Selective Cytotoxicity in Chronic Myeloid Leukemia (K-562) Cells Induced by 532 nm LASER Irradiation Without Exogenous Photosensitizers
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Line and Culture Conditions
2.2. Spectroscopic Characterization and Definition of the Optical Therapeutic Window (OTW)
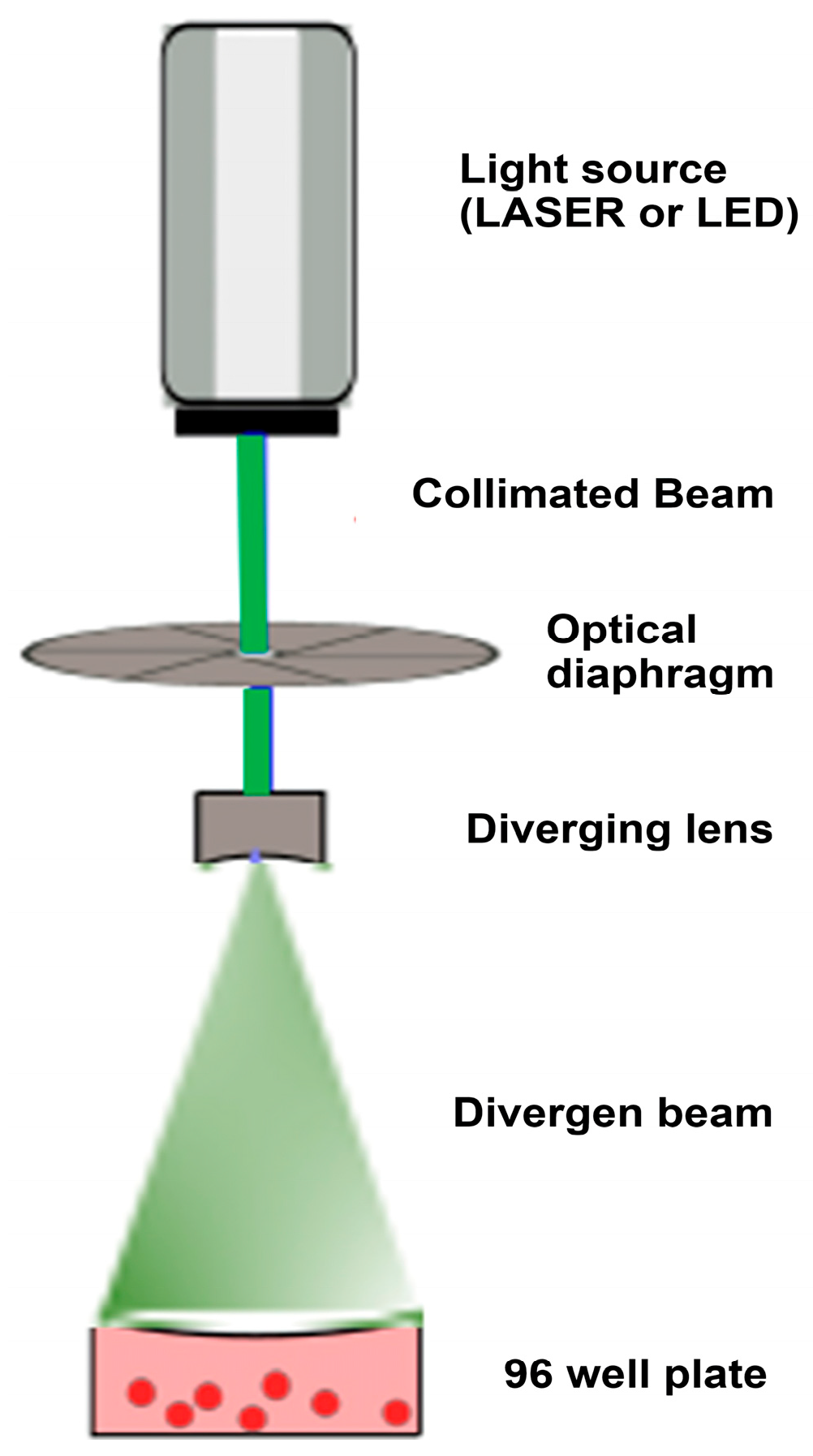
2.3. Optical Setup and Irradiation Protocol
2.4. Experimental Design
2.5. Quantification of Cytotoxicity and Cell Viability
2.6. Processing Scientific Images
2.7. Statistical Analysis
2.8. Ethical Considerations
3. Results
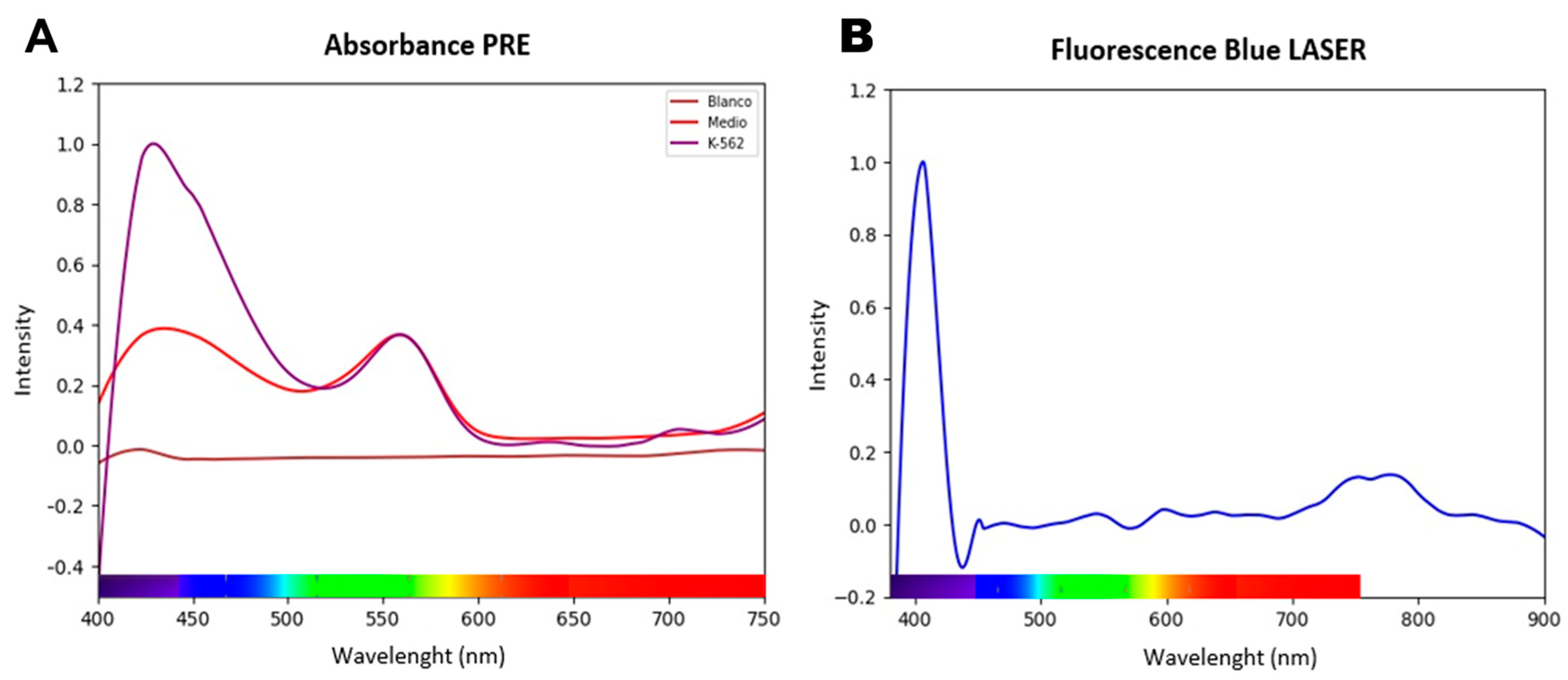
3.1. The Intrinsic Optical Properties of K-562 Cells Reveal a Therapeutic Window in the Visible Spectrum
3.2. Selective Irradiation with a 532 nm LASER Induces Significant and Dose-Dependent Cytotoxicity
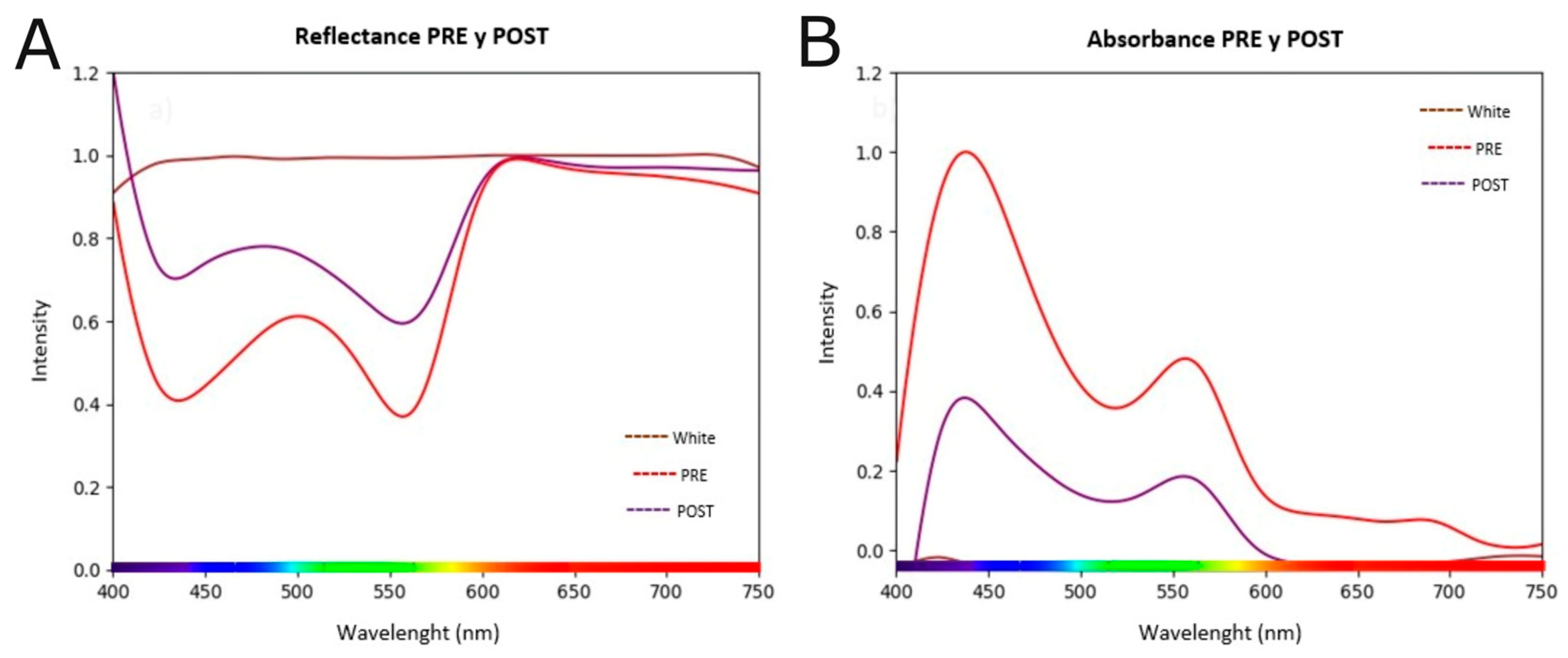
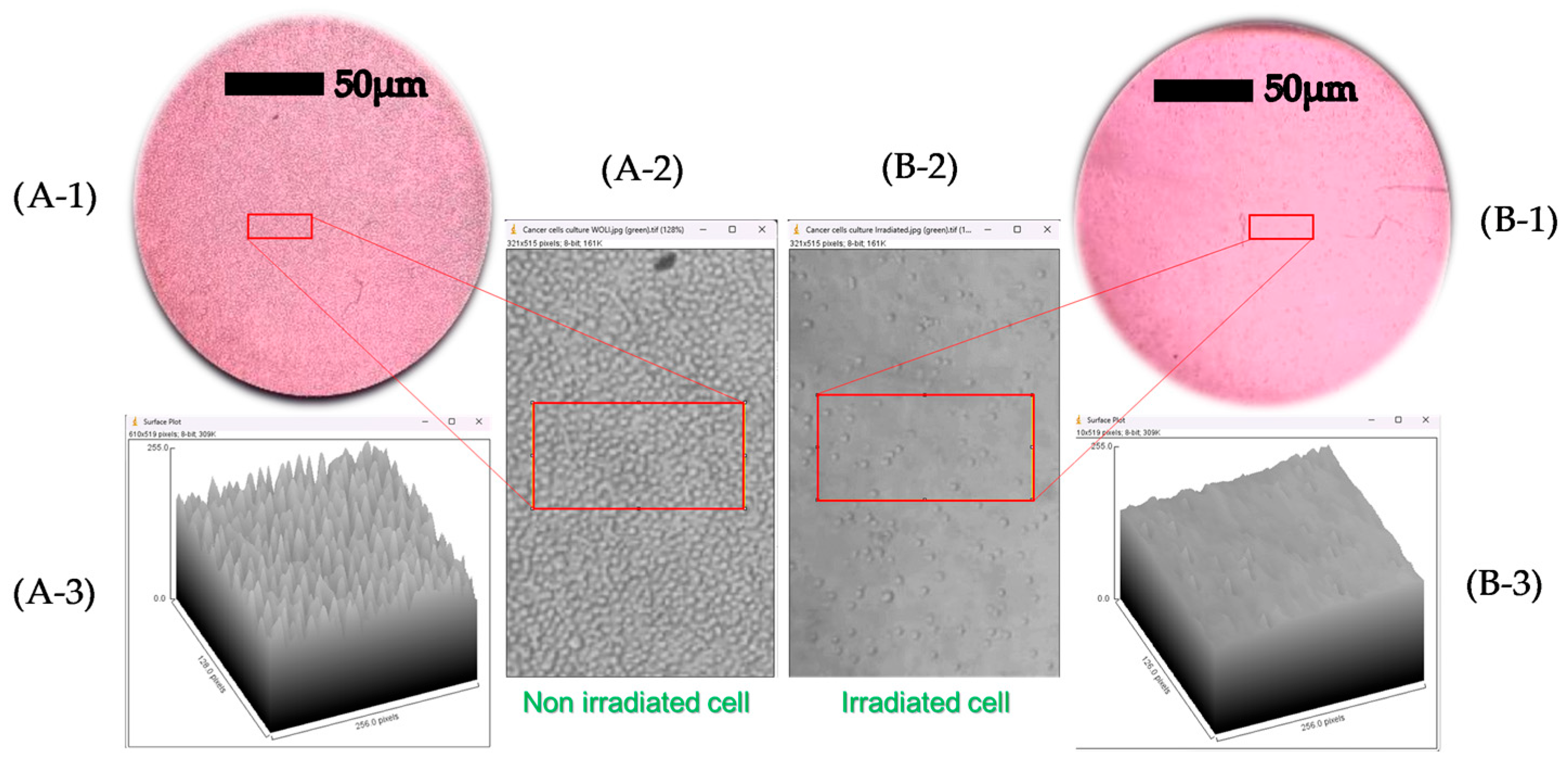
3.3. Irradiation Induces Profound Changes in the Spectroscopic Signature and Cell Morphology
3.4. Cross-Verification Through Multiple Assays Confirms the Robustness of the Cytotoxic Effect
4. Discussion
4.1. Main Findings and Postulated Mechanisms
4.2. Comparison with Current Literature
4.3. Clinical and Therapeutic Implications
4.4. Study Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CML | Chronic Myeloid Leukemia |
| TKI | Tyrosine Kinase Inhibitor |
| DLS | Direct Light Stimulation |
| OTW | Optical Therapeutic Window |
| PDT | Photodynamic Therapy |
| ECP | Extracorporeal Photopheresis |
| ROS | Reactive Oxygen Species |
| SD | Standard Deviation |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay |
References
- Andretta, E.; Costa, C.; Longobardi, C.; Damiano, S.; Giordano, A.; Pagnini, F.; Montagnaro, S.; Quintiliani, M.; Lauritano, C.; Ciarcia, R. Potential Approaches Versus Approved or Developing Chronic Myeloid Leukemia Therapy. Front. Oncol. 2021, 11, 801779. [Google Scholar] [CrossRef]
- Montoya-Quintero, K.F.; Galván-Barrios, J.; Martinez-Guevara, D.; Dueñas, D.; Montenegro, J.; Liscano, Y. Bridging the Gap: Cancer Scientific Equity, Global Child Health, and Distribution of CAR T-Cell Therapy Clinical Trials in Childhood Cancer. Front. Pediatr. 2025, 13, 1611187. [Google Scholar] [CrossRef]
- Poudel, G.; Tolland, M.G.; Hughes, T.P.; Pagani, I.S. Mechanisms of Resistance and Implications for Treatment Strategies in Chronic Myeloid Leukaemia. Cancers 2022, 14, 3300. [Google Scholar] [CrossRef]
- Rangraze, I.R.; El-Tanani, M.; Wali, A.F.; Rizzo, M. Beyond TKIs: Advancing Therapeutic Frontiers with Immunotherapy, Targeted Agents, and Combination Strategies in Resistant Chronic Myeloid Leukemia. Hemato 2025, 6, 6. [Google Scholar] [CrossRef]
- Restrepo, J.C.; Martínez Guevara, D.; Pareja López, A.; Montenegro Palacios, J.F.; Liscano, Y. Identification and Application of Emerging Biomarkers in Treatment of Non-Small-Cell Lung Cancer: Systematic Review. Cancers 2024, 16, 2338. [Google Scholar] [CrossRef] [PubMed]
- Schirrmacher, V. From Chemotherapy to Biological Therapy: A Review of Novel Concepts to Reduce the Side Effects of Systemic Cancer Treatment (Review). Int. J. Oncol. 2018, 54, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Khan, M.J.; Abu, J.; Naeem, A. Revolutionizing Cancer Care Strategies: Immunotherapy, Gene Therapy, and Molecular Targeted Therapy. Mol. Biol. Rep. 2024, 51, 219. [Google Scholar] [CrossRef] [PubMed]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef] [PubMed]
- Manda, G.; Hinescu, M.E.; Neagoe, I.V.; Ferreira, L.F.V.; Boscencu, R.; Vasos, P.R.; Basaga, S.H.; Cuadrado, A. Emerging Therapeutic Targets in Oncologic Photodynamic Therapy. Curr. Pharm. Des. 2019, 24, 5268–5295. [Google Scholar] [CrossRef] [PubMed]
- Soleimany, A.; Aghmiouni, D.K.; Amirikhah, M.; Shokrgozar, M.A.; Khoee, S.; Sarmento, B. Two-Photon Mediated Cancer Therapy: A Comprehensive Review on Two-Photon Photodynamic Therapy and Two-Photon-Activated Therapeutic Delivery Systems. Adv. Funct. Mater. 2024, 34, 2408594. [Google Scholar] [CrossRef]
- Ferro, A.P.; Guirro, R.R.d.J.; Orellana, M.D.; de Santis, G.C.; Júnior, J.A.F.; Guirro, E.C.d.O. Photobiomodulation with Laser and Led on Mesenchymal Stem Cells Viability and Wound Closure in Vitro. Lasers Med. Sci. 2024, 39, 205. [Google Scholar]
- Naderi, M.S.; Razzaghi, M.; Esmaeeli Djavid, G.; Hajebrahimi, Z. A Comparative Study of 660 Nm Low-Level Laser and Light Emitted Diode in Proliferative Effects of Fibroblast Cells. J. Lasers Med. Sci. 2017, 8, S46–S50. [Google Scholar] [CrossRef]
- Oka, T.; Matsuoka, K.; Utsunomiya, A. Sensitive Photodynamic Detection of Adult T-Cell Leukemia/Lymphoma and Specific Leukemic Cell Death Induced by Photodynamic Therapy: Current Status in Hematopoietic Malignancies. Cancers 2020, 12, 335. [Google Scholar] [CrossRef]
- Sando, Y.; Matsuoka, K.; Sumii, Y.; Kondo, T.; Ikegawa, S.; Sugiura, H.; Nakamura, M.; Iwamoto, M.; Meguri, Y.; Asada, N.; et al. 5-Aminolevulinic Acid-Mediated Photodynamic Therapy Can Target Aggressive Adult T Cell Leukemia/Lymphoma Resistant to Conventional Chemotherapy. Sci. Rep. 2020, 10, 17237. [Google Scholar] [CrossRef]
- Ochoa Arbeláez, D.V.; SolarteRodríguez, E.; Gutiérrez Montes, J.O. Design, Assembly and Start-up of an Experimental Set-up for Optical Characterization in Biological Tissues. JER J. Eng. Res. 2023, 3, 2–8. [Google Scholar] [CrossRef]
- Hopkins, S.L.; Siewert, B.; Askes, S.H.C.; Veldhuizen, P.; Zwier, R.; Heger, M.; Bonnet, S. An in Vitro Cell Irradiation Protocol for Testing Photopharmaceuticals and the Effect of Blue, Green, and Red Light on Human Cancer Cell Lines. Photochem. Photobiol. Sci. 2016, 15, 644–653. [Google Scholar] [CrossRef]
- Heidari, A. A Bio-Spectroscopic Study of DNA Density and Color Role as Determining Factor for Absorbed Irradiation in Cancer Cells. Adv. Cancer Prev. 2016, 1, e102. [Google Scholar] [CrossRef]
- MTT Cell Growth Assay Kit MSDS—CT02—Merck. Available online: https://www.merckmillipore.com/CO/es/product/msds/MM_NF-CT02?Origin=PDP (accessed on 7 September 2025).
- Karu, T.I.; Kolyakov, S.F. Exact Action Spectra for Cellular Responses Relevant to Phototherapy. Photomed. Laser Surg. 2005, 23, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic Therapy of Cancer: An Update. CA A Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Demidova, T.N. Mechanisms of Low Level Light Therapy; Hamblin, M.R., Waynant, R.W., Anders, J., Eds.; SPIE: San Jose, CA, USA, 2006; p. 614001. [Google Scholar]
- Pruitt, T.; Carter, C.; Wang, X.; Wu, A.; Liu, H. Photobiomodulation at Different Wavelengths Boosts Mitochondrial Redox Metabolism and Hemoglobin Oxygenation: Lasers vs. Light-Emitting Diodes In Vivo. Metabolites 2022, 12, 103. [Google Scholar] [CrossRef]
- Allison, R.R.; Downie, G.H.; Cuenca, R.; Hu, X.-H.; Childs, C.J.; Sibata, C.H. Photosensitizers in Clinical PDT. Photodiagnosis Photodyn. Ther. 2004, 1, 27–42. [Google Scholar] [CrossRef]
- De Freitas, L.F.; Hamblin, M.R. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J. Select. Topics Quantum Electron. 2016, 22, 348–364. [Google Scholar] [CrossRef] [PubMed]
- Ash, C.; Dubec, M.; Donne, K.; Bashford, T. Effect of Wavelength and Beam Width on Penetration in Light-Tissue Interaction Using Computational Methods. Lasers Med. Sci. 2017, 32, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Valero, J.M.V.; Escobar, N.A.; Fernández-Durán, D.d.A.; Pérez, L.V.; Ruiz, J.M.; Penco, J.M.M.; Gómez, R.B.; Miranda, M.P. Photopheresis: New Immunomodulatory Therapy for T-Lymphocite Mediated Diseases. An. De Med. Interna 2003, 20, 421–426. [Google Scholar]


| Quantification Method | Light Source | Wavelength (nm) | Dose (J/cm2) | Cell Death (%) |
|---|---|---|---|---|
| Microscopy (Trypan Blue) | LASER | 532 | 10 | 67.8 |
| Spectroscopic (Absorbance) | LASER | 532 | 10 | 70.6 |
| Cytotoxicity Assay (MTT) | LASER | 532 | 10 | 59.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochoa-Arbeláez, D.V.; Solarte-Rodríguez, E.; Liscano, Y. Selective Cytotoxicity in Chronic Myeloid Leukemia (K-562) Cells Induced by 532 nm LASER Irradiation Without Exogenous Photosensitizers. Biomedicines 2025, 13, 2649. https://doi.org/10.3390/biomedicines13112649
Ochoa-Arbeláez DV, Solarte-Rodríguez E, Liscano Y. Selective Cytotoxicity in Chronic Myeloid Leukemia (K-562) Cells Induced by 532 nm LASER Irradiation Without Exogenous Photosensitizers. Biomedicines. 2025; 13(11):2649. https://doi.org/10.3390/biomedicines13112649
Chicago/Turabian StyleOchoa-Arbeláez, Danielle Viviana, Efraín Solarte-Rodríguez, and Yamil Liscano. 2025. "Selective Cytotoxicity in Chronic Myeloid Leukemia (K-562) Cells Induced by 532 nm LASER Irradiation Without Exogenous Photosensitizers" Biomedicines 13, no. 11: 2649. https://doi.org/10.3390/biomedicines13112649
APA StyleOchoa-Arbeláez, D. V., Solarte-Rodríguez, E., & Liscano, Y. (2025). Selective Cytotoxicity in Chronic Myeloid Leukemia (K-562) Cells Induced by 532 nm LASER Irradiation Without Exogenous Photosensitizers. Biomedicines, 13(11), 2649. https://doi.org/10.3390/biomedicines13112649






