Pilot Study of PIVKA-II in the Prognostic Assessment of Hepatocellular Carcinoma in Chronic Viral Hepatitis: Comparative Findings from HBV and HCV Cohorts from a Single Center in Serbia
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Hepatocellular Carcinoma, Stage, Incidence and Survival
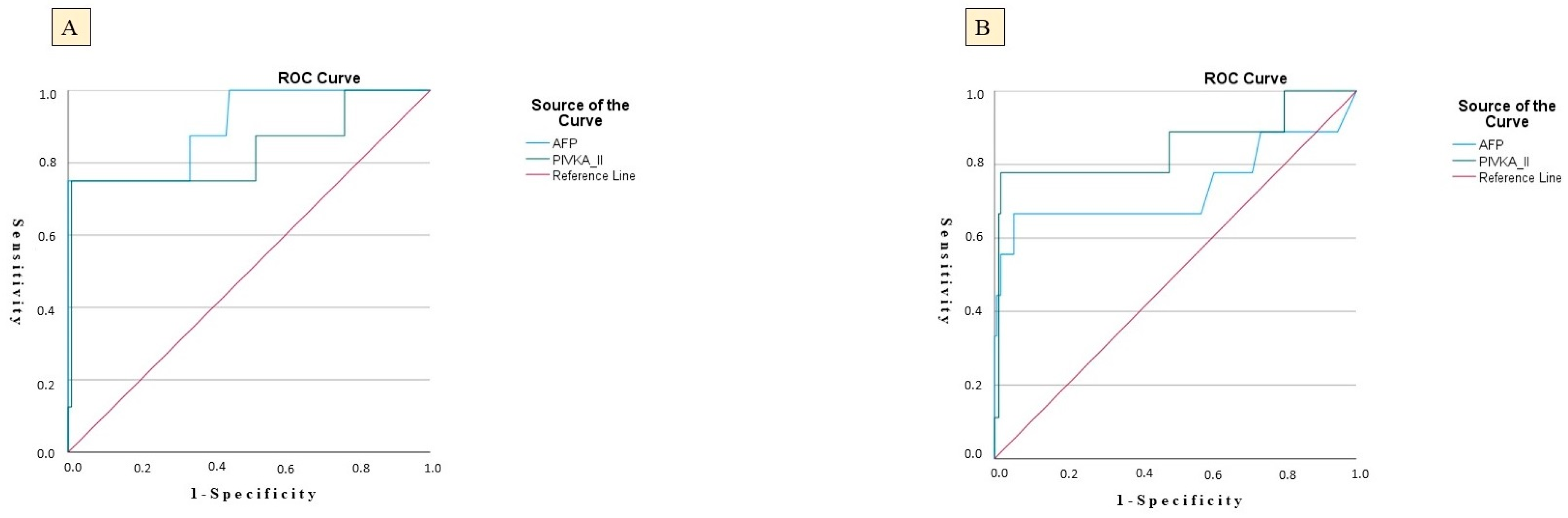
3.2. Predictive Performance of Biomarkers and Risk Modeling
3.3. HCC Predictors in HBV and HCV Cohorts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Samant, H.; Amiri, H.S.; Zibari, G.B. Addressing the Worldwide Hepatocellular Carcinoma: Epidemiology, Prevention and Management. J. Gastrointest. Oncol. 2021, 12 (Suppl. S2), S361–S373. [Google Scholar] [CrossRef]
- Foglia, B.; Turato, C.; Cannito, S. Hepatocellular Carcinoma: Latest Research in Pathogenesis, Detection and Treatment. Int. J. Mol. Sci. 2023, 24, 12224. [Google Scholar] [CrossRef]
- Ivancovsky Wajcman, D.; Nicolàs, A.; Picchio, C.A.; van Selm, L.; Dusheiko, G.; Younossi, Z.M.; Dillon, J.F.; Alqahtani, S.A.; Razavi, H.; Colombo, M.G.; et al. Prioritising viral hepatitis elimination to prevent hepatocellular carcinoma: A public health approach for effective preventive hepatology. JHEP Rep. 2025, 7, 101436. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The Global Epidemiology of NAFLD and NASH in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef]
- Xu, H.Q.; Wang, C.G.; Zhou, Q.; Gao, Y.H. Effects of alcohol consumption on viral hepatitis B and C. World J. Clin. Cases 2021, 9, 10052–10063. [Google Scholar] [CrossRef] [PubMed]
- Milosevic, I.; Todorovic, N.; Filipovic, A.; Simic, J.; Markovic, M.; Stevanovic, O.; Malinic, J.; Katanic, N.; Mitrovic, N.; Nikolic, N. HCV and HCC Tango-Deciphering the Intricate Dance of Disease: A Review Article. Int. J. Mol. Sci. 2023, 24, 16048. [Google Scholar] [CrossRef] [PubMed]
- Melendez-Torres, J.; Singal, A.G. Early detection of hepatocellular carcinoma: Roadmap for improvement. Expert. Rev. Anticancer Ther. 2022, 22, 621–632. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parikh, N.D.; Mehta, A.S.; Singal, A.G.; Block, T.; Marrero, J.A.; Lok, A.S. Biomarkers for the Early Detection of Hepatocellular Carcinoma. Cancer Epidemiol. Biomark. Prev. 2020, 29, 2495–2503. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singal, A.G.; Llovet, J.M.; Yarchoan, M.; Mehta, N.; Heimbach, J.K.; Dawson, L.A.; Jou, J.H.; Kulik, L.M.; Agopian, V.G.; Marrero, J.A.; et al. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology 2023, 78, 1922–1965. [Google Scholar] [CrossRef]
- Dong, L.; Qiu, X.; Gao, F.; Wang, K.; Xu, X. Protein induced by vitamin K absence or antagonist II: Experience to date and future directions. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 189016. [Google Scholar] [CrossRef]
- Svobodova, S.; Karlikova, M.; Topolcan, O.; Fiala, O.; Pecen, L.; Svoboda, T.; Holubec, L. PIVKA-II as a potential new biomarker for hepatocellular carcinoma: A pilot study. Anticancer Res. 2018, 38, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Suttichaimongkol, T.; Mitpracha, M.; Tangvoraphonkchai, K.; Sadee, P.; Sawanyawisuth, K.; Sukeepaisarnjaroen, W. PIVKA-II or AFP has better diagnostic properties for hepatocellular carcinoma diagnosis in high-risk patients. World J. Gastroenterol. 2023, 29, 826–839. [Google Scholar] [CrossRef]
- Hadi, H.; Wan Shuaib, W.M.A.; Raja Ali, R.A.; Othman, H. Utility of PIVKA-II and AFP in differentiating hepatocellular carcinoma from benign liver diseases. Medicina 2022, 58, 1015. [Google Scholar] [CrossRef]
- Si, Y.-Q.; Wang, X.-Q.; Fan, G.; Wang, C.-Y.; Zheng, Y.-W.; Song, X.; Pan, C.-C.; Chu, F.-L.; Liu, Z.-F.; Lu, B.-R.; et al. Value of AFP and PIVKA-II in diagnosis of HBV-related hepatocellular carcinoma and prediction of vascular invasion and tumor differentiation. Infect. Agents Cancer 2020, 15, 70. [Google Scholar] [CrossRef]
- Feng, H.; Li, B.; Li, Z.; Wei, Q.; Ren, L. PIVKA-II serves as a potential biomarker that complements AFP for the diagnosis of hepatocellular carcinoma. BMC Cancer 2021, 21, 401. [Google Scholar] [CrossRef]
- Park, S.J.; Jang, J.Y.; Jeong, S.W.; Cho, Y.K.; Lee, S.H.; Kim, S.G.; Cha, S.W.; Kim, Y.S.; Cho, Y.D.; Kim, H.S.; et al. Usefulness of AFP, AFP-L3, and PIVKA-II, and their combinations in diagnosing early HCC. Medicine 2017, 96, e6652. [Google Scholar]
- Koike, Y.; Shiratori, Y.; Sato, S.; Obi, S.; Teratani, T.; Imamura, M.; Yoshida, H.; Shiina, S.; Omata, M. Des-gamma-carboxy prothrombin as a useful predisposing factor for the development of portal venous invasion in patients with hepatocellular carcinoma: A prospective analysis of 227 patients. Cancer 2001, 91, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Lau, G.K.; Wei, L.; Moriyama, M.; Yu, M.L.; Chuang, W.L.; Ibrahim, A.; Lesmana, C.R.A.; Sollano, J.; Kumar, M.; et al. APASL HCV guidelines of virus-eradicated patients by DAA on how to monitor HCC occurrence and HBV reactivation. Hepatol. Int. 2019, 13, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Association for the Study of the Liver. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J. Hepatol. 2021, 75, 659–689. [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on the management of hepatocellular carcinoma. J. Hepatol. 2025, 82, 315–374. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Takemura, N.; Yamashita, T.; Watadani, T.; Kaibori, M.; Kubo, S.; Shimada, M.; Nagano, H.; Hatano, E.; Aikata, H.; et al. Clinical Practice Guidelines for Hepatocellular Carcinoma: The Japan Society of Hepatology 2021 version (5th JSH-HCC Guidelines). Hepatol. Res. 2023, 53, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Tzartzeva, K.; Obi, J.; Rich, N.E.; Parikh, N.D.; Marrero, J.A.; Yopp, A.; Waljee, A.K.; Singal, A.G. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients with Cirrhosis: A Meta-analysis. Gastroenterology 2018, 154, 1706–1718.e1. [Google Scholar] [CrossRef]
- Ge, C.; Luo, M.; Guo, K.; Zhu, D.; Han, N.; Wang, T.; Zhao, X. Role of PIVKA-II in screening for malignancies at a hepatobiliary and pancreatic disease center: A large-scale real-world study. ILIVER 2022, 1, 209–216. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Bao Nguyen, T.; Tan, C.K.; Hasan, I.; Setiawan, L.; Yu, M.-L.; Izumi, N.; Nguyen, H.; Pierce Kah-Hoe, C.; Mohamed, R.; et al. Utility of combining PIVKA-II and AFP in the surveillance and monitoring of hepatocellular carcinoma in the Asia-Pacific region. Clin. Mol. Hepatol. 2023, 29, 277–292. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, S.; Sun, L.; Yao, L.; Zhu, H.; Diao, Y.; Wang, M.; Xing, H.; Lau, W.Y.; Guan, M.; Pawlik, T.M.; et al. Diagnostic Performance of AFP, AFP-L3, or PIVKA-II for Hepatitis C Virus-Associated Hepatocellular Carcinoma: A Multicenter Analysis. J. Clin. Med. 2022, 11, 5075. [Google Scholar] [CrossRef]
- Kobeissy, A.; Merza, N.; Al-Hillan, A.; Boujemaa, S.; Ahmed, Z.; Nawras, M.; Albaaj, M.; Dahiya, D.S.; Alastal, Y.; Hassan, M. Protein Induced by Vitamin K Absence or Antagonist-II Versus Alpha-Fetoprotein in the Diagnosis of Hepatocellular Carcinoma: A Systematic Review with Meta-Analysis. J. Clin. Med. Res. 2023, 15, 343–359. [Google Scholar] [CrossRef]
- Kurniawan, J.; Djianzonie, J.A.C.; Mulyana, E.; Tahapary, D.L.; Sulaiman, A.S.; Wijaya, I.P.; Nasution, S.A.; Setiati, S. Updating AFP Level in Chronic Hepatitis B to Evaluate the Risk of Hepatocellular Carcinoma Occurrence. Acta Med. Indones. 2024, 56, 282–290. [Google Scholar]
- Seo, S.I.; Kim, H.S.; Kim, W.J.; Shin, W.G.; Kim, D.J.; Kim, K.H.; Jang, M.K.; Lee, J.H.; Kim, J.S.; Kim, H.Y.; et al. Diagnostic value of PIVKA-II and alpha-fetoprotein in hepatitis B virus-associated hepatocellular carcinoma. World J. Gastroenterol. 2015, 21, 3928–3935. [Google Scholar] [CrossRef]
- Jefferies, M.; Rauff, B.; Rashid, H.; Lam, T.; Rafiq, S. Update on Global Epidemiology of Viral Hepatitis and Preventive Strategies. World J. Clin. Cases 2018, 6, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Fattovich, G.; Stroffolini, T.; Zagni, I.; Donato, F. Hepatocellular Carcinoma in Cirrhosis: Incidence and Risk Factors. Gastroenterology 2004, 127, S35–S50. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.Q.; Mathurin, P.; Cortez-Pinto, H.; Loomba, R. Global Epidemiology of Alcohol-Associated Cirrhosis and HCC: Trends, Projections and Risk Factors. Nat. Rev. Gastroenterol. Hepatol. 2022, 20, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Russo, F.P.; Zanetto, A.; Pinto, E.; Battistella, S.; Penzo, B.; Burra, P.; Farinati, F. Hepatocellular Carcinoma in Chronic Viral Hepatitis: Where Do We Stand? Int. J. Mol. Sci. 2022, 23, 500. [Google Scholar] [CrossRef] [PubMed]
- Darvishi, M.; Nouri, M.; Rahimi, R.; Heidari-Soureshjani, S.; Hashemi Rafsanjani, S.M.R. A Systematic Review of the Impact of Resveratrol on Viral Hepatitis and Chronic Viral Hepatitis-Related Hepatocellular Carcinoma. Curr. Mol. Med. 2025, 25, 589–604. [Google Scholar] [CrossRef]
- D’souza, S.; Lau, K.C.; Coffin, C.S.; Patel, T.R. Molecular Mechanisms of Viral Hepatitis Induced Hepatocellular Carcinoma. World J. Gastroenterol. 2020, 26, 5759–5783. [Google Scholar] [CrossRef] [PubMed]
- Westbrook, R.H.; Dusheiko, G. Natural History of Hepatitis C. J. Hepatol. 2014, 61, S58–S68. [Google Scholar] [CrossRef]
- John, B.V.; Dang, Y.; Kaplan, D.E.; Jou, J.H.; Taddei, T.H.; Spector, S.A.; Martin, P.; Bastaich, D.R.; Chao, H.-H.; Dahman, B. Liver Stiffness Measurement and Risk Prediction of Hepatocellular Carcinoma after HCV Eradication in Veterans with Cirrhosis. Clin. Gastroenterol. Hepatol. 2024, 22, 778–788.e7. [Google Scholar] [CrossRef]
- Davitkov, P.; Hoffman, K.; Falck-Ytter, Y.; Wilson, B.; Stojadinovikj, G.; Anthony, D.D.; Cohen, S.M.; Cooper, G. Increasing Liver Stiffness Is Associated with Higher Incidence of Hepatocellular Carcinoma in Hepatitis c Infection and Non-Alcoholic Fatty Liver Disease-A Population-Based Study. PLoS ONE 2023, 18, e0280647. [Google Scholar] [CrossRef]
- Ha, N.B.; Yao, F. Alcohol and Hepatocellular Carcinoma. Clin. Liver Dis. 2024, 28, 633–646. [Google Scholar] [CrossRef]
- Yu, M.C.; Yuan, J.-M.; Lu, S.C. Alcohol, Cofactors and the Genetics of Hepatocellular Carcinoma. J. Gastroenterol. Hepatol. 2008, 23 (Suppl. S1), S92–S97. [Google Scholar] [CrossRef]
- Purohit, V.; Rapaka, R.; Kwon, O.S.; Song, B.J. Roles of Alcohol and Tobacco Exposure in the Development of Hepatocellular Carcinoma. Life Sci. 2013, 92, 3–9. [Google Scholar] [CrossRef]
- Ogunwobi, O.O.; Harricharran, T.; Huaman, J.; Galuza, A.; Odumuwagun, O.; Tan, Y.; Ma, G.X.; Nguyen, M.T. Mechanisms of Hepatocellular Carcinoma Progression. J. Gastroenterol. Hepatol. 2019, 25, 2279–2293. [Google Scholar] [CrossRef] [PubMed]
- Ohhira, M.; Ohtake, T.; Saito, H.; Ikuta, K.; Tanaka, K.; Tanabe, H.; Kawashima, T.; Fujimoto, Y.; Naraki, T.; Ono, M.; et al. Increase of serum des-gamma-carboxy prothrombin in alcoholic liver disease without hepatocellular carcinoma. Alcohol. Clin. Exp. Res. 1999, 23 (Suppl. S4), 67S–70S. [Google Scholar] [CrossRef]
- Kang, K.H.; Kim, J.H.; Kang, S.H.; Lee, B.J.; Seo, Y.S.; Yim, H.J.; Yeon, J.E.; Park, J.J.; Kim, J.S.; Bak, Y.T.; et al. The influence of alcoholic liver disease on serum PIVKA-II levels in patients without hepatocellular carcinoma. Gut Liver 2015, 9, 224–230. [Google Scholar] [CrossRef]
- Honda, T.; Ichikawa, T.; Yamashima, M.; Yamamichi, S.; Koike, M.; Nakano, Y.; Honda, T.; Yajima, H.; Miyazaki, O.; Kuribayashi, Y.; et al. PIVKA II is associated with liver function, bone metabolism, and muscle function in patients with liver disease. Biomed. Rep. 2023, 20, 2. [Google Scholar] [CrossRef] [PubMed]
- Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: A modelling study. Lancet Gastroenterol. Hepatol. 2017, 2, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.; Silini, E.; Crosignani, A.; Borzio, F.; Leandro, G.; Bono, F.; Asti, M.; Rossi, S.; Larghi, A.; Cerino, A.; et al. Hepatitis c Virus Genotypes and Risk of Hepatocellular Carcinoma in Cirrhosis: A Prospective Study. Hepatology 1997, 25, 754–758. [Google Scholar] [CrossRef]
- Raimondi, S.; Bruno, S.; Mondelli, M.U.; Maisonneuve, P. Hepatitis c Virus Genotype 1b as a Risk Factor for Hepatocellular Carcinoma Development: A Meta-Analysis. J. Hepatol. 2009, 50, 1142–1154. [Google Scholar] [CrossRef]
- Milosevic, I.; Delic, D.; Lazarevic, I.; Pavlovic, I.P.; Korac, M.; Bojovic, K.; Jevtovic, D. The significance of hepatitis B virus (HBV) genotypes for the disease and treatment outcome among patients with chronic hepatitis B in Serbia. J. Clin. Virol. 2013, 58, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Sunbul, M. Hepatitis B virus genotypes: Global distribution and clinical importance. World J. Gastroenterol. 2014, 20, 5427–5434. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Padarath, K.; Deroubaix, A.; Kramvis, A. The Complex Role of HBeAg and Its Precursors in the Pathway to Hepatocellular Carcinoma. Viruses 2023, 15, 857. [Google Scholar] [CrossRef]
- Chen, C.Y.; Crowther, C.; Kew, M.C.; Kramvis, A. A valine to phenylalanine mutation in the precore region of hepatitis B virus causes intracellular retention and impaired secretion of HBe-antigen. Hepatol. Res. 2008, 38, 580–592. [Google Scholar] [CrossRef]
- Bhoola, N.H.; Kramvis, A. Hepatitis B e Antigen Expression by Hepatitis B Virus Subgenotype A1 Relative to Subgenotypes A2 and D3 in Cultured Hepatocellular Carcinoma (Huh7) Cells. Intervirology 2016, 59, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Mitra, B.; Wang, J.; Kim Elena, S.; Mao, R.; Dong, M.; Liu, Y.; Zhang, J.; Guo, H. Hepatitis B Virus Precore Protein p22 Inhibits Alpha Interferon Signaling by Blocking STAT Nuclear Translocation. J. Virol. 2019, 93, e00196-19. [Google Scholar] [CrossRef] [PubMed]
- Cornberg, M.; Sandmann, L.; Jaroszewicz, J.; Kennedy, P.; Lampertico, P.; Lemoine, M.; Lens, S.; Testoni, B.; Wong, G.L.H.; Russo, F.P. EASL Clinical Practice Guidelines on the Management of Hepatitis B Virus Infection. J. Hepatol. 2025, 83, 502–583. [Google Scholar] [CrossRef] [PubMed]
- Papatherodoridis, G.; Lampertico, P. HCC Risk in Patients Who Are HBeAg-Positive and Have Chronic Hepatitis B under Long-Term Nucleos(T)Ide Analogue Therapy: New Insights from Asia. Hepatology 2024, 80, 260–262. [Google Scholar] [CrossRef]
| Variable | CHB Cohort N = 242; n (57.2%) | CHC Cohort N = 181; n (42.8%) | p | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Sex | Male | 117 (48.3%) | 106 (58.6) | 0.037 |
| Female | 125 (51.7%) | 75 (41.4%) | ||
| Age | 53.10 ± 15.33 | 51.31 ± 13.89 | 0.254 | |
| Vital status (deceased) | 4 (1.6%) | 3 (1.6%) | 0.852 | |
| Body mass index [kg/m2] | 25.83 ± 4.21 | 21.92 ± 3.87 | 0.043 | |
| Chronic alcohol abuse | 21 (8.6%) | 34 (18.8%) | 0.025 | |
| Intravenous drug use | 13 (5.3%) | 67 (37.0%) | <0.001 | |
| Only non-intravenous drug use | 7 (2.9%) | 21 (11.6%) | 0.004 | |
| Smoking | 81 (33.5%) | 82 (45.3%) | 0.043 | |
| Liver fibrosis and steatosis parameters | ||||
| F0/F1—No/mild fibrosis | 130 (53.9%) | 72 (39.8%) | 0.042 | |
| F2—Moderate fibrosis | 44 (18.3%) | 45 (24.9%) | 0.186 | |
| F3—Severe fibrosis | 23 (9.5%) | 15 (8.3%%) | 0.694 | |
| F4—Cirrhosis | 45 (18.7%) | 49 (27.1%) | 0.097 | |
| FibroScan® stiffness [kPa] | 8.37±7.58 | 9.17±8.66 | 0.197 | |
| S0—No steatosis | 127 (52.5%) | 89 (49.1%) | 0.510 | |
| S1—Mild steatosis | 62 (25.6%) | 56 (30.9%) | 0.287 | |
| S2—Moderate steatosis | 26 (10.7%) | 19 (10.5%) | 0.698 | |
| S3—Severe steatosis | 27 (11.1%) | 17 (9.3%) | 0.458 | |
| FibroScan® CAP [dB/m] | 241.5 ± 29.58 | 248.5 ± 32.34 | 0.575 | |
| Cirrhosis patient profile | ||||
| Child–Pugh class A | 29 (11.9%) | 28 (15.4%) | 0.125 | |
| Child–Pugh class B | 11 (4.5%) | 13 (7.1%) | 0.059 | |
| Child–Pugh class C | 5 (2.1%) | 8 (4.4%) | 0.074 | |
| Ascites | 6 (2.5%) | 11 (6.0%) | 0.028 | |
| Hepatic encephalopathy | 4 (1.6%) | 4 (2.2%) | 0.512 | |
| Portal hypertension | 15 (6.1%) | 17 (9.3%) | 0.408 | |
| Esophageal varices | 15 (6.1%) | 16 (8.8%) | 0.263 | |
| Comorbidities | ||||
| Hypertension | 72 (29.7%) | 64 (35.4%) | 0.221 | |
| Cerebrovascular insult | 5 (2.1%) | 3 (1.7%) | 0.801 | |
| Other cardiovascular diseases | 14 (5.8%) | 8 (4.4%) | 0.572 | |
| Diabetes mellitus | 34 (14.1%) | 21 (11.6%) | 0.503 | |
| OAK vitamin K antagonist therapy | 18 (7.4%) | 12 (6.6%) | 0.138 | |
| Respiratory diseases | 14 (5.8%) | 13 (7.2%) | 0.573 | |
| Chronic kidney disease | 15 (6.2%) | 12 (6.6%) | 0.863 | |
| Malignant diseases | 27 (11.2%) | 22 (12.2%) | 0.746 | |
| Systemic connective tissue diseases | 6 (2.5%) | 4 (2.2%) | 0.891 | |
| Neurological diseases | 6 (2.5%) | 10 (5.5%) | 0.083 | |
| Hypo/hyperthyroidism | 7 (2.9%) | 9 (5.0%) | 0.261 | |
| Psychiatric disorders | 13 (5.4%) | 30 (16.6%) | 0.001 | |
| Variable | CHB Cohort N = 242; n (57.2%) | CHC Cohort N = 181; n (42.8%) |
|---|---|---|
| HBV DNA PCR [IU/mL] | 15,213.0 ± 5412.0 | n/a |
| HBeAg positivity | 61 (25.2%) | |
| Anti-HBe positivity | 180 (74.3%) | |
| TDF therapy | 94 (38.8%) | |
| TAF therapy | 67 (27.6%) | |
| HCV RNA RT PCR [IU/mL] | n/a | 1,359,520.0 ± 825.2 |
| Genotype 1a | 67 (37.0%) | |
| Genotype 1b | 14 (7.7%) | |
| Genotype 2 | 12 (6.6%) | |
| Genotype 3 | 70 (38.7%) | |
| Genotype 4 | 19 (10.5%) | |
| Sofosbuvir/velpatasvir | 78 (43.1%) | |
| Glecaprevir/pibrentasvir | 92 (50.8%) | |
| Elbasvir/grazoprevir | 11 (6.1%) | |
| Prior PEG-IFN therapy | 17 (9.4%) |
| Variable | CHB Cohort N = 242 n (57.2%) | CHC Cohort N = 181 n (42.8%) | p |
|---|---|---|---|
| Hepatocellular carcinoma | 12 (5.0%) | 10 (5.5%) | 0.697 |
| HCC stage I | 1 (0.4%) | 1 (0.5%) | 0.841 |
| HCC stage II | 2 (0.8%) | 3 (1.6%) | 0.160 |
| HCC stage III | 6 (2.5%) | 2 (1.1%) | 0.098 |
| HCC stage IV | 3 (1.2%) | 4 (2.2%) | 0.341 |
| Vital status (deceased) | 3 (1.2%) | 4 (2.2%) | 0.970 |
| Time from diagnosis to death [months] | 4.6 ± 3.8 | 7.1 ± 4.1 | 0.016 |
| Surgical resection | 7 (2.9%) | 6 (3.3%) | 0.947 |
| Microwave ablation | 2 (0.8%) | 1 (0.5%) | 0.989 |
| TACE treatment | 1 (0.4%) | 0 | n/a |
| Sorafenib therapy | 5 (2.1%) | 3 (1.7%) | 0.997 |
| Factors analyzed for predicting the occurrence of HCC | |||
| Initial values of PIVKA-II [ng/mL] | 129.5 (53.0–206.5) | 116.0 (71.0–161.0) | 0.257 |
| Initial values of AFP [μg/L] | 9.0 (2.0–6.0) | 7.5 (1.0–4.0) | 0.136 |
| HCC as an Outcome Variable–CHB Cohort | ||||
|---|---|---|---|---|
| Area under the curve | Specificity | NPV | p | |
| PIVKA-II | 0.809 | 64.5% | 98.15% | 0.024 |
| Cut-off | Sensitivity | PPV | Standard error | |
| 47.0 | 84.5% | 15.18% | 0.048 | |
| Area under the curve | Specificity | NPV | p | |
| AFP | 0.830 | 65.5% | 98.31% | 0.019 |
| Cut-off | Sensitivity | PPV | Standard error | |
| 11.2 | 82.9% | 11.91% | 0.057 | |
| HCC as an Outcome Variable–CHC Cohort | ||||
| Area under the curve | Specificity | NPV | p | |
| PIVKA-II | 0.812 | 61.2% | 98.09% | 0.012 |
| Cut-off | Sensitivity | PPV | Standard error | |
| 46.0 | 82.0% | 16.23% | 0.006 | |
| Area under the curve | Specificity | NPV | p | |
| AFP | 0.824 | 76.5% | 97.98% | 0.020 |
| Cut-off | Sensitivity | PPV | Standard error | |
| 11.6 | 75.3% | 15.21% | 0.051 | |
| CHB Cohort | CHC Cohort | |||||||||||
| Model 1 | Univariate Cox Model | Multivariate Cox Model | Univariate Cox Model | Multivariate Cox Model | ||||||||
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | |
| Sex | 1.16 | 0.82–1.64 | 0.376 | 1.45 | 0.92–2.30 | 0.108 | 1.36 | 0.81–2.14 | 0.152 | |||
| Age | 1.01 | 0.98–1.03 | 0.260 | 1.03 | 1.01–1.05 | 0.004 | 1.01 | 1.01–1.06 | 0.012 | |||
| BMI [kg/m2] | 0.98 | 0.93–1.03 | 0.399 | 1.02 | 0.98–1.07 | 0.271 | ||||||
| Chronic alcohol abuse | 2.29 | 1.85–2.97 | 0.036 | 2.21 | 1.74–2.93 | 0.028 | 1.72 | 1.12–2.64 | 0.013 | 1.69 | 1.09–2.67 | 0.024 |
| Intravenous drug use | 1.35 | 0.78–2.34 | 0.281 | 1.58 | 0.93–2.67 | 0.086 | ||||||
| Non-intravenous drug use | 1.12 | 0.72–1.75 | 0.614 | 1.21 | 0.74–1.99 | 0.438 | ||||||
| Smoking (current) | 1.09 | 0.78–1.52 | 0.625 | 1.35 | 0.88–2.06 | 0.170 | ||||||
| F0/F1—No/mild fibrosis | 1.05 | 0.70–1.58 | 0.807 | 1.00 | 0.63–1.59 | 0.990 | ||||||
| F2—Moderate fibrosis | 1.22 | 0.78–1.90 | 0.372 | 1.43 | 0.85–2.41 | 0.177 | ||||||
| F3—Severe fibrosis | 1.41 | 0.90–2.22 | 0.119 | 1.52 | 0.98–2.31 | 0.156 | 2.12 | 1.19–3.77 | 0.025 | 1.81 | 0.98–3.14 | 0.084 |
| F4—Cirrhosis | 1.58 | 0.95–2.64 | 0.080 | 1.68 | 0.99–2.81 | 0.054 | 3.65 | 2.01–6.63 | 0.001 | 3.60 | 1.98–6.35 | 0.001 |
| FibroScan® stiffness [kPa] | 1.02 | 0.99–1.06 | 0.163 | 1.04 | 1.02–1.06 | 0.011 | 1.02 | 1.01–1.05 | 0.008 | |||
| S0—No steatosis | 1.00 | 0.65–1.56 | 0.991 | 1.00 | 0.61–1.64 | 0.992 | ||||||
| S1—Mild steatosis | 0.92 | 0.58–1.47 | 0.736 | 0.88 | 0.55–1.39 | 0.582 | ||||||
| S2—Moderate steatosis | 1.08 | 0.68–1.72 | 0.747 | 0.97 | 0.60–1.58 | 0.902 | ||||||
| S3—Severe steatosis | 1.19 | 0.75–1.88 | 0.456 | 1.15 | 0.69–1.93 | 0.589 | ||||||
| FibroScan® CAP [dB/m] | 1.00 | 0.99–1.01 | 0.282 | 1.45 | 0.92–2.30 | 0.138 | ||||||
| Model 2 | Univariate Cox Model | Multivariate Cox Model | Univariate Cox Model | Multivariate Cox Model | ||||||||
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | |
| Sex | 1.16 | 0.82–1.64 | 0.376 | 1.20 | 0.98–1.41 | 0.205 | 1.45 | 0.92–2.30 | 0.108 | |||
| Age | 1.01 | 0.98–1.03 | 0.260 | 1.02 | 0.79–1.24 | 0.331 | 1.03 | 1.01–1.05 | 0.004 | 1.04 | 1.01–1.06 | 0.012 |
| Child–Pugh class A | 1.08 | 0.69–1.70 | 0.733 | 1.16 | 0.91–1.42 | 0.368 | 1.13 | 0.96–1.33 | 0.128 | |||
| Child–Pugh class B | 1.25 | 0.80–1.95 | 0.325 | 1.23 | 1.00–1.45 | 0.527 | 1.21 | 0.90–1.63 | 0.084 | 1.14 | 0.87–1.98 | 0.159 |
| Child–Pugh class C | 1.52 | 0.92–2.51 | 0.106 | 1.51 | 1.27–1.74 | 0.315 | 1.88 | 1.67–2.15 | 0.033 | 1.64 | 1.21–1.81 | 0.041 |
| Ascites | 1.31 | 0.87–1.97 | 0.227 | 1.36 | 1.13–1.59 | 0.172 | 1.07 | 0.91–1.26 | 0.425 | |||
| Hepatic encephalopathy | 1.28 | 0.81–2.01 | 0.281 | 1.29 | 1.03–1.55 | 0.466 | 0.99 | 0.83–1.19 | 0.966 | |||
| Portal hypertension | 1.18 | 0.78–1.77 | 0.436 | 1.25 | 0.97–1.52 | 0.451 | 1.15 | 0.93–1.42 | 0.198 | |||
| Esophageal varices | 1.14 | 0.70–1.69 | 0.520 | 1.18 | 0.97–1.40 | 0.292 | 1.06 | 0.87–1.29 | 0.531 | |||
| CHB Cohort | CHC Cohort | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 3 | Univariate Cox Model | Multivariate Cox Model | Univariate Cox Model | Multivariate Cox Model | ||||||||
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | |
| Sex | 1.16 | 0.82–1.64 | 0.376 | 1.45 | 0.92–2.30 | 0.108 | ||||||
| Age | 1.01 | 0.98–1.03 | 0.260 | 1.03 | 1.01–1.05 | 0.004 | 1.04 | 1.01–1.06 | 0.006 | |||
| Initial values of PIVKA-II | 1.01 | 1.00–1.01 | 0.004 | 1.02 | 1.01–1.03 | 0.006 | 1.05 | 1.00–1.10 | 0.015 | 1.03 | 1.01–1.05 | 0.002 |
| Initial values of AFP | 1.13 | 1.11–1.17 | 0.018 | 1.14 | 1.10–1.18 | 0.024 | 1.11 | 1.03–1.20 | 0.006 | 1.16 | 1.04–1.24 | 0.012 |
| Combined AFP/PIVKA-II | 1.26 | 1.06–1.38 | 0.001 | 1.38 | 1.20–1.46 | 0.001 | 1.31 | 1.15–1.4 | 0.001 | 1.36 | 1.21–1.37 | 0.001 |
| Model 4 | Univariate Cox Model | Multivariate Cox Model | Multivariate Cox Model | Multivariate Cox Model | ||||||||
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | |
| Sex | 1.16 | 0.82–1.64 | 0.376 | 1.45 | 0.92–2.30 | 0.108 | ||||||
| Age | 1.01 | 0.98–1.03 | 0.260 | 1.03 | 1.01–1.05 | 0.004 | ||||||
| HBV DNA PCR | 1.98 | 0.65–2.54 | 0.620 | n/a | ||||||||
| HBeAg positivity | 1.87 | 1.24–2.46 | 0.024 | 1.56 | 1.17–2.31 | 0.027 | ||||||
| anti-HBe positivity | 0.67 | 0.41–1.01 | 0.111 | 0.75 | 0.51–1.08 | 0.156 | ||||||
| TDF therapy | 0.59 | 0.25–1.06 | 0.412 | |||||||||
| TAF therapy | 0.78 | 0.69–1.05 | 0.236 | |||||||||
| HCV RNA RT PCR | n/a | 1.12 | 0.68–1.85 | 0.417 | ||||||||
| Genotype 1a | 1.29 | 0.78–2.15 | 0.293 | |||||||||
| Genotype 1b | 1.41 | 1.01–1.96 | 0.034 | 1.38 | 1.02–2.08 | 0.020 | ||||||
| Genotype 2 | 1.67 | 0.94–2.95 | 0.084 | 1.52 | 0.91–2.19 | 0.284 | ||||||
| Genotype 3 | 1.03 | 0.99–1.07 | 0.134 | |||||||||
| Genotype 4 | 0.95 | 0.58–1.56 | 0.832 | |||||||||
| Sofosbuvir/velpatasvir | 0.91 | 0.59–1.42 | 0.077 | |||||||||
| Glecaprevir/pibrentasvir | 1.02 | 0.66–1.57 | 0.938 | |||||||||
| Elbasvir/grazoprevir | 0.98 | 0.96–1.01 | 0.089 | 0.97 | 0.94–1.06 | 0.165 | ||||||
| Prior PEG-IFN therapy | 1.33 | 0.86–2.05 | 0.199 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milošević, I.; Nikolić, N.; Stanković, S.; Filipović, A.; Ranin, J.; Paunović, I.; Simić, J.; Beronja, B. Pilot Study of PIVKA-II in the Prognostic Assessment of Hepatocellular Carcinoma in Chronic Viral Hepatitis: Comparative Findings from HBV and HCV Cohorts from a Single Center in Serbia. Biomedicines 2025, 13, 2653. https://doi.org/10.3390/biomedicines13112653
Milošević I, Nikolić N, Stanković S, Filipović A, Ranin J, Paunović I, Simić J, Beronja B. Pilot Study of PIVKA-II in the Prognostic Assessment of Hepatocellular Carcinoma in Chronic Viral Hepatitis: Comparative Findings from HBV and HCV Cohorts from a Single Center in Serbia. Biomedicines. 2025; 13(11):2653. https://doi.org/10.3390/biomedicines13112653
Chicago/Turabian StyleMilošević, Ivana, Nataša Nikolić, Sanja Stanković, Ana Filipović, Jovana Ranin, Irena Paunović, Jelena Simić, and Branko Beronja. 2025. "Pilot Study of PIVKA-II in the Prognostic Assessment of Hepatocellular Carcinoma in Chronic Viral Hepatitis: Comparative Findings from HBV and HCV Cohorts from a Single Center in Serbia" Biomedicines 13, no. 11: 2653. https://doi.org/10.3390/biomedicines13112653
APA StyleMilošević, I., Nikolić, N., Stanković, S., Filipović, A., Ranin, J., Paunović, I., Simić, J., & Beronja, B. (2025). Pilot Study of PIVKA-II in the Prognostic Assessment of Hepatocellular Carcinoma in Chronic Viral Hepatitis: Comparative Findings from HBV and HCV Cohorts from a Single Center in Serbia. Biomedicines, 13(11), 2653. https://doi.org/10.3390/biomedicines13112653







