Abstract
Background: Lipoprotein(a) [Lp(a)] is a modifier of cardiovascular risk, and it should be determined at least once in a lifetime. Methods: Subjects with low or moderate cardiovascular risk, estimated by SCORE2, were invited to have a determination of Lp(a), and those with Lp(a) > 50 mg/dL were classified into a higher-risk category. Eligibility of statins was assessed according to treatment targets. Results: We analyzed 140 subjects, with a mean age of 54.3 (8.1) years and 62.9% women. The median Lp(a) was 15.2 (interquartile range: 6.7–44.5) mg/dL, and 22.1% of the cohort had Lp(a) > 50 mg/dL. No differences were observed in mean age, sex, or lipid profile in subjects with Lp(a) below or above 50 mg/dL; alkaline phosphatase (ALP) was significantly higher in subjects with Lp(a) > 50 mg/dL. After incorporating Lp(a) values into the SCORE-2 assessment, 22.6% of individuals initially of low risk were reclassified as moderate risk, and 77.4% were reclassified from moderate to high risk; moreover, 61.4% (86 subjects) were considered eligible for treatment with statins. Conclusions: Our results highlight that 22.1% of the subjects classified as low or moderate cardiovascular risk by SCORE-2 are reclassified to higher risk, and 61.4% were eligible for statin treatment as a result of Lp(a) testing.
1. Introduction
Individualized cardiovascular risk stratification is recommended for analyzing the risk of developing major cardiovascular complications and for guiding the treatment of different cardiovascular risk factors [1]. The European Society of Cardiology recommends assessing SCORE-2 in people without cardiovascular disease. This provides an estimation of individuals’ 10-year risk of developing or dying from cardiovascular disease, and subjects are classified as having low (<5% at 10 years), intermediate (5–10%), or high cardiovascular risk (>10%) [1]. Age, gender, systolic blood pressure, smoking, and non-HDL cholesterol are used for the assessment of the SCORE-2 although some situations reclassify the risk obtained by the SCORE2 scale, such as the detection of atherosclerotic plaques in the carotid arteries, microalbuminuria, or glomerular filtration rates of <30 mL/min/1.72 m2, and these subjects are categorized as high risk [1].
Lipoprotein(a) (Lp(a)) is a low-density lipoprotein (LDL) particle that contains an additional protein, apoLipoprotein(a) [2]. Values of Lp(a) > 50 mg/dL are associated with an increased risk of cardiovascular complications, while values > 180 mg/dL confer a risk similar to that of familial hypercholesterolemia [3,4,5]. It is estimated that approximately 6–8% of people without cardiovascular disease have values > 50 mg/dL; however, this percentage can be as high as 20% among patients with premature cardiovascular disease [6,7,8]. Lp(a) levels are genetically determined in more than 80% of cases and do not change with age or lifestyle; therefore, a single measurement in a person’s lifetime is considered sufficient to determine the cardiovascular risk associated with Lp(a) [2,3,9]. Nonetheless, several reports have highlighted the low rate of Lp(a) measurement in the population [6,7]. The 2025 update on the clinical guidelines for the management of dyslipidemias endorsed Lp(a) as an established modifier of cardiovascular risk [10].
The objective of our study was to implement Lp(a) measurements for people with low or moderate cardiovascular risk in our healthcare setting in order to assess the reclassification rate and the implications of initiating lipid-lowering strategies.
2. Materials and Methods
We designed a pilot study as an observational, cross-sectional study. Since early 2023, SCORE-2 has been automatically calculated for all routine test requests made by primary care practitioners in the Health Department of the Hospital de San Juan [11]. Patients with low or intermediate risk were identified as candidates for Lp(a) screening and were invited to participate in the study. Once they signed the informed consent form, a blood sample was taken to measure only Lp(a) and high-sensitivity troponin I (hs-cTnI) [12]. Patients with Lp(a) > 50 mg were reclassified into a higher-risk category. Eligibility for statin treatment was assessed according to the treatment targets recommended in clinical guidelines: LDLc > 155 mg/dL for low risk, LDLc > 100 mg/dL for moderate risk, and LDLc > 70 for high risk [10,13]. The study protocol and informed consent were approved by the ethics committee of the hospital. The costs related to Lp(a) and hs-cTnI determinations were covered by the investigators’ research funds with no external funding.
The inclusion criteria were age >40 and <69 years, low or intermediate risk estimated by SCORE-2, and signing the informed consent form. Exclusion criteria were active neoplastic disease, a life expectancy of <1 year due to previous pathologies, and pregnancy.
Blood samples were obtained from a brachial vein puncture, frozen at −80 °C, and processed together. Lp(a) concentrations were quantified by particle-enhanced immunoturbidimetry on an Alinity c analyzer (Abbott Diagnostics, Abbott Park, IL 60064-3500, USA) according to the manufacturer’s instructions. The results are reported in mg/dL. Elevated Lp(a) was defined as >50 mg/dL, which is the threshold used to reclassify individuals as high risk. Determination of hs-cTnI was quantified by a chemiluminescent microparticle immunoassay (CMIA) on an Alinity i-analyzer (Abbott Diagnostics, Abbott Park, IL 60064-3500, USA) following the manufacturer’s instructions. The results are reported in ng/L [14].
We performed a sample size estimation to determine the number of patients required for the study to have 80% power to detect differences in the contrast of the null hypothesis (H0: μ1 = μ2) using a two-tailed Student’s t-test for proportions. Taking into account a significance level of 5%, a high prevalence of Lp(a) of 8%, and a 10% loss to follow-up, we estimated that the study would require at least 137 patients. These calculations were performed using G*Power 3.1. Quantitative variables are presented as median and interquartile range (IQR); differences among the four groups were assessed by the non-parametric Kruskal–Wallis test. Qualitative variables are presented as percentages, and differences were analyzed by Chi2. Glomerular filtration rates were calculated using the CKD-EPI formula [14]. Reclassification was represented by Sankey graphs. Statistical difference was accepted at p < 0.05. All analyses were performed using STATA 14.3 (StataCorp, 2009. Stata Statistical Software: Release 14. College Station, TX, USA: StataCorp LP).
3. Results
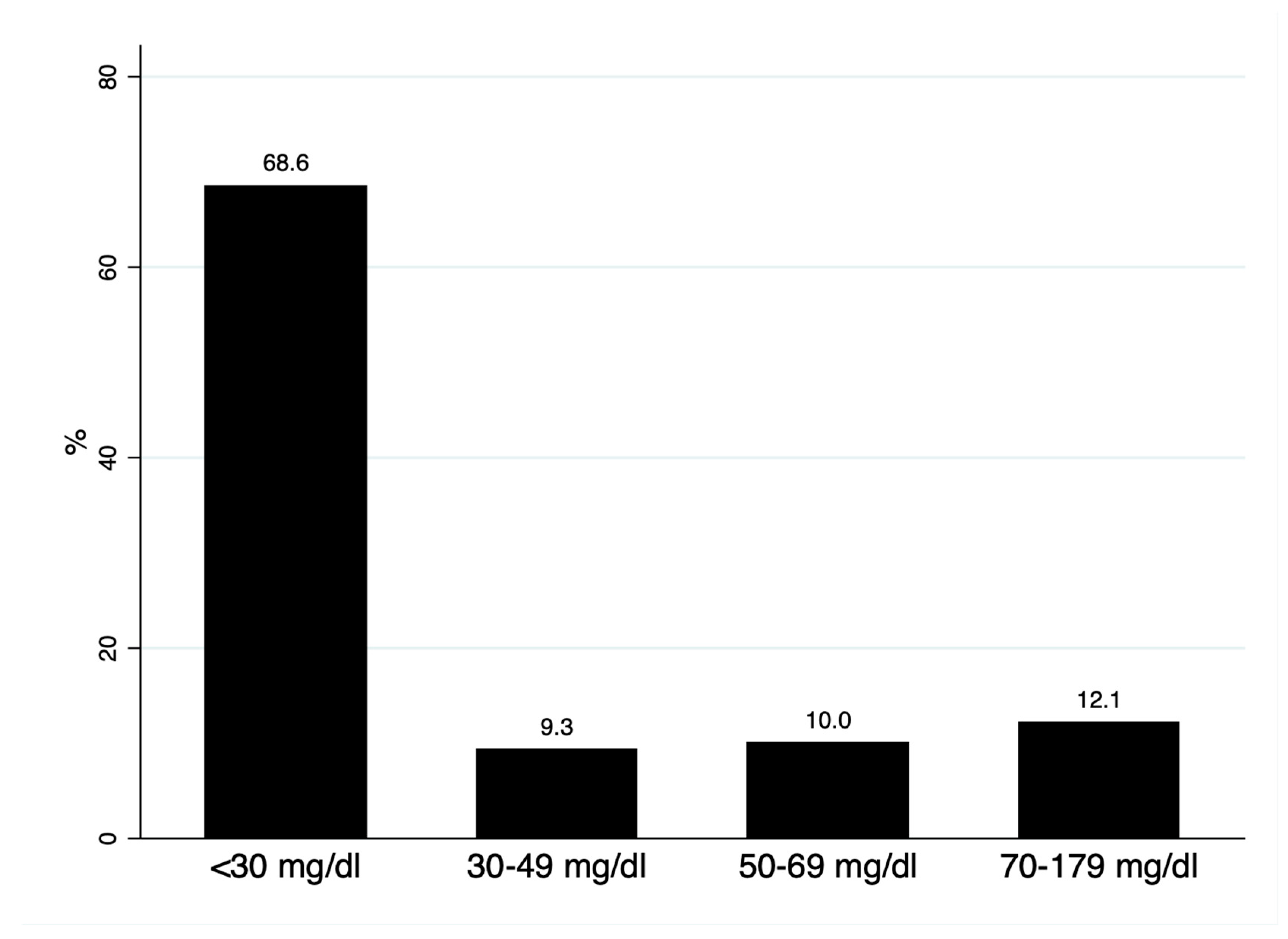
We analyzed 140 subjects, with a mean age of 54.3 (8.1) years and 62.9% women. The median Lp(a) was 15.2 (interquartile range: 6.7–44.5) mg/dL; 68.6% had Lp(a) < 30 mg/dL, 9.3% presented with levels of 30–49 mg/d, and 22.1% of the cohort had Lp(a) > 50 mg/dL (Figure 1). As shown in Table 1, no differences were observed in mean age, sex, or lipid profile in subjects with Lp(a) below or above 50 mg/dL; alkaline phosphatase (ALP) was significantly higher in subjects with Lp(a) > 50 mg/dL.
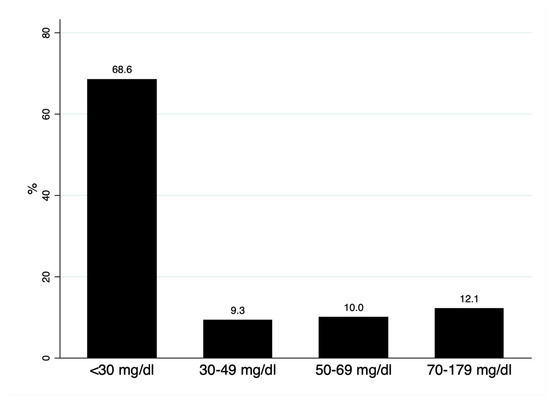
Figure 1.
Distribution of lipoprotein(a) concentrations.

Table 1.
Clinical characteristics of the population according to Lipoprotein(a) [Lp(a)] levels.
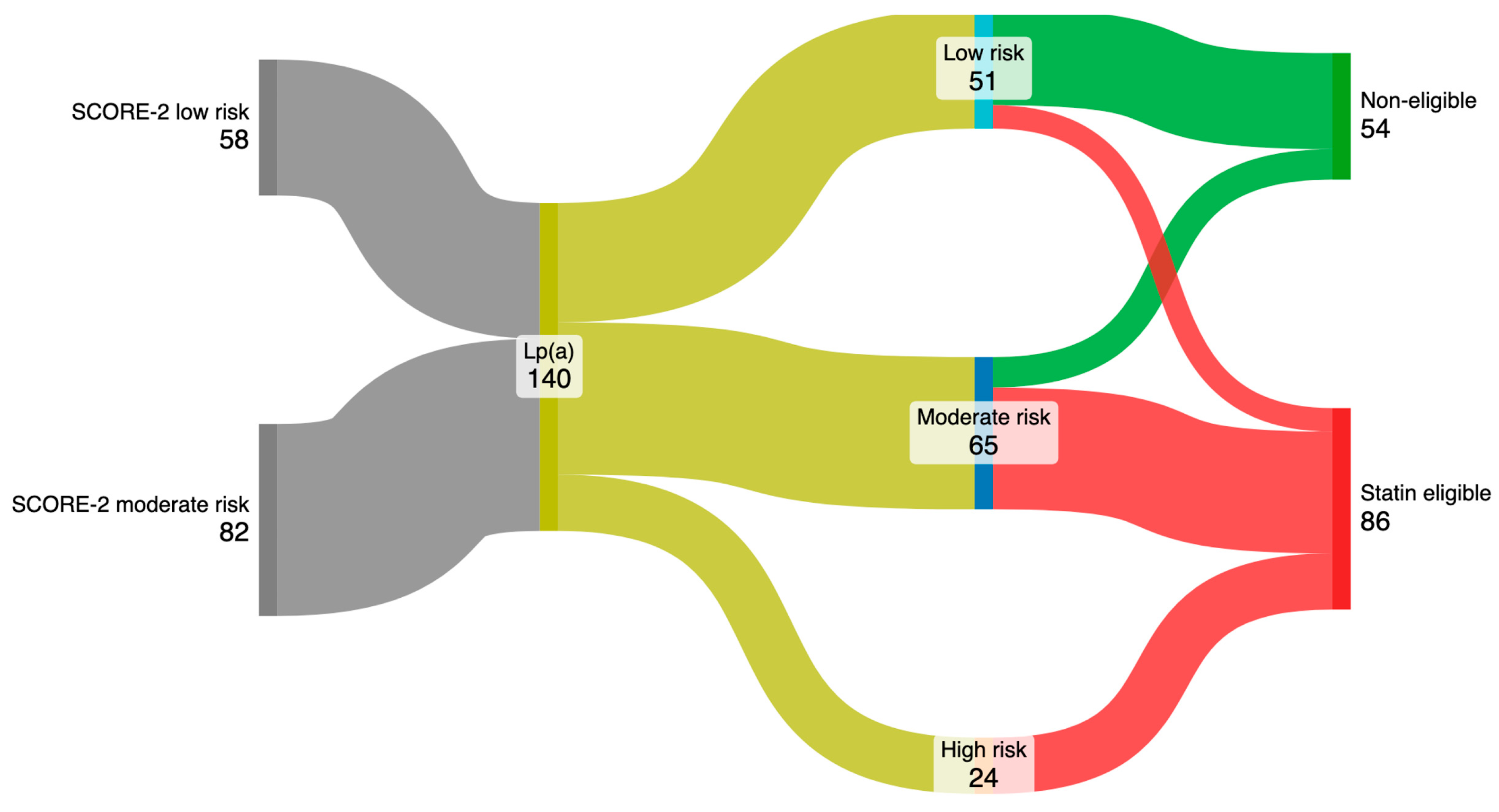
After incorporating Lp(a) values into the SCORE-2 assessment, 22.6% of individuals initially of low risk were reclassified to moderate risk, and 77.4% were reclassified from moderate to high risk (Figure 2). Furthermore, 19.6% of patients reclassified as low risk had LDLc > 150 mg/dL, 80% of those reclassified to moderate risk had LDLc > 100 mg/dL, and all subjects reclassified to the high-risk group had LDL > 70 mg/dL. As a consequence, 61.4% (86 subjects) were considered eligible for treatment with statins.
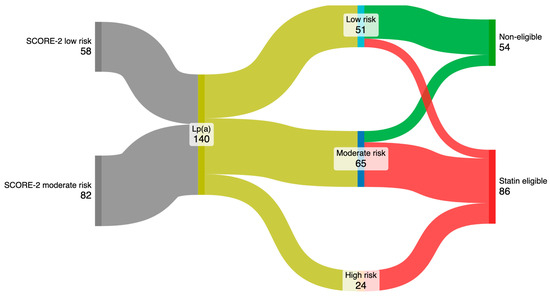
Figure 2.
Reclassification of cardiovascular risk representation and identification of patients eligible for statins.
4. Discussion
The results of our community-level study show that one-fifth of subjects categorized as low or moderate risk by SCORE2 were reclassified as having a higher cardiovascular risk, and that 61.4% were eligible for statins following the Lp(a) results. These results could significantly impact the management of cardiovascular risk factors. Since our clinical features are similar to previous reports [6,7,15,16,17], we believe that these results might be representative and clinically applicable.
There is a broad consensus that Lp(a) should be determined at least once in a lifetime, since a single determination has high predictive value for the incidence of cardiovascular disease [3,9]. Lp(a) levels < 30 mg/l might be considered as non-elevated, 30–50 mg/dL as slightly elevated, and >50 mg/dL as elevated [2]; nonetheless, the inclusion criterion for some of the clinical trials was Lp(a) > 70 mg/dL [18]. Despite the evidence supporting the role of Lp(a) in cardiovascular risk, it is not widely measured due to the lack of availability or the skepticism regarding what to do with the results. We designed a pilot study for the screening of Lp(a) in our healthcare area that revealed the high prevalence of non-normal Lp(a) values, the elevated reclassification rate, and the percentage of subjects that would be eligible for statins. Similarly, once a subject has elevated Lp(a), it is recommended to test in first-line relatives [2,4]. A recent study performed in Spain showed that 60% of the relatives of patients with premature myocardial infarction and elevated Lp(a) had levels > 50 mg/dL [15]. Determining Lp(a) also has clinical implications for patients with established cardiovascular disease; while it may not change lipid treatment targets, it is associated with lower LDLc control [16] and a higher risk of recurrent ischemic events [17] and could guide prolonged dual antiplatelet therapy [19]. There are currently no therapies available to reduce Lp(a) levels below 50 mg/dL, although PCSK9-directed therapies decrease Lp(a) by 20–30%, and they could be another mechanism for reductions in major cardiovascular events observed in clinical trials [20,21,22].
One of the most direct implications of our results is that more than half of the subjects would be eligible for statins. This result underscores the potential benefits of incorporating Lp(a) screening into routine cardiovascular risk evaluation, enabling the identification of patients who may require lipid-lowering strategies even in the absence of other major risk factors. Our results are in concordance with an analysis of 10,000 subjects aged 40–69 years from the UK Biobank that found that 18% of patients have Lp(a) > 50 mg/dL (>105 nmol/L). That study achieved an estimation that revealed that the risk reclassification could induce the initiation of statin and blood pressure-lowering therapies, which would reduce the cost and burden and cardiovascular disease in this population [23]. Given the pro-atherogenic and pro-thrombotic properties of Lp(a) [24], its coexistence with elevated LDLc may accelerate vascular injury, further strengthening the rationale for early and sustained LDL-lowering interventions [10]. We conducted this pilot study prior to including Lp(a) as a routine test, which helped us to establish a protocol to detect subjects with elevated Lp(a), as is achieved with other results from the laboratory [25]. Lp(a) is mostly determined genetically and has no clear associations with lifestyle habits or other clinical conditions. Subjects with Lp(a) > 50 mg/dL in our cohort, in concordance with previous evidence [8,15,16], have similar clinical characteristics to the other subjects, which increases the relevance of an active screening plan. Subjects with Lp(a) > 50 mg/dL had a higher prevalence of moderate cardiovascular risk, and, therefore, the screening might be even more effective in this group.
Our study has some limitations. Firstly, it was a cross-sectional and single-center study that can only describe associations but not causality. Secondly, subjects were invited to participate, which induced a selection bias. Thirdly, although Lp(a) was measured using a standardized immunoturbidimetric assay, the results were expressed in mg/dL; molar units (nmol/L) are increasingly recommended to improve comparability across studies and to align with emerging therapeutic trial criteria. Fourthly, sample size was relatively modest and the participants were from a specific geographical region; this may not reflect the distribution of Lp(a) levels or reclassification rates in other populations with different ethnic, genetic, or lifestyle backgrounds; similarly, we could not adjust for certain potential confounding factors, specific dietary habits, family histories of premature cardiovascular disease, or concomitant inflammatory conditions, which may influence cardiovascular risk independently of Lp(a). Finally, there was no longitudinal follow-up available to assess whether reclassification based on Lp(a) translated into improved clinical outcomes, such as reductions in cardiovascular events, following changes in lipid-lowering therapy; this is planned for the coming years. Since clinical features are similar to previous reports [6,7,8,15,16], we believe that our results might be representative and clinically meaningful.
5. Conclusions
Our results show that, as a result of Lp(a) testing, 22.1% of subjects classified as having low or moderate cardiovascular risk were reclassified into a higher-risk category, and 62.4% were considered eligible for statin treatment. The results underscore the potential benefits of incorporating Lp(a) screening into routine cardiovascular risk evaluation, enabling the identification of patients who may require lipid-lowering strategies even in the absence of other major risk factors.
Author Contributions
Conceptualization, A.C. and E.F.; methodology, A.C. and E.F.; software, J.M.S. and E.F.; validation, M.A.Q., J.M.L.-A., E.F. and A.C.; formal analysis, A.C.; investigation, A.C. and E.F.; resources, A.C.; data curation, E.F. and Á.B.; writing—original draft preparation, A.C.; writing—review and editing, A.C. and E.F.; visualization, A.C. and E.F.; supervision, A.C. and E.F.; project administration, A.C. and E.F.; funding acquisition, A.C. and E.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of Hospital Universitario de San Juan (protocol code 23/036, date: 31 May 2023) for studies involving humans.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent was obtained from the patient(s) to publish this paper.
Data Availability Statement
The datasets presented in this article are not readily available because they were collected by the investigators. Requests to access the datasets should be directed to acorderofort@gmail.com.
Acknowledgments
A.C. received support from the Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Spain.
Conflicts of Interest
The authors declare no conflicts of interest. Alberto Cordero reports the following, which are not related to the results of this study: (a) honoraria for lectures from AstraZeneca, AMGEN, Bristol-Myers Squibb, Ferrer, Boehringer Ingelheim, MSD, Daiichy Sankio, Novartis, Novo Nordisk, Sanofi, and Amarin; (b) consulting fees from AstraZeneca, Ferrer, Sanofi, AMGEN, Novartis, Lilly, Novo Nordisk, Daiichy Sankio, and Amarin.
Abbreviations
The following abbreviations are used in this manuscript:
| hs-cTnI | High-sensitivity troponin |
| LDLc | Low-density lipoprotein cholesterol |
| Lp(a) | Lipoprotein(a) |
References
- Visseren, F.L.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: Developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies With the special contribution of the European Association of Preventive Cardiology (EAPC). Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, F.; Mora, S.; Stroes, E.S.; Ference, B.A.; Arsenault, B.J.; Berglund, L.; Dweck, M.R.; Koschinsky, M.; Lambert, G.; Mach, F.; et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: A European Atherosclerosis Society consensus statement. Eur. Heart J. 2022, 43, 3925–3946. [Google Scholar] [CrossRef]
- Trinder, M.; Paruchuri, K.; Haidermota, S.; Bernardo, R.; Zekavat, S.M.; Gilliland, T.; Januzzi, J., Jr.; Natarajan, P. Repeat Measures of Lipoprotein(a) Molar Concentration and Cardiovascular Risk. J. Am. Coll. Cardiol. 2022, 79, 617–628. [Google Scholar] [CrossRef]
- Reyes-Soffer, G.; Ginsberg, H.N.; Berglund, L.; Duell, P.B.; Heffron, S.P.; Kamstrup, P.R.; Lloyd-Jones, D.M.; Marcovina, S.M.; Yeang, C.; Koschinsky, M.L.; et al. Lipoprotein(a): A Genetically Determined, Causal, and Prevalent Risk Factor for Atherosclerotic Cardiovascular Disease: A Scientific Statement From the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 2022, 42, E48–E60. [Google Scholar] [CrossRef]
- Raitakari, O.; Kartiosuo, N.; Pahkala, K.; Hutri-Kähönen, N.; Bazzano, L.A.; Chen, W.; Urbina, E.M.; Jacobs, D.R., Jr.; Sinaiko, A.; Steinberger, J.; et al. Lipoprotein(a) in Youth and Prediction of Major Cardiovascular Outcomes in Adulthood. Circulation 2023, 147, 23–31. [Google Scholar] [CrossRef]
- Arrobas Velilla, T.; Fabiani de la Iglesia, J.; Martín Pérez, S.; Calbo Caballos, L.; Gómez Barrado, J.J.; León Justel, A. Lipoproteína (a) en una selección de hospitales de Andalucía y Extremadura. ¿Infradiagnosticada e infrautilizada? Rev. Esp. Cardiol. 2022, 75, 845–846. [Google Scholar] [CrossRef]
- Stürzebecher, P.E.; Schorr, J.J.; Klebs, S.H.G.; Laufs, U. Trends and consequences of lipoprotein(a) testing: Cross-sectional and longitudinal health insurance claims database analyses. Atherosclerosis 2023, 367, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Man, S.; Zu, Y.; Yang, X.; Deng, Y.; Shen, D.; Ma, Y.; Fu, J.; Du, J.; Yu, C.; Lv, J.; et al. Prevalence of Elevated Lipoprotein(a) and its Association With Subclinical Atherosclerosis in 2.9 Million Chinese Adults. J. Am. Coll. Cardiol. 2025, 85, 1979–1992. [Google Scholar] [CrossRef]
- Trinder, M.; Uddin, M.M.; Finneran, P.; Aragam, K.G.; Natarajan, P. Clinical Utility of Lipoprotein(a) and LPA Genetic Risk Score in Risk Prediction of Incident Atherosclerotic Cardiovascular Disease. JAMA Cardiol. 2021, 6, 287–295. [Google Scholar] [CrossRef]
- Mach, F.; Koskinas, K.C.; Roeters van Lennep, J.E.; Tokgözoğlu, L.; Badimon, L.; Baigent, C.; Benn, M.; Binder, C.J.; Catapano, A.L.; De Backer, G.G.; et al. 2025 Focused Update of the 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Developed by the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur. Heart J. 2025, ehaf190. [Google Scholar] [CrossRef]
- Salinas, M.; Flores, E.; Ahumada, M.; Leiva-Salinas, M.; Blasco, A.; Leiva-Salinas, C. Advancing cardiovascular risk assessment: Real-time SCORES2 calculation through CDSS in primary care patients. Clin. Biochem. 2025, 137, 110922. [Google Scholar] [CrossRef]
- Sharrod-Cole, H.; Ford, C. Multicenter Evaluation of a High-Sensitivity Troponin I Assay and Verification of an Early Rule-Out Algorithm. J. Appl. Lab. Med. 2019, 4, 95–100. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2019, 41, 111–188. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.; Castro, A.F., III; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Olmo, M.R.; Bailen, M.C.; Martínez Quesada, M.; Rus Mansilla, C.; Martin Toro, M.; López Suarez, A.; Lucas García, M.; Cortez Quiroga, G.; Calvo Bernal, B.; Ortiz Cruces, S.; et al. Lp(a) Levels in Relatives of Patients with Acute Coronary Syndrome and Elevated Lp(a): HER(a) Study. J. Clin. Med. 2024, 13, 2256. [Google Scholar] [CrossRef] [PubMed]
- Olmo, R.F.; Cortez, G.; Toro, M.M.; Sandín, M.; Mora, J.; Oterino, A.; Bailen, M.C.; Quiles-Granado, J.; Urbiola, P.; Ruz, L.F.; et al. A machine learning algorithm for the identification elevated Lp(a) in patients with, or high-risk of having, coronary heart disease. Int. J. Cardiol. 2024, 418, 132612. [Google Scholar] [CrossRef] [PubMed]
- Miñana, G.; Cordero, A.; Fácila, L.; Company, M.; Fernández-Cisnal, A.; Valero, E.; Carratalá, A.; Navarro, J.; Llergo, J.T.; Fernández-Olmo, R.; et al. Lipoprotein(a) and Long-Term Recurrent Infarction After an Acute Myocardial Infarction. Am. J. Cardiol. 2024, 211, 9–16. [Google Scholar] [CrossRef]
- Greco, A.; Finocchiaro, S.; Spagnolo, M.; Faro, D.C.; Mauro, M.S.; Raffo, C.; Sangiorgio, G.; Imbesi, A.; Laudani, C.; Mazzone, P.M.; et al. Lipoprotein(a) as a Pharmacological Target: Premises, Promises, and Prospects. Circulation 2025, 151, 400–415. [Google Scholar] [CrossRef]
- Patel, S.M.; Bonaca, M.P.; Morrow, D.A.; Palazzolo, M.G.; Jarolim, P.; Steg, P.G.; Bhatt, D.L.; Storey, R.F.; Sabatine, M.S.; O’Donoghue, M.L. Lipoprotein(a) and Benefit of Antiplatelet Therapy: Insights From the PEGASUS-TIMI 54 Trial. JACC Adv. 2023, 2, 100675. [Google Scholar] [CrossRef]
- Stiekema, L.C.; Stroes, E.S.; Verweij, S.L.; Kassahun, H.; Chen, L.; Wasserman, S.M.; Sabatine, M.S.; Mani, V.; Fayad, Z.A. Persistent arterial wall inflammation in patients with elevated lipoprotein(a) despite strong low-density lipoprotein cholesterol reduction by proprotein convertase subtilisin/kexin type 9 antibody treatment. Eur. Heart J. 2019, 40, 2775–2781. [Google Scholar] [CrossRef]
- Mszar, R.; Cainzos-Achirica, M.; Valero-Elizondo, J.; Lahan, S.; Al-Kindi, S.G.; Quispe, R.; Ali, S.S.; Arias, L.; Saxena, A.; Shah, S.H.; et al. Lipoprotein(a) and Coronary Plaque in Asymptomatic Individuals: The Miami Heart Study at Baptist Health South Florida. Circ. Cardiovasc. Imaging 2024, 17, e016152. [Google Scholar] [CrossRef] [PubMed]
- Nurmohamed, N.S.; Gaillard, E.L.; Malkasian, S.; de Groot, R.J.; Ibrahim, S.; Bom, M.J.; Kaiser, Y.; Earls, J.P.; Min, J.K.; Kroon, J.; et al. Lipoprotein(a) and Long-Term Plaque Progression, Low-Density Plaque, and Pericoronary Inflammation. JAMA Cardiol. 2024, 9, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Morton, J.I.; Kronenberg, F.; Daccord, M.; Bedlington, N.; Geanta, M.; Silberzahn, T.; Chen, Z.; Eisele, J.L.; Eliasen, B.; Harada-Shiba, M.; et al. Lp(a) testing for the primary prevention of cardiovascular disease in high-income countries: A cost-effectiveness analysis. Atherosclerosis 2025, 409, 120447. [Google Scholar] [CrossRef] [PubMed]
- Björnson, E.; Adiels, M.; Taskinen, M.R.; Burgess, S.; Chapman, M.J.; Packard, C.J.; Borén, J. Lipoprotein(a) Is Markedly More Atherogenic Than LDL: An Apolipoprotein B-Based Genetic Analysis. J. Am. Coll. Cardiol. 2024, 83, 385–395. [Google Scholar] [CrossRef]
- Flores, E.; Martínez-Racaj, L.; Torreblanca, R.; Blasco, A.; Lopez-Garrigós, M.; Gutiérrez, I.; Salinas, M. Clinical Decision Support System in laboratory medicine. Clin. Chem. Lab. Med. (CCLM) 2023, 62, 1277–1282. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).