Abstract
Background: Hepatocellular carcinoma (HCC) frequently develops in patients with chronic hepatitis B and C. Early detection is critical, but current methods, including ultrasound and AFP, have suboptimal accuracy. Objectives: This study aimed to evaluate the predictive performance of protein induced by vitamin K absence or antagonist-II (PIVKA-II) and alpha-fetoprotein (AFP) testing, alone and in combination, for HCC development. Methods: A retrospective cohort study at a single university center included 242 CHB and 181 CHC patients. Data on demographics, clinical status, laboratory parameters, and imaging were collected, with fibrosis and steatosis assessed by FibroScan®. Serum AFP and PIVKA-II were measured, but measurements of PIVKA-II in patients receiving vitamin K antagonists were excluded from the analysis. HCC diagnosis and staging followed clinical guidelines. Cox regression and ROC analyses identified independent predictors and evaluated biomarker accuracy for HCC detection. Results: HCC incidence was comparable between cohorts (5.0% in CHB vs. 5.5% in CHC). Both AFP and PIVKA-II independently predicted HCC development in multivariate models adjusted for age and sex. The combined biomarker score (AFP × PIVKA-II) showed superior predictive accuracy with hazard ratios of 1.38 (CHB) and 1.36 (CHC). ROC analyses demonstrated high discriminative ability for PIVKA-II (AUC ~0.81) and AFP (AUC ~0.83) in both cohorts. Additional independent predictors were chronic alcohol abuse, cirrhosis, and higher liver stiffness measurements. Specific viral factors such as HBeAg positivity and HCV subgenotype 1b were also associated with increased HCC risk. Conclusions: AFP and PIVKA-II are independent, valuable biomarkers for HCC risk in chronic hepatitis B and C. Combined use improves early detection, aiding timely treatment. These results support adding PIVKA-II to AFP in surveillance, but larger studies are needed to confirm the findings and refine cut-off values.
1. Introduction
Hepatocellular carcinoma (HCC) is the sixth most commonly diagnosed cancer and the third leading cause of cancer-related death worldwide, accounting for over 900,000 new cases and more than 800,000 deaths annually [1,2]. Historically, chronic viral hepatitis B (CHB) and hepatitis C (CHC) have been among the predominant etiologic factors, together accounting for more than 75% of HCC cases globally. Despite the availability of effective antiviral therapies and the widespread implementation of HBV vaccination programs, chronic viral hepatitis continues to be a major contributor to the global HCC burden, particularly in regions with limited access to screening and sustained virological suppression or response [3]. Furthermore, monitoring for additional risk factors for HCC remains insufficient. This is particularly important given that the burden of viral hepatitis is further compounded by the rising global prevalence of steatotic liver disease and alcohol-associated liver disease, both of which may act synergistically with chronic viral hepatitis to accelerate liver fibrosis progression and increase the risk of hepatocarcinogenesis [4,5,6].
HCC is characterized by poor survival outcomes, largely due to asymptomatic progression and diagnosis at advanced stages when curative treatment options are limited [7]. Early detection is essential for improving prognosis, but current surveillance strategies—based on ultrasound and alpha-fetoprotein (AFP) testing—have suboptimal sensitivity and specificity, especially for early stage tumors [8,9].
Protein induced by vitamin K absence or antagonist-II (PIVKA-II), also known as des-gamma-carboxy prothrombin (DCP), has emerged as a promising biomarker in this setting. PIVKA-II is an abnormal prothrombin molecule produced by malignant hepatocytes due to impaired vitamin K-dependent carboxylation. Its biological role in hepatocarcinogenesis is linked to tumor growth, angiogenesis, and invasion, making it not only a diagnostic but also a relevant marker of HCC progression [10,11,12,13]. Several studies have demonstrated that PIVKA-II, when used in combination with AFP, enhances the accuracy of HCC detection and improves risk stratification compared with either marker alone [14,15,16]. In addition, serum PIVKA-II level was demonstrated to be the most useful parameter predisposing to portal venous invasion, an important factor adversely affecting prognosis in HCC [17]. Importantly, most data come from Asian cohorts, while evidence in European patients with chronic viral hepatitis remains scarce [18].
This study aimed to compare the clinical characteristics of patients with CHB and CHC, with a particular focus on evaluating the predictive value of PIVKA-II and AFP for HCC development. The overarching goal was to assess whether the addition of PIVKA-II could improve risk stratification and support more effective surveillance strategies in patients with chronic viral hepatitis.
2. Materials and Methods
This study was a retrospective cohort analysis conducted at a single university clinical center involving 242 patients with CHB and 181 patients with CHC. The study included adults (≥18 years) with confirmed CHB or CHC based on serological and molecular testing. Only patients who had available demographic, clinical, and laboratory data, and who provided informed consent, were included in the analysis. Exclusion criteria were strict to avoid confounding factors; patients with HIV, HBV, HCV, or HDV co-infection were excluded. Additionally, patients with a prior diagnosis of HCC or those who had received prior chemotherapy, immunotherapy, or other cancer-directed treatments were excluded from the study. Importantly, patients on vitamin K antagonists (VKAs) were excluded from the analysis of PIVKA-II levels, as these medications interfere with the measurement of vitamin K-dependent proteins and could therefore influence the results. Patients with incomplete medical records or missing key data related to baseline liver function, fibrosis stages, or biomarker measurements were also excluded.
Demographic information, including age and sex, were obtained from medical records. Behavioral factors such as smoking, alcohol consumption, and drug use (intravenous and non-intravenous) were documented through patient questionnaires. Chronic alcohol abuse was classified according to the World Health Organization (WHO) guidelines, and current smoking was defined as smoking within the past 30 days. Intravenous drug use (IVDU) was particularly noted as a risk factor for both viral hepatitis and liver disease progression.
Liver fibrosis and steatosis were assessed using FibroScan® (Echosens, Paris, France), a non-invasive vibration-controlled transient elastography (VCTE) technique that simultaneously measures liver stiffness (LSM, in kilopascals) and the controlled attenuation parameter (CAP, in dB/m). Interpretation of liver stiffness values was based on the EASL Clinical Practice Guidelines (2021), with etiology-specific cut-offs applied. For chronic hepatitis B (CHB), significant fibrosis (≥F2) was defined as LSM ≥ 7.9 kPa, advanced fibrosis (≥F3) as ≥8.8 kPa, and cirrhosis (F4) as ≥11.7 kPa. For chronic hepatitis C (CHC), the corresponding thresholds were ≥7.2 kPa for ≥F2, ≥9.5 kPa for ≥F3, and ≥12.5 kPa for cirrhosis. Steatosis grading was based on the CAP values, with ≥238 dB/m indicating mild steatosis (S1), ≥260 dB/m moderate steatosis (S2), and ≥290 dB/m severe steatosis (S3), regardless of underlying liver disease etiology [11]. Cirrhotic patients were further categorized according to the Child–Pugh classification, which assesses liver function and complications.
Molecular analyses included the measurement of HBV DNA in CHB patients and HCV RNA in CHC patients using standard real-time PCR assays on the Abbott m2000 RealTime System (Abbott Laboratories®, Chicago, IL, USA). Serum levels of AFP and PIVKA-II were quantified using commercially available enzyme-linked immunosorbent assays: AFP was determined using the Abbott assay (Abbott Laboratories®, Chicago, IL, USA), and PIVKA-II was measured using the Roche Diagnostics assay (Roche Diagnostics®, Basel, Switzerland).The use of these biomarkers was based on the current recommendations from the European Association for the Study of the Liver (EASL) and the Japan Society of Hepatology (JSH), which support their role in the early detection and risk assessment of HCC [19,20,21]. Data addressing the patients’ comorbidities (hypertension, diabetes mellitus, cardiovascular, renal, neurological and psychiatric disorders) were gathered using medical records. Hepatic complications such as ascites, hepatic encephalopathy, and portal hypertension were also recorded. Treatment approaches, including surgical resection, microwave ablation, transarterial chemoembolization (TACE), and sorafenib therapy, were noted for patients diagnosed with HCC.
For CHB patients, antiviral treatment regimens included tenofovir disoproxil fumarate (TDF) (94 patients, 38.8%) and tenofovir alafenamide (TAF) (67 patients, 27.6%). For CHC patients, antiviral treatments included sofosbuvir/velpatasvir (78 patients, 43.1%), glecaprevir/pibrentasvir (92 patients, 50.8%), and elbasvir/grazoprevir (11 patients, 6.1%). Patients with prior exposure to pegylated interferon (PEG-IFN) were also included as long as they met the study’s inclusion criteria. All patients were closely monitored for adherence to treatment as well as for viral suppression and liver function.
The diagnosis of HCC was established in accordance with the EASL Clinical Practice Guidelines on the management of hepatocellular carcinoma [20]. HCC staging followed the Barcelona Clinic Liver Cancer (BCLC) system [22]. All patients underwent serum AFP and PIVKA-II testing as part of routine surveillance. In individuals at risk for HCC, detection of a focal liver lesion ≥ 10 mm on the surveillance ultrasound prompted further assessment with contrast-enhanced multiphasic computed tomography (CT) or magnetic resonance imaging (MRI). The diagnosis of HCC was confirmed non-invasively in cases where lesions demonstrated the characteristic radiological hallmarks of arterial phase hyperenhancement (APHE) followed by washout in the portal venous or delayed phases. If imaging was inconclusive, percutaneous liver biopsy was performed for histopathological confirmation. All imaging and diagnostic procedures were interpreted by experienced hepatologists and radiologists specialized in liver disease.
In patients exhibiting elevated serum levels of AFP and/or PIVKA-II, supplementary imaging, typically contrast-enhanced multiphasic CT or MRI, was undertaken to confirm or exclude HCC in accordance with the guideline-based diagnostic criteria. The biomarkers PIVKA-II and AFP were analyzed to evaluate their predictive role for HCC development. The combined effect of PIVKA-II and AFP was also evaluated by calculating a composite variable derived from the product of the two biomarker values (PIVKA-II × AFP).
Statistical analyses were performed using IBM SPSS Statistics, version 23.0 (IBM Corp, Armonk, NY, USA). Prior to inferential testing, the distribution of all scalar (continuous) variables was assessed for normality using the Kolmogorov–Smirnov and Shapiro–Wilk tests. Variables with a normal distribution were expressed as means with standard deviations (SD), while non-normally distributed variables were presented as medians with interquartile ranges (IQR). Categorical variables were reported as absolute and relative frequencies. For comparisons between the CHB and CHC cohorts, the independent samples t-test was used for normally distributed continuous variables, and the Mann–Whitney U test for non-normally distributed variables. The Chi-square test or Fisher’s exact test, as appropriate, was applied for categorical data.
Univariate Cox proportional hazards regression analysis was performed to identify variables associated with the development of hepatocellular carcinoma (HCC). Variables with p-values < 0.05 as well as those approaching statistical significance (p < 0.125) were subsequently included in the multivariate Cox regression model to identify independent predictors of HCC occurrence. In instances where no variables reached statistical significance in univariate analysis, all candidate variables were included in the multivariate model. A total of six regression models were constructed for the purposes of this study, each adjusted for patient age and sex. To assess the discriminatory performance of individual biomarkers, including PIVKA-II and AFP, receiver operating characteristic (ROC) curve analysis was conducted. The area under the curve (AUC) was calculated to evaluate predictive accuracy, with values approaching 1.0 indicating greater discriminatory power. To assess the discriminatory performance of AFP and PIVKA-II, ROC curve analysis was performed. Cut-off values were determined by identifying the threshold that maximized the Youden index (sensitivity + specificity − 1), representing the point of optimal diagnostic accuracy in our sample. This approach ensured that the selected thresholds were not arbitrarily chosen but statistically derived based on the characteristics of the study population. All statistical tests were two-sided, and a p-value of less than 0.05 was considered statistically significant.
This study was conducted in accordance with the principles of the Declaration of Helsinki. The study protocol was reviewed and approved by the Ethics Committee of the University Clinical Center of Serbia where the patients were treated (Approval No. 1228/20). Given the retrospective design, informed consent was obtained, and all patient data were fully anonymized prior to analysis, ensuring confidentiality and compliance with the institutional and international ethical standards.
3. Results
A total of 242 patients with CHB and 181 patients with CHC were compared according to their demographic, behavioral, histological, biochemical, and prognostic parameters. In the CHB cohort, 117 individuals (48.3%) were male and 125 (51.7%) were female, whereas the CHC cohort comprised 106 males (58.6%) and 75 females (41.4%) (p = 0.037). No statistically significant difference in age between sexes was observed in either cohort (CHC p = 0.510, CHB p = 0.684). Mean age did not differ significantly, measuring 53.10 ± 15.33 years in CHB versus 51.31 ± 13.89 years in CHC patients (p = 0.254). Body mass index (BMI) was higher among the CHB patients (25.83 ± 4.21 kg/m2) compared with the CHC patients (21.92 ± 3.87 kg/m2; p = 0.043). Chronic alcohol abuse was documented in 21 CHB patients (8.6%) and 34 CHC patients (18.8%; p = 0.025), while intravenous drug use occurred in 13 CHB patients (5.3%) versus 67 CHC patients (37.0%; p < 0.001). Non-intravenous drug use was noted in 7 CHB (2.9%) and 21 CHC (11.6%) patients (p = 0.004). Current smoking prevalence stood at 33.5% in CHB and 45.3% in CHC (p = 0.043) (Table 1).

Table 1.
Overview of the characteristics of the studied cohorts—chronic hepatitis B cohort and chronic hepatitis C cohort.
Mild or absent liver fibrosis (F0/F1) was observed in 130 CHB patients (53.9%) and 72 CHC patients (39.8%; p = 0.042). Moderate fibrosis to cirrhosis (F2–F4) showed no statistically significant differences between cohorts. CAP values averaged 241.5 ± 29.58 dB/m in CHB and 248.5 ± 32.34 dB/m in CHC (p = 0.575). Among the cirrhotic patients, ascites was present in 6 CHB cases (2.5%) versus 11 CHC cases (6.0%; p = 0.028), whereas the rates of hepatic encephalopathy, portal hypertension, and esophageal varices did not differ significantly (Table 1).
Hypertension was reported in 72 CHB patients (29.7%) and 64 CHC patients (35.4%; p = 0.221), and diabetes mellitus in 34 CHB (14.1%) versus 21 CHC (11.6%; p = 0.503). Psychiatric disorders were documented in 13 CHB patients (5.4%) compared with 30 CHC patients (16.6%; p = 0.001). All other assessed comorbid conditions (cardiovascular, respiratory, renal, malignant, connective tissue, neurological, thyroid) showed no significant inter-group differences (Table 1). Differences in laboratory parameters between the CHB and CHC cohorts are presented in tabular form (Supplementary Table S1).
Among the CHB patients, the mean HBV DNA level was 15,213.0 ± 5412.0 IU/mL. HBeAg was positive in 61 individuals (25.2%), while anti-HBe antibodies were detected in 180 (74.3%) patients. Regarding therapy, 94 patients (38.8%) received TDF, and 67 (27.6%) received TAF. In the CHC cohort, the mean HCV RNA level was 1,359,520.0 ± 825.2 IU/mL. Genotype distribution followed the pattern commonly reported in European countries, with genotype 1 as the most prevalent and genotype 3 as the second most frequent. Detailed data are presented in Table 2. Antiviral therapy was administered according to the current guidelines, with detailed regimens outlined in Table 2.

Table 2.
Virological and therapeutic characteristics of the cohorts.
3.1. Hepatocellular Carcinoma, Stage, Incidence and Survival
HCC developed in 12 (5.0%) CHB patients and 10 CHC patients (5.5%; p = 0.697). Mortality among those diagnosed with HCC was 1.2% (3 patients) in CHB and 2.2% (4 patients) in CHC (p = 0.970). Mean interval from HCC diagnosis to death was longer in CHB (8.2 ± 4.2 months) than in CHC (7.1 ± 4.1 months; p = 0.016). Rates of surgical resection, microwave ablation, TACE, and sorafenib therapy did not differ significantly (Table 3). Distribution across HCC stages I to IV showed no statistically significant differences.

Table 3.
Overview of the population characteristics in patients with hepatocellular carcinoma.
Patients receiving VKAs comprised 12.5% (n = 30) of the CHB cohort and 5.5% (n = 10) of the CHC cohort. This subgroup exhibited significantly elevated PIVKA II levels (385.7 ± 78.7) compared with other patients (118.8 ± 38.9). Notably, no cases of HCC were observed among patients receiving this particular oral anticoagulant therapy. Due to the known interference of VKAs with PIVKA-II measurements, these patients were excluded from the analysis assessing the predictive value of PIVKA-II levels.
3.2. Predictive Performance of Biomarkers and Risk Modeling
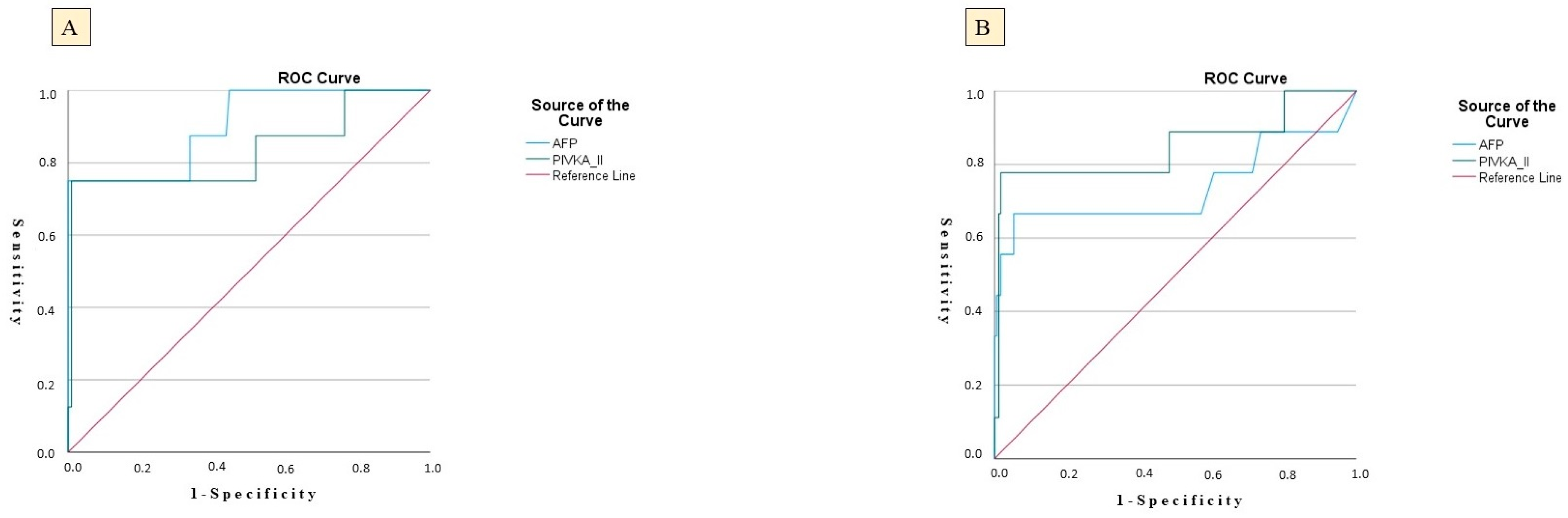
In the CHB group, PIVKA-II achieved an AUC of 0.809 (SE = 0.048; p = 0.024), indicating substantial ability to differentiate between patients who did and did not develop HCC. At a threshold of 48.5 ng/mL, PIVKA-II demonstrated a sensitivity of 81.5%—correctly identifying 81.5% of eventual HCC cases—and a specificity of 51.5%, reflecting a moderate false-positive rate. The positive predictive value (PPV) for PIVKA-II was 15.18%, while the negative predictive value (NPV) was 98.1%. AFP yielded an even higher AUC of 0.830 (SE = 0.057; p = 0.039), suggesting slightly superior discriminative capacity compared with PIVKA-II. Using an AFP cut-off of 11.2 µg/L, sensitivity reached 82.9% and specificity 65.5%, indicating that AFP correctly classified over 80% of HCC outcomes while excluding nearly two-thirds of non-cases. The PPV for AFP was 11.9%, and the NPV was 97.8%, reflecting a more robust ability to exclude non-cases and a moderate ability to predict true positives (Table 4, Figure 1).

Table 4.
Parameters on the receiver operating characteristics curve analysis.
Figure 1.
ROC curves showing the diagnostic performance of serum PIVKA-II and AFP for predicting HCC; (A) CHC cohort; (B) CHB cohort.
Parallel trends were observed in the CHC cohort. PIVKA-II achieved an AUC of 0.812 (SE = 0.046; p = 0.012) at a threshold of 47.2 ng/mL, yielding a sensitivity of 82.0% and specificity of 53.9%, mirroring its performance in CHB. The PPV for PIVKA-II in the CHC cohort was 16.23%, while the NPV was 98.1%, indicating a similar predictive performance. AFP in CHC patients produced an AUC of 0.824 (SE = 0.051; p = 0.020), with a sensitivity of 83.3% and specificity of 66.5% at a cut-off of 11.6 µg/L. The PPV for AFP was 9.49%, and the NPV was 98.1%, reinforcing AFP’s strong predictive accuracy (Table 4, Figure 1).
3.3. HCC Predictors in HBV and HCV Cohorts
In the CHB cohort, the univariate regression analysis (Model 1) revealed that chronic alcohol abuse, severe fibrosis, and cirrhosis were significantly associated with the development of HCC, and these variables were subsequently included in the multivariate model. In the multivariate analysis, chronic alcohol abuse remained an independent predictor of HCC occurrence (HR 2.21; 95% CI 1.74–2.93; p = 0.028), corresponding to a 121% increase in HCC risk. In the CHC cohort, univariate analysis identified older age, chronic alcohol abuse, the presence of severe fibrosis, cirrhosis, and higher FibroScan® stiffness values [kPa] as significant predictors of HCC development. In the multivariate model, independent predictors of HCC included older age (HR 1.01; p = 0.012), representing a 1% increase in HCC risk per additional year of age; chronic alcohol abuse (HR 1.69; p = 0.024), associated with a 69% increase in risk; presence of cirrhosis (HR 3.60; p = 0.001), corresponding to a 260% increase in risk; and elevated FibroScan® stiffness values (HR 1.02; p = 0.028), with each 1 kPa increase associated with a 2% increase in HCC risk (Table 5).

Table 5.
Results of the multivariate Cox proportional hazards model: factors associated with the development of HCC.
In Model 2, univariate Cox regression identified age and Child–Pugh class C as significant predictors of HCC development in the CHC cohort. These variables were subsequently included in the multivariate analysis. The multivariate model was adjusted for sex and age. Child–Pugh class C was independently associated with a higher risk of HCC (HR 1.64; 95% CI 1.21–1.81; p = 0.041), corresponding to a 64% increased risk compared with patients not in class C. In the CHB cohort, no variable demonstrated statistical significance in the univariate analysis; consequently, all candidate predictors were tested in the multivariate model, where none showed a significant association with HCC development (all p-values > 0.05) (Table 5).
In the CHB cohort, the univariate analysis (Model 3) identified the baseline PIVKA-II levels, AFP levels, and combined use of AFP and PIVKA-II levels score as significant predictors of HCC development. In the multivariate model, adjusted for age and sex, both PIVKA-II (HR 1.05; 95% CI 1.00–1.10; p = 0.015) and AFP (HR 1.11; 95% CI 1.03–1.20; p = 0.006) remained independent predictors of HCC, indicating that each unit increase in PIVKA-II and AFP was associated with a 5% and 11% increase in HCC risk, respectively. Additionally, in the CHB cohort, the combined use of AFP and PIVKA-II showed strong predictive performance, with a hazard ratio of 1.38 (95% CI 1.20–1.46; p = 0.001) in the multivariate model, suggesting a 38% increase in HCC risk per unit increase in the combined score (Table 6).

Table 6.
Results of the multivariate Cox proportional hazards model: factors associated with the development of HCC.
In the CHC cohort, univariate analysis identified the baseline PIVKA-II, AFP, and combined use of AFP and PIVKA-II levels as significant predictors. In the multivariate model adjusted for age and sex, independent predictors of HCC were age, PIVKA-II (HR 1.03; 95% CI 1.01–1.05; p = 0.002), and AFP (HR 1.16; 95% CI 1.04–1.24; p = 0.012). These results indicate that each unit increase in age, PIVKA-II, AFP, or risk model score was associated with a 4%, 3%, 16%, and 24% increase in HCC risk, respectively. Additionally, in the CHC cohort, the combined use of AFP and PIVKA-II showed strong predictive performance, with a hazard ratio of 1.36 (95% CI 1.21–1.37; p = 0.001) in the multivariate model, suggesting a 36% increase in HCC risk per unit increase in the combined score (Table 6).
In the HBV group, HBeAg positivity remained a statistically significant factor (HR 1.56; 95% CI 1.17–2.31; p = 0.027), corresponding to a 56% increased risk of HCC. In the HCV cohort, genotype 1b showed a consistent and significant association with HCC risk (HR 1.38; 95% CI 1.02–2.08; p = 0.020), corresponding to a 38% increase in risk (Table 6). In univariate models 5 and 6, none of the analyzed variables showed a statistically significant association with the development of HCC in either the CHB or CHC cohorts. All variables included in these models were subsequently analyzed in multivariate Cox regression; however, none remained statistically significant in the adjusted analyses (Supplementary Table S2).
4. Discussion
Liver cancer is the third leading cause of cancer-related mortality and the sixth most commonly diagnosed cancer worldwide, with HCC accounting for approximately 90% of all primary liver malignancies [20]. Given that viral hepatitis remains a significant global public health challenge, and that HCC represents one of its most severe long-term complications, this study investigated factors associated with HCC development in patients with CHB and CHC. In addition, it evaluated the sensitivity and specificity of standard surveillance methods—namely ultrasound combined with AFP—and explored the diagnostic performance of PIVKA-II, a biomarker not yet incorporated into routine HCC surveillance protocols in Europe.
Despite advancements in curative and palliative therapies, including surgical resection, liver transplantation, and systemic treatments, HCC prognosis remains poor due to late-stage diagnosis in a large proportion of patients. Early detection is therefore critical and depends on effective surveillance strategies in high-risk populations. Standard surveillance, as recommended by EASL and AASLD, consists of ultrasound with AFP every six months [9,19]. In a meta-analysis, Tzartzeva et al. showed that the addition of AFP to ultrasound significantly improved the sensitivity for detecting early stage HCC, increasing it from 40% with ultrasound alone to 60% with the combined approach [23]. The fact that this strategy misses one in three early stage HCC cases highlights a critical gap in existing surveillance protocols and reinforces the need for enhanced detection tools. The optimal scenario would involve the availability of blood-based biomarkers with adequate diagnostic accuracy to facilitate the early stage detection of HCC. PIVKA-II, also known as des-γ-carboxy prothrombin (DCP), is an abnormal form of prothrombin produced by malignant hepatocytes due to impaired post-translational carboxylation. Unlike normal prothrombin, which requires vitamin K-dependent carboxylation, PIVKA-II lacks γ-carboxyglutamic acid residues, making it a promising tumor marker in HCC [24].
The underlying etiology of HCC should be taken into account, as it can significantly influence the diagnostic performance of both AFP and PIVKA-II [25]. In our study, the diagnostic performance of AFP and PIVKA-II in the CHB cohort was comparable to that observed in the CHC cohort, suggesting that both biomarkers demonstrate similar utility across chronic viral hepatitis etiologies. Our findings demonstrate that both biomarkers possess significant predictive value for HCC development in patients with CHB and CHC, with the AUC values consistently exceeding 0.80 in both cohorts. These findings are consistent with previous reports highlighting the utility of each biomarker in HCC surveillance. Analyzing the available literature on the diagnostic performance of AFP and PIVKA-II in patients with CHB and CHC, PIVKA-II has consistently demonstrated superior accuracy compared with AFP. Liu et al. reported that PIVKA-II alone achieved an AUC of 0.90 for the detection of HCC in HCV-infected patients, outperforming AFP (AUC = 0.80), while the combination of AFP and PIVKA-II further improved the diagnostic accuracy (AUC = 0.93) [26]. Similarly, previous studies have shown that PIVKA-II offers better diagnostic performance than AFP in HBV-related HCC, with reported AUCs of 0.901 for PIVKA-II and 0.765 for AFP [24]. In addition, a large-scale meta-analysis reported pooled AUCs of 0.89 for PIVKA-II and 0.78 for AFP across populations with HBV-related, HCV-related, or mixed-etiology HCC [14].
The findings of this study demonstrate that both AFP and PIVKA-II exhibit high NPV, supporting their clinical utility in ruling out HCC and underscoring their reliability as surveillance tools in at-risk populations. Although higher than the PPV observed for AFP (11.9% in the CHB cohort and 9.49% in the CHC cohort), the PPV for PIVKA-II (15.18% and 16.23% in the CHB and CHC cohorts, respectively) remains suboptimal, highlighting the limitations of relying on a single biomarker for early detection. Parikh et al. emphasized that while AFP and PIVKA-II offer value for the early detection of HCC, neither marker alone provides sufficient sensitivity or specificity to be used as a standalone surveillance tool [8]. PPV of PIVKA-II arises from the low prevalence of HCC in the general population, which is characteristic of screening biomarkers. Although PIVKA-II may exhibit high sensitivity and specificity, its PPV remains limited when applied to cohorts with a low absolute risk such as patients without cirrhosis (81.3% and 72.9% in CHB and CHC cohorts, respectively) or those in the early stages of HCC. The cut-off of 48.5 ng/mL used for PIVKA-II in our CHB cohort yielded a high sensitivity (81.5%), albeit at the expense of reduced specificity, which is consistent with findings from Dong et al., who reported moderate false-positive rates at comparable PIVKA-II thresholds [10]. In contrast, AFP at a cut-off of 11.2 µg/L showed slightly higher specificity (65.5%) with a similar sensitivity (82.9%), suggesting that combining both biomarkers may improve diagnostic accuracy and reduce false positives in HBV related HCC surveillance. This supports the growing body of evidence advocating for dual-marker strategies in high-risk populations to enhance early detection without overburdening follow-up investigations. In comparison to previously published data using a lower AFP cut-off of 8.7 ng/mL, our study used a slightly higher threshold (11.2 µg/L) and achieved a higher sensitivity (82.9% vs. 58%) but lower specificity (65.5% vs. 94%) [27]. While the lower threshold improved the specificity and PPV, it may have resulted in missed early cases. These differences highlight the trade-offs between sensitivity and specificity depending on the chosen cut-off, reflecting the need for context-specific optimization of biomarker thresholds in HCC surveillance strategies for CHB patients.
In the CHC cohort, our findings mirrored the trends observed in CHB. PIVKA-II demonstrated a sensitivity of 82.0% and specificity of 53.9% at a cut-off value of 47.2 ng/mL. AFP performed similarly, with a slightly higher specificity (66.5%) and comparable sensitivity (83.3%) at a cut-off of 11.6 µg/L. Previous evidence supports the role of PIVKA-II as a useful diagnostic biomarker in CHC, consistent with our observations [27]. Although PIVKA-II and AFP demonstrated a high sensitivity and NPV in our HCV cohort, the relatively low specificity and PPV raise concerns about false positives and unnecessary diagnostic follow-up. There is a clear need to establish individualized surveillance strategies in patients with CHC, ensuring that biomarker thresholds are optimized according to clinical context and risk stratification. This limitation reinforces the importance of using a combination of biomarkers to optimize performance. Incorporating dual-marker strategies, such as combining AFP and PIVKA-II, appears to improve sensitivity while balancing specificity.
Our findings indicate that the combination of AFP and PIVKA-II provides superior predictive value for HCC in patients with CHB compared with either biomarker alone. This aligns with the results of Kurniawan et al. and Seo et al. who demonstrated that while AFP and PIVKA-II had similar individual diagnostic performance in differentiating HCC from nonmalignant CHB, their combined use significantly improved the diagnostic accuracy, particularly in cirrhotic patients [28,29]. Notably, both studies identified similar cut-off values for AFP and PIVKA-II, further supporting the consistency and potential clinical applicability of these biomarkers in early HCC detection and risk stratification within CHB cohorts [30].
In our CHC cohort, the combination of AFP and PIVKA-II demonstrated superior predictive performance for HCC compared with the individual use of either marker. These findings are consistent with the study by Liu et al., which highlighted that among the HCV-associated HCC patients, PIVKA-II alone showed the highest individual diagnostic accuracy, and its combination with AFP yielded the best overall performance [26].
In our study, higher liver stiffness values on FibroScan® and the presence of cirrhosis were identified as independent predictors of HCC in the CHC cohort, consistent with the traditionally accepted concept that HCC predominantly arises in the context of end stage liver disease (ESLD) [31,32,33]. Previous studies have shown that HCC in chronic HCV infection develops on a cirrhotic background in 80–90% of cases, while less frequently in non-cirrhotic livers with pronounced inflammation [34,35]. Cirrhosis promotes the development of HCC through chronic hepatocyte injury, regenerative proliferation, fibrotic remodeling, sustained inflammation, oxidative stress, and HCV protein-mediated oncogenic signaling [6,36,37]. Elevated liver stiffness above 12.5–14 kPa reflects advanced fibrosis and indicates increased HCC risk, with values >20–25 kPa correlating with a substantially higher tumor incidence [38,39]. Additionally, chronic alcohol abuse was identified as an independent predictor of HCC in both cohorts. This finding is expected, considering the pathogenic mechanisms through which alcohol promotes tumorigenesis including DNA and protein adduct formation, mutations in tumor suppressor genes (p53, PTEN), activation of oncogenic pathways (c-MYC, β-catenin), oxidative stress, NF-κB and STAT3 signaling, pro-inflammatory cytokine expression (TNF-α, IL-6), and mitochondrial dysfunction [40,41,42]. It is important to emphasize that both alcohol-related liver disease (ALD) and recent antibiotic use may act as confounding factors when interpreting elevated serum PIVKA-II levels [43,44]. Therefore, PIVKA-II measurements in patients with ALD or those treated with antibiotics should be interpreted with caution. In the present study, none of the patients had received antibiotics prior to PIVKA-II testing. However, a proportion of patients had ALD, underscoring the need for further research to establish the optimal PIVKA-II cut-off value for diagnosing hepatocellular HCC in this subgroup. The mechanism by which alcohol affects the serum PIVKA-II levels remains unclear. Although vitamin K deficiency has been proposed as a possible explanation in chronic alcohol users, previous studies have not confirmed a consistent relationship between serum vitamin K concentrations and PIVKA-II levels. This discrepancy underscores a significant gap in understanding and highlights the need for further investigation.
The challenge of establishing a valid cut-off value for PIVKA-II, beyond the factors already mentioned, lies in the fact that other conditions and clinical entities may also lead to elevated levels such as Child–Pugh classes B and C cirrhosis [45]. This subgroup warrants particular attention in HCC screening, as their substantially increased risk of HCC and altered biomarker dynamics may necessitate the definition of distinct cut-off thresholds to ensure optimal diagnostic accuracy. Moreover, elevated PIVKA-II levels have also been reported in association with abnormal bone physiology in females and muscle weakness in males, further underscoring the complexity of interpreting this biomarker [45].
HCV genotypes 1 and 3 predominated in this study, representing the two most common genotypes in Serbia, in line with distribution patterns reported in Europe and globally [46]. This study identified HCV genotype 1b infection as an independent predictor of HCC development, with an HR of 1.38 (95% CI: 1.02–2.08). In an effort to clarify this issue, a prospective study by B. Savino et al., conducted before the availability of DAA therapy, including 163 consecutive HCV-positive patients with cirrhosis, demonstrated that HCV genotype 1b was independently associated with HCC development, with an HR of 3.02 (95% CI: 1.40–6.53) [47]. A meta-analysis by Raimondi S. et al., analyzing 57 studies and focusing on age-adjusted risk estimates, showed that HCV genotype 1b infection is associated with an increased risk of HCC compared with other genotypes, with relative risks ranging from 1.60 to 2.46 depending on the presence of cirrhosis [48]. Considering that our analysis did not evaluate the predictive significance of HCV genotype 1b infection exclusively in patients with cirrhosis, but across all stages of fibrosis, this may explain the somewhat lower HR observed compared with the aforementioned studies.
In Serbia, HBV genotype D is predominant, while a much smaller proportion of patients carry genotype A; other genotypes have not been documented [49]. Importantly, genotype D, similar to genotype C, which is typical for Asia, has been associated with more rapid progression to cirrhosis and HCC, which has important implications for prognosis and clinical management [50]. HBeAg is a crucial marker of active HBV replication and plays a significant role in modulating the host immune response. It can impact both innate and adaptive immune mechanisms, thereby acting as a tolerogen that facilitates HBV persistence within the host [51,52]. Through mechanisms that include immune evasion and interference with apoptotic pathways, HBeAg contributes to the chronicity of HBV infection [51,52]. Furthermore, its ability to evade immune detection and modulate cellular processes may enhance the hepatocarcinogenic potential of HBV, increasing the risk of HCC [53]. HBeAg and its precursors interfere and also stimulate intracellular signaling, which promotes hepatocyte proliferation [53,54]. Together, these mechanisms contribute to key cancer hallmarks, including tumor-promoting inflammation, resistance to cell death, and sustained proliferative signaling, implicating HBeAg as a potential viral oncoprotein [53,54]. The role of HBeAg has been increasingly recognized in relation to disease complications, leading to changes in treatment recommendations. Data support the initiation of NA therapy in all HBeAg-positive patients above the age of 30, as already advised by European guidelines [55]. More recently, Chinese guidelines have even extended these indications to patients younger than 30 years with minimal biochemical activity and no clear clinical evidence of active liver disease [56].
5. Conclusions
The results of this study demonstrate that both AFP and PIVKA-II have independent diagnostic value in the detection of HCC among patients with chronic hepatitis B and C. Importantly, the combined use of these two biomarkers yielded superior predictive accuracy compared with either marker alone, consistently across both etiological cohorts.
The study also highlights the need for an individualized approach to patients at risk of HCC, ideally through the development of a scoring system that integrates additional relevant factors, such as HBeAg positivity, HCV genotype and subgenotype, LSM values in kPa, and alcohol abuse, which could be incorporated into existing scoring models. Beyond the additive value of biomarkers, this study highlights that HCC risk is strongly influenced by disease etiology and host-related factors. In the CHC cohort, higher liver stiffness values and the presence of cirrhosis were confirmed as independent predictors of HCC, in line with the long-established association between advanced fibrosis and carcinogenesis. Chronic alcohol consumption emerged as a key risk factor in both the CHB and CHC cohorts, not only contributing to hepatocarcinogenesis through multiple pathogenic mechanisms, but also complicating the interpretation of serum PIVKA-II levels, as alcohol-related liver disease may act as a confounder. In addition, the study identified HCV genotype 1b infection as an independent predictor of HCC development, corroborating previous evidence of its higher oncogenic potential compared with other genotypes. Furthermore, HBeAg positivity was highlighted as a biologically plausible driver of hepatocarcinogenesis due to its immunomodulatory and oncogenic properties, providing additional rationale for guideline-supported initiation of nucleos(t)ide analogue therapy in specific subgroups.
Collectively, these results indicate that future surveillance strategies for HCC should not rely solely on single biomarkers but rather on integrated risk-based models. Such models could incorporate biomarker profiles (AFP, PIVKA-II), viral factors (HBV genotype, HBeAg status, HCV genotype/subgenotype), host-related parameters (liver stiffness, cirrhosis, alcohol exposure), and other clinical cofactors. This approach would allow for the development of individualized scoring systems that are capable of refining surveillance intervals, optimizing cut-off values, and prioritizing high-risk patients for intensified monitoring.
Given the relatively limited sample size, further large-scale, multicenter prospective studies are warranted to validate these findings, standardize biomarker thresholds across diverse populations, and formally integrate AFP and PIVKA-II into international surveillance algorithms. Ultimately, the integration of biomarker-based strategies with clinical and virological risk factors represents a crucial step toward precision surveillance, with the potential to substantially improve early detection and survival outcomes in patients at risk of HCC.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines13112653/s1, Table S1: Laboratory parameters of the investigated cohorts; Table S2: Results of the multivariate Cox proportional hazards model: factors associated with the development of HCC.
Author Contributions
Conceptualization, I.M., N.N., and B.B.; Methodology, I.M. and S.S.; Software, B.B.; Validation, N.N., I.P., and J.S.; Formal analysis, B.B. and J.R.; Investigation, A.F. and J.S.; Resources, I.M. and S.S.; Data curation, A.F., S.S., and I.P.; Writing—original draft preparation, I.M., N.N., and B.B.; Writing—review and editing, I.M.; Visualization, A.F., J.R., J.S., and I.P.; Supervision, I.M.; Project administration, I.M. and N.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the University Clinical Center of Serbia (protocol code 1228/20, approved on 3 June 2025).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data supporting the reported results in this study are not publicly available due to privacy and confidentiality concerns as they consist of the patients’ medical histories. Access to these data is restricted to protect patient confidentiality and comply with ethical regulations. Requests for data access can be directed to the corresponding author, subject to institutional and ethical approvals.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Samant, H.; Amiri, H.S.; Zibari, G.B. Addressing the Worldwide Hepatocellular Carcinoma: Epidemiology, Prevention and Management. J. Gastrointest. Oncol. 2021, 12 (Suppl. S2), S361–S373. [Google Scholar] [CrossRef]
- Foglia, B.; Turato, C.; Cannito, S. Hepatocellular Carcinoma: Latest Research in Pathogenesis, Detection and Treatment. Int. J. Mol. Sci. 2023, 24, 12224. [Google Scholar] [CrossRef]
- Ivancovsky Wajcman, D.; Nicolàs, A.; Picchio, C.A.; van Selm, L.; Dusheiko, G.; Younossi, Z.M.; Dillon, J.F.; Alqahtani, S.A.; Razavi, H.; Colombo, M.G.; et al. Prioritising viral hepatitis elimination to prevent hepatocellular carcinoma: A public health approach for effective preventive hepatology. JHEP Rep. 2025, 7, 101436. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The Global Epidemiology of NAFLD and NASH in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef]
- Xu, H.Q.; Wang, C.G.; Zhou, Q.; Gao, Y.H. Effects of alcohol consumption on viral hepatitis B and C. World J. Clin. Cases 2021, 9, 10052–10063. [Google Scholar] [CrossRef] [PubMed]
- Milosevic, I.; Todorovic, N.; Filipovic, A.; Simic, J.; Markovic, M.; Stevanovic, O.; Malinic, J.; Katanic, N.; Mitrovic, N.; Nikolic, N. HCV and HCC Tango-Deciphering the Intricate Dance of Disease: A Review Article. Int. J. Mol. Sci. 2023, 24, 16048. [Google Scholar] [CrossRef] [PubMed]
- Melendez-Torres, J.; Singal, A.G. Early detection of hepatocellular carcinoma: Roadmap for improvement. Expert. Rev. Anticancer Ther. 2022, 22, 621–632. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parikh, N.D.; Mehta, A.S.; Singal, A.G.; Block, T.; Marrero, J.A.; Lok, A.S. Biomarkers for the Early Detection of Hepatocellular Carcinoma. Cancer Epidemiol. Biomark. Prev. 2020, 29, 2495–2503. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singal, A.G.; Llovet, J.M.; Yarchoan, M.; Mehta, N.; Heimbach, J.K.; Dawson, L.A.; Jou, J.H.; Kulik, L.M.; Agopian, V.G.; Marrero, J.A.; et al. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology 2023, 78, 1922–1965. [Google Scholar] [CrossRef]
- Dong, L.; Qiu, X.; Gao, F.; Wang, K.; Xu, X. Protein induced by vitamin K absence or antagonist II: Experience to date and future directions. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 189016. [Google Scholar] [CrossRef]
- Svobodova, S.; Karlikova, M.; Topolcan, O.; Fiala, O.; Pecen, L.; Svoboda, T.; Holubec, L. PIVKA-II as a potential new biomarker for hepatocellular carcinoma: A pilot study. Anticancer Res. 2018, 38, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Suttichaimongkol, T.; Mitpracha, M.; Tangvoraphonkchai, K.; Sadee, P.; Sawanyawisuth, K.; Sukeepaisarnjaroen, W. PIVKA-II or AFP has better diagnostic properties for hepatocellular carcinoma diagnosis in high-risk patients. World J. Gastroenterol. 2023, 29, 826–839. [Google Scholar] [CrossRef]
- Hadi, H.; Wan Shuaib, W.M.A.; Raja Ali, R.A.; Othman, H. Utility of PIVKA-II and AFP in differentiating hepatocellular carcinoma from benign liver diseases. Medicina 2022, 58, 1015. [Google Scholar] [CrossRef]
- Si, Y.-Q.; Wang, X.-Q.; Fan, G.; Wang, C.-Y.; Zheng, Y.-W.; Song, X.; Pan, C.-C.; Chu, F.-L.; Liu, Z.-F.; Lu, B.-R.; et al. Value of AFP and PIVKA-II in diagnosis of HBV-related hepatocellular carcinoma and prediction of vascular invasion and tumor differentiation. Infect. Agents Cancer 2020, 15, 70. [Google Scholar] [CrossRef]
- Feng, H.; Li, B.; Li, Z.; Wei, Q.; Ren, L. PIVKA-II serves as a potential biomarker that complements AFP for the diagnosis of hepatocellular carcinoma. BMC Cancer 2021, 21, 401. [Google Scholar] [CrossRef]
- Park, S.J.; Jang, J.Y.; Jeong, S.W.; Cho, Y.K.; Lee, S.H.; Kim, S.G.; Cha, S.W.; Kim, Y.S.; Cho, Y.D.; Kim, H.S.; et al. Usefulness of AFP, AFP-L3, and PIVKA-II, and their combinations in diagnosing early HCC. Medicine 2017, 96, e6652. [Google Scholar]
- Koike, Y.; Shiratori, Y.; Sato, S.; Obi, S.; Teratani, T.; Imamura, M.; Yoshida, H.; Shiina, S.; Omata, M. Des-gamma-carboxy prothrombin as a useful predisposing factor for the development of portal venous invasion in patients with hepatocellular carcinoma: A prospective analysis of 227 patients. Cancer 2001, 91, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Lau, G.K.; Wei, L.; Moriyama, M.; Yu, M.L.; Chuang, W.L.; Ibrahim, A.; Lesmana, C.R.A.; Sollano, J.; Kumar, M.; et al. APASL HCV guidelines of virus-eradicated patients by DAA on how to monitor HCC occurrence and HBV reactivation. Hepatol. Int. 2019, 13, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Association for the Study of the Liver. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J. Hepatol. 2021, 75, 659–689. [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on the management of hepatocellular carcinoma. J. Hepatol. 2025, 82, 315–374. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Takemura, N.; Yamashita, T.; Watadani, T.; Kaibori, M.; Kubo, S.; Shimada, M.; Nagano, H.; Hatano, E.; Aikata, H.; et al. Clinical Practice Guidelines for Hepatocellular Carcinoma: The Japan Society of Hepatology 2021 version (5th JSH-HCC Guidelines). Hepatol. Res. 2023, 53, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Tzartzeva, K.; Obi, J.; Rich, N.E.; Parikh, N.D.; Marrero, J.A.; Yopp, A.; Waljee, A.K.; Singal, A.G. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients with Cirrhosis: A Meta-analysis. Gastroenterology 2018, 154, 1706–1718.e1. [Google Scholar] [CrossRef]
- Ge, C.; Luo, M.; Guo, K.; Zhu, D.; Han, N.; Wang, T.; Zhao, X. Role of PIVKA-II in screening for malignancies at a hepatobiliary and pancreatic disease center: A large-scale real-world study. ILIVER 2022, 1, 209–216. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Bao Nguyen, T.; Tan, C.K.; Hasan, I.; Setiawan, L.; Yu, M.-L.; Izumi, N.; Nguyen, H.; Pierce Kah-Hoe, C.; Mohamed, R.; et al. Utility of combining PIVKA-II and AFP in the surveillance and monitoring of hepatocellular carcinoma in the Asia-Pacific region. Clin. Mol. Hepatol. 2023, 29, 277–292. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, S.; Sun, L.; Yao, L.; Zhu, H.; Diao, Y.; Wang, M.; Xing, H.; Lau, W.Y.; Guan, M.; Pawlik, T.M.; et al. Diagnostic Performance of AFP, AFP-L3, or PIVKA-II for Hepatitis C Virus-Associated Hepatocellular Carcinoma: A Multicenter Analysis. J. Clin. Med. 2022, 11, 5075. [Google Scholar] [CrossRef]
- Kobeissy, A.; Merza, N.; Al-Hillan, A.; Boujemaa, S.; Ahmed, Z.; Nawras, M.; Albaaj, M.; Dahiya, D.S.; Alastal, Y.; Hassan, M. Protein Induced by Vitamin K Absence or Antagonist-II Versus Alpha-Fetoprotein in the Diagnosis of Hepatocellular Carcinoma: A Systematic Review with Meta-Analysis. J. Clin. Med. Res. 2023, 15, 343–359. [Google Scholar] [CrossRef]
- Kurniawan, J.; Djianzonie, J.A.C.; Mulyana, E.; Tahapary, D.L.; Sulaiman, A.S.; Wijaya, I.P.; Nasution, S.A.; Setiati, S. Updating AFP Level in Chronic Hepatitis B to Evaluate the Risk of Hepatocellular Carcinoma Occurrence. Acta Med. Indones. 2024, 56, 282–290. [Google Scholar]
- Seo, S.I.; Kim, H.S.; Kim, W.J.; Shin, W.G.; Kim, D.J.; Kim, K.H.; Jang, M.K.; Lee, J.H.; Kim, J.S.; Kim, H.Y.; et al. Diagnostic value of PIVKA-II and alpha-fetoprotein in hepatitis B virus-associated hepatocellular carcinoma. World J. Gastroenterol. 2015, 21, 3928–3935. [Google Scholar] [CrossRef]
- Jefferies, M.; Rauff, B.; Rashid, H.; Lam, T.; Rafiq, S. Update on Global Epidemiology of Viral Hepatitis and Preventive Strategies. World J. Clin. Cases 2018, 6, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Fattovich, G.; Stroffolini, T.; Zagni, I.; Donato, F. Hepatocellular Carcinoma in Cirrhosis: Incidence and Risk Factors. Gastroenterology 2004, 127, S35–S50. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.Q.; Mathurin, P.; Cortez-Pinto, H.; Loomba, R. Global Epidemiology of Alcohol-Associated Cirrhosis and HCC: Trends, Projections and Risk Factors. Nat. Rev. Gastroenterol. Hepatol. 2022, 20, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Russo, F.P.; Zanetto, A.; Pinto, E.; Battistella, S.; Penzo, B.; Burra, P.; Farinati, F. Hepatocellular Carcinoma in Chronic Viral Hepatitis: Where Do We Stand? Int. J. Mol. Sci. 2022, 23, 500. [Google Scholar] [CrossRef] [PubMed]
- Darvishi, M.; Nouri, M.; Rahimi, R.; Heidari-Soureshjani, S.; Hashemi Rafsanjani, S.M.R. A Systematic Review of the Impact of Resveratrol on Viral Hepatitis and Chronic Viral Hepatitis-Related Hepatocellular Carcinoma. Curr. Mol. Med. 2025, 25, 589–604. [Google Scholar] [CrossRef]
- D’souza, S.; Lau, K.C.; Coffin, C.S.; Patel, T.R. Molecular Mechanisms of Viral Hepatitis Induced Hepatocellular Carcinoma. World J. Gastroenterol. 2020, 26, 5759–5783. [Google Scholar] [CrossRef] [PubMed]
- Westbrook, R.H.; Dusheiko, G. Natural History of Hepatitis C. J. Hepatol. 2014, 61, S58–S68. [Google Scholar] [CrossRef]
- John, B.V.; Dang, Y.; Kaplan, D.E.; Jou, J.H.; Taddei, T.H.; Spector, S.A.; Martin, P.; Bastaich, D.R.; Chao, H.-H.; Dahman, B. Liver Stiffness Measurement and Risk Prediction of Hepatocellular Carcinoma after HCV Eradication in Veterans with Cirrhosis. Clin. Gastroenterol. Hepatol. 2024, 22, 778–788.e7. [Google Scholar] [CrossRef]
- Davitkov, P.; Hoffman, K.; Falck-Ytter, Y.; Wilson, B.; Stojadinovikj, G.; Anthony, D.D.; Cohen, S.M.; Cooper, G. Increasing Liver Stiffness Is Associated with Higher Incidence of Hepatocellular Carcinoma in Hepatitis c Infection and Non-Alcoholic Fatty Liver Disease-A Population-Based Study. PLoS ONE 2023, 18, e0280647. [Google Scholar] [CrossRef]
- Ha, N.B.; Yao, F. Alcohol and Hepatocellular Carcinoma. Clin. Liver Dis. 2024, 28, 633–646. [Google Scholar] [CrossRef]
- Yu, M.C.; Yuan, J.-M.; Lu, S.C. Alcohol, Cofactors and the Genetics of Hepatocellular Carcinoma. J. Gastroenterol. Hepatol. 2008, 23 (Suppl. S1), S92–S97. [Google Scholar] [CrossRef]
- Purohit, V.; Rapaka, R.; Kwon, O.S.; Song, B.J. Roles of Alcohol and Tobacco Exposure in the Development of Hepatocellular Carcinoma. Life Sci. 2013, 92, 3–9. [Google Scholar] [CrossRef]
- Ogunwobi, O.O.; Harricharran, T.; Huaman, J.; Galuza, A.; Odumuwagun, O.; Tan, Y.; Ma, G.X.; Nguyen, M.T. Mechanisms of Hepatocellular Carcinoma Progression. J. Gastroenterol. Hepatol. 2019, 25, 2279–2293. [Google Scholar] [CrossRef] [PubMed]
- Ohhira, M.; Ohtake, T.; Saito, H.; Ikuta, K.; Tanaka, K.; Tanabe, H.; Kawashima, T.; Fujimoto, Y.; Naraki, T.; Ono, M.; et al. Increase of serum des-gamma-carboxy prothrombin in alcoholic liver disease without hepatocellular carcinoma. Alcohol. Clin. Exp. Res. 1999, 23 (Suppl. S4), 67S–70S. [Google Scholar] [CrossRef]
- Kang, K.H.; Kim, J.H.; Kang, S.H.; Lee, B.J.; Seo, Y.S.; Yim, H.J.; Yeon, J.E.; Park, J.J.; Kim, J.S.; Bak, Y.T.; et al. The influence of alcoholic liver disease on serum PIVKA-II levels in patients without hepatocellular carcinoma. Gut Liver 2015, 9, 224–230. [Google Scholar] [CrossRef]
- Honda, T.; Ichikawa, T.; Yamashima, M.; Yamamichi, S.; Koike, M.; Nakano, Y.; Honda, T.; Yajima, H.; Miyazaki, O.; Kuribayashi, Y.; et al. PIVKA II is associated with liver function, bone metabolism, and muscle function in patients with liver disease. Biomed. Rep. 2023, 20, 2. [Google Scholar] [CrossRef] [PubMed]
- Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: A modelling study. Lancet Gastroenterol. Hepatol. 2017, 2, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.; Silini, E.; Crosignani, A.; Borzio, F.; Leandro, G.; Bono, F.; Asti, M.; Rossi, S.; Larghi, A.; Cerino, A.; et al. Hepatitis c Virus Genotypes and Risk of Hepatocellular Carcinoma in Cirrhosis: A Prospective Study. Hepatology 1997, 25, 754–758. [Google Scholar] [CrossRef]
- Raimondi, S.; Bruno, S.; Mondelli, M.U.; Maisonneuve, P. Hepatitis c Virus Genotype 1b as a Risk Factor for Hepatocellular Carcinoma Development: A Meta-Analysis. J. Hepatol. 2009, 50, 1142–1154. [Google Scholar] [CrossRef]
- Milosevic, I.; Delic, D.; Lazarevic, I.; Pavlovic, I.P.; Korac, M.; Bojovic, K.; Jevtovic, D. The significance of hepatitis B virus (HBV) genotypes for the disease and treatment outcome among patients with chronic hepatitis B in Serbia. J. Clin. Virol. 2013, 58, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Sunbul, M. Hepatitis B virus genotypes: Global distribution and clinical importance. World J. Gastroenterol. 2014, 20, 5427–5434. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Padarath, K.; Deroubaix, A.; Kramvis, A. The Complex Role of HBeAg and Its Precursors in the Pathway to Hepatocellular Carcinoma. Viruses 2023, 15, 857. [Google Scholar] [CrossRef]
- Chen, C.Y.; Crowther, C.; Kew, M.C.; Kramvis, A. A valine to phenylalanine mutation in the precore region of hepatitis B virus causes intracellular retention and impaired secretion of HBe-antigen. Hepatol. Res. 2008, 38, 580–592. [Google Scholar] [CrossRef]
- Bhoola, N.H.; Kramvis, A. Hepatitis B e Antigen Expression by Hepatitis B Virus Subgenotype A1 Relative to Subgenotypes A2 and D3 in Cultured Hepatocellular Carcinoma (Huh7) Cells. Intervirology 2016, 59, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Mitra, B.; Wang, J.; Kim Elena, S.; Mao, R.; Dong, M.; Liu, Y.; Zhang, J.; Guo, H. Hepatitis B Virus Precore Protein p22 Inhibits Alpha Interferon Signaling by Blocking STAT Nuclear Translocation. J. Virol. 2019, 93, e00196-19. [Google Scholar] [CrossRef] [PubMed]
- Cornberg, M.; Sandmann, L.; Jaroszewicz, J.; Kennedy, P.; Lampertico, P.; Lemoine, M.; Lens, S.; Testoni, B.; Wong, G.L.H.; Russo, F.P. EASL Clinical Practice Guidelines on the Management of Hepatitis B Virus Infection. J. Hepatol. 2025, 83, 502–583. [Google Scholar] [CrossRef] [PubMed]
- Papatherodoridis, G.; Lampertico, P. HCC Risk in Patients Who Are HBeAg-Positive and Have Chronic Hepatitis B under Long-Term Nucleos(T)Ide Analogue Therapy: New Insights from Asia. Hepatology 2024, 80, 260–262. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).