Estrogen Receptors as Key Factors in Carcinogenesis
Abstract
1. Introduction
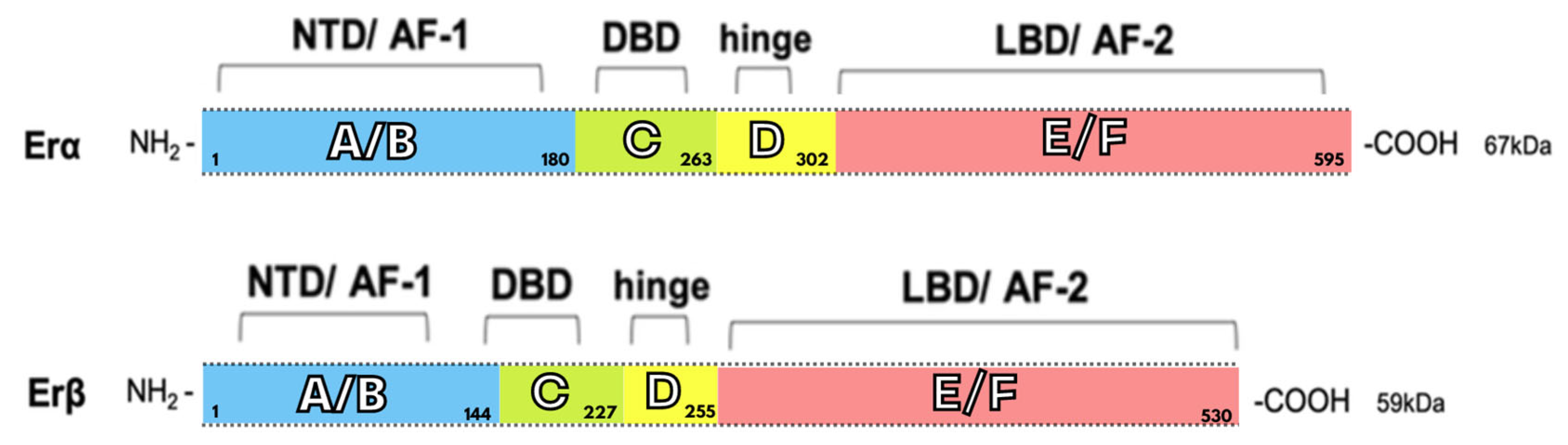
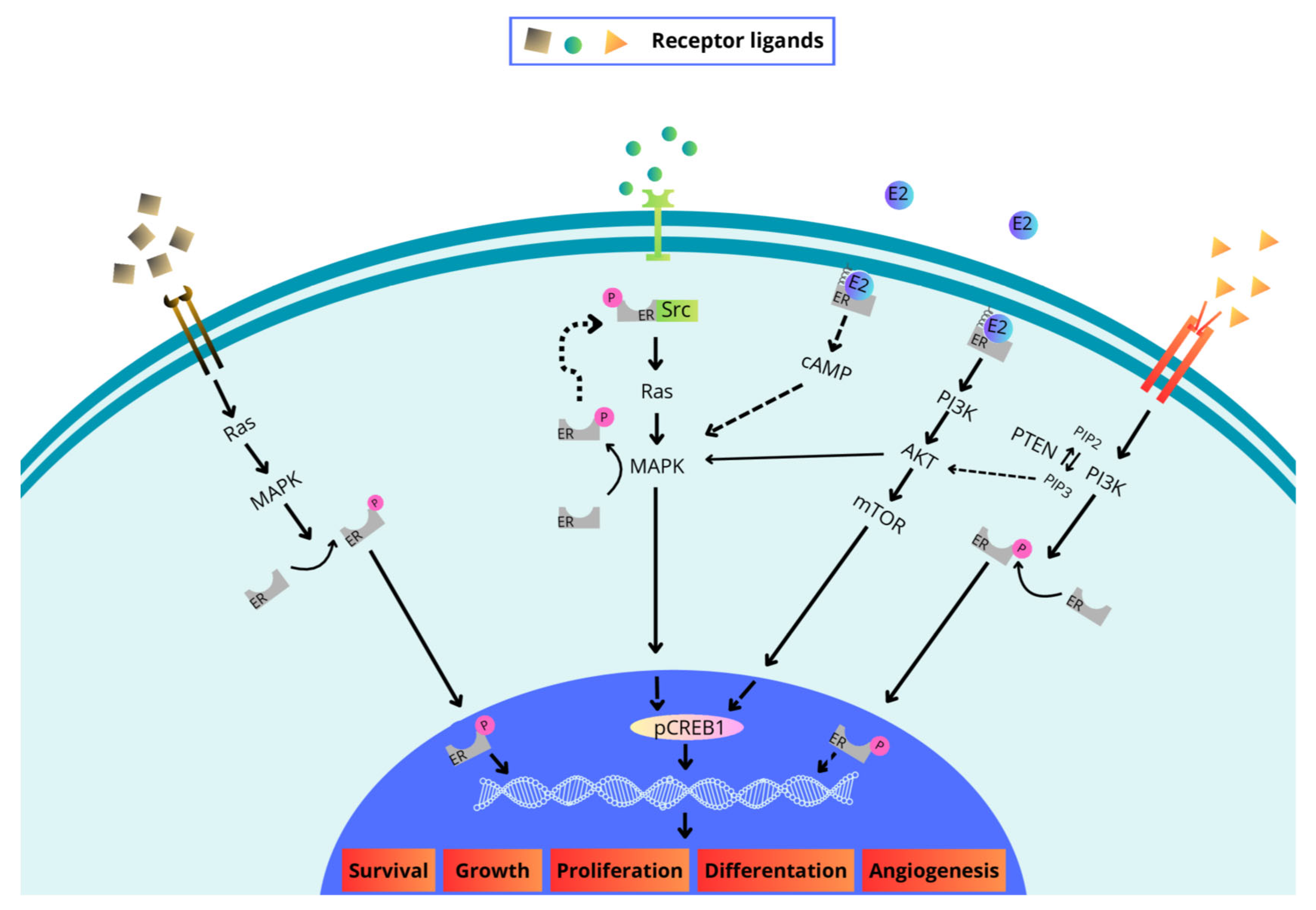
2. Structure and Signaling of the Estrogen Receptor
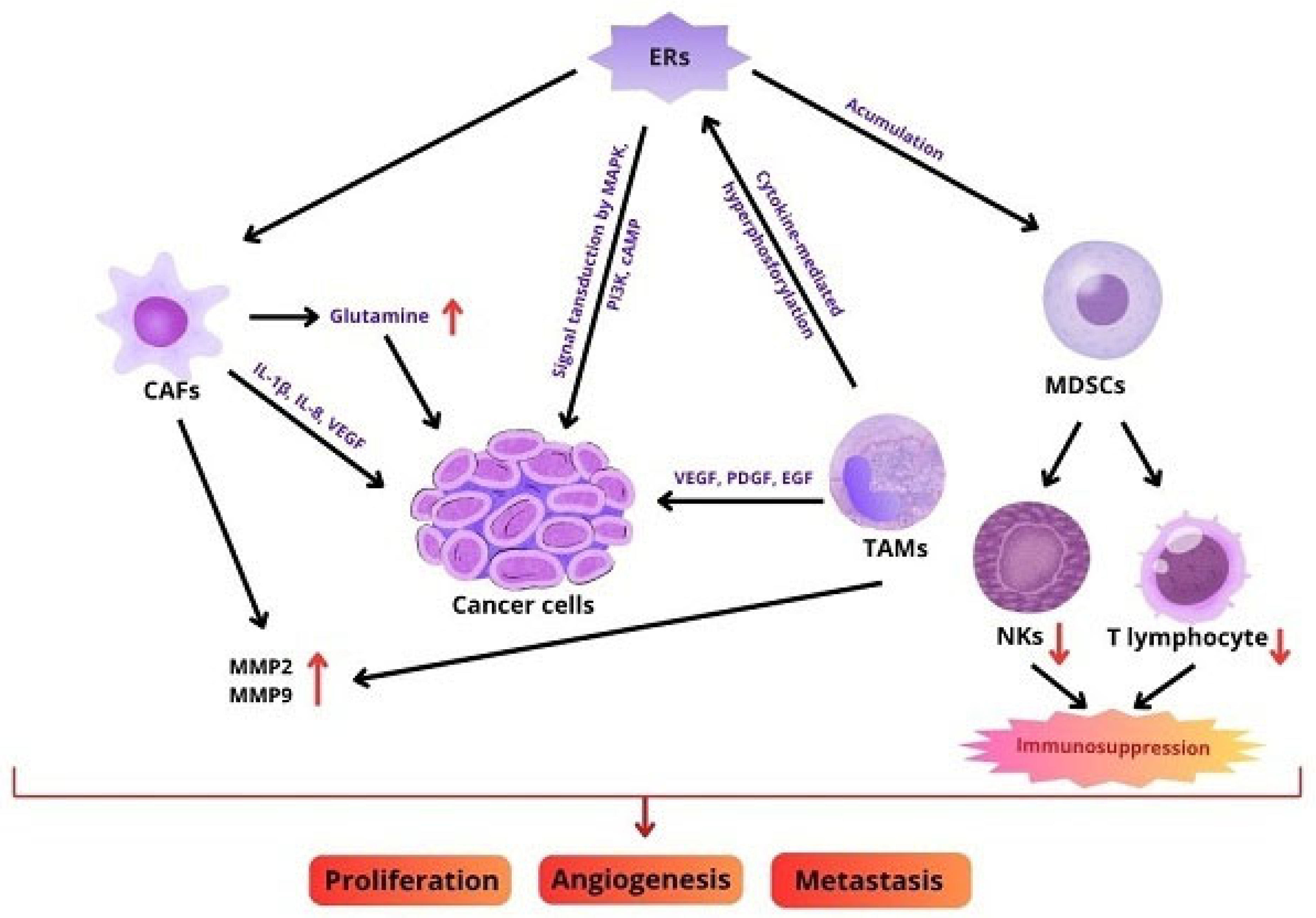
3. Role of TME
4. Role of ER in TME
5. The Role of the ERs in Specific Cancers
5.1. Ovarian Cancer
5.2. Breast Cancer
5.3. Thyroid Cancer
5.4. Colorectal Cancer
6. SERMs/SERDs/AIs
7. Hormone Therapy Resistance
8. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AF | Activation function domains |
| Akt | Protein kinase B |
| AR | Androgen receptor |
| AIs | Aromatase inhibitors |
| AP1 | Activating factor 1 |
| AR | Aromatase |
| BC | Breast cancer |
| BMI | Body mass index |
| CAFs | Cancer-associated fibroblasts |
| cAMP | Cyclic adenosine monophosphate |
| CCL2 | CC-chemokine ligand 2 |
| CCNA2 | Cyclin A2 |
| CDK4/6 | Cyclin-dependent kinases 4 and 6 |
| CDK4/6i | Cyclin-dependent kinase 4/6 inhibitor |
| CERANs | Complete estrogen receptor antagonists |
| COX-2 | Cyclooxygenase-2 |
| CRC | Colorectal cancer |
| CREB | Cyclic AMP-responsive element-binding protein |
| CRPC | Castration-resistant prostate cancer |
| CSCs | Cancer stem cells |
| CysLT1R | Cysteinyl leukotriene receptor 1 |
| DBD | DNA-binding domain |
| ECM | Extracellular matrix |
| EGF | Epidermal growth factor |
| EGFR | Epidermal growth factor receptor |
| EMT | Epithelial–mesenchymal transition |
| ER | Estrogen receptor |
| ER+ | Estrogen receptor-positive |
| ERα | Estrogen receptor alpha |
| ERβ | Estrogen receptor beta |
| ERE | Estrogen response elements |
| ERK-1 | Extracellular signal-regulated kinase 1 |
| ER-positive | Estrogen receptor-positive |
| ESR1 | Estrogen receptor 1 gene |
| ESR2 | Estrogen receptor 2 gene |
| E2 | 17β-estradiol |
| FGFR | Fibroblast growth factor receptor |
| FBLN-1C | Fibulin-1C |
| FOXP3 | Forkhead box P3 gene |
| GLI1 | Glioma-associated oncogene homolog 1 |
| GLI2 | Glioma-associated oncogene homolog 2 |
| GnRH | Gonadotropin-releasing hormone |
| GPER | G protein-coupled estrogen receptor |
| HA | Hyaluronic acid |
| HAS2 | Hyaluronan synthase 2 |
| HDAC3 | Histone deacetylase 3 |
| HDAC6 | Histone deacetylase 6 |
| HER2 | Human epidermal growth factor receptor 2 |
| HGF | Hepatocyte growth factor |
| HHIP | Hedgehog-interacting protein |
| HO-1 | Heme oxygenase-1 |
| HRT | Hormone replacement therapy |
| H3K4me3 | Histone H3 lysine 4 trimethylation |
| IGF | Insulin-like growth factor |
| IGF-1 | Insulin-like growth factor 1 |
| IGF1R | Insulin-like growth factor 1 receptor |
| IL-1β | Interleukin-1 beta |
| IL1R1 | Interleukin 1 type I receptor gene |
| IL-2 | Interleukin-2 |
| IL-4 | Interleukin-4 |
| IL-6 | Interleukin-6 |
| IL-7 | Interleukin-7 |
| IL-8 | Interleukin-8 |
| IL-11 | Interleukin-11 |
| ITGA5 | Integrin 5α |
| LBD | Ligand-binding domain |
| MAPK | Mitogen-activated protein kinase |
| MBC | Male breast cancer |
| MCAM | Melanoma cell adhesion molecule |
| MDSC | Myeloid-derived suppressor cell |
| MMPs | Matrix metalloproteinases |
| MMP2 | Matrix metalloproteinase-2 |
| MMP9 | Matrix metalloproteinase-9 |
| mTOR | Mammalian target of rapamycin |
| NF-κB | Nuclear factor kappa-light-chain-enhancer |
| NKs | Natural killer cells |
| NR | Nuclear receptor |
| NTD | N-terminal nucleotide binding domain |
| OC | Ovarian cancer |
| PC | Prostate cancer |
| pCREB1 | Phosphorylated cAMP-responsive element-binding protein 1 |
| PD-1 | Programmed cell death protein-1 |
| PDGF | Platelet-derived growth factor |
| PD-L1 | Programmed cell death-ligand 1 |
| PIP2 | Phosphatidylinositol 4,5-bisphosphate |
| PIP3 | Phosphatidylinositol 3,4,5-trisphosphate |
| PI3K | Phosphatidylinositol 3-kinase |
| PI3KCA | Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha |
| PR | Progesterone receptor |
| PROTACs | proteolysis-targeting chimeras |
| PTC | Papillary thyroid carcinoma |
| PTCH1 | Proteins patched 1 |
| PTCH2 | Proteins patched 2 |
| PTEN | Phosphatidylinositol-3,4,5-trisphosphate 3-phosphatase |
| Ras | GTPase HRas |
| RRE | Raloxifene responding element |
| RTKs | Receptor tyrosine kinases RTKs |
| SERCAs | Selective covalent estrogen receptor antagonists |
| SERDs | Selective estrogen receptor downregulators |
| SERMs | Selective estrogen receptor modulators |
| Src | Proto-oncogene tyrosine-protein kinase |
| STAT3 | Signal transducer and activator of transcription 3 |
| STAT5 | Signal transducer and activator of transcription 5 |
| TC | Thyroid cancer |
| Treg | Regulatory T lymphocyte |
| TAMs | Tumour-associated macrophages |
| TANs | Tumour-associated neutrophils |
| TGF-β | Transforming growth factor beta |
| TGFβ1 | Transforming growth factor beta 1 |
| TIME | Tumor immune microenvironment |
| TIMPs | Tissue inhibitors of metalloproteinases |
| TME | Tumour microenvironment |
| TNBC | Triple-negative breast cancer |
| TNF-α | Tumour necrosis factor alpha |
| TSH | Thyroid-stimulating hormone |
| VEGF | Vascular endothelial growth factor |
References
- Bray, F.; Laversanne, M.; Sung, H. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Dee, E.C.; Wu, J.F.; Feliciano, E.J.G.; Ting, F.I.L.; Willmann, J.; Ho, F.D.V.; Jain, B.; Jain, U.; Chen, J.; Moraes, F.Y.; et al. National Cancer System Characteristics and Global Pan-Cancer Outcomes. JAMA Oncol. 2025, 11, 650–654. [Google Scholar] [CrossRef]
- Jafari, H.; Hussain, S.; Campbell, M.J. Nuclear Receptor Coregulators in Hormone-Dependent Cancers. Cancers 2022, 14, 2402. [Google Scholar] [CrossRef]
- Hamid, A.A.; Issa, M.B.; Nizar, N.N.A. Hormones. In Preparation and Processing of Religious and Cultural Foods; Elsevier: Amsterdam, The Netherlands, 2018; pp. 253–277. [Google Scholar]
- Montaruli, A.; Castelli, L.; Mulè, A.; Scurati, R.; Esposito, F.; Galasso, L.; Roveda, E. Biological Rhythm and Chronotype: New Perspectives in Health. Biomolecules 2021, 11, 487. [Google Scholar] [CrossRef]
- Liu, Y.C.; Yeh, C.T.; Lin, K.H. Molecular Functions of Thyroid Hormone Signaling in Regulation of Cancer Progression and Anti-Apoptosis. Int. J. Mol. Sci. 2019, 20, 4986. [Google Scholar] [CrossRef]
- Chu, L.; Shu, X.; Huang, Y.; Chu, T.; Ge, M.; Lu, Q. Sex steroid hormones in urinary exosomes as biomarkers for the prediction of prostate cancer. Clin. Chim. Acta 2022, 531, 389–398. [Google Scholar] [CrossRef]
- Costa, A.R.; Lança de Oliveira, M.; Cruz, I.; Gonçalves, I.; Cascalheira, J.F.; Santos, C.R.A. The Sex Bias of Cancer. Trends Endocrinol. Metab. 2020, 31, 785–799. [Google Scholar] [CrossRef]
- Boye, A.; Osei, S.; Brah, A. Therapeutic prospects of sex hormone receptor signaling in hormone-responsive cancers. Biomed. Pharmacother. 2024, 180, 117473. [Google Scholar] [CrossRef]
- Pakdel, F. The Role of Estrogen Receptors in Health and Disease. Int. J. Mol. Sci. 2023, 24, 11354. [Google Scholar] [CrossRef] [PubMed]
- Pakdel, F. Molecular Pathways of Estrogen Receptor Action. Int. J. Mol. Sci. 2018, 19, 2591. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; He, H.; Indukuri, R.; Huang, Z.; Stepanauskaite, L.; Sinha, I.; Haldosén, L.A.; Zhao, C.; Williams, C. ERα and ERβ Homodimers in the Same Cellular Context Regulate Distinct Transcriptomes and Functions. Front. Endocrinol. 2022, 13, 930227. [Google Scholar] [CrossRef]
- Rae, J.M.; Lippman, M.E. The role of estrogen receptor signaling in suppressing the immune response to cancer. J. Clin. Investig. 2021, 131, e155476. [Google Scholar] [CrossRef]
- Chen, P.; Li, B.; Ou-Yang, L. Role of estrogen receptors in health and disease. Front. Endocrinol. 2022, 13, 839005. [Google Scholar] [CrossRef] [PubMed]
- Langdon, S.P.; Herrington, C.S.; Hollis, R.L.; Gourley, C. Estrogen Signaling and Its Potential as a Target for Therapy in Ovarian Cancer. Cancers 2020, 12, 1647. [Google Scholar] [CrossRef]
- Arneth, B. Tumor Microenvironment. Medicina 2019, 56, 15. [Google Scholar] [CrossRef]
- Kozieł, M.J.; Piastowska-Ciesielska, A.W. Estrogens, Estrogen Receptors and Tumor Microenvironment in Ovarian Cancer. Int. J. Mol. Sci. 2023, 24, 14673. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, N.; Silveyra, P. Estrogen receptor signaling mechanisms. Adv. Protein Chem. Struct. Biol. 2019, 116, 135–170. [Google Scholar] [PubMed]
- Patel, H.K.; Bihani, T. Selective estrogen receptor modulators (SERMs) and selective estrogen receptor degraders (SERDs) in cancer treatment. Pharmacol. Ther. 2018, 186, 1–24. [Google Scholar] [CrossRef]
- Brett, J.O.; Spring, L.M.; Bardia, A.; Wander, S.A. ESR1 mutation as an emerging clinical biomarker in metastatic hormone receptor-positive breast cancer. Breast Cancer Res. 2021, 23, 85. [Google Scholar] [CrossRef]
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef]
- Neupane, N.; Bawek, S.; Gurusinghe, S.; Ghaffary, E.M.; Mirmosayyeb, O.; Thapa, S.; Falkson, C.; O’Regan, R.; Dhakal, A. Oral SERD, a Novel Endocrine Therapy for Estrogen Receptor-Positive Breast Cancer. Cancers 2024, 16, 619. [Google Scholar] [CrossRef]
- Bardia, A.; Cortés, J.; Bidard, F.C.; Neven, P.; Garcia-Sáenz, J.; Aftimos, P.; O’Shaughnessy, J.; Lu, J.; Tonini, G.; Scartoni, S.; et al. Elacestrant in ER+, HER2- Metastatic Breast Cancer with ESR1-Mutated Tumors: Subgroup Analyses from the Phase III EMERALD Trial by Prior Duration of Endocrine Therapy plus CDK4/6 Inhibitor and in Clinical Subgroups. Clin. Cancer Res. 2024, 30, 4299–4309. [Google Scholar] [PubMed]
- Gong, Z.; Yang, S.; Wei, M.; Vlantis, A.C.; Chan, J.Y.K.; van Hasselt, C.A.; Li, D.; Zeng, X.; Xue, L.; Tong, M.C.F.; et al. The Isoforms of Estrogen Receptor Alpha and Beta in Thyroid Cancer. Front. Oncol. 2022, 12, 916804. [Google Scholar] [CrossRef]
- Belluti, S.; Imbriano, C.; Casarini, L. Nuclear Estrogen Receptors in Prostate Cancer: From Genes to Function. Cancers 2023, 15, 4653. [Google Scholar] [CrossRef]
- Arao, Y.; Korach, K.S. The physiological role of estrogen receptor functional domains. Essays Biochem. 2021, 65, 867–875. [Google Scholar] [CrossRef]
- Bano, A.; Stevens, J.H.; Modi, P.S.; Gustafsson, J.Å.; Strom, A.M. Estrogen Receptor β4 Regulates Chemotherapy Resistance and Induces Cancer Stem Cells in Triple Negative Breast Cancer. Int. J. Mol. Sci. 2023, 24, 5867. [Google Scholar] [CrossRef]
- Tirado-Garibay, A.C.; Falcón-Ruiz, E.A.; Ochoa-Zarzosa, A.; López-Meza, J.E. GPER: An Estrogen Receptor Key in Metastasis and Tumoral Microenvironments. Int. J. Mol. Sci. 2023, 24, 14993. [Google Scholar] [CrossRef]
- Barton, M.; Filardo, E.J.; Lolait, S.J.; Thomas, P.; Maggiolini, M.; Prossnitz, E.R. Twenty years of the G protein-coupled estrogen receptor GPER: Historical and personal perspectives. J. Steroid Biochem. Mol. Biol. 2018, 176, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Périan, S.; Vanacker, J.M. GPER as a Receptor for Endocrine-Disrupting Chemicals (EDCs). Front. Endocrinol. 2020, 11, 545. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Gieniec, K.A.; Lannagan, T.R.M.; Wang, T.; Asai, N.; Mizutani, Y.; Iida, T.; Ando, R.; Thomas, E.M.; Sakai, A.; et al. The Origin and Contribution of Cancer-Associated Fibroblasts in Colorectal Carcinogenesis. Gastroenterology 2022, 162, 890–906. [Google Scholar] [CrossRef]
- Chuangchot, N.; Jamjuntra, P.; Yangngam, S.; Luangwattananun, P.; Thongchot, S.; Junking, M.; Thuwajit, P.; Yenchitsomanus, P.T.; Thuwajit, C. Enhancement of PD-L1-attenuated CAR-T cell function through breast cancer-associated fibroblasts-derived IL-6 signaling via STAT3/AKT pathways. Breast Cancer Res. 2023, 25, 86. [Google Scholar] [CrossRef]
- Shin, E.; Koo, J.S. Cell Component and Function of Tumor Microenvironment in Thyroid Cancer. Int. J. Mol. Sci. 2022, 23, 12578. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Li, Z.; Zheng, B.; Lin, X.; Pan, Y.; Gong, P.; Zhuo, W.; Hu, Y.; Chen, C.; Chen, L.; et al. Cancer-associated fibroblasts in breast cancer: Challenges and opportunities. Cancer Commun. 2022, 42, 401–434. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhong, F.; Liu, L. Single-cell transcriptional atlas of tumor-associated macrophages in breast cancer. Breast Cancer Res. 2024, 26, 129. [Google Scholar] [CrossRef]
- Gok Yavuz, B.; Gunaydin, G.; Gedik, M.E.; Kosemehmetoglu, K.; Karakoc, D.; Ozgur, F.; Guc, D. Cancer associated fibroblasts sculpt tumour microenvironment by recruiting monocytes and inducing immunosuppressive PD-1+ TAMs. Sci. Rep. 2019, 9, 3172. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, C.; Li, X.; Yang, F.; Wang, N.; Ji, G.; Liu, Q.; Zhu, H.; Xu, S.; Li, H. Unraveling the role of M2 TAMs in ovarian cancer dynamics: A systematic review. J. Transl. Med. 2025, 23, 623. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Yang, Z.; Xu, S.; Li, X.; Yang, X.; Jin, P.; Liu, Y.; Zhou, X.; Zhang, T.; Gong, C.; et al. Heterotypic CAF-tumor spheroids promote early peritoneal metastatis of ovarian cancer. J. Exp. Med. 2019, 216, 688–703. [Google Scholar] [CrossRef]
- Cho, J.G.; Byeon, H.K.; Oh, K.H.; Baek, S.K.; Kwon, S.Y.; Jung, K.Y.; Woo, J.S. Clinicopathological significance of cancer-associated fibroblasts in papillary thyroid carcinoma: A predictive marker of cervical lymph node metastasis. Eur. Arch. Otorhinolaryngol. 2018, 275, 2355–2361. [Google Scholar] [CrossRef]
- Jaroszewski, A.; Geysels, R.C.; Volpini, X.; Pellizas, C.G.; Motran, C.C.; Stempin, C.C.; Nicola, J.P.; Cheng, S.Y.; Fozzatti, L. Anaplastic thyroid cancer cell-secreted TGFβ1 plays a key role in inducing macrophage polarization of human monocytes. Am. J. Cancer Res. 2024, 14, 3626–3638. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.; Sinha, S.; Roy, R.; Biswas, N. Autophagy mediated immune response regulation and drug resistance in cancer. Mol. Biol. Rep. 2025, 52, 492. [Google Scholar] [CrossRef]
- Qin, Q.; Ji, H.; Li, D.; Zhang, H.; Zhang, Z.; Zhang, Q. Tumor-associated macrophages increase COX-2 expression promoting endocrine resistance in breast cancer via the PI3K/Akt/mTOR pathway. Neoplasma 2021, 68, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ji, H.; Niu, X.; Yin, L.; Wang, Y.; Gu, Y.; Wang, J.; Zhou, X.; Zhang, H.; Zhang, Q. Tumor-associated macrophages secrete CC-chemokine ligand 2 and induce tamoxifen resistance by activating PI3K/Akt/mTOR in breast cancer. Cancer Sci. 2020, 111, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Febrero, B.; Ruiz-Manzanera, J.J.; Ros-Madrid, I.; Hernández, A.M.; Orenes-Piñero, E.; Rodríguez, J.M. Tumor microenvironment in thyroid cancer: Immune cells, patterns, and novel treatments. Head Neck. 2024, 46, 1486–1499. [Google Scholar] [CrossRef]
- Mirzaei, M.; Sheikholeslami, S.A.; Jalili, A.; Bereimipour, A.; Sharbati, S.; Kaveh, V.; Salari, S. Investigating the molecular mechanisms of Tamoxifen on the EMT pathway among patients with breast cancer. J. Med. Life 2022, 15, 835–844. [Google Scholar] [CrossRef]
- Hassn Mesrati, M.; Syafruddin, S.E.; Mohtar, M.A.; Syahir, A. CD44: A Multifunctional Mediator of Cancer Progression. Biomolecules 2021, 11, 1850. [Google Scholar] [CrossRef]
- Soliman, F.; Ye, L.; Jiang, W.; Hargest, R. Targeting Hyaluronic Acid and Peritoneal Dissemination in Colorectal Cancer. Clin. Color. Cancer. 2022, 21, e126–e134. [Google Scholar] [CrossRef]
- Affo, S.; Nair, A.; Brundu, F.; Ravichandra, A.; Bhattacharjee, S.; Matsuda, M.; Chin, L.; Filliol, A.; Wen, W.; Song, X.; et al. Promotion of cholangiocarcinoma growth by diverse cancer-associated fibroblast subpopulations. Cancer Cell 2021, 39, 866–882.e11. [Google Scholar] [CrossRef] [PubMed]
- Kellett-Clarke, H.; Stegmann, M.; Barclay, A.N.; Metcalfe, C. CD44 Binding to Hyaluronic Acid Is Redox Regulated by a Labile Disulfide Bond in the Hyaluronic Acid Binding Site. PLoS ONE 2015, 10, e0138137. [Google Scholar] [CrossRef]
- Hanoux, V.; Eguida, J.; Fleurot, E.; Levallet, J.; Bonnamy, P.J. Increase in hyaluronic acid degradation decreases the expression of estrogen receptor alpha in MCF7 breast cancer cell line. Mol. Cell. Endocrinol. 2018, 476, 185–197. [Google Scholar] [CrossRef]
- Trnkova, L.; Buocikova, V.; Mego, M.; Cumova, A.; Burikova, M.; Bohac, M.; Miklikova, S.; Cihova, M.; Smolkova, B. Epigenetic deregulation in breast cancer microenvironment: Implications for tumor progression and therapeutic strategies. Biomed. Pharmacother. 2024, 174, 116559. [Google Scholar] [CrossRef]
- Li, X.; Jin, Y.; Xue, J. Unveiling Collagen’s Role in Breast Cancer: Insights into Expression Patterns, Functions and Clinical Implications. Int. J. Gen. Med. 2024, 17, 1773–1787. [Google Scholar] [CrossRef]
- JingSong, H.; Hong, G.; Yang, J.; Duo, Z.; Li, F.; Wei Cai, C.; Xue Ying, L.; You Sheng, M.; Yi Wen, O.; Yue, P.; et al. siRNA-mediated suppression of collagen type iv alpha 2 (COL4A2) mRNA inhibits triple-negative breast cancer cell proliferation and migration. Oncotarget 2017, 8, 2585–2593. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, A.; Tiidus, P.M.; Vandenboom, R. Mechanisms of Estrogen Influence on Skeletal Muscle: Mass, Regeneration, and Mitochondrial Function. Sports Med. 2022, 52, 2853–2869. [Google Scholar] [CrossRef]
- Inoue, C.; Miki, Y.; Suzuki, T. New Perspectives on Sex Steroid Hormones Signaling in Cancer-Associated Fibroblasts of Non-Small Cell Lung Cancer. Cancers 2023, 15, 3620. [Google Scholar] [CrossRef] [PubMed]
- Reid, S.E.; Pantaleo, J.; Bolivar, P.; Bocci, M.; Sjölund, J.; Morsing, M.; Cordero, E.; Larsson, S.; Malmberg, M.; Seashore-Ludlow, B.; et al. Cancer-associated fibroblasts rewire the estrogen receptor response in luminal breast cancer, enabling estrogen independence. Oncogene 2024, 43, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Meng, L.H. Emerging roles of class I PI3K inhibitors in modulating tumor microenvironment and immunity. Acta Pharmacol. Sin. 2020, 41, 1395–1402. [Google Scholar] [CrossRef]
- Fabris, L.; Sato, K.; Alpini, G.; Strazzabosco, M. The Tumor Microenvironment in Cholangiocarcinoma Progression. Hepatology 2021, 73 (Suppl. S1), 75–85. [Google Scholar] [CrossRef]
- Basak, U.; Sarkar, T.; Mukherjee, S.; Chakraborty, S.; Dutta, A.; Dutta, S.; Nayak, D.; Kaushik, S.; Das, T.; Sa, G. Tumor-associated macrophages: An effective player of the tumor microenvironment. Front. Immunol. 2023, 14, 1295257. [Google Scholar] [CrossRef]
- Swanton, C.; Bernard, E.; Abbosh, C.; André, F.; Auwerx, J.; Balmain, A.; Bar-Sagi, D.; Bernards, R.; Bullman, S.; DeGregori, J.; et al. Embracing cancer complexity: Hallmarks of systemic disease. Cell 2024, 187, 1589–1616. [Google Scholar] [CrossRef]
- Harris, M.A.; Savas, P.; Virassamy, B.; O’Malley, M.M.R.; Kay, J.; Mueller, S.N.; Mackay, L.K.; Salgado, R.; Loi, S. Towards targeting the breast cancer immune microenvironment. Nat. Rev. Cancer 2024, 24, 554–577. [Google Scholar] [CrossRef]
- Bae, W.J.; Kim, S.; Ahn, J.M.; Han, J.H.; Lee, D. Estrogen-responsive cancer-associated fibroblasts promote invasive property of gastric cancer in a paracrine manner via CD147 production. FASEB J. 2022, 36, e22597. [Google Scholar] [CrossRef]
- Cirillo, F.; Pellegrino, M.; Talia, M.; Perrotta, I.D.; Rigiracciolo, D.C.; Spinelli, A.; Scordamaglia, D.; Muglia, L.; Guzzi, R.; Miglietta, A.M.; et al. Estrogen receptor variant ERα46 and insulin receptor drive in primary breast cancer cells growth effects and interleukin 11 induction prompting the motility of cancer-associated fibroblasts. Clin. Transl. Med. 2021, 11, e516. [Google Scholar] [CrossRef]
- Nagel, A.; Popeda, M.; Muchlinska, A.; Sadej, R.; Szade, J.; Zielinski, J.; Skokowski, J.; Niemira, M.; Kretowski, A.; Markiewicz, A.; et al. ERα36-High Cancer-Associated Fibroblasts as an Unfavorable Factor in Triple-Negative Breast Cancer. Cancers 2022, 14, 2005. [Google Scholar] [CrossRef]
- He, C.; Peng, M.; Zeng, X.; Dong, H.; Sun, Z.; Xu, J.; Liu, M.; Liu, L.; Huang, Y.; Peng, Z.; et al. Microenvironmental G protein-coupled estrogen receptor-mediated glutamine metabolic coupling between cancer-associated fibroblasts and triple-negative breast cancer cells governs tumour progression. Clin. Transl. Med. 2024, 14, e70131. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Wang, Y.; Ma, D.; Cheng, W.; Liu, J.; Yong, T.; Chen, H.; Wang, C. Immunotherapy: Reshape the Tumor Immune Microenvironment. Front. Immunol. 2022, 13, 844142. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liang, Y.; Wang, L. Shaping Polarization of Tumor-Associated Macrophages in Cancer Immunotherapy. Front. Immunol. 2022, 13, 888713. [Google Scholar] [CrossRef] [PubMed]
- Castellaro, A.M.; Rodriguez-Baili, M.C.; Di Tada, C.E.; Gil, G.A. Tumor-Associated Macrophages Induce Endocrine Therapy Resistance in ER+ Breast Cancer Cells. Cancers 2019, 11, 189. [Google Scholar] [CrossRef]
- Jayshree, R.S. The Immune Microenvironment in Human Papilloma Virus-Induced Cervical Lesions-Evidence for Estrogen as an Immunomodulator. Front. Cell. Infect. Microbiol. 2021, 11, 649815. [Google Scholar]
- Sharpe, M.A.; Baskin, D.S.; Jenson, A.V.; Baskin, A.M. Hijacking Sexual Immuno-Privilege in GBM-An Immuno-Evasion Strategy. Int. J. Mol. Sci. 2021, 22, 10983. [Google Scholar] [CrossRef]
- O’Leary, K.A.; Bates, A.M.; Jin, W.J.; Burkel, B.M.; Sriramaneni, R.N.; Emma, S.E.; Nystuen, E.J.; Sumiec, E.G.; Ponik, S.M.; Morris, Z.S.; et al. Estrogen receptor blockade and radiation therapy cooperate to enhance the response of immunologically cold ER+ breast cancer to immunotherapy. Breast Cancer Res. 2023, 25, 68. [Google Scholar] [CrossRef]
- Lasser, S.A.; Ozbay Kurt, F.G.; Arkhypov, I.; Utikal, J.; Umansky, V. Myeloid-derived suppressor cells in cancer and cancer therapy. Nat. Rev. Clin. Oncol. 2024, 21, 147–164. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhen, Y.; Hu, C.; Yi, H. Myeloid-Derived Suppressor Cell-Derived Arginase-1 Oppositely Modulates IL-17A and IL-17F Through the ESR/STAT3 Pathway During Colitis in Mice. Front. Immunol. 2020, 11, 687. [Google Scholar] [CrossRef] [PubMed]
- Kozasa, K.; Mabuchi, S.; Matsumoto, Y.; Kuroda, H.; Yokoi, E.; Komura, N.; Kawano, M.; Takahashi, R.; Sasano, T.; Shimura, K.; et al. Estrogen stimulates female cancer progression by inducing myeloid-derived suppressive cells: Investigations on pregnant and non-pregnant experimental models. Oncotarget 2019, 10, 1887–1902. [Google Scholar] [CrossRef]
- Mangani, S.; Piperigkou, Z.; Koletsis, N.E.; Ioannou, P.; Karamanos, N.K. Estrogen receptors and extracellular matrix: The critical interplay in cancer development and progression. FEBS J. 2025, 292, 1558–1572. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Li, J.P.; Guo, W.; Wang, D.Q.; Yao, A.; Zhang, H.M.; Huang, F.; Li, H.H.; Dai, Z.T.; Zhang, Z.; et al. Novel interactions between ERα-36 and STAT3 mediate breast cancer cell migration. Cell Commun. Signal. 2019, 17, 93. [Google Scholar] [CrossRef]
- Piperigkou, Z.; Karamanos, N.K. Estrogen receptor-mediated targeting of the extracellular matrix network in cancer. Semin. Cancer Biol. 2020, 62, 116–124. [Google Scholar] [CrossRef]
- Barcus, C.E.; O’Leary, K.A.; Brockman, J.L.; Rugowski, D.E.; Liu, Y.; Garcia, N.; Yu, M.; Keely, P.J.; Eliceiri, K.W.; Schuler, L.A. Elevated collagen-I augments tumor progressive signals, intravasation and metastasis of prolactin-induced estrogen receptor alpha positive mammary tumor cells. Breast Cancer Res. 2017, 19, 9. [Google Scholar] [CrossRef]
- Filho, A.M.; Laversanne, M.; Ferlay, J.; Colombet, M.; Piñeros, M.; Znaor, A.; Parkin, D.M.; Soerjomataram, I.; Bray, F. The GLOBOCAN 2022 cancer estimates: Data sources, methods, and a snapshot of the cancer burden worldwide. Int. J. Cancer 2025, 156, 1336–1346. [Google Scholar] [CrossRef]
- Trabert, B.; Coburn, S.B.; Falk, R.T.; Manson, J.E.; Brinton, L.A.; Gass, M.L.; Kuller, L.H.; Rohan, T.E.; Pfeiffer, R.M.; Qi, L.; et al. Circulating estrogens and postmenopausal ovarian and endometrial cancer risk among current hormone users in the Women’s Health Initiative Observational Study. Cancer Causes Control 2019, 30, 1201–1211. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Wang, Y.; Zhao, X.; Qi, X. Hormone therapy for ovarian cancer: Emphasis on mechanisms and applications (Review). Oncol. Rep. 2021, 46, 223. [Google Scholar] [CrossRef]
- Smolarz, B.; Biernacka, K.; Łukasiewicz, H.; Samulak, D.; Piekarska, E.; Romanowicz, H.; Makowska, M. Ovarian Cancer-Epidemiology, Classification, Pathogenesis, Treatment, and Estrogen Receptors’ Molecular Backgrounds. Int. J. Mol. Sci. 2025, 26, 4611. [Google Scholar] [CrossRef]
- Salvati, A.; Gigantino, V.; Nassa, G.; Giurato, G.; Alexandrova, E.; Rizzo, F.; Tarallo, R.; Weisz, A. The Histone Methyltransferase DOT1L Is a Functional Component of Estrogen Receptor Alpha Signaling in Ovarian Cancer Cells. Cancers 2019, 11, 1720. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, S.; Ohta, T.; Yamanouchi, K.; Liu, Z.; Sudo, T.; Kojimahara, T.; Seino, M.; Narumi, M.; Tsutsumi, S.; Takahashi, T.; et al. Activation of estrogen receptor α by estradiol and cisplatin induces platinum-resistance in ovarian cancer cells. Cancer Biol. Ther. 2017, 18, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Bogush, T.A.; Basharina, A.A.; Bogush, E.A.; Ryabinina, O.M.; Tjulandina, A.S.; Tjulandin, S.A. Estrogen Receptors alpha and beta in Ovarian Cancer: Expression Level and Prognosis. Dokl. Biochem. Biophys. 2018, 482, 249–251. [Google Scholar] [CrossRef]
- Chauvin, S.; Cohen-Tannoudji, J.; Guigon, C.J. Estradiol Signaling at the Heart of Folliculogenesis: Its Potential Deregulation in Human Ovarian Pathologies. Int. J. Mol. Sci. 2022, 23, 512. [Google Scholar] [CrossRef]
- Andreinie, R.; Nita, S.; Saleh, I. Relationship of estrogen beta (ERβ) receptor genes polymorphism with epithelial ovarian cancer. J. Phys. 2019, 1246, 012005. [Google Scholar]
- Sajeev, A.; BharathwajChetty, B.; Manickasamy, M.K.; Alqahtani, M.S.; Abbas, M.; Shakibaei, M.; Sethi, G.; Ma, Z.; Kunnumakkara, A.B. Nuclear receptors in ovarian cancer: Changing paradigms in cancer therapeutics. Front. Oncol. 2024, 14, 1383939. [Google Scholar] [CrossRef]
- Reichenbach, J.; Fraungruber, P.; Mayr, D.; Buschmann, C.; Kraus, F.B.T.; Topalov, N.E.; Chelariu-Raicu, A.; Kolben, T.; Burges, A.; Mahner, S.; et al. Nuclear receptor co-repressor NCOR2 and its relation to GPER with prognostic impact in ovarian cancer. J. Cancer Res. Clin. Oncol. 2023, 149, 8719–8728. [Google Scholar] [CrossRef] [PubMed]
- Jung, J. Role of G Protein-Coupled Estrogen Receptor in Cancer Progression. Toxicol. Res. 2019, 35, 209–214. [Google Scholar] [CrossRef]
- Schüler-Toprak, S.; Skrzypczak, M.; Ignatov, T.; Ignatov, A.; Ortmann, O.; Treeck, O. G protein-coupled estrogen receptor 1 (GPER-1) and agonist G-1 inhibit growth of ovarian cancer cells by activation of anti-tumoral transcriptome responses: Impact of GPER-1 mRNA on survival. J. Cancer Res. Clin. Oncol. 2020, 146, 3175–3188. [Google Scholar] [CrossRef]
- Han, N.; Heublein, S.; Jeschke, U.; Kuhn, C.; Hester, A.; Czogalla, B.; Mahner, S.; Rottmann, M.; Mayr, D.; Schmoeckel, E.; et al. The G-Protein-Coupled Estrogen Receptor (GPER) Regulates Trimethylation of Histone H3 at Lysine 4 and Represses Migration and Proliferation of Ovarian Cancer Cells In Vitro. Cells 2021, 10, 619. [Google Scholar] [CrossRef]
- Toàn, N.M. Novel Molecular Classification of Breast Cancer with PET Imaging. Medicina 2024, 60, 2099. [Google Scholar] [CrossRef]
- Zhang, X. Molecular Classification of Breast Cancer: Relevance and Challenges. Arch. Pathol. Lab. Med. 2023, 147, 46–51. [Google Scholar] [CrossRef]
- Vang, A.; Salem, K.; Fowler, A.M. Progesterone Receptor Gene Polymorphisms and Breast Cancer Risk. Endocrinology 2023, 164, bqad020. [Google Scholar] [CrossRef]
- Gosain, R.; Pollock, Y.; Jain, D. Age-related Disparity: Breast Cancer in the Elderly. Curr. Oncol. Rep. 2016, 18, 69. [Google Scholar] [CrossRef] [PubMed]
- Giordano, S.H. Breast Cancer in Men. N. Engl. J. Med. 2018, 378, 2311–2320. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.; Speirs, V.; Shaaban, A.M. Male breast cancer: An update. Virchows Arch. 2022, 480, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Heller, S.L. Breast Cancer Screening in Men. J. Breast Imaging 2023, 5, 104–111. [Google Scholar] [CrossRef]
- Loibl, S.; Poortmans, P.; Morrow, M.; Denkert, C.; Curigliano, G. Breast cancer. Lancet 2021, 397, 1750–1769. [Google Scholar] [CrossRef]
- Orrantia-Borunda, E.; Anchondo-Nuñez, P.; Acuña-Aguilar, L.E.; Gómez-Valles, F.O.; Ramírez-Valdespino, C.A. Subtypes of Breast Cancer. In Breast Cancer; Mayrovitz, H.N., Ed.; Exon Publications: Brisbane, Australia, 2022. [Google Scholar]
- Tecalco-Cruz, A.C.; Ramírez-Jarquín, J.O.; Cruz-Ramos, E. Estrogen Receptor Alpha and its Ubiquitination in Breast Cancer Cells. Curr. Drug Targets 2019, 20, 690–704. [Google Scholar] [CrossRef]
- Rugo, H.S.; Rumble, R.B.; Macrae, E.; Barton, D.L.; Connolly, H.K.; Dickler, M.N.; Fallowfield, L.; Fowble, B.; Ingle, J.N.; Jahanzeb, M.; et al. Endocrine Therapy for Hormone Receptor-Positive Metastatic Breast Cancer: American Society of Clinical Oncology Guideline. J. Clin. Oncol. 2016, 34, 3069–3103. [Google Scholar] [CrossRef] [PubMed]
- Porras, L.; Ismail, H.; Mader, S. Positive Regulation of Estrogen Receptor Alpha in Breast Tumorigenesis. Cells 2021, 10, 2966. [Google Scholar] [CrossRef]
- Clusan, L.; Ferrière, F.; Flouriot, G.; Pakdel, F. A Basic Review on Estrogen Receptor Signaling Pathways in Breast Cancer. Int. J. Mol. Sci. 2023, 24, 6834. [Google Scholar] [CrossRef]
- Pagano, M.T.; Ortona, E.; Dupuis, M.L. A Role for Estrogen Receptor alpha36 in Cancer Progression. Front. Endocrinol. 2020, 11, 506. [Google Scholar] [CrossRef]
- Maczis, M.A.; Maceyka, M.; Waters, M.R.; Newton, J.; Singh, M.; Rigsby, M.F.; Turner, T.H.; Alzubi, M.A.; Harrell, J.C.; Milstien, S.; et al. Sphingosine kinase 1 activation by estrogen receptor α36 contributes to tamoxifen resistance in breast cancer. J. Lipid Res. 2018, 59, 2297–2307. [Google Scholar] [CrossRef]
- Clusan, L.; Le Goff, P.; Flouriot, G.; Pakdel, F. A Closer Look at Estrogen Receptor Mutations in Breast Cancer and Their Implications for Estrogen and Antiestrogen Responses. Int. J. Mol. Sci. 2021, 22, 756. [Google Scholar] [CrossRef]
- Wu, W.; Warner, M.; Wang, L.; He, W.W.; Zhao, R.; Guan, X.; Botero, C.; Huang, B.; Ion, C.; Coombes, C.; et al. Drivers and suppressors of triple-negative breast cancer. Proc. Natl. Acad. Sci. USA 2021, 118, e2104162118. [Google Scholar] [CrossRef]
- Choi, Y. Estrogen Receptor β Expression and Its Clinical Implication in Breast Cancers: Favorable or Unfavorable? J. Breast Cancer 2022, 25, 75–93. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Ma, D.; Chen, S.; Tang, R.; Yang, J.; Meng, C.; Feng, Y.; Liu, L.; Wang, J.; Luo, H.; et al. High GPER expression in triple-negative breast cancer is linked to pro-metastatic pathways and predicts poor patient outcomes. npj Breast Cancer 2022, 8, 100. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, F.; Talia, M.; Santolla, M.F.; Pellegrino, M.; Scordamaglia, D.; Spinelli, A.; De Rosis, S.; Giordano, F.; Muglia, L.; Zicarelli, A.; et al. GPER deletion triggers inhibitory effects in triple negative breast cancer (TNBC) cells through the JNK/c-Jun/p53/Noxa transduction pathway. Cell Death Discov. 2023, 9, 353. [Google Scholar] [CrossRef]
- Chen, Z.J.; Wei, W.; Jiang, G.M.; Liu, H.; Wei, W.D.; Yang, X.; Wu, Y.M.; Liu, H.; Wong, C.K.; Du, J.; et al. Activation of GPER suppresses epithelial mesenchymal transition of triple negative breast cancer cells via NF-κB signals. Mol. Oncol. 2016, 10, 775–788. [Google Scholar] [CrossRef]
- Kampa, M.; Lappano, R.; Grande, F.; Rizzuti, B.; Maggiolini, M.; Castanas, E.; Jacquot, Y. Promising Perspectives of the Antiproliferative GPER Inverse Agonist ERα17p in Breast Cancer. Cells 2023, 12, 653. [Google Scholar] [CrossRef]
- Lappano, R.; Talia, M.; Cirillo, F.; Rigiracciolo, D.C.; Scordamaglia, D.; Guzzi, R.; Miglietta, A.M.; De Francesco, E.M.; Belfiore, A.; Sims, A.H.; et al. The IL1β-IL1R signaling is involved in the stimulatory effects triggered by hypoxia in breast cancer cells and cancer-associated fibroblasts (CAFs). J. Exp. Clin. Cancer Res. 2020, 39, 153. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-López, I.G.; Ramírez de Arellano, A.; Jave-Suárez, L.F.; Hernández-Silva, C.D.; García-Chagollan, M.; Hernández-Bello, J.; Lopez-Pulido, E.I.; Macias-Barragan, J.; Montoya-Buelna, M.; Muñoz-Valle, J.F.; et al. Interaction between 17β-estradiol, prolactin and human papillomavirus induce E6/E7 transcript and modulate the expression and localization of hormonal receptors. Cancer Cell Int. 2019, 19, 227. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.G.; Lebot, M.N.; Sukkarn, B.; Ball, G.; Green, A.R.; Rakha, E.A.; Ellis, I.O.; Storr, S.J. Low expression of G protein-coupled oestrogen receptor 1 (GPER) is associated with adverse survival of breast cancer patients. Oncotarget 2018, 9, 25946–25956. [Google Scholar] [CrossRef]
- Li, W.; Liang, H.; Wang, W.; Liu, J.; Liu, X.; Lao, S.; Liang, W.; He, J. Global cancer statistics for adolescents and young adults: Population based study. J. Hematol. Oncol. 2024, 17, 99. [Google Scholar] [CrossRef] [PubMed]
- Pizzato, M.; Li, M.; Vignat, J.; Laversanne, M.; Singh, D.; La Vecchia, C.; Vaccarella, S. The epidemiological landscape of thyroid cancer worldwide: GLOBOCAN estimates for incidence and mortality rates in 2020. Lancet Diabetes Endocrinol. 2022, 10, 264–272. [Google Scholar] [CrossRef]
- Liu, J.; Xu, T.; Ma, L.; Chang, W. Signal Pathway of Estrogen and Estrogen Receptor in the Development of Thyroid Cancer. Front. Oncol. 2021, 11, 593479. [Google Scholar] [CrossRef]
- Wang, H.; Li, S.; Shi, J.; Feng, C.; Wang, Y.; Zhang, F. Unbalanced bidirectional causal association between thyroid cancer and ER-positive breast cancer: Should we recommend screening for thyroid cancer in breast cancer patients? BMC Genom. 2023, 24, 762. [Google Scholar] [CrossRef]
- Qiu, Y.B.; Liao, L.Y.; Jiang, R.; Xu, M.; Xu, L.W.; Chen, G.G.; Liu, Z.M. PES1 promotes the occurrence and development of papillary thyroid cancer by upregulating the ERα/ERβ protein ratio. Sci. Rep. 2019, 9, 1032. [Google Scholar] [CrossRef]
- Xu, L.W.; Gou, X.; Yang, J.Y.; Jiang, R.; Jiang, X.; Chen, G.G.; Liu, Z.M. Methylation of ERβ 5′-untranslated region attenuates its inhibitory effect on ERα gene transcription and promotes the initiation and progression of papillary thyroid cancer. FASEB J. 2021, 35, e21516. [Google Scholar] [CrossRef] [PubMed]
- Rubio, G.A.; Catanuto, P.; Glassberg, M.K.; Lew, J.I.; Elliot, S.J. Estrogen receptor subtype expression and regulation is altered in papillary thyroid cancer after menopause. Surgery 2018, 163, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Zhang, H.; Li, J.; Guan, H.; He, L.; Wang, Z.; Shan, Z.; Teng, W. Estrogen Induces Metastatic Potential of Papillary Thyroid Cancer Cells through Estrogen Receptor α and β. Int. J. Endocrinol. 2013, 2013, 941568. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Li, Z.; Ma, X.; Wu, L.; Fu, L.; Qin, G. ETV5 overexpression contributes to tumor growth and progression of thyroid cancer through PIK3CA. Life Sci. 2020, 253, 117693. [Google Scholar] [CrossRef]
- Chou, C.K.; Chi, S.Y.; Hung, Y.Y.; Yang, Y.C.; Fu, H.C.; Wang, J.H.; Chen, C.C.; Kang, H.Y. Decreased Expression of Estrogen Receptors Is Associated with Tumorigenesis in Papillary Thyroid Carcinoma. Int. J. Mol. Sci. 2022, 23, 1015. [Google Scholar] [CrossRef]
- Ahn, H.Y.; Song, R.Y.; Ahn, H.S.; Kim, H.S. Expression of Estrogen and Progesterone Receptors in Papillary Thyroid Carcinoma in Korea. Cancer Res. Treat. 2021, 53, 1204–1212. [Google Scholar] [CrossRef]
- Manfroi, P.A.; Bertoni, A.P.S.; Furlanetto, T.W. GPER1 in the thyroid: A systematic review. Life Sci. 2020, 241, 117112. [Google Scholar] [CrossRef]
- Bertoni, A.P.S.; Manfroi, P.A.; Tomedi, J.; Assis-Brasil, B.M.; de Souza Meyer, E.L.; Furlanetto, T.W. The gene expression of GPER1 is low in fresh samples of papillary thyroid carcinoma (PTC), and in silico analysis. Mol. Cell. Endocrinol. 2021, 535, 111397. [Google Scholar] [CrossRef]
- Laha, D.; Nilubol, N.; Boufraqech, M. New Therapies for Advanced Thyroid Cancer. Front. Endocrinol. 2020, 11, 82. [Google Scholar] [CrossRef]
- Yuan, Y.; Sun, P.; Xiao, H.; Li, L.; Li, J.; Ai, X. Risk of second primary thyroid cancer in cancer survivors. Sci. Rep. 2024, 14, 12478. [Google Scholar] [CrossRef]
- Forma, A.; Kłodnicka, K.; Pająk, W.; Flieger, J.; Teresińska, B.; Januszewski, J.; Baj, J. Thyroid Cancer: Epidemiology, Classification, Risk Factors, Diagnostic and Prognostic Markers, and Current Treatment Strategies. Int. J. Mol. Sci. 2025, 26, 5173. [Google Scholar] [CrossRef]
- Wu, K.; Chen, Y.; Guo, R.; Zeng, Q.; Yu, Y. Leptin and insulin synergize with PIK3CA mutation to enhance PD-L1 mediated immunosuppression in thyroid cancer. Exp. Cell Res. 2024, 442, 114229. [Google Scholar] [CrossRef]
- Abancens, M.; Bustos, V.; Harvey, H.; McBryan, J.; Harvey, B.J. Sexual Dimorphism in Colon Cancer. Front. Oncol. 2020, 10, 607909. [Google Scholar] [CrossRef]
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Refaat, B.; Aslam, A.; Idris, S.; Almalki, A.H.; Alkhaldi, M.Y.; Asiri, H.A.; Almaimani, R.A.; Mujalli, A.; Minshawi, F.; Alamri, S.A.; et al. Profiling estrogen, progesterone, and androgen receptors in colorectal cancer in relation to gender, menopausal status, clinical stage, and tumour sidedness. Front. Endocrinol. 2023, 14, 1187259. [Google Scholar] [CrossRef] [PubMed]
- Maingi, J.W.; Tang, S.; Liu, S.; Ngenya, W.; Bao, E. Targeting estrogen receptors in colorectal cancer. Mol. Biol. Rep. 2020, 47, 4087–4091. [Google Scholar] [CrossRef]
- Ditonno, I.; Losurdo, G.; Rendina, M.; Pricci, M.; Girardi, B.; Ierardi, E.; Di Leo, A. Estrogen Receptors in Colorectal Cancer: Facts, Novelties and Perspectives. Curr. Oncol. 2021, 28, 4256–4263. [Google Scholar] [CrossRef]
- Topi, G.; Ghatak, S.; Satapathy, S.R.; Ehrnström, R.; Lydrup, M.L.; Sjölander, A. Combined Estrogen Alpha and Beta Receptor Expression Has a Prognostic Significance for Colorectal Cancer Patients. Front. Med. 2022, 9, 739620. [Google Scholar] [CrossRef] [PubMed]
- Topi, G.; Satapathy, S.R.; Ghatak, S.; Hellman, K.; Ek, F.; Olsson, R.; Ehrnström, R.; Lydrup, M.L.; Sjölander, A. High Oestrogen receptor alpha expression correlates with adverse prognosis and promotes metastasis in colorectal cancer. Cell Commun. Signal. 2024, 22, 198. [Google Scholar] [CrossRef]
- Hases, L.; Indukuri, R.; Birgersson, M.; Nguyen-Vu, T.; Lozano, R.; Saxena, A.; Hartman, J.; Frasor, J.; Gustafsson, J.Å.; Katajisto, P.; et al. Intestinal estrogen receptor beta suppresses colon inflammation and tumorigenesis in both sexes. Cancer Lett. 2020, 492, 54–62. [Google Scholar] [CrossRef]
- Indukuri, R.; Hases, L.; Archer, A.; Williams, C. Estrogen Receptor Beta Influences the Inflammatory p65 Cistrome in Colon Cancer Cells. Front. Endocrinol. 2021, 12, 650625. [Google Scholar] [CrossRef] [PubMed]
- Božović, A.; Mandušić, V.; Todorović, L.; Krajnović, M. Estrogen Receptor Beta: The Promising Biomarker and Potential Target in Metastases. Int. J. Mol. Sci. 2021, 22, 1656. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhang, Q.; Shen, X.; Zhen, P.; Marin, A.; Garcia-Milian, R.; Roper, J.; Khan, S.A.; Johnson, C.H. Asparagine synthetase and G-protein coupled estrogen receptor are critical responders to nutrient supply in KRAS mutant colorectal cancer. bioRxiv 2023. [Google Scholar] [CrossRef]
- Qiu, Y.A.; Xiong, J.; Yu, T. Role of G Protein-Coupled Estrogen Receptor in Digestive System Carcinomas: A Minireview. OncoTargets Ther. 2021, 14, 2611–2622. [Google Scholar] [CrossRef]
- Abancens, M.; Harvey, B.J.; McBryan, J. GPER Agonist G1 Prevents Wnt-Induced JUN Upregulation in HT29 Colorectal Cancer Cells. Int. J. Mol. Sci. 2022, 23, 12581. [Google Scholar] [CrossRef]
- Ionescu, V.A.; Gheorghe, G.; Bacalbasa, N.; Chiotoroiu, A.L.; Diaconu, C. Colorectal Cancer: From Risk Factors to Oncogenesis. Medicina 2023, 59, 1646. [Google Scholar] [CrossRef]
- Sninsky, J.A.; Shore, B.M.; Lupu, G.V.; Crockett, S.D. Risk Factors for Colorectal Polyps and Cancer. Gastrointest. Endosc. Clin. N. Am. 2022, 32, 195–213. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G.; van de Velde, C.J.; Watanabe, T. Colorectal cancer. Nat. Rev. Dis. Primers 2015, 1, 15065. [Google Scholar] [CrossRef]
- Hormone Therapy for Brest Cancer. Available online: https://www.cancer.gov/types/breast/breast-hormone-therapy-fact-sheet (accessed on 22 September 2025).
- Lobo-Martins, S.; Arecco, L.; Cabral, T.P.; Agostinetto, E.; Dauccia, C.; Franzoi, M.A.; Del Mastro, L.; Lambertini, M.; Piccart, M.; de Azambuja, E. Extended adjuvant endocrine therapy in early breast cancer: Finding the individual balance. ESMO Open 2025, 10, 105057. [Google Scholar] [CrossRef]
- Homewood, D.; Fu, M.H.; Sathianathen, N.; La Bianca, S.; Tran, B.; Corcoran, N.M. Evolution of hormonal therapy for prostate cancer. Aust. J. Gen. Pract. 2024, 53, 291–300. [Google Scholar] [CrossRef]
- Mitra, S.; Lami, M.S.; Ghosh, A.; Das, R.; Tallei, T.E.; Fatimawali Islam, F.; Dhama, K.; Begum, M.Y.; Aldahish, A.; Chidambaram, K.; et al. Hormonal Therapy for Gynecological Cancers: How Far Has Science Progressed toward Clinical Applications? Cancers 2022, 14, 759. [Google Scholar] [CrossRef] [PubMed]
- Biondi, B.; Filetti, S.; Schlumberger, M. Thyroid-hormone therapy and thyroid cancer: A reassessment. Nat. Clin. Pract. Endocrinol. Metab. 2005, 1, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Duan, X.; Huang, Z.; Dong, Y.; Zhu, J.; Guo, H.; Tian, H.; Zou, C.G.; Xie, K. Nuclear receptors in health and disease: Signaling pathways, biological functions and pharmaceutical interventions. Signal Transduct. Target. Ther. 2025, 10, 228. [Google Scholar] [CrossRef] [PubMed]
- Pickar, J.H.; MacNeil, T.; Ohleth, K. SERMs: Progress and future perspectives. Maturitas 2010, 67, 129–138. [Google Scholar] [CrossRef]
- Mirkin, S.; Pickar, J.H. Selective estrogen receptor modulators (SERMs): A review of clinical data. Maturitas 2015, 80, 52–57. [Google Scholar] [CrossRef]
- Pickar, J.H.; Boucher, M.; Morgenstern, D. Tissue selective estrogen complex (TSEC): A review. Menopause 2018, 25, 1033–1045. [Google Scholar] [CrossRef]
- Lumachi, F.; Santeufemia, D.A.; Basso, S.M. Current medical treatment of estrogen receptor-positive breast cancer. World J. Biol. Chem. 2015, 6, 231–239. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, L.; Liu, Y.; Zhang, J.; Zheng, L.; Zheng, M. Adverse Event Profiles of the Third-Generation Aromatase Inhibitors: Analysis of Spontaneous Reports Submitted to FAERS. Biomedicines 2024, 12, 1708. [Google Scholar] [CrossRef]
- Davies, C.; Pan, H.; Godwin, J.; Gray, R.; Arriagada, R.; Raina, V.; Abraham, M.; Medeiros Alencar, V.H.; Badran, A.; Bonfill, X.; et al. Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) Collaborative Group. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 2013, 381, 805–816. [Google Scholar] [CrossRef]
- Available online: https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2817 (accessed on 22 September 2025).
- Maximov, P.Y.; McDaniel, R.E.; Fernandes, D.J.; Korostyshevskiy, V.R.; Bhatta, P.; Mürdter, T.E.; Flockhart, D.A.; Jordan, V.C. Simulation with cells in vitro of tamoxifen treatment in premenopausal breast cancer patients with different CYP2D6 genotypes. Br. J. Pharmacol. 2014, 171, 5624–5635. [Google Scholar] [CrossRef]
- Rahmawati, D.R.; Nurrochmad, A.; Meiyanto, E.; Jenie, R.I. Persistent synergistic antiproliferative and apoptosis induction effects of PGV-1 and tamoxifen or 4-hydroxytamoxifen on ER-positive breast cancer cells. J. Appl. Pharm. Sci. 2025, 15, 205–215. [Google Scholar] [CrossRef]
- Martinkovich, S.; Shah, D.; Planey, S.L.; Arnott, J.A. Selective estrogen receptor modulators: Tissue specificity and clinical utility. Clin. Interv. Aging 2014, 9, 1437–1452. [Google Scholar] [CrossRef] [PubMed]
- Brufsky, A.M. Understanding the estrogen receptor signaling pathway: Focus on current endocrine agents for breast cancer in postmenopausal women. Community Oncol. 2011, 8, 343–352. [Google Scholar] [CrossRef]
- Wong, M.M.; Guo, C.; Zhang, J. Nuclear receptor corepressor complexes in cancer: Mechanism, function and regulation. Am. J. Clin. Exp. Urol. 2014, 2, 169–187. [Google Scholar]
- Shang, Y.; Brown, M. Molecular determinants for the tissue specificity of SERMs. Science 2002, 295, 2465–2468. [Google Scholar] [CrossRef]
- Ali, S.; Rasool, M.; Chaoudhry, H.; NPushparaj, P.; Jha, P.; Hafiz, A.; Mahfooz, M.; Abdus Sami, G.; Azhar Kamal, M.; Bashir, S.; et al. Molecular mechanisms and mode of tamoxifen resistance in breast cancer. Bioinformation 2016, 12, 135–139. [Google Scholar] [CrossRef]
- Légaré, S.; Basik, M. Minireview: The Link Between ERα Corepressors and Histone Deacetylases in Tamoxifen Resistance in Breast Cancer. Mol. Endocrinol. 2016, 30, 965–976. [Google Scholar] [CrossRef]
- Ghanavati, M.; Khorshidi, Y.; Shadnoush, M.; Akbari, M.E.; Ardehali, S.H.; Chavarri-Guerra, Y.; Akbari, A.; Barragan-Carrillo, R.; Amin Amlashi, M.; Javid, Z.; et al. Tamoxifen use and risk of endometrial cancer in breast cancer patients: A systematic review and dose-response meta-analysis. Cancer Rep. 2023, 6, e1806. [Google Scholar] [CrossRef]
- Mustonen, M.V.; Pyrhönen, S.; Kellokumpu-Lehtinen, P.L. Toremifene in the treatment of breast cancer. World J. Clin. Oncol. 2014, 5, 393–405. [Google Scholar] [CrossRef]
- Berthou, F.; Dreano, Y.; Belloc, C.; Kangas, L.; Gautier, J.C.; Beaune, P. Involvement of cytochrome P450 3A enzyme family in the major metabolic pathways of toremifene in human liver microsomes. Biochem. Pharmacol. 1994, 47, 1883–1895. [Google Scholar] [CrossRef]
- Kim, J.; Coss, C.C.; Barrett, C.M.; Mohler, M.L.; Bohl, C.E.; Li, C.M.; He, Y.; Veverka, K.A.; Dalton, J.T. Role and pharmacologic significance of cytochrome P-450 2D6 in oxidative metabolism of toremifene and tamoxifen. Int. J. Cancer 2013, 132, 1475–1485. [Google Scholar] [CrossRef]
- Wiseman, L.R.; Goa, K. L Toremifene: A review of its pharmacological properties and clinical efficacy in the management of advanced breast cancer. Drugs 1997, 54, 141–160. [Google Scholar] [CrossRef]
- Kangas, L. Biochemical and pharmacological effects of toremifene metabolites. Cancer Chemother. Pharmacol. 1990, 27, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Provinciali, N.; Suen, C.; Dunn, B.K.; DeCensi, A. Raloxifene hydrochloride for breast cancer risk reduction in postmenopausal women. Expert. Rev. Clin. Pharmacol. 2016, 9, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Jones, N.R.; Manni, A.; Lazarus, P. Characterization of raloxifene glucuronidation: Potential role of UGT1A8 genotype on raloxifene metabolism in vivo. Cancer Prev. Res. 2013, 6, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Dodge, J.A.; Lugar, C.W.; Cho, S.; Short, L.L.; Sato, M.; Yang, N.N.; Spangle, L.A.; Martin, M.J.; Phillips, D.L.; Glasebrook, A.L.; et al. Evaluation of the major metabolites of raloxifene as modulators of tissue selectivity. J. Steroid Biochem. Mol. Biol. 1997, 61, 97–106. [Google Scholar] [CrossRef]
- Bryant, H.U.; Dere, W.H. Selective estrogen receptor modulators: An alternative to hormone replacement therapy. Proc. Soc. Exp. Biol. Med. 1998, 217, 45–52. [Google Scholar] [CrossRef]
- Snyder, K.R.; Sparano, N.; Malinowski, J.M. Raloxifene hydrochloride. Am. J. Health Syst. Pharm. 2000, 57, 1669–1678. [Google Scholar] [CrossRef]
- Rey, J.R.; Cervino, E.V.; Rentero, M.L.; Crespo, E.C.; Alvaro, A.O.; Casillas, M. Raloxifene: Mechanism of action, effects on bone tissue, and applicability in clinical traumatology practice. Open Orthop. J. 2009, 3, 14–21. [Google Scholar] [CrossRef]
- Yang, N.N.; Venugopalan, M.; Hardikar, S.; Glasebrook, A. Identification of an estrogen response element activated by metabolites of 17beta-estradiol and raloxifene. Science 1996, 273, 1222–1225. [Google Scholar] [CrossRef]
- Han, K.O.; Kang, Y.S.; Hwang, C.S.; Moon, I.G.; Yim, C.H.; Chung, H.Y.; Jang, H.C.; Yoon, H.K.; Han, I.K.; Choi, Y.K. Identification of a mutation in the human raloxifene response element of the transforming growth factor-beta 3 gene. J. Korean Med. Sci. 2001, 16, 549–552. [Google Scholar] [CrossRef]
- Curran, M.; Wiseman, L. Fulvestrant. Drugs 2001, 61, 807–814. [Google Scholar] [CrossRef]
- Kim, N.; Lukong, K.E. Treating ER-positive breast cancer: A review of the current FDA-approved SERMs and SERDs and their mechanisms of action. Oncol. Rev. 2025, 19, 1564642. [Google Scholar] [CrossRef]
- Robertson, J.F.; Harrison, M. Fulvestrant: Pharmacokinetics and pharmacology. Br. J. Cancer 2004, 90 (Suppl. S1), S7–S10. [Google Scholar] [CrossRef]
- Carlson, R.W. The history and mechanism of action of fulvestrant. Clin. Breast Cancer. 2005, 6 (Suppl. S1), S5–S8. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, R.I.; Gee, J.M.; Manning, D.L.; Wakeling, A.E.; Montano, M.M.; Katzenellenbogen, B.S. Responses to pure antiestrogens (ICI 164384, ICI 182780) in estrogen-sensitive and -resistant experimental and clinical breast cancer. Ann. N. Y. Acad. Sci. 1995, 761, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Nephew, K.P. Fulvestrant (ICI 182,780)-dependent interacting proteins mediate immobilization and degradation of estrogen receptor-alpha. J. Biol. Chem. 2006, 281, 9607–9615. [Google Scholar] [CrossRef]
- Chumsri, S.; Howes, T.; Bao, T.; Sabnis, G.; Brodie, A. Aromatase, aromatase inhibitors, and breast cancer. J. Steroid Biochem. Mol. Biol. 2011, 125, 13–22. [Google Scholar] [CrossRef]
- Hong, Y.; Chen, S. Aromatase inhibitors: Structural features and biochemical characterization. Ann. N. Y. Acad. Sci. 2006, 1089, 237–251. [Google Scholar] [CrossRef]
- Bhutani, K.; Vishwakarma, S.; Yadav, P.; Yadav, M.K. The current landscape of aromatase inhibitors for the treatment of estrogen receptor-positive breast carcinoma. J. Steroid Biochem. Mol. Biol. 2025, 250, 106729. [Google Scholar] [CrossRef] [PubMed]
- Moharrer Navaei, N.; Moharrer Navaei, N. Unlocking the Potential of Aromatase Inhibitors: Recent Advances in Drug Design, Synthesis, Docking Activity, and in Vitro Bioactivity Evaluations. Synth. Sinter. 2023, 3, 234–240. [Google Scholar] [CrossRef]
- Sinn, H.P.; Kreipe, H. A Brief Overview of the WHO Classification of Breast Tumors, 4th Edition, Focusing on Issues and Updates from the 3rd Edition. Breast Care 2013, 8, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Kong, D.; Liu, J.; Zhan, L.; Luo, L.; Zheng, W.; Zheng, Q.; Chen, C.; Sun, S. Breast cancer heterogeneity and its implication in personalized precision therapy. Exp. Hematol. Oncol. 2023, 12, 3. [Google Scholar] [CrossRef]
- Testa, U.; Castelli, G.; Pelosi, E. Breast Cancer: A Molecularly Heterogenous Disease Needing Subtype-Specific Treatments. Med. Sci. 2020, 8, 18. [Google Scholar] [CrossRef]
- Ferreira Almeida, C.; Correia-da-Silva, G.; Teixeira, N.; Amaral, C. Influence of tumor microenvironment on the different breast cancer subtypes and applied therapies. Biochem. Pharmacol. 2024, 223, 116178. [Google Scholar] [CrossRef]
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef]
- Akinpelu, A.; Akinsipe, T.; Avila, L.A.; Arnold, R.D.; Mistriotis, P. The impact of tumor microenvironment: Unraveling the role of physical cues in breast cancer progression. Cancer Metastasis Rev. 2024, 43, 823–844. [Google Scholar] [CrossRef]
- Deepak, K.G.K.; Vempati, R.; Nagaraju, G.P.; Dasari, V.R.; Nagini, S.; Rao, D.N.; Malla, R.R. Tumor microenvironment: Challenges and opportunities in targeting metastasis of triple negative breast cancer. Pharmacol. Res. 2020, 153, 104683. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Su, Y.; Zhong, S.; Cong, L.; Liu, B.; Yang, J.; Tao, Y.; He, Z.; Chen, C.; Jiang, Y. Exosomes: Key players in cancer and potential therapeutic strategy. Signal Transduct. Target. Ther. 2020, 5, 145. [Google Scholar] [CrossRef]
- Xie, F.; Zhou, X.; Fang, M.; Li, H.; Su, P.; Tu, Y.; Zhang, L.; Zhou, F. Extracellular Vesicles in Cancer Immune Microenvironment and Cancer Immunotherapy. Adv. Sci. 2019, 6, 1901779. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, S.; Tang, W.; Huang, Q.; Mei, Y.; Yang, H. Exosomes from tamoxifen-resistant breast cancer cells transmit drug resistance partly by delivering miR-9-5p. Cancer Cell Int. 2021, 21, 55. [Google Scholar] [CrossRef]
- La Camera, G.; Gelsomino, L.; Caruso, A.; Panza, S.; Barone, I.; Bonofiglio, D.; Andò, S.; Giordano, C.; Catalano, S. The Emerging Role of Extracellular Vesicles in Endocrine Resistant Breast Cancer. Cancers 2021, 13, 1160. [Google Scholar] [CrossRef]
- Patel, R.; Klein, P.; Tiersten, A.; Sparano, J.A. An emerging generation of endocrine therapies in breast cancer: A clinical perspective. npj Breast Cancer 2023, 9, 20. [Google Scholar] [CrossRef]
- Hanker, A.B.; Sudhan, D.R.; Arteaga, C.L. Overcoming Endocrine Resistance in Breast Cancer. Cancer Cell 2020, 37, 496–513. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Paluch-Shimon, S.; Senkus, E.; Curigliano, G.; Aapro, M.S.; André, F.; Barrios, C.H.; Bergh, J.; Bhattacharyya, G.S.; Biganzoli, L.; et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann. Oncol. 2020, 31, 1623–1649. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Extending the duration of endocrine treatment for early breast cancer: Patient-level meta-analysis of 12 randomised trials of aromatase inhibitors in 22,031 postmenopausal women already treated with at least 5 years of endocrine therapy. Lancet 2025, 406, 603–614. [Google Scholar] [CrossRef]
- Eapen, J.V.; George, P.; Thomas, S.; Antony, J. Integrative Strategies in Breast Cancer Therapy: Conventional Approaches, Emerging Advances, and Future Challenges. ASPET Discov. 2025, 1, 100012. [Google Scholar] [CrossRef]
- Berry, D.A.; Muss, H.B.; Thor, A.D.; Dressler, L.; Liu, E.T.; Broadwater, G.; Budman, D.R.; Henderson, I.C.; Barcos, M.; Hayes, D.; et al. HER-2/neu and p53 expression versus tamoxifen resistance in estrogen receptor-positive, node-positive breast cancer. J. Clin. Oncol. 2000, 18, 3471–3479. [Google Scholar] [CrossRef] [PubMed]
- Parisot, J.P.; Hu, X.F.; DeLuise, M.; Zalcberg, J.R. Altered expression of the IGF-1 receptor in a tamoxifen-resistant human breast cancer cell line. Br. J. Cancer 1999, 79, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Ojo, D.; Wei, F.; Liu, Y.; Wang, E.; Zhang, H.; Lin, X.; Wong, N.; Bane, A.; Tang, D. Factors Promoting Tamoxifen Resistance in Breast Cancer via Stimulating Breast Cancer Stem Cell Expansion. Curr. Med. Chem. 2015, 22, 2360–2374. [Google Scholar] [CrossRef] [PubMed]
- Pietras, R.J.; Arboleda, J.; Reese, D.M.; Wongvipat, N.; Pegram, M.D.; Ramos, L.; Gorman, C.M.; Parker, M.G.; Sliwkowski, M.X.; Slamon, D.J. HER-2 tyrosine kinase pathway targets estrogen receptor and promotes hormone-independent growth in human breast cancer cells. Oncogene 1995, 10, 2435–2446. [Google Scholar] [PubMed]
- Girault, I.; Bièche, I.; Lidereau, R. Role of estrogen receptor alpha transcriptional coregulators in tamoxifen resistance in breast cancer. Maturitas 2006, 54, 342–351. [Google Scholar] [CrossRef]
- Nass, N.; Kalinski, T. Tamoxifen resistance: From cell culture experiments towards novel biomarkers. Pathol. Res. Pract. 2015, 211, 189–197. [Google Scholar] [CrossRef]
- Miller, D.L.; Kern, F.G. Growth factor signal transduction and hormone independence in breast cancer. Adv. Oncobiol. 1999, 2, 11–80. [Google Scholar]
- Gago, F.E.; Fanelli, M.A.; Ciocca, D.R. Co-expression of steroid hormone receptors (estrogen receptor alpha and/or progesterone receptors) and Her2/neu (c-erbB-2) in breast cancer: Clinical outcome following tamoxifen-based adjuvant therapy. J. Steroid Biochem. Mol. Biol. 2006, 98, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Arpino, G.; Wiechmann, L.; Osborne, C.K.; Schiff, R. Crosstalk between the estrogen receptor and the HER tyrosine kinase receptor family: Molecular mechanism and clinical implications for endocrine therapy resistance. Endocr. Rev. 2008, 29, 217–233. [Google Scholar] [CrossRef]
- Pietras, R.J.; Márquez-Garbán, D.C. Membrane-associated estrogen receptor signaling pathways in human cancers. Clin. Cancer Res. 2007, 13, 4672–4676. [Google Scholar] [CrossRef] [PubMed]
- Fuqua, S.A.; Wiltschke, C.; Zhang, Q.X.; Borg, A.; Castles, C.G.; Friedrichs, W.E.; Hopp, T.; Hilsenbeck, S.; Mohsin, S.; O’Connell, P.; et al. A hypersensitive estrogen receptor-alpha mutation in premalignant breast lesions. Cancer Res. 2000, 60, 4026–4029. [Google Scholar]
- Yang, X.; Phillips, D.L.; Ferguson, A.T.; Nelson, W.G.; Herman, J.G.; Davidson, N.E. Synergistic activation of functional estrogen receptor (ER)-alpha by DNA methyltransferase and histone deacetylase inhibition in human ER-alpha-negative breast cancer cells. Cancer Res. 2001, 61, 7025–7029. [Google Scholar]
- Parl, F.F. Multiple mechanisms of estrogen receptor gene repression contribute to ER-negative breast cancer. Pharmacogenom. J. 2003, 3, 251–253. [Google Scholar] [CrossRef]
- Prossnitz, E.R.; Barton, M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat. Rev. Endocrinol. 2011, 7, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Filardo, E.J.; Quinn, J.A.; Bland, K.I.; Frackelton, A.R., Jr. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol. Endocrinol. 2000, 14, 1649–1660. [Google Scholar] [CrossRef]
- Revankar, C.M.; Cimino, D.F.; Sklar, L.A.; Arterburn, J.B.; Prossnitz, E.R. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 2005, 307, 1625–1630. [Google Scholar] [CrossRef]
- Bryś, M. Androgens and androgen receptor: Do they play a role in breast cancer? Med. Sci. Monit. 2000, 6, 433–438. [Google Scholar]
- De Amicis, F.; Thirugnansampanthan, J.; Cui, Y.; Selever, J.; Beyer, A.; Parra, I.; Weigel, N.L.; Herynk, M.H.; Tsimelzon, A.; Lewis, M.T.; et al. Androgen receptor overexpression induces tamoxifen resistance in human breast cancer cells. Breast Cancer Res. Treat. 2010, 121, 1–11. [Google Scholar] [CrossRef]
- Ciupek, A.; Rechoum, Y.; Gu, G.; Gelsomino, L.; Beyer, A.R.; Brusco, L.; Covington, K.R.; Tsimelzon, A.; Fuqua, S.A. Androgen receptor promotes tamoxifen agonist activity by activation of EGFR in ERα-positive breast cancer. Breast Cancer Res. Treat. 2015, 154, 225–237. [Google Scholar] [CrossRef]
- Chang, C.; Saltzman, A.; Yeh, S.; Young, W.; Keller, E.; Lee, H.J.; Wang, C.; Mizokami, A. Androgen receptor: An overview. Crit. Rev. Eukaryot. Gene Expr. 1995, 5, 97–125. [Google Scholar] [CrossRef]
- Higa, G.M.; Fell, R.G. Sex hormone receptor repertoire in breast cancer. Int. J. Breast Cancer 2013, 2013, 284036. [Google Scholar] [CrossRef] [PubMed]
- Tarulli, G.A.; Butler, L.M.; Tilley, W.D.; Hickey, T.E. Bringing androgens up a NOTCH in breast cancer. Endocr. Relat. Cancer 2014, 21, T183–T202. [Google Scholar] [CrossRef]
- Cuenca-López, M.D.; Montero, J.C.; Morales, J.C.; Prat, A.; Pandiella, A.; Ocana, A. Phospho-kinase profile of triple negative breast cancer and androgen receptor signaling. BMC Cancer. 2014, 14, 302. [Google Scholar] [CrossRef] [PubMed]
- Heinlein, C.A.; Chang, C. The roles of androgen receptors and androgen-binding proteins in nongenomic androgen actions. Mol. Endocrinol. 2002, 16, 2181–2187. [Google Scholar] [CrossRef] [PubMed]
- Stecca, B.; Ruiz i Altaba, A. Context-dependent regulation of the GLI code in cancer by HEDGEHOG and non-HEDGEHOG signals. J. Mol. Cell Biol. 2010, 2, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.W.; Nguyen, M.P.; Padalecki, S.S.; Grubbs, B.G.; Merkel, A.R.; Oyajobi, B.O.; Matrisian, L.M.; Mundy, G.R.; Sterling, J.A. TGF-beta promotion of Gli2-induced expression of parathyroid hormone-related protein, an important osteolytic factor in bone metastasis, is independent of canonical Hedgehog signaling. Cancer Res. 2011, 71, 822–831. [Google Scholar] [CrossRef] [PubMed]
- Javelaud, D.; Alexaki, V.I.; Dennler, S.; Mohammad, K.S.; Guise, T.A.; Mauviel, A. TGF-β/SMAD/GLI2 signaling axis in cancer progression and metastasis. Cancer Res. 2011, 71, 5606–5610. [Google Scholar] [CrossRef]
- Ramaswamy, B.; Lu, Y.; Teng, K.Y.; Nuovo, G.; Li, X.; Shapiro, C.L.; Majumder, S. Hedgehog signaling is a novel therapeutic target in tamoxifen-resistant breast cancer aberrantly activated by PI3K/AKT pathway. Cancer Res. 2012, 72, 5048–5059. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, S.; Xin, Q.; Zhang, Y.; Wang, K.; Li, M. Recent progress of CDK4/6 inhibitors′ current practice in breast cancer. Cancer Gene Ther. 2024, 31, 1283–1291. [Google Scholar] [CrossRef]
- Gallanis, G.T.; Sharif, G.M.; Schmidt, M.O.; Friedland, B.N.; Battina, R.; Rahhal, R.; Davis, J.E.; Khan, I.S., Jr.; Wellstein, A.; Riegel, A.T. Stromal Senescence following Treatment with the CDK4/6 Inhibitor Palbociclib Alters the Lung Metastatic Niche and Increases Metastasis of Drug-Resistant Mammary Cancer Cells. Cancers 2023, 15, 1908. [Google Scholar] [CrossRef]
- Roberto, M.; Astone, A.; Botticelli, A.; Carbognin, L.; Cassano, A.; D’Auria, G.; Fabbri, A.; Fabi, A.; Gamucci, T.; Krasniqi, E.; et al. CDK4/6 Inhibitor Treatments in Patients with Hormone Receptor Positive, Her2 Negative Advanced Breast Cancer: Potential Molecular Mechanisms, Clinical Implications and Future Perspectives. Cancers 2021, 13, 332. [Google Scholar] [CrossRef]
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-mutated, hormone receptor–positive advanced breast cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef]
- Schwartzberg, L.S.; Yardley, D.A.; Elias, A.D.; Patel, M.; LoRusso, P.; Burris, H.A.; Gucalp, A.; Peterson, A.C.; Blaney, M.E.; Steinberg, J.L.; et al. A Phase I/Ib Study of Enzalutamide Alone and in Combination with Endocrine Therapies in Women with Advanced Breast Cancer. Clin. Cancer Res. 2017, 23, 4046–4054. [Google Scholar] [CrossRef]
- Lu, Q.; Xia, W.; Lee, K.; Zhang, J.; Yuan, H.; Yuan, Z.; Shi, Y.; Wang, S.; Xu, F. Bicalutamide plus Aromatase Inhibitor in Patients with Estrogen Receptor-Positive/Androgen Receptor-Positive Advanced Breast Cancer. Oncologist 2020, 25, 21–e15. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lin, Y.; Zhu, H.; Zhou, Y.; Mao, F.; Huang, X.; Sun, Q.; Li, C. Efficacy and Safety Profile of Histone Deacetylase Inhibitors for Metastatic Breast Cancer: A Meta-Analysis. Front. Oncol. 2022, 12, 901152. [Google Scholar] [CrossRef] [PubMed]
- Wawruszak, A.; Borkiewicz, L.; Okon, E.; Kukula-Koch, W.; Afshan, S.; Halasa, M. Vorinostat (SAHA) and Breast Cancer: An Overview. Cancers 2021, 13, 4700. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, A.; Haluska, P.; Tolcher, A.W.; Erlichman, C.; Papadopoulos, K.P.; Lensing, J.L.; Beeram, M.; Molina, J.R.; Rasco, D.W.; Arcos, R.R.; et al. A First-in-Human Phase I Study of the Oral p38 MAPK Inhibitor, Ralimetinib (LY2228820 Dimesylate), in Patients with Advanced Cancer. Clin. Cancer Res. 2016, 22, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Zaman, K.; Winterhalder, R.; Mamot, C.; Hasler-Strub, U.; Rochlitz, C.; Mueller, A.; Berset, C.; Wiliders, H.; Perey, L.; Rudolf, C.B.; et al. Fulvestrant with or without selumetinib, a MEK 1/2 inhibitor, in breast cancer progressing after aromatase inhibitor therapy: A multicentre randomised placebo-controlled double-blind phase II trial, SAKK 21/08. Eur. J. Cancer 2015, 51, 1212–1220. [Google Scholar] [CrossRef]
- Kaboli, P.J.; Imani, S.; Jomhori, M.; Ling, K.H. Chemoresistance in breast cancer: PI3K/Akt pathway inhibitors vs the current chemotherapy. Am. J. Cancer Res. 2021, 11, 5155–5183. [Google Scholar]
- McDonnell, D.P.; Wardell, S.E.; Chang, C.Y.; Norris, J.D. Next-Generation Endocrine Therapies for Breast Cancer. J. Clin. Oncol. 2021, 39, 1383–1388. [Google Scholar] [CrossRef]
- Razavi, P.; Chang, M.T.; Xu, G.; Bandlamudi, C.; Ross, D.S.; Vasan, N.; Cai, Y.; Bielski, C.M.; Donoghue, M.T.A.; Jonsson, P.; et al. The Genomic Landscape of Endocrine-Resistant Advanced Breast Cancers. Cancer Cell 2018, 34, 427–438.e6. [Google Scholar] [CrossRef]
- Goetz, M.P.; Bagegni, N.A.; Batist, G.; Brufsky, A.; Cristofanilli, M.A.; Damodaran, S.; Daniel, B.R.; Fleming, G.F.; Gradishar, W.J.; Graff, S.L.; et al. Lasofoxifene versus fulvestrant for ER+/HER2- metastatic breast cancer with an ESR1 mutation: Results from the randomized, phase II ELAINE 1 trial. Ann. Oncol. 2023, 34, 1141–1151. [Google Scholar] [CrossRef]
- Goetz, M.P.; Wander, S.A.; Bachelot, T.; de Nonneville, A.; Gal-Yam, E.N.; Sammons, S.L.; Shen, S.; Twelves, C.; Boruta, G.; Portman, D.J.; et al. ELAINE 3: Phase 3 study of lasofoxifene plus abemaciclib to treat ER+/HER2-, ESR1-mutated, metastatic breast cancer. Future Oncol. 2025, 21, 1317–1324. [Google Scholar] [CrossRef]
- McDonnell, D.P.; Wardell, S.E. The molecular mechanisms underlying the pharmacological actions of ER modulators: Implications for new drug discovery in breast cancer. Curr. Opin. Pharmacol. 2010, 10, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Wardell, S.E.; Nelson, E.R.; Chao, C.A.; Alley, H.M.; McDonnell, D.P. Evaluation of the pharmacological activities of RAD1901, a selective estrogen receptor degrader. Endocr. Relat. Cancer. 2015, 22, 713–724. [Google Scholar] [CrossRef]
- Beumer, J.H.; Foldi, J. Pharmacology and pharmacokinetics of elacestrant. Cancer Chemother. Pharmacol. 2023, 92, 157–163. [Google Scholar] [CrossRef]
- Sarfraz, A.; Sarfraz, M.; Javad, F.; Khalid, M.; Shah, B.; Gul, A.; Ganiyani, M.A.; Ismail, A.; Cheema, K. Elacestrant in hormone receptor-positive metastatic breast cancer: A post-hoc analysis. Explor. Target. Antitumor Ther. 2025, 6, 1002293. [Google Scholar] [CrossRef]
- Ciruelos, E.M.; Hamilton, E.P.; Kim, S.-B.; Im, Y.-H.; Segui Solis, E.; García Saenz., J.Á.; Di Sanzo, A.; Domínguez Lizarbe, M.; Wasserman, T.; Theall, K.P.; et al. 364P Elacestrant in combination with abemaciclib in patients (pts) with brain metastasis (mets) from estrogen receptor-positive (ER+), HER2-negative (HER2-) breast cancer: Preliminary data from ELECTRA, an open-label, multicenter, phase Ib/II study. Ann. Oncol. 2024, 35, S370. [Google Scholar] [CrossRef]
- Oliveira, M.; Hamilton, E.P.; Incorvati, J.; de la Heras, B.B.; Calvo, E.; Garcia-Corbacho, J.; Ruiz-Borrego, M.; Vaklavas, C.; Turner, N.C.; Ciruelos, E.M.; et al. Serena-1: Updated analyses from a phase 1 study (parts C/D) of the next-generation oral SERD camizestrant (AZD9833) in combination with palbociclib, in women with ER-positive, HER2-negative advanced breast cancer. J. Clin. Oncol. 2022, 40, 1032. [Google Scholar] [CrossRef]
- Bhagwat, S.V.; Zhao, B.; Shen, W.; Mur, C.; Barr, R.; Kindler, L.J.; Rubio, A.; Bastian, J.A.; Cohen, J.D.; Mattioni, B.E.; et al. Preclinical characterization of LY3484356, a novel, potent and orally bioavailable selective estrogen receptor degrader (SERD). Cancer Res. 2021, 81, 1236. [Google Scholar] [CrossRef]
- Bondeson, D.P.; Mares, A.; Smith, I.E.; Ko, E.; Campos, S.; Miah, A.H.; Mulholland, K.E.; Routly, N.; Buckley, D.L.; Gustafson, J.L.; et al. Catalytic in vivo protein knockdown by small-molecule PROTACs. Nat. Chem. Biol. 2015, 11, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Xiang, H.; Luo, G. Targeting estrogen receptor α for degradation with PROTACs: A promising approach to overcome endocrine resistance. Eur. J. Med. Chem. 2020, 206, 112689. [Google Scholar] [CrossRef]
- Flanagan, J.J.; Qian, Y.; Gough, S.M.; Andreoli, M.; Bookbinder, M.; Cadelina, G.; Bradley, J.; Rousseau, E.; Willard, R.; Pizzano, J.; et al. Abstract P5-04-18: ARV-471, an oral estrogen receptor PROTAC degrader for breast cancer. Cancer Res. 2019, 79 (Suppl. S4), P5-04-18. [Google Scholar] [CrossRef]
- Hodges-Gallaghe, L.; Sun, R.; Myles, D.C.; Harmon, C.L.; Kushner, P.J. Abstract P5-05-02: Preclinical development of OP-1250, an oral complete estrogen receptor antagonist (CERAN) that shrinks ER-positive breast tumors in xenograft models. Cancer Res. 2020, 80 (Suppl. S4), P5-05-02. [Google Scholar] [CrossRef]
- Parisian, A.D.; Barratt, S.A.; Hodges-Gallagher, L.; Ortega, F.E.; Peña, G.; Sapugay, J.; Robello, B.; Sun, R.; Kulp, D.; Palanisamy, G.S.; et al. Palazestrant (OP-1250), A Complete Estrogen Receptor Antagonist, Inhibits Wild-type and Mutant ER-positive Breast Cancer Models as Monotherapy and in Combination. Mol. Cancer Ther. 2024, 23, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Puyang, X.; Furman, C.; Zheng, G.Z.; Wu, Z.J.; Banka, D.; Aithal, K.; Agoulnik, S.; Bolduc, D.M.; Buonamici, S.; Caleb, B.; et al. Discovery of Selective Estrogen Receptor Covalent Antagonists for the Treatment of ERαWT and ERαMUT Breast Cancer. Cancer Discov. 2018, 8, 1176–1193. [Google Scholar] [CrossRef] [PubMed]
- Al Hashami, Z.S.; van der Vegt, B.; Mourits, M.J.E.; Kluiver, J.; van den Berg, A. miRNA-dependent resistance mechanisms to anti-hormonal therapies in estrogen receptor-positive breast cancer patients. Mol. Ther. Oncol. 2025, 33, 200941. [Google Scholar] [CrossRef]
- Huang, L.; Liang, G.; Zhang, Q.; Zhao, W. The Role of Long Noncoding RNAs in Antiestrogen Resistance in Breast Cancer: An Overview and Update. J. Breast Cancer 2020, 23, 129–140. [Google Scholar] [CrossRef]
- Leppert, W.; Strąg-Lemanowicz, A. Rola leczenia hormonalnego u pacjentów z zaawansowaną chorobą nowotworową. Med. Paliatywna Prakt. 2015, 9, 30–38. [Google Scholar]
- Nourmoussavi, M.; Pansegrau, G.; Popesku, J.; Hammond, G.L.; Kwon, J.S.; Carey, M.S. Ovarian ablation for premenopausal breast cancer: A review of treatment considerations and the impact of premature menopause. Cancer Treat. Rev. 2017, 55, 26–35. [Google Scholar] [CrossRef]
- Castellón, E.A.; Indo, S.; Contreras, H.R. Cancer Stemness/Epithelial-Mesenchymal Transition Axis Influences Metastasis and Castration Resistance in Prostate Cancer: Potential Therapeutic Target. Int. J. Mol. Sci. 2022, 23, 14917. [Google Scholar] [CrossRef]
- Deli, T.; Orosz, M.; Jakab, A. Hormone Replacement Therapy in Cancer Survivors-Review of the Literature. Pathol. Oncol. Res. 2020, 26, 63–78. [Google Scholar] [CrossRef]
- Rozenberg, S.; Di Pietrantonio, V.; Vandromme, J.; Gilles, C. Menopausal hormone therapy and breast cancer risk. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101577. [Google Scholar] [CrossRef]
- van Bommel, M.H.D.; IntHout, J.; Veldmate, G.; Kets, C.M.; de Hullu, J.A.; van Altena, A.M.; Harmsen, M.G. Contraceptives and cancer risks in BRCA1/2 pathogenic variant carriers: A systematic review and meta-analysis. Hum. Reprod. Update 2023, 29, 197–217. [Google Scholar] [CrossRef]
- Loizzi, V.; Cerbone, M.; Arezzo, F.; Silvestris, E.; Damiani, G.R.; Cazzato, G.; Cicinelli, E.; Cormio, G. Contraception as chemoprevention of ovarian cancer in BRCA1 and BRCA2 women. Hormones 2024, 23, 277–286. [Google Scholar] [CrossRef]
- Wang, X.; Ha, D.; Yoshitake, R.; Chan, Y.S.; Sadava, D.; Chen, S. Exploring the Biological Activity and Mechanism of Xenoestrogens and Phytoestrogens in Cancers: Emerging Methods and Concepts. Int. J. Mol. Sci. 2021, 22, 8798. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, A.K.; Dhiman, N.; Kumar, A.; Jaitak, V. Engagement of phytoestrogens in breast cancer suppression: Structural classification and mechanistic approach. Eur. J. Med. Chem. 2021, 213, 113037. [Google Scholar] [CrossRef] [PubMed]
- Adeel, M.; Zain, M.; Shakoor, N.; Azeem, I.; Hussain, M.; Ahmad, M.A.; Chaudhary, S.; Zaheer, U.; Aziz, M.A.; Ahmar, S.; et al. Estrogens in plants and emerging risks to human health. Environ. Int. 2023, 178, 107985. [Google Scholar] [CrossRef] [PubMed]
| Estrogen Receptor Expression | Estrogen Receptor Level Compared to Normal Tissue | Poor Prognosis Connection | References | |
|---|---|---|---|---|
| Ovarian cancer | ERα+ ERβ+ GPER+ | ERα ˜ Erβ↓ GPER ˜ | Erβ↓ ERβ ND GPER↑/↓ | [85,86,87,89,90,91] |
| Breast cancer | ERα+/- ERβ+/- GPER+ | ERα↑ ERβ ND GPER ND | Erα↓ ERβ ND GPER↑/↓ | [104,105,106,107,109,111,112,113] |
| Thyroid cancer | ERα+ ERβ+ GPER+ | Erα↑ Erβ↓ GPER ND | Erα↑ Erβ↓ GPER↑/↓ | [120,124,129,130] |
| Colorectal cancer | ERα+ ERβ+ GPER+ | Erα↑ Erβ↓ GPER ND | Erα↑ Erβ↓ GPER↑/↓ | [138,139,140,145,146,147] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gruszka, O.; Jurzak, M.; Bednarek, I.A. Estrogen Receptors as Key Factors in Carcinogenesis. Biomedicines 2025, 13, 2620. https://doi.org/10.3390/biomedicines13112620
Gruszka O, Jurzak M, Bednarek IA. Estrogen Receptors as Key Factors in Carcinogenesis. Biomedicines. 2025; 13(11):2620. https://doi.org/10.3390/biomedicines13112620
Chicago/Turabian StyleGruszka, Oliwia, Magdalena Jurzak, and Ilona Anna Bednarek. 2025. "Estrogen Receptors as Key Factors in Carcinogenesis" Biomedicines 13, no. 11: 2620. https://doi.org/10.3390/biomedicines13112620
APA StyleGruszka, O., Jurzak, M., & Bednarek, I. A. (2025). Estrogen Receptors as Key Factors in Carcinogenesis. Biomedicines, 13(11), 2620. https://doi.org/10.3390/biomedicines13112620





