Multi-Center National Study of Genotype–Phenotype Correlation and Clinical Characteristics in Children and Young Adults with Friedreich’s Ataxia from Serbia
Abstract
1. Introduction
2. Materials and Methods
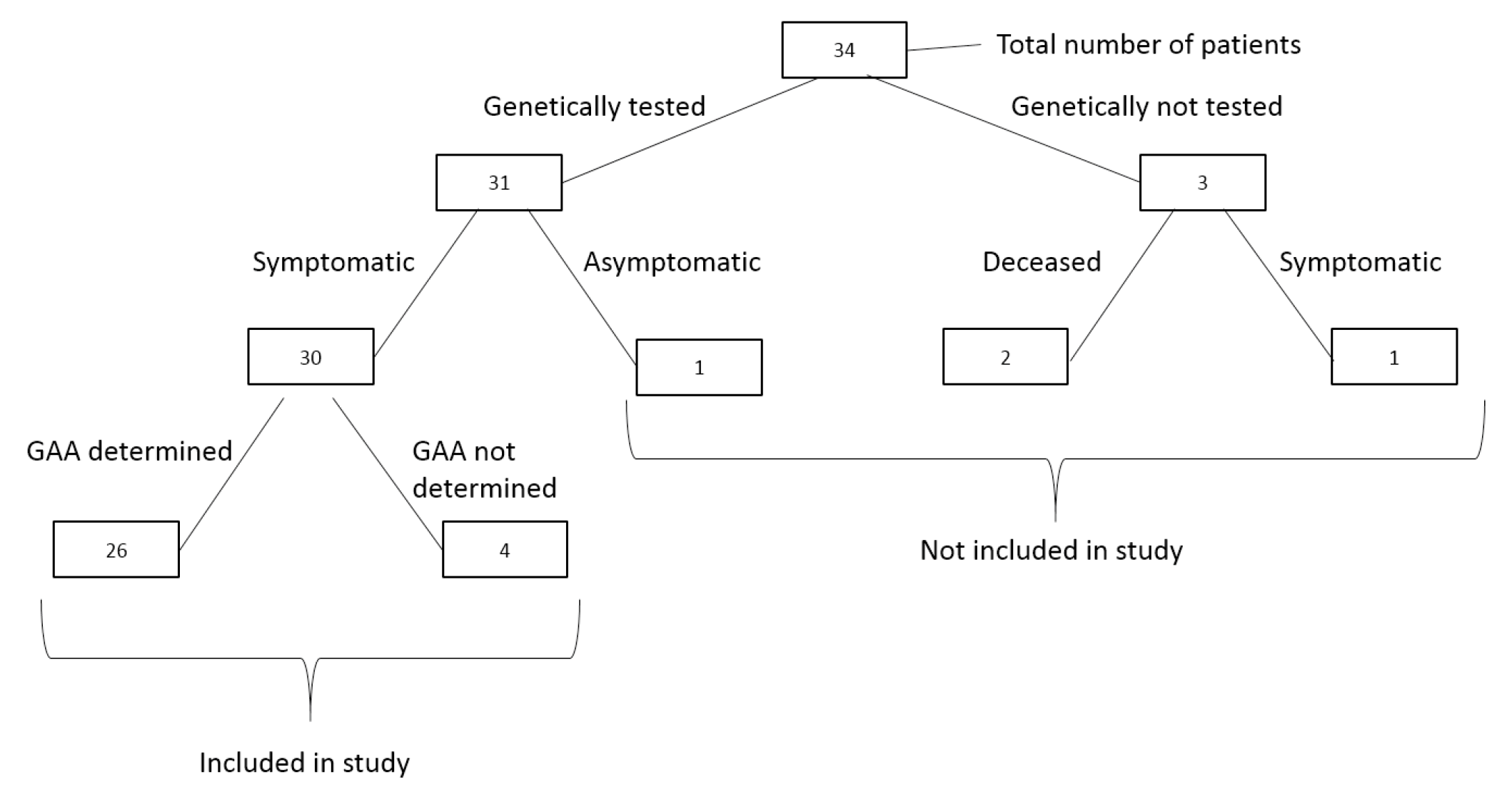
2.1. Study Design and Participants
2.2. Clinical Assessment
2.3. Genomic Analysis
2.4. Statistical Analysis
3. Results
3.1. Patient Demographics and Clinical Characteristics
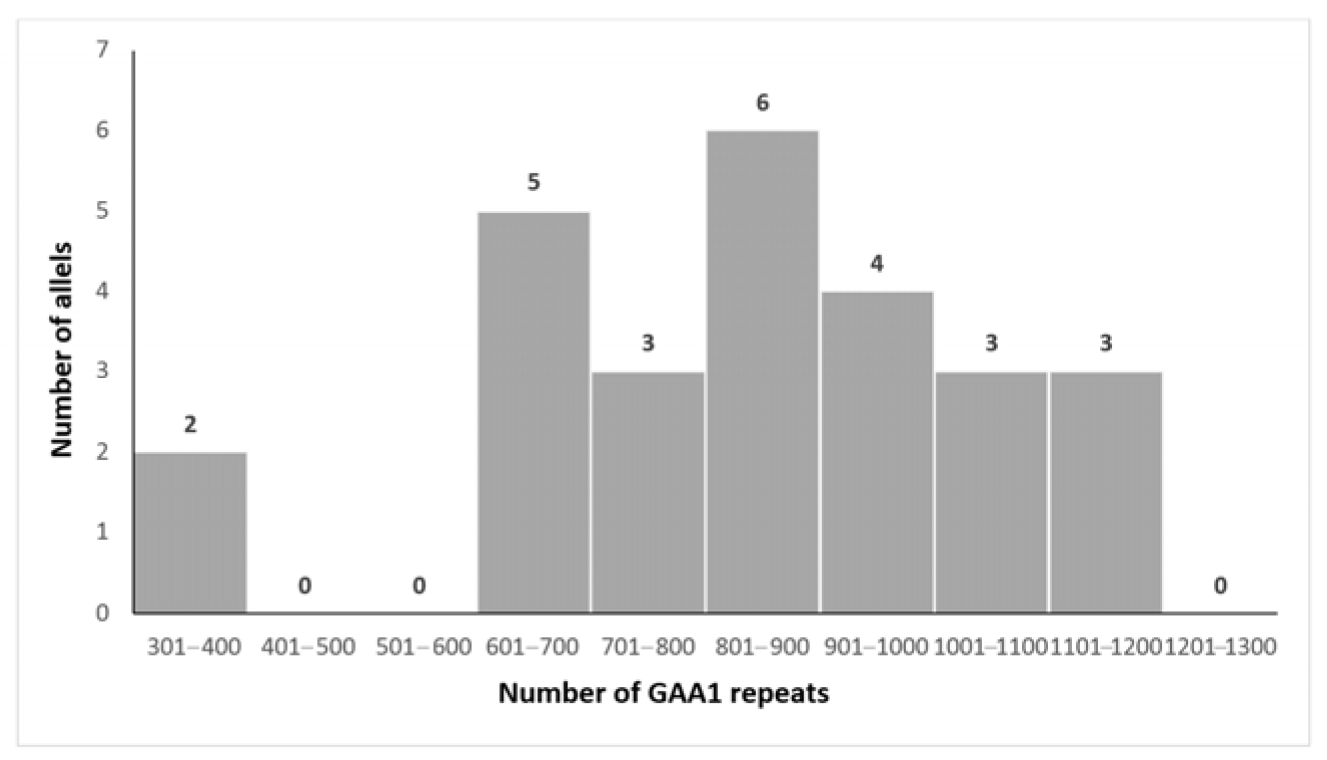
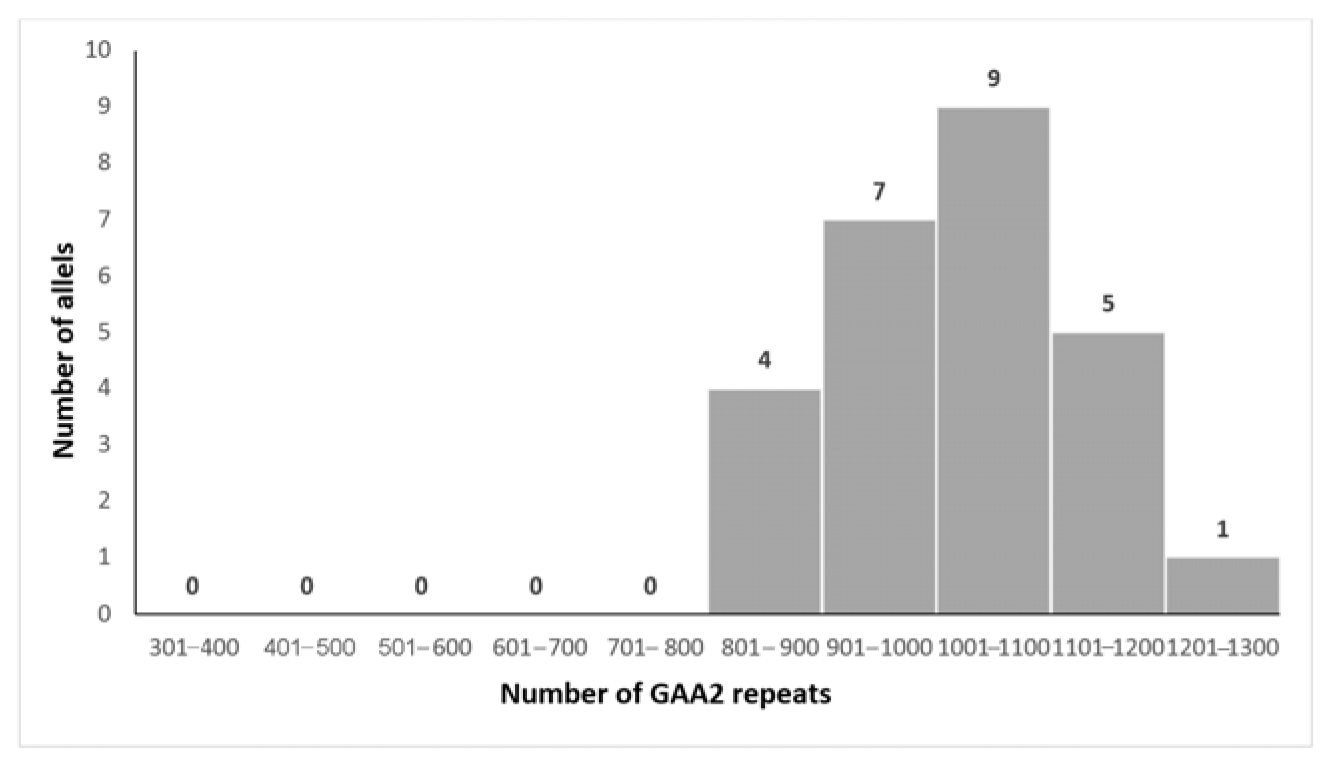
3.2. Genetic Analysis
3.3. Genotype–Phenotype Correlations
3.4. Intrafamilial Variability
4. Discussion
5. Limitation
6. Conclusions and Further Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FA | Friedreich’s ataxia |
| GAA | Trinucleotide guanine–adenine–adenine sequence |
| FXN | Frataxin gene |
| SIRT6 | Sirtuin 6 gene |
| S46N | S46N denotes a missense mutation where serine (S) at amino acid position 46 is replaced by asparagine (N) in the protein sequence |
| AOO | Age of onset |
| ECG | Electrocardiography |
| TTE | Transthoracic echocardiography |
| OGTT | Oral glucose tolerance test |
| HCM | Hypertrophic cardiomyopathy |
| DM | Diabetes mellitus |
| NS | Nephrotic syndrome |
| ROS | Reactive oxygen species |
References
- Cossée, M.; Schmitt, M.; Campuzano, V.; Reutenauer, L.; Moutou, C.; Mandel, J.L.; Koenig, M. Evolution of the Friedreich’s ataxia trinucleotide repeat expansion: Founder effect and premutations. Proc. Natl. Acad. Sci. USA 1997, 94, 7452–7457. [Google Scholar] [CrossRef]
- Buesch, K.; Zhang, R. A systematic review of disease prevalence, health-related quality of life, and economic outcomes associated with Friedreich’s ataxia. Curr. Med. Res. Opin. 2022, 38, 1739–1749. [Google Scholar] [CrossRef]
- Cook, A.; Giunti, P. Friedreich’s ataxia: Clinical features, pathogenesis and management. Br. Med. Bull. 2017, 124, 19–30. [Google Scholar] [CrossRef]
- Bürk, K. Friedreich Ataxia: Current status and future prospects. Cerebellum Ataxias 2017, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Harding, A.E. Friedreich’s ataxia: A clinical and genetic study of 90 families with an analysis of early diagnostic criteria and intrafamilial clustering of clinical features. Brain 1981, 104, 589–620. [Google Scholar] [CrossRef] [PubMed]
- Durr, A.; Cossee, M.; Agid, Y.; Campuzano, V.; Mignard, C.; Penet, C.; Mandel, J.-L.; Brice, A.; Koenig, M. Clinical and genetic abnormalities in patients with Friedreich’s ataxia. N. Engl. J. Med. 1996, 335, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Reetz, K.; Lischewski, S.A.; Dogan, I.; Didszun, C.; Pishnamaz, M.; Konrad, K.; Marx-Schütt, K.; Farmer, J.; Lynch, D.R.; Corben, L.A.; et al. Friedreich’s ataxia—A rare multisystem disease. Lancet Neurol. 2025, 24, 614–624. [Google Scholar] [CrossRef]
- Reetz, K.; Dogan, I.; Hohenfeld, C.; Didszun, C.; Giunti, P.; Mariotti, C.; Durr, A.; Boesch, S.; Garrido, F.J.R.d.R.; Schöls, L.; et al. Nonataxia symptoms in Friedreich Ataxia: Report from the registry of the European Friedreich’s Ataxia Consortium for Translational Studies (EFACTS). Neurology 2018, 91, e917–e930. [Google Scholar] [CrossRef]
- Delatycki, M.B.; Bidichandani, S.I. Friedreich ataxia—Pathogenesis and implications for therapies. Neurobiol. Dis. 2019, 132, 104606. [Google Scholar] [CrossRef]
- Indelicato, E.; Nachbauer, W.; Eigentler, A.; Amprosi, M.; Matteucci Gothe, P.; Giunti, P.; Mariotti, C.; Arpa, J.; Durr, A.; Klopstock, T.; et al. Onset features and time to diagnosis in Friedreich’s Ataxia. Orphanet J. Rare Dis. 2020, 15, 198. [Google Scholar] [CrossRef]
- Shen, M.M.; Rummey, C.; Lynch, D.R. Phenotypic variation of FXN compound heterozygotes in a Friedreich ataxia cohort. Ann. Clin. Transl. Neurol. 2024, 11, 1110–1121. [Google Scholar] [CrossRef]
- Campuzano, V.; Montermini, L.; Molto, M.D.; Pianese, L.; Cossee, M.; Cavalcanti, F.; Monros, E.; Rodius, F.; Duclos, F.; Monticelli, A.; et al. Friedreich’s ataxia: Autosomal recessive disease caused by intronic GAA triplet repeat expansion. Science 1996, 271, 1423–1427. [Google Scholar] [CrossRef]
- Montermini, L.; Andermann, E.; Labuda, M.; Richter, A.; Pandolfo, M.; Cavalcanti, F.; Pianese, L.; Iodice, L.; Farina, G.; Monticelli, A.; et al. The Friedreich ataxia GAA triplet repeat: Premutation and normal alleles. Hum. Mol. Genet. 1997, 6, 1261–1266. [Google Scholar] [CrossRef]
- Cossee, M.; Durr, A.; Schmidt, M.; Dahl, N.; Trouvillas, P.; Allison, P.; Kostrzewa, M.; Nivelon-Chevallier, A.; Gustavson, K.-H.; Kohlschütter, A.; et al. Friedreich’s ataxia: Point mutations and clinical presentation of compound heterozygotes. Ann. Neurol. 1999, 45, 200–206. [Google Scholar] [CrossRef]
- Galea, C.A.; Huq, A.; Lockhart, P.J.; Tai, G.; Corben, L.A.; Yiu, E.M.; Gurrin, L.C.; Lynch, D.R.; Gelbard, S.; Durr, A.; et al. Compound heterozygous FXN mutations and clinical outcome in Friedreich ataxia. Ann. Neurol. 2016, 79, 485–495. [Google Scholar] [CrossRef]
- Epplen, C.; Epplen, J.T.; Frank, G.; Miterski, B.; Santos, E.J.M.; Schöls, L. Differential stability of the (GAA)n tract in the Friedreich ataxia (STM7) gene. Hum. Genet. 1997, 99, 83. [Google Scholar] [CrossRef]
- Nethisinghe, S.; Kesavan, M.; Ging, H.; Labrum, R.; Polke, J.M.; Islam, S.; Garcia-Moreno, H.; Callaghan, M.F.; Cavalcanti, F.; Pook, M.A.; et al. Interruptions of the FXN GAA repeat tract delay the age at onset of Friedreich’s ataxia in a location dependent manner. Int. J. Mol. Sci. 2021, 22, 7507. [Google Scholar] [CrossRef] [PubMed]
- Rodden, L.N.; Rummey, C.; Dong, Y.N.; Lagedrost, S.; Regner, S.; Brocht, A.; Bushara, K.; Delatycki, M.B.; Gomez, C.M.; Mathews, K.; et al. A non-synonymous single nucleotide polymorphism in SIRT6 predicts neurological severity in Friedreich ataxia. Front. Mol. Biosci. 2022, 9, 933788. [Google Scholar] [CrossRef] [PubMed]
- Filla, A.; De Michele, G.; Cavalcanti, F.; Pianese, L.; Monticelli, A.; Campanella, G. The relationship between trinucleotide (GAA) repeat length and clinical features in Friedreich ataxia. Am. J. Hum. Genet. 1996, 59, 554–560. [Google Scholar] [PubMed]
- Delatycki, M.B.; Paris, D.B.; Gardner, R.J.; Nicholson, G.A.; Nassif, N.; Storey, E.; MacMillan, J.C.; Collins, V.; Williamson, R.; Forrest, S.M. Clinical and genetic study of Friedreich ataxia in an Australian population. Am. J. Med. Genet. 1999, 87, 168–174. [Google Scholar] [CrossRef]
- Schools, L.; Amoiridis, G.; Przuntek, H. Friedreich’s ataxia: Revision of phenotype according to molecular genetics. Brain 1997, 120, 2131–2140. [Google Scholar] [CrossRef]
- Lamont, P.J.; Davis, M.B.; Wood, N.W. Identification and sizing of the GAA trinucleotide repeat expansion of Friedreich’s ataxia in 56 patients. Brain 1997, 120, 673–680. [Google Scholar] [CrossRef]
- McCabe, D.J.; Ryan, F.; Moore, D.P.; McQuaid, S.; King, M.D.; Kelly, A.; Daly, K.; Barton, D.; Murphy, R. Typical Friedreich’s ataxia without GAA expansions and GAA expansion without typical Friedreich’s ataxia. J. Neurol. 2000, 247, 346–355. [Google Scholar] [CrossRef]
- Montermini, L.; Richter, A.; Morgan, K.; Justice, C.M.; Julien, D.; Castellotti, B.; Mercier, J.; Poirier, J.; Capozzoli, F.; Bouchard, J.; et al. Phenotypic variability in Friedreich ataxia: Role of the associated GAA triplet repeat expansion. Ann. Neurol. 1997, 41, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Monros, E.; Molto, M.D.; Martinez, F.; Canizares, J.; Blanca, J.; Vilchez, J.J.; Prieto, F.; de Frutos, R.; Palau, F. Phenotype correlation and intergenerational dynamics of the Friedreich ataxia GAA trinucleotide repeat. Am. J. Hum. Genet. 1997, 61, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Mateo, I.; Llorca, J.; Volpini, V.; Corral, J.; Berciano, J.; Combarros, O. Expanded GAA repeats and clinical variation in Friedreich’s ataxia. Acta Neurol. Scand. 2004, 109, 75–78. [Google Scholar] [CrossRef] [PubMed]
- De Michele, G.; Filla, A.; Criscuolo, C.; Scarano, V.; Cavalcanti, F.; Pianese, L.; Monticelli, A.; Cocozza, S. Determinants of onset age in Friedreich ataxia. J. Neurol. 1998, 245, 166–168. [Google Scholar] [CrossRef]
- Reetz, K.; Dogan, I.; Costa, A.S.; Dafotakis, M.; Fedosov, K.; Giunti, P.; Parkinson, M.H.; Sweeney, M.G.; Mariotti, C.; Panzeri, M.; et al. Biological and clinical characteristics of the European Friedreich’s Ataxia Consortium for Translational Studies (EFACTS) cohort: A cross-sectional analysis of baseline data. Lancet Neurol. 2015, 14, 174–182. [Google Scholar] [CrossRef]
- Al-Mahdawi, S.; Ging, H.; Bayot, A.; Cavalcanti, F.; La Cognata, V.; Cavallaro, S.; Giunti, P.; Pook, M.A. Large interruptions of GAA repeat expansion mutations in Friedreich ataxia are very rare. Front. Cell. Neurosci. 2018, 12, 443. [Google Scholar] [CrossRef]
- Rummey, C.; Corben, L.A.; Delatycki, M.; Wilmot, G.; Subramony, S.H.; Corti, M.; Bushara, K.; Duquette, A.; Gomez, C.; Hoyle, J.C.; et al. Natural history of Friedreich ataxia: Heterogeneity of neurologic progression and consequences for clinical trial design. Neurology 2022, 99, e1499–e1510. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014; Available online: http://www.R-project.org/ (accessed on 29 August 2025).
- Kovacevic, G.; Todorovic, S.; Novakovic, I.; Savic-Pavicevic, D.; Rasic, V.M.; Dobricic, V. Genotype-phenotype correlation in Friedreich’s ataxia. Eur. J. Paediatr. Neurol. 2017, 21 (Suppl. S1), e205–e206. [Google Scholar] [CrossRef]
- Kovacevic, G.; Todorovic, S.; Novakovic, I.; Savic-Pavicevic, D.; Rasic, V.M.; Dobricic, V. Genotype-phenotype correlation in Friedreich’s ataxia. In Proceedings of the 12th European Paediatric Neurology Society Congress, Lyon, France, 20–24 June 2017. [Google Scholar]
- Filla, A.; De Michele, G.; Coppola, G.; Federico, A.; Vita, G.; Toscano, A.; Uncini, A.; Pisanelli, P.; Barone, P.; Scarano, V.; et al. Accuracy of clinical diagnostic criteria for Friedreich’s ataxia. Mov. Disord. 2000, 15, 1255–1258. [Google Scholar] [CrossRef] [PubMed]
- Hanson, E.; Sheldon, M.; Pacheco, B.; Alkubeysi, M.; Raizada, V. Heart disease in Friedreich’s ataxia. World J. Cardiol. 2019, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pousset, F.; Legrand, L.; Monin, M.L.; Ewenczyk, C.; Charles, P.; Komajda, M.; Brice, A.; Pandolfo, M.; Isnard, R.; du Montcel, S.T.; et al. 22-year follow-up study of long-term cardiac outcome and predictors of survival in Friedreich ataxia. JAMA Neurol. 2015, 72, 1334–1341. [Google Scholar] [CrossRef]
- Norrish, G.; Rance, T.; Montanes, E.; Field, E.; Brown, E.; Bhole, V.; Stuart, G.; Uzun, O.; McLeod, K.A.; Ilina, M.; et al. Friedreich’s ataxia-associated childhood hypertrophic cardiomyopathy: A national cohort study. Arch. Dis. Child. 2022, 107, 450–455. [Google Scholar] [CrossRef]
- Schadt, K.A.; Friedman, L.S.; Regner, S.R.; Mark, G.E.; Lynch, D.R.; Lin, K.Y. Cross-sectional analysis of electrocardiograms in a large heterogeneous cohort of Friedreich ataxia subjects. J. Child Neurol. 2012, 27, 1187–1192. [Google Scholar] [CrossRef]
- McCormick, A.; Farmer, J.; Perlman, S.; Delatycki, M.; Wilmot, G.; Matthews, K.; Yoon, G.; Hoyle, C.; Subramony, S.H.; Zesiewicz, T.; et al. Impact of diabetes in the Friedreich ataxia clinical outcome measures study. Ann. Clin. Transl. Neurol. 2017, 4, 622–631. [Google Scholar] [CrossRef]
- Tamaroff, J.; DeDio, A.; Wade, K.; Wells, M.; Park, C.; Leavens, K.; Rummey, C.; Kelly, A.; Lynch, D.R.; McCormack, S.E. Friedreich’s Ataxia related diabetes: Epidemiology and management practices. Diabetes Res. Clin. Pract. 2022, 186, 109828. [Google Scholar] [CrossRef]
- Greeley, N.R.; Regner, S.; Willi, S.; Lynch, D.R. Cross-sectional analysis of glucose metabolism in Friedreich ataxia. J. Neurol. Sci. 2014, 342, 29–35. [Google Scholar] [CrossRef]
- Patel, M.; Isaacs, C.J.; Seyer, L.; Brigatti, K.; Gelbard, S.; Strawser, C.; Foerster, D.; Shinnick, J.; Schadt, K.; Yiu, E.M.; et al. Progression of Friedreich ataxia: Quantitative characterization over 5 years. Ann. Clin. Transl. Neurol. 2016, 3, 684–694. [Google Scholar] [CrossRef]
- Watters, G.V.; Zlotkin, S.H.; Kaplan, B.S.; Humphreys, P.; Drummond, K.N. Friedreich’s ataxia with nephrotic syndrome and convulsive disorder: Clinical and neurophysiological studies with renal and nerve biopsies and an autopsy. Can. J. Neurol. Sci. 1981, 8, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Shinnick, J.E.; Isaacs, C.J.; Vivaldi, S.; Schadt, K.; Lynch, D.R. Friedreich ataxia and nephrotic syndrome: A series of two patients. BMC Neurol. 2016, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.; Sindgikar, S.P.; Shenoy, R.D.; Shenoy, V. Oxidative stress in childhood steroid sensitive nephrotic syndrome and its correlation with DNA damage. Int. J. Contemp. Pediatr. 2016, 3, 768–772. [Google Scholar] [CrossRef]
- Ercanbrack, W.S.; Ramirez, M.; Dungan, A.; Gaul, E.; Ercanbrack, S.J.; Wingert, R.A. Frataxin deficiency and the pathology of Friedreich’s Ataxia across tissues. Tissue Barriers 2025, 2462357. [Google Scholar] [CrossRef]
- Pandolfo, M. Neurologic outcomes in Friedreich ataxia: Study of a single-site cohort. Neurol. Genet. 2020, 6, e415. [Google Scholar] [CrossRef]
- Reetz, K.; Dogan, I.; Hilgers, R.D.; Giunti, P.; Mariotti, C.; Durr, A.; Boesch, S.; de Rivera, F.J.R.; Schöls, L.; Klockgether, T.; et al. Progression characteristics of the Euro pean Friedreich’s Ataxia Consortium for Translational Studies (EFACTS): A 2 year cohort study. Lancet Neurol. 2016, 15, 1346–1354. [Google Scholar] [CrossRef]
- Rummey, C.; Farmer, J.M.; Lynch, D.R. Predictors of loss of ambulation in Friedreich’s ataxia. EClinicalMedicine 2020, 18, 100213. [Google Scholar] [CrossRef]
- Badhwar, A.; Jansen, A.; Andermann, F.; Pandolfo, M.; Andermann, E. Striking intrafamilial phenotypic variability and spastic paraplegia in the presence of similar homozygous expansions of the FRDA1 gene. Mov. Disord. 2004, 19, 1424–1431. [Google Scholar] [CrossRef]
- Eshaghi, K.; Rao, P.H.; Shen, M.M.; Lynch, D.R. Genetic and phenotypic variability in siblings with Friedreich ataxia. Neurol. Genet. 2025, 11, e200234. [Google Scholar] [CrossRef]
| Clinical Characteristics | Number of Patients | Percentage |
|---|---|---|
| 1. Neurological | ||
| Ataxia | 30/30 | 100% |
| Romberg sign | 30/30 | 100% |
| Lower limb areflexia | 30/30 | 100% |
| Upper limb areflexia | 28/30 | 93.3% |
| Extensor plantar response | 20/28 | 71.4% |
| Dysarthria | 16/30 | 53.3% |
| Nystagmus | 10/30 | 33.3% |
| 2. Orthopedic | ||
| Scoliosis | 26/30 | 86.7% |
| Foot deformities | 23/28 | 76.7% |
| 3. Cardiometabolic | ||
| Cardiomyopathy | 22/29 | 73.7% |
| Diabetes mellitus | 2/30 | 6.7% |
| 4. Comorbidity | ||
| Nephrotic syndrome | 2/30 | 6.7% |
| GAA1 | GAA2 | GAA1-GAA2 Total | Age at Onset | |||||
|---|---|---|---|---|---|---|---|---|
| Rho | p-Value | Rho | p-Value | Rho | p-Value | Rho | p-Value | |
| Age at onset | −0.122 | 0.552 | −0.112 | 0.953 | −0.078 | 0.706 | n.a. | n.a |
| Disease duration to loss of independent walking | −0.143 | 0.658 | −0.046 | 0.886 | −0.061 | 0.851 | −0.718 | 0.006 |
| Disease duration to becoming wheelchair-bound | 0.286 | 0.556 | 0.286 | 0.556 | 0.286 | 0.556 | −0.642 | 0.086 |
| Family I | Family II | Family III | ||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | I-1 | I-2 | I-3 | II-1 | II-2 | III-1 | III-2 | III-3 |
| GAA1 | 850 | 890 | 880 | 876 | 858 | 659 | 919 | - |
| GAA2 | 1070 | 1071 | 1066 | 943 | 1014 | 1168 | 1148 | - |
| Age at onset (years) | 8 | 14 | 14 | 8 | 6 | 13 | 12 | 15 |
| Age at examination | 16 | 21 | 21 | 18 | 11 | 18 | 23 | 21 |
| Independent walk * | - (16) | - (21) | - (21) | + | + | + | - (19) | + |
| Positive Babinski sign | + | + | + | - | - | - | + | + |
| Pes cavus | + | + | + | + | + | - | + | - |
| Scoliosis | + | + | + | + | + | + | + | - |
| Cardiomyopathy $ | + (10) | + (15) | + (19) | + (15) | - | - | - | + (18) |
| Dysarthria | + | + | + | - | - | - | + | - |
| Author | Patient’s Number | Age at FA Onset | Number of GAA1/ GAA2 | Age at NS Onset | Clinical Type of NS | Pathohistological Changes | Course | Outcome |
|---|---|---|---|---|---|---|---|---|
| Watters, et al. Can J Neurol Sci, 1981 [43] | 1 | 6 years | NA | 5 years | SSNS | MCD | Frequent relapses | Steroid-dependent NS, died at 20 |
| 2 | 5.5 years | NA | 12 years | SSNS | MCD | Several relapses | Stable remission | |
| Shinnick, et al. BMC Neurol. 2016 [44] | 3 | 10 years | 650/850 | 2 years | SSNS | Biopsy not indicated | Several relapses | Stable remission |
| 4 | 7 years | 650/1000 | 5 years | SSNS | Biopsy not indicated | Several relapses | Stable remission | |
| Our study | 5 | 6 years | 329/824 | 18 months | SSNS | Biopsy not indicated | A few relapses | Stable remission |
| 6 | 6 years | NA | 17 years | SSNS | MCD | Frequent relapses | Remission, but GFR has declined at 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovacevic, G.; Todorovic, S.; Novakovic, I.; Dobricic, V.; Savic-Pavicevic, D.; Milic Rasic, V.; Svetel, M.; Brkusanin, M.; Vukomanovic, V.; Vucinic, D.; et al. Multi-Center National Study of Genotype–Phenotype Correlation and Clinical Characteristics in Children and Young Adults with Friedreich’s Ataxia from Serbia. Biomedicines 2025, 13, 2646. https://doi.org/10.3390/biomedicines13112646
Kovacevic G, Todorovic S, Novakovic I, Dobricic V, Savic-Pavicevic D, Milic Rasic V, Svetel M, Brkusanin M, Vukomanovic V, Vucinic D, et al. Multi-Center National Study of Genotype–Phenotype Correlation and Clinical Characteristics in Children and Young Adults with Friedreich’s Ataxia from Serbia. Biomedicines. 2025; 13(11):2646. https://doi.org/10.3390/biomedicines13112646
Chicago/Turabian StyleKovacevic, Gordana, Slobodanka Todorovic, Ivana Novakovic, Valerija Dobricic, Dusanka Savic-Pavicevic, Vedrana Milic Rasic, Marina Svetel, Milos Brkusanin, Vladislav Vukomanovic, Dragana Vucinic, and et al. 2025. "Multi-Center National Study of Genotype–Phenotype Correlation and Clinical Characteristics in Children and Young Adults with Friedreich’s Ataxia from Serbia" Biomedicines 13, no. 11: 2646. https://doi.org/10.3390/biomedicines13112646
APA StyleKovacevic, G., Todorovic, S., Novakovic, I., Dobricic, V., Savic-Pavicevic, D., Milic Rasic, V., Svetel, M., Brkusanin, M., Vukomanovic, V., Vucinic, D., Ostojic, S., Putnik, J., & Kosac, A. (2025). Multi-Center National Study of Genotype–Phenotype Correlation and Clinical Characteristics in Children and Young Adults with Friedreich’s Ataxia from Serbia. Biomedicines, 13(11), 2646. https://doi.org/10.3390/biomedicines13112646






