open 2025, 8, e255235. [Google Scholar] [CrossRef]
- Catherino, W.H.; Eltoukhi, H.M.; Al-Hendy, A. Racial and ethnic differences in the pathogenesis and clinical manifestations of uterine leiomyoma. Semin. Reprod. Med. 2013, 31, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Magaoay, B.; Rosen, M.P.; Cedars, M.I. Presence of Fibroids on Transvaginal Ultrasonography in a Community-Based, Diverse Cohort of 996 Reproductive-Age Female Participants. JAMA Netw. Open 2023, 6, e2312701. [Google Scholar] [CrossRef] [PubMed]
- Othman, E.E.; Al-Hendy, A. Molecular genetics and racial disparities of uterine leiomyomas. Best. Pract. Res. Clin. Obstet. Gynaecol. 2008, 22, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Giaquinto, A.N.; Sung, H.; Newman, L.A.; Freedman, R.A.; Smith, R.A.; Star, J.; Jemal, A.; Siegel, R.L. Breast cancer statistics 2024. CA Cancer J. Clin. 2024, 74, 477–495. [Google Scholar] [CrossRef]
- Walters, R.G.; Millwood, I.Y.; Lin, K.; Schmidt Valle, D.; McDonnell, P.; Hacker, A.; Avery, D.; Edris, A.; Fry, H.; Cai, N.; et al. Genotyping and population characteristics of the China Kadoorie Biobank. Cell Genom. 2023, 3, 100361. [Google Scholar] [CrossRef]
- Sakaue, S.; Kanai, M.; Tanigawa, Y.; Karjalainen, J.; Kurki, M.; Koshiba, S.; Narita, A.; Konuma, T.; Yamamoto, K.; Akiyama, M.; et al. A cross-population atlas of genetic associations for 220 human phenotypes. Nat. Genet. 2021, 53, 1415–1424. [Google Scholar] [CrossRef]
- Burgess, S.; Butterworth, A.; Thompson, S.G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 2013, 37, 658–665. [Google Scholar] [CrossRef]
- Bowden, J.; Del Greco, M.F.; Minelli, C.; Davey Smith, G.; Sheehan, N.; Thompson, J. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat. Med. 2017, 36, 1783–1802. [Google Scholar] [CrossRef]
- Burgess, S.; Davey Smith, G.; Davies, N.M.; Dudbridge, F.; Gill, D.; Glymour, M.M.; Hartwig, F.P.; Kutalik, Z.; Holmes, M.V.; Minelli, C.; et al. Guidelines for performing Mendelian randomization investigations: Update for summer 2023. Wellcome Open Res. 2019, 4, 186. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Del Greco, M.F.; Minelli, C.; Davey Smith, G.; Sheehan, N.A.; Thompson, J.R. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: The role of the I2 statistic. Int. J. Epidemiol. 2016, 45, 1961–1974. [Google Scholar] [CrossRef]
- Greco, M.F.; Minelli, C.; Sheehan, N.A.; Thompson, J.R. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat. Med. 2015, 34, 2926–2940. [Google Scholar] [CrossRef]
- Bowden, J.; Holmes, M.V. Meta-analysis and Mendelian randomization: A review. Res. Synth. Methods 2019, 10, 486–496. [Google Scholar] [CrossRef]
- Verbanck, M.; Chen, C.Y.; Neale, B.; Do, R. Publisher Correction: Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 1196. [Google Scholar] [CrossRef]
- Au Yeung, S.L.; Gill, D. Standardizing the reporting of Mendelian randomization studies. BMC Med. 2023, 21, 187. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, M.; Xu, F.; Jiang, S. Wnt signaling in breast cancer: Biological mechanisms, challenges and opportunities. Mol. Cancer 2020, 19, 165. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Deng, C.X. Fibroblast Growth Factor Receptor 2 Signaling in Breast Cancer. Int. J. Biol. Sci. 2017, 13, 1163–1171. [Google Scholar] [CrossRef]
- Jin, H.; Lee, S.; Won, S. Causal Evaluation of Laboratory Markers in Type 2 Diabetes on Cancer and Vascular Diseases Using Various Mendelian Randomization Tools. Front. Genet. 2020, 11, 597420. [Google Scholar] [CrossRef]
- Choi, E.J.; Cho, S.B.; Lee, S.R.; Lim, Y.M.; Jeong, K.; Moon, H.S.; Chung, H. Comorbidity of gynecological and non-gynecological diseases with adenomyosis and endometriosis. Obstet. Gynecol. Sci. 2017, 60, 579–586. [Google Scholar] [CrossRef]
- Lin, K.Y.; Yang, C.Y.; Lam, A.; Chang, C.Y.; Lin, W.C. Uterine leiomyoma is associated with the risk of developing endometriosis: A nationwide cohort study involving 156,195 women. PLoS ONE 2021, 16, e0256772. [Google Scholar] [CrossRef]
- Nam, K.; Kim, J.; Lee, S. Genome-wide study on 72,298 individuals in Korean biobank data for 76 traits. Cell Genom. 2022, 2, 100189. [Google Scholar] [CrossRef]
- Englund, K.; Blanck, A.; Gustavsson, I.; Lundkvist, U.; Sjoblom, P.; Norgren, A.; Lindblom, B. Sex steroid receptors in human myometrium and fibroids: Changes during the menstrual cycle and gonadotropin-releasing hormone treatment. J. Clin. Endocrinol. Metab. 1998, 83, 4092–4096. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Yee, K.A.; Kaplan, C.; Siddiqui, J.F. Estrogen receptor alpha expression in normal human breast epithelium is consistent over time. Int. J. Cancer 2002, 102, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xiao, C.; Han, Z.; Zhang, L.; Zhao, X.; Hao, Y.; Xiao, J.; Gallagher, C.S.; Kraft, P.; Morton, C.C.; et al. Investigating the shared genetic architecture of uterine leiomyoma and breast cancer: A genome-wide cross-trait analysis. Am. J. Hum. Genet. 2022, 109, 1272–1285. [Google Scholar] [CrossRef]
- Mohanty, S.S.; Mohanty, P.K. Obesity as potential breast cancer risk factor for postmenopausal women. Genes. Dis. 2021, 8, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Wielsoe, M.; Kern, P.; Bonefeld-Jorgensen, E.C. Serum levels of environmental pollutants is a risk factor for breast cancer in Inuit: A case control study. Environ. Health 2017, 16, 56. [Google Scholar] [CrossRef]
- Stewart, E.A.; Cookson, C.L.; Gandolfo, R.A.; Schulze-Rath, R. Epidemiology of uterine fibroids: A systematic review. BJOG 2017, 124, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Uimari, O.; Nazri, H.; Tapmeier, T. Endometriosis and Uterine Fibroids (Leiomyomata): Comorbidity, Risks and Implications. Front. Reprod. Health 2021, 3, 750018. [Google Scholar] [CrossRef] [PubMed]
- Surrey, E.S.; Soliman, A.M.; Johnson, S.J.; Davis, M.; Castelli-Haley, J.; Snabes, M.C. Risk of Developing Comorbidities Among Women with Endometriosis: A Retrospective Matched Cohort Study. J. Womens Health 2018, 27, 1114–1123. [Google Scholar] [CrossRef] [PubMed]

| Traits | Data Source | No. of Participants | Population | No. of Variants | URL |
|---|---|---|---|---|---|
| Uterine fibroids | BBJ | 80,208 (14,475 cases + 65,733 controls) | East Asian | 13,401,454 | https://pheweb.jp/, accessed on 27 October 2022 |
| Breast cancer | BBJ | 79,550 (6325 cases + 73,225 controls) | East Asian | 13,401,000 | |
| Uterine fibroids | CKB | 45,427 (877 cases + 44,550 controls) | East Asian | 8,929,108 | https://pheweb.ckbiobank.org/, accessed on 14 January 2025 |
| Breast cancer | CKB | 45,386 (503 cases + 44,883 controls) | East Asian | 8,578,343 |
| Exposure | Outcome | Heterogeneity | Horizontal Pleiotropy | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Cochran’s Q Test
from IVW |
Rücker’s Q’
Test
from MR-Egger |
MR-PRESSO
Global Test | MR-Egger | MR-Egger (SIMEX) | |||||||
| N | F | I2 (%) | p | p | p | Intercept, β (SE) | p | Intercept, β (SE) | p | ||
| Uterine fibroid | Breast cancer | 16 | 81.51 | 88.27 | 0.338 | 0.339 | 0.388 | −0.045 (0.045) | 0.339 | −0.048 (0.047) | 0.326 |
| Breast cancer | Uterine fibroid | 7 | 149.34 | 76.47 | 0.978 | 0.996 | 0.976 | −0.084 (0.093) | 0.405 | −0.107 (0.027) | 0.011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.; Seo, J.H. Bidirectional Mendelian Randomization Analysis of the Causal Relationship Between Uterine Fibroids and Breast Cancer in East Asian Women. Biomedicines 2025, 13, 2654. https://doi.org/10.3390/biomedicines13112654
Lee Y, Seo JH. Bidirectional Mendelian Randomization Analysis of the Causal Relationship Between Uterine Fibroids and Breast Cancer in East Asian Women. Biomedicines. 2025; 13(11):2654. https://doi.org/10.3390/biomedicines13112654
Chicago/Turabian StyleLee, Young, and Je Hyun Seo. 2025. "Bidirectional Mendelian Randomization Analysis of the Causal Relationship Between Uterine Fibroids and Breast Cancer in East Asian Women" Biomedicines 13, no. 11: 2654. https://doi.org/10.3390/biomedicines13112654
APA StyleLee, Y., & Seo, J. H. (2025). Bidirectional Mendelian Randomization Analysis of the Causal Relationship Between Uterine Fibroids and Breast Cancer in East Asian Women. Biomedicines, 13(11), 2654. https://doi.org/10.3390/biomedicines13112654






