Context-Dependent Roles of Siglec-F+ Neutrophils
Abstract
1. Introduction
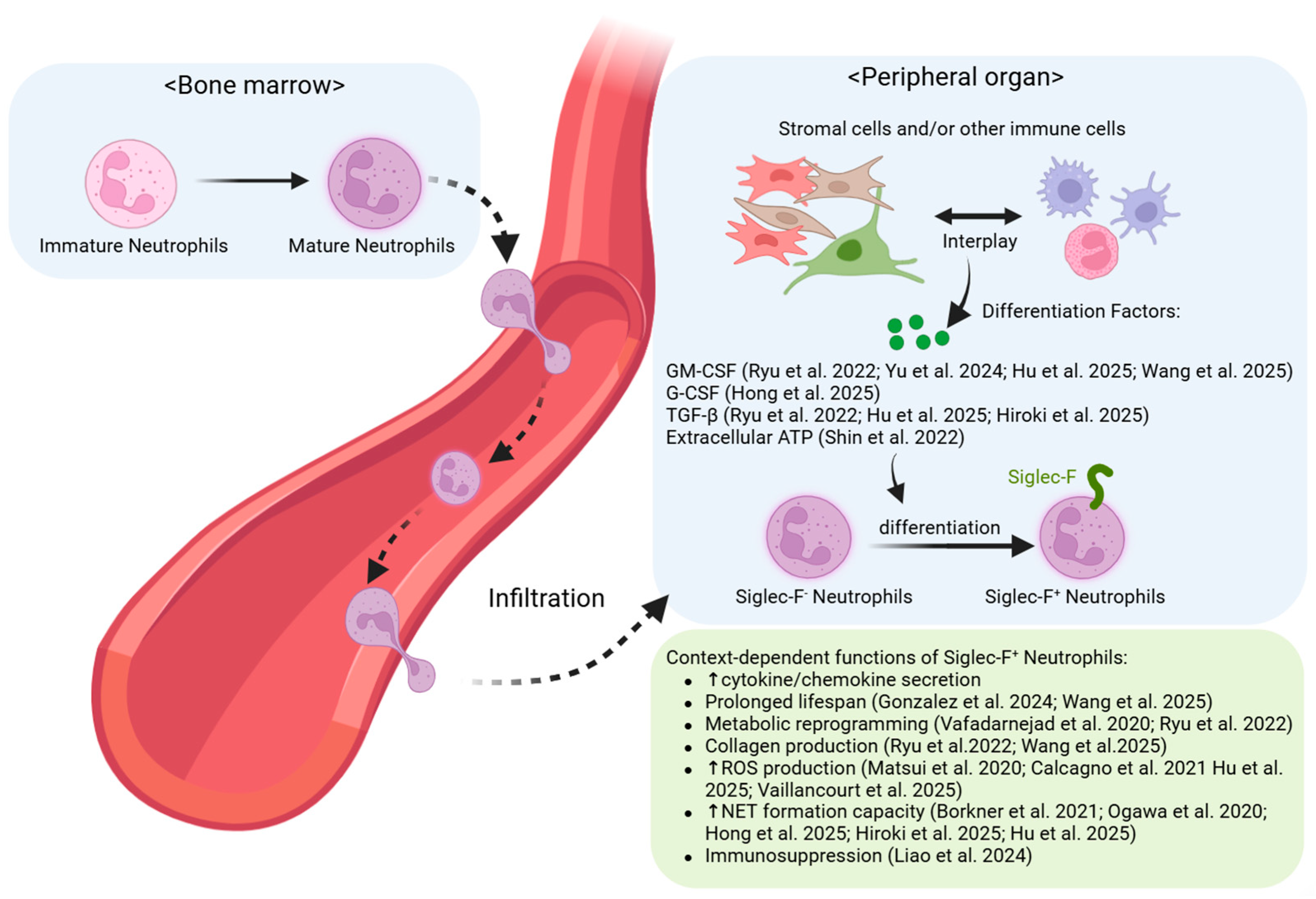
2. Mechanisms of Siglec-F+ Neutrophil Generation
2.1. Normal Neutrophil Development and Tissue-Specific Conversion
2.2. Existing Neutrophil Classification Systems and the Position of Siglec-F+ Subsets
2.3. Molecular Mechanisms of Siglec-F Signaling
3. Siglec-F+ Neutrophils in Noncancerous Diseases: Organ-Specific Manifestations
3.1. Nasal Cavity: Protective Surveillance and Homeostasis
3.2. Lung: Pathogenic Inflammation and Tissue Destruction
3.3. Cardiovascular System: Consistent Late-Phase Accumulation and Fibrosis
3.4. Kidney: Profibrotic Conversion and Collagen-Secretion
3.5. Central Nervous System: Th17-Driven Autoimmune
3.6. Spleen: Immunosuppressive Accumulation and Systemic Tolerance in Sepsis
3.7. Common Mechanistic Themes and Tissue-Specific Variations
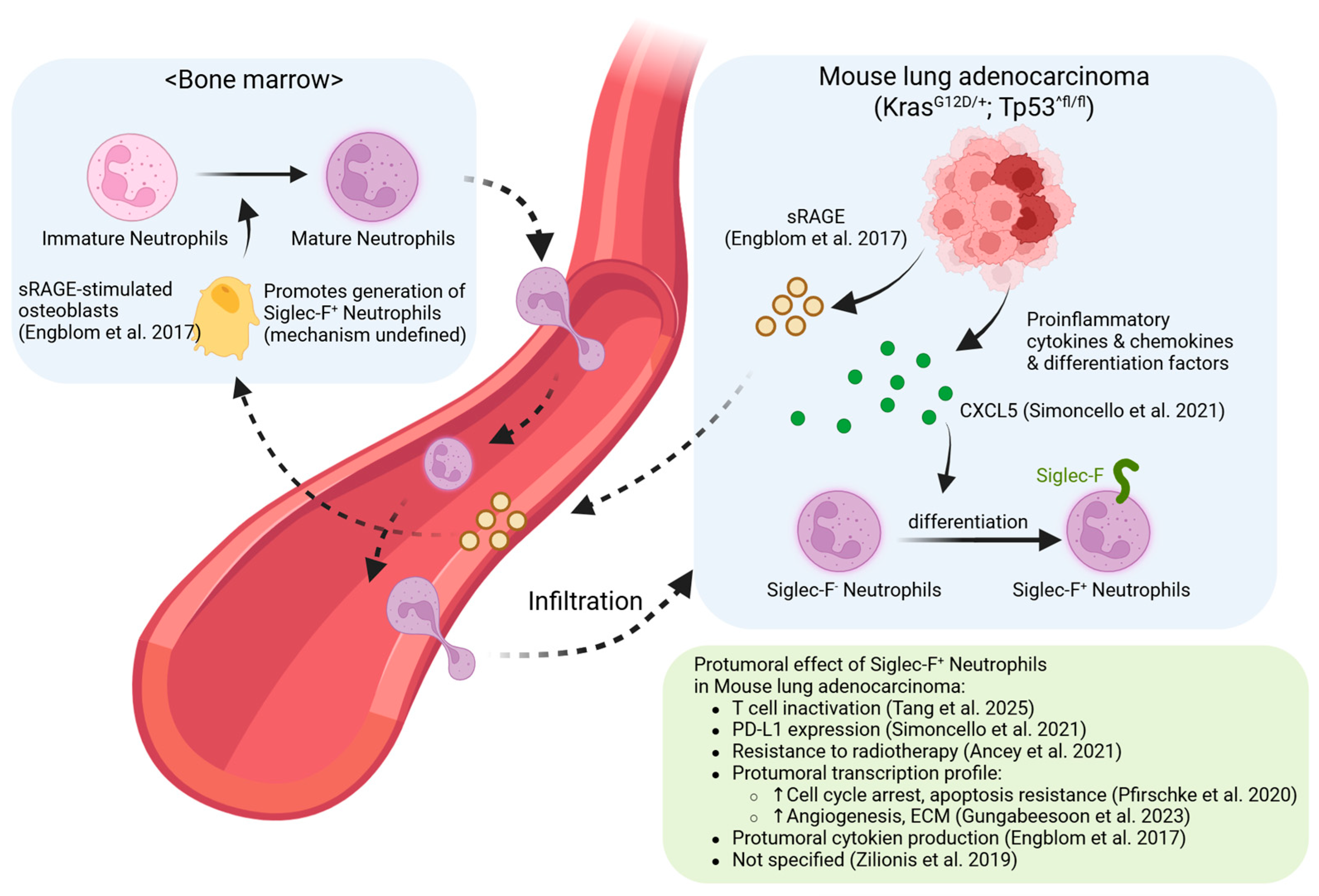
4. Siglec-F+ Neutrophils in Cancer: The Paradox of Pro-Tumor Default and Immunotherapy-Induced Reprogramming
4.1. The Pro-Tumor Default: A Stable and Dominant Paradigm
4.2. Modulation During Therapy: From Cytokine Paradoxes to Immunotherapy-Induced Reprogramming
5. Therapeutic Implications of Siglec-F+ Neutrophil Biology
5.1. Therapeutic Strategies in Non-Cancer Contexts
5.2. Therapeutic Strategies in Cancer
5.2.1. The Choice Between Depletion and Reprogramming
5.2.2. Targeting Metabolic and Functional Vulnerabilities
5.2.3. Overcoming Immunotherapy Resistance
6. Future Directions: Bridging the Translational Gap
6.1. Fundamental Questions in Siglec-F+ Neutrophil Biology
6.2. The Critical Translational Gap: Human Siglec-8+ Neutrophils
6.3. Roadmap for Clinical Translation
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PMN | Polymorphonuclear neutrophil |
| Siglec-F | Sialic acid-binding immunoglobulin-like lectin F |
| TAN | Tumor-associated neutrophil |
| HCC | Hepatocellular carcinoma |
| G-CSF | Granulocyte colony-stimulating factor |
| NET | Neutrophil extracellular trap |
| PMN-MDSC | Polymorphonuclear myeloid-derived suppressor cell |
| BALF | Bronchoalveolar lavage fluid |
| LDN | Low-density neutrophil |
| HDN | High-density neutrophil |
| ITIM | Immunoreceptor tyrosine-based inhibitory motif |
| ROS | Reactive oxygen species |
| DEP | Diesel exhaust particle |
| UUO | Unilateral ureteral obstruction |
| EAE | Experimental autoimmune encephalomyelitis |
| CLP | Cecal ligation and puncture |
| PICS | Persistent inflammation, immunosuppression, and catabolism syndrome |
| sRAGE | Soluble receptor for advanced glycation end-products |
| MASH | Metabolic dysfunction-associated steatohepatitis |
References
- Borregaard, N. Neutrophils, from Marrow to Microbes. Immunity 2010, 33, 657–670. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Silvestre-Roig, C.; Hidalgo, A.; Soehnlein, O. Neutrophil heterogeneity: Implications for homeostasis and pathogenesis. Blood 2016, 127, 2173–2181. [Google Scholar] [CrossRef]
- Liu, S.; Wu, W.; Du, Y.; Yin, H.; Chen, Q.; Yu, W.; Wang, W.; Yu, J.; Liu, L.; Lou, W.; et al. The evolution and heterogeneity of neutrophils in cancers: Origins, subsets, functions, orchestrations and clinical applications. Mol. Cancer 2023, 22, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Yang, T.; Chen, D.; Zhang, P.; Luo, J.; Chen, S.; Gu, S.; Shen, Y.; Tang, T.; Chang, T.; et al. Integrating Machine Learning and Multi-Omics to Explore Neutrophil Heterogeneity. Biomedicines 2025, 13, 2171. [Google Scholar] [CrossRef] [PubMed]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009, 16, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Antuamwine, B.B.; Bosnjakovic, R.; Hofmann-Vega, F.; Wang, X.; Theodosiou, T.; Iliopoulos, I.; Brandau, S. N1 versus N2 and PMN-MDSC: A critical appraisal of current concepts on tumor-associated neutrophils and new directions for human oncology. Immunol. Rev. 2022, 314, 250–279. [Google Scholar] [CrossRef]
- Bronte, V.; Brandau, S.; Chen, S.-H.; Colombo, M.P.; Frey, A.B.; Greten, T.F.; Mandruzzato, S.; Murray, P.J.; Ochoa, A.; Ostrand-Rosenberg, S.; et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016, 7, 12150. [Google Scholar] [CrossRef]
- Zhou, Y.; Shen, G.; Zhou, X.; Li, J. Therapeutic potential of tumor-associated neutrophils: Dual role and phenotypic plasticity. Signal Transduct. Target. Ther. 2025, 10, 1–21. [Google Scholar] [CrossRef]
- Fu, Y.; Wen, Z.; Fan, J. Interaction of low-density neutrophils with other immune cells in the mechanism of inflammation. Mol. Med. 2025, 31, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Engblom, C.; Pfirschke, C.; Zilionis, R.; Da Silva Martins, J.; Bos, S.A.; Courties, G.; Rickelt, S.; Severe, N.; Baryawno, N.; Faget, J.; et al. Osteoblasts remotely supply lung tumors with cancer-promoting SiglecFhigh neutrophils. Science 2017, 358, eaal5081. [Google Scholar] [CrossRef] [PubMed]
- Zilionis, R.; Engblom, C.; Pfirschke, C.; Savova, V.; Zemmour, D.; Saatcioglu, H.D.; Krishnan, I.; Maroni, G.; Meyerovitz, C.V.; Kerwin, C.M.; et al. Single-Cell Transcriptomics of Human and Mouse Lung Cancers Reveals Conserved Myeloid Populations across Individuals and Species. Immunity 2019, 50, 1317–1334.e10. [Google Scholar] [CrossRef] [PubMed]
- Pfirschke, C.; Engblom, C.; Gungabeesoon, J.; Lin, Y.; Rickelt, S.; Zilionis, R.; Messemaker, M.; Siwicki, M.; Gerhard, G.M.; Kohl, A.; et al. Tumor-Promoting Ly-6G+ SiglecFhigh Cells Are Mature and Long-Lived Neutrophils. Cell Rep. 2020, 32, 108164. [Google Scholar] [CrossRef]
- Ancey, P.-B.; Contat, C.; Boivin, G.; Sabatino, S.; Pascual, J.; Zangger, N.; Perentes, J.Y.; Peters, S.; Abel, E.D.; Kirsch, D.G.; et al. GLUT1 Expression in Tumor-Associated Neutrophils Promotes Lung Cancer Growth and Resistance to Radiotherapy. Cancer Res. 2021, 81, 2345–2357. [Google Scholar] [CrossRef]
- Simoncello, F.; Piperno, G.M.; Caronni, N.; Battini, T.; Cappelletto, A.; Bicciato, S.; Montaldo, E.; Artuso, A.; Jemos, C.; Benvenuti, F.; et al. SiglecFhigh neutrophils in lung tumor tissues suppress local CD8 T cell responses and limit the efficacy of anti PD-L1 antibodies. bioRxiv 2021. [Google Scholar] [CrossRef]
- Gungabeesoon, J.; Gort-Freitas, N.A.; Kiss, M.; Bolli, E.; Messemaker, M.; Siwicki, M.; Hicham, M.; Bill, R.; Koch, P.; Cianciaruso, C.; et al. A neutrophil response linked to tumor control in immunotherapy. Cell 2023, 186, 1448–1464.e20. [Google Scholar] [CrossRef]
- Hirschhorn, D.; Budhu, S.; Kraehenbuehl, L.; Gigoux, M.; Schröder, D.; Chow, A.; Ricca, J.M.; Gasmi, B.; De Henau, O.; Mangarin, L.M.B.; et al. T cell immunotherapies engage neutrophils to eliminate tumor antigen escape variants. Cell 2023, 186, 1432–1447.e17. [Google Scholar] [CrossRef]
- Veglia, F.; Hashimoto, A.; Dweep, H.; Sanseviero, E.; De Leo, A.; Tcyganov, E.; Kossenkov, A.; Mulligan, C.; Nam, B.; Masters, G.; et al. Analysis of classical neutrophils and polymorphonuclear myeloid-derived suppressor cells in cancer patients and tumor-bearing mice. J. Exp. Med. 2021, 218, e20201803. [Google Scholar] [CrossRef]
- Teo, J.M.N.; Chen, Z.; Chen, W.; Tan, R.J.Y.; Cao, Q.; Chu, Y.; Ma, D.; Chen, L.; Yu, H.; Lam, K.-H.; et al. Tumor-associated neutrophils attenuate the immunosensitivity of hepatocellular carcinoma. J. Exp. Med. 2024, 222, e20241442. [Google Scholar] [CrossRef]
- Tang, Z.; Hu, J.; Li, X.-C.; Wang, W.; Zhang, H.-Y.; Guo, Y.-Y.; Shuai, X.; Chu, Q.; Xie, C.; Lin, D.; et al. A subset of neutrophils activates anti-tumor immunity and inhibits non-small-cell lung cancer progression. Dev. Cell 2024, 60, 379–395.e8. [Google Scholar] [CrossRef]
- Zhu, X.; Heng, Y.; Ma, J.; Zhang, D.; Tang, D.; Ji, Y.; He, C.; Lin, H.; Ding, X.; Zhou, J.; et al. Prolonged Survival of Neutrophils Induced by Tumor-Derived G-CSF/GM-CSF Promotes Immunosuppression and Progression in Laryngeal Squamous Cell Carcinoma. Adv. Sci. 2024, 11, e2400836. [Google Scholar] [CrossRef]
- Ugolini, A.; De Leo, A.; Yu, X.; Scirocchi, F.; Liu, X.; Peixoto, B.; Scocozza, D.; Pace, A.; Perego, M.; Gardini, A.; et al. Functional Reprogramming of Neutrophils within the Brain Tumor Microenvironment by Hypoxia-Driven Histone Lactylation. Cancer Discov. 2025, 15, 1270–1296. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Asano, K.; Yotsumoto, S.; Yamane, T.; Arita, M.; Hayashi, Y.; Harada, H.; Makino-Okamura, C.; Fukuyama, H.; Kondo, K.; et al. Frontline Science: Conversion of neutrophils into atypical Ly6G+SiglecF+ immune cells with neurosupportive potential in olfactory neuroepithelium. J. Leukoc. Biol. 2020, 109, 481–496. [Google Scholar] [CrossRef]
- Matsui, M.; Nagakubo, D.; Satooka, H.; Hirata, T. A novel Siglec-F+ neutrophil subset in the mouse nasal mucosa exhibits an activated phenotype and is increased in an allergic rhinitis model. Biochem. Biophys. Res. Commun. 2020, 526, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Kim, J.; Ham, S.; Choi, S.M.; Lee, C.-H.; Lee, J.C.; Kim, J.H.; Cho, S.-H.; Kang, H.R.; Kim, Y.-M.; et al. A unique population of neutrophils generated by air pollutant–induced lung damage exacerbates airway inflammation. J. Allergy Clin. Immunol. 2022, 149, 1253–1269.e8. [Google Scholar] [CrossRef] [PubMed]
- Mizugaki, A.; Wada, T.; Tsuchida, T.; Oda, Y.; Kayano, K.; Yamakawa, K.; Tanaka, S. Neutrophil phenotypes implicated in the pathophysiology of post-traumatic sepsis. Front. Med. 2022, 9, 982399. [Google Scholar] [CrossRef]
- Yu, J.; Kim, S.; Song, H.S.; Kim, H.Y.; Kim, Y.M.; Lee, H.; Yang, S.; Kim, H.W.; Mun, S.J.; Jung, B.; et al. GPR43 in eosinophils prevents the emergence of pathogenic Siglec-Fhi neutrophils in allergic airway inflammation. bioRxiv 2024. [Google Scholar] [CrossRef]
- Hu, W.; Kwon, L.; Jeong, Y.S.; Bae, G.H.; Kim, Y.S.; Zabel, B.A.; Bae, Y.-S. SiglecF Expressing Neutrophils Exacerbate Th17-Mediated Autoimmune Neuroinflammation. Immune Netw. 2025, 25, e19. [Google Scholar] [CrossRef]
- Hong, J.; Kang, M.-H.; Lee, J.; Cha, M.-S.; Bae, Y.-S.; Kim, H.Y.; Lim, Y.T.; Bae, Y.-S. γδ+ T-cell-derived IL-17A stimulates airway epithelial/stromal cells to secrete G-CSF, promoting lung-specific pathogenic Siglec-F+ neutrophil development in PPE-induced emphysema. Cell. Mol. Immunol. 2025, 22, 791–805. [Google Scholar] [CrossRef]
- Hiroki, C.H.; Hassanabad, M.F.; Defaye, M.; Sarden, N.; Bartlett, A.; Farias, R.; Nguyen, A.P.; Guerrero-Fonseca, I.M.; Yoon, G.; Brown, L.; et al. Nociceptor neurons suppress alveolar macrophage-induced Siglec-F+ neutrophil-mediated inflammation to protect against pulmonary fibrosis. Immunity 2025, 58, 2054–2068.e6. [Google Scholar] [CrossRef]
- Borkner, L.; Curham, L.M.; Wilk, M.M.; Moran, B.; Mills, K.H. IL-17 mediates protective immunity against nasal infection with Bordetella pertussis by mobilizing neutrophils, especially Siglec-F+ neutrophils. Mucosal Immunol. 2021, 14, 1218–1219. [Google Scholar] [CrossRef]
- Liao, C.; Luo, S.; Liu, X.; Zhang, L.; Xie, P.; Zhou, W.; Lu, Y.; Zhong, H.; Zhang, X.; Xiong, Z.; et al. Siglec-F+ neutrophils in the spleen induce immunosuppression following acute infection. Theranostics 2024, 14, 2589–2604. [Google Scholar] [CrossRef]
- Vaillancourt, M.; Fernandes, S.E.; Aguilar, D.; Galdino, A.C.M.; Jorth, P. A chronic Pseudomonas aeruginosa mouse lung infection modeling the mucus obstruction, lung function, and inflammation of human cystic fibrosis. Infect. Immun. 2025, 93, e0023025. [Google Scholar] [CrossRef] [PubMed]
- Vafadarnejad, E.; Rizzo, G.; Krampert, L.; Arampatzi, P.; Arias-Loza, A.-P.; Nazzal, Y.; Rizakou, A.; Knochenhauer, T.; Bandi, S.R.; Nugroho, V.A.; et al. Dynamics of Cardiac Neutrophil Diversity in Murine Myocardial Infarction. Circ. Res. 2020, 127, E232–E249. [Google Scholar] [CrossRef]
- Calcagno, D.M.; Zhang, C.; Toomu, A.; Huang, K.; Ninh, V.K.; Miyamoto, S.; Aguirre, A.D.; Fu, Z.; Brown, J.H.; King, K.R. SiglecF(HI) Marks Late-Stage Neutrophils of the Infarcted Heart: A Single-Cell Transcriptomic Analysis of Neutrophil Diversification. J. Am. Hear. Assoc. 2021, 10, e019019. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, Y.; Yu, C.; Deng, R.; Chen, Y.; Jiang, K.; Liang, J.; Hu, C.; Yang, X.; Zhang, B.; et al. Periodontitis-related myocardial fibrosis by expansion of collagen-producing SiglecF+ neutrophils. Eur. Hear. J. 2025, 46, 2223–2238. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Shin, J.W.; Kwon, S.; Lee, J.; Kim, Y.C.; Bae, Y.-S.; Bae, Y.-S.; Kim, D.K.; Kim, Y.S.; Yang, S.H.; et al. Siglec-F–expressing neutrophils are essential for creating a profibrotic microenvironment in renal fibrosis. J. Clin. Investig. 2022, 132. [Google Scholar] [CrossRef]
- Gonzalez, R.J.; Hanč, P.; Alvarez, D.; Kazer, S.W.; Messou, M.A.; Mazo, I.B.; Levan, S.R.; Muenker, C.A.; Reeves, R.K.; von Andrian, U.H.; et al. Constitutive immune surveillance of nasal mucosa by three neutrophil subsets with distinct origin, phenotype, and function. bioRxiv 2024. [Google Scholar] [CrossRef]
- Kim, H.K.; Sierra, M.D.L.L.; Williams, C.K.; Gulino, A.V.; Tosato, G. G-CSF down-regulation of CXCR4 expression identified as a mechanism for mobilization of myeloid cells. Blood 2006, 108, 812–820. [Google Scholar] [CrossRef]
- Tak, T.; Tesselaar, K.; Pillay, J.; Borghans, J.A.M.; Koenderman, L. What’s your age again? Determination of human neutrophil half-lives revisited. J. Leukoc. Biol. 2013, 94, 595–601. [Google Scholar] [CrossRef]
- Eash, K.J.; Greenbaum, A.M.; Gopalan, P.K.; Link, D.C. CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J. Clin. Investig. 2010, 120, 2423–2431. [Google Scholar] [CrossRef]
- Evrard, M.; Kwok, I.W.H.; Chong, S.Z.; Teng, K.W.W.; Becht, E.; Chen, J.; Sieow, J.L.; Penny, H.L.; Ching, G.C.; Devi, S.; et al. Developmental Analysis of Bone Marrow Neutrophils Reveals Populations Specialized in Expansion, Trafficking, and Effector Functions. Immunity 2018, 48, 364–379.e8. [Google Scholar] [CrossRef]
- Manz, M.G.; Boettcher, S. Emergency granulopoiesis. Nat. Rev. Immunol. 2014, 14, 302–314. [Google Scholar] [CrossRef]
- Wang, T.-T.; Zhao, Y.-L.; Peng, L.-S.; Chen, N.; Chen, W.; Lv, Y.-P.; Mao, F.-Y.; Zhang, J.-Y.; Cheng, P.; Teng, Y.-S.; et al. Tumour-activated neutrophils in gastric cancer foster immune suppression and disease progression through GM-CSF-PD-L1 pathway. Gut 2017, 66, 1900–1911. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, H.; Deng, Y.; Tai, Y.; Zeng, K.; Zhang, Y.; Liu, W.; Zhang, Q.; Yang, Y. Cancer-associated fibroblasts induce PDL1+ neutrophils through the IL6-STAT3 pathway that foster immune suppression in hepatocellular carcinoma. Cell Death Dis. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Mao, H.; Kano, G.; A Hudson, S.; Brummet, M.; Zimmermann, N.; Zhu, Z.; Bochner, B.S. Mechanisms of Siglec-F-Induced Eosinophil Apoptosis: A Role for Caspases but Not for SHP-1, Src Kinases, NADPH Oxidase or Reactive Oxygen. PLOS ONE 2013, 8, e68143. [Google Scholar] [CrossRef] [PubMed]
- Wahnou, H.; El Kebbaj, R.; Hba, S.; Ouadghiri, Z.; El Faqer, O.; Pinon, A.; Liagre, B.; Limami, Y.; Duval, R.E. Neutrophils and Neutrophil-Based Drug Delivery Systems in Anti-Cancer Therapy. Cancers 2025, 17, 1232. [Google Scholar] [CrossRef]
- Liu, Y.; He, W.; Li, X.; Lu, X.; Wu, C.; Gao, Y.; Fan, D.; Dong, C.; Zhao, H. Neutrophil membrane-coated circular RNA nanoparticles for targeted immunotherapy in HER2-positive breast cancer brain metastasis. Cell Commun. Signal. 2025, 23, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Tateno, H.; Crocker, P.R.; Paulson, J.C. Mouse Siglec-F and human Siglec-8 are functionally convergent paralogs that are selectively expressed on eosinophils and recognize 6′-sulfo-sialyl Lewis X as a preferred glycan ligand. Glycobiology 2005, 15, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Gabrilovich, D.I. Hypoxia-inducible factors in regulation of immune responses in tumour microenvironment. Immunology 2014, 143, 512–519. [Google Scholar] [CrossRef]
- Brand, A.; Singer, K.; Koehl, G.E.; Kolitzus, M.; Schoenhammer, G.; Thiel, A.; Matos, C.; Bruss, C.; Klobuch, S.; Peter, K.; et al. LDHA-Associated Lactic Acid Production Blunts Tumor Immunosurveillance by T and NK Cells. Cell Metab. 2016, 24, 657–671. [Google Scholar] [CrossRef]
- Steggerda, S.M.; Bennett, M.K.; Chen, J.; Emberley, E.; Huang, T.; Janes, J.R.; Li, W.; MacKinnon, A.L.; Makkouk, A.; Marguier, G.; et al. Inhibition of arginase by CB-1158 blocks myeloid cell-mediated immune suppression in the tumor microenvironment. J. Immunother. Cancer 2017, 5, 101. [Google Scholar] [CrossRef] [PubMed]
- Steele, C.W.; Karim, S.A.; Leach, J.D.G.; Bailey, P.; Upstill-Goddard, R.; Rishi, L.; Foth, M.; Bryson, S.; McDaid, K.; Wilson, Z.; et al. CXCR2 Inhibition Profoundly Suppresses Metastases and Augments Immunotherapy in Pancreatic Ductal Adenocarcinoma. Cancer Cell 2016, 29, 832–845. [Google Scholar] [CrossRef] [PubMed]
- Bazzazi, H.; Shahraz, A. A mechanistic systems pharmacology modeling platform to investigate the effect of PD-L1 expression heterogeneity and dynamics on the efficacy of PD-1 and PD-L1 blocking antibodies in cancer. J. Theor. Biol. 2021, 522, 110697. [Google Scholar] [CrossRef] [PubMed]
- Dunsmore, G.; Guo, W.; Li, Z.; Bejarano, D.A.; Pai, R.; Yang, K.; Kwok, I.; Tan, L.; Ng, M.; Fabregat, C.D.L.C.; et al. Timing and location dictate monocyte fate and their transition to tumor-associated macrophages. Sci. Immunol. 2024, 9, eadk3981. [Google Scholar] [CrossRef]
- O’sUllivan, J.A.; Carroll, D.J.; Bochner, B.S. Glycobiology of Eosinophilic Inflammation: Contributions of Siglecs, Glycans, and Other Glycan-Binding Proteins. Front. Med. 2017, 4, 116. [Google Scholar] [CrossRef]
| Location | Context | Key Drivers | Key Features | Outcome | References |
|---|---|---|---|---|---|
| Nasal cavity | Steady-State | Not identified | Long-lived; Hyper-segmented nuclei with dendritic processes; APC-like cross-presentations to CD8+ T cells | Homeostatic | Gonzalez et al. (2024) [38] |
| B. pertussis Infection | IL-17A | Tissue-resident; Enhanced NETosis; Produce antimicrobial peptides (S100A8, LCN2). | Protective | Borkner et al. (2021) [31] | |
| OVA-induced Rhinitis | Ovalbumin | “Botryoid” nuclei; Enhanced phagocytosis and ROS production. | Unclear | Matsui et al. (2020) [24] | |
| Olfactory Neuroepithelium | LPS (for conversion); MMZ (for accumulation) | Bone marrow-derived, local conversion; Retain NETosis capacity; Express neurogenesis-related genes (Efna5, Sox11, Il33). | Reparative | Ogawa et al. (2021) [23] | |
| Lung | PPE-induced Emphysema | G-CSF secreted by stromal cells, which is induced by IL-17A from γδ+ T cells | Pro-inflammatory cytokine profile (↑TNF-α, IL-6, IL-1β; ↓IL-10); Enhanced NETosis and phagocytosis. | Pathogenic | Hong et al. (2024) [29] |
| DEP-induced Asthma | Extracellular ATP (via P2X1 receptor) | Spontaneous NET release; High cysteinyl leuko-triene production (via Ltc4s). | Pathogenic | Shin et al. [25] | |
| Cystic Fibrosis-like Disease (Scnn1b-Tg mice) | Chronic P. aeruginosa infection/Mucus obstruction | Presence correlates with exacerbated inflammation and tissue damage. | Unclear | Vaillancourt et al. [33] | |
| Allergic Airway Inflammation | Eosinophil-derived IL-4 and GM-CSF | PECAM-1 co-expression; Enhanced Th17-promoting cytokine activity (Il1a, Il23a, Tnf). | Pathogenic | Yu et al. (2024) [27] | |
| Heart | Myocardial Infarction (MI) | Not identified | Pro-inflammatory transcriptome (TNF, Myc, NF-κB, OxPhos) [35]; Aged/activated phenotype; Enhanced phagocytosis and ROS [34]. | Not classified (pro-inflammatory) | Calcagno et al. (2021) [35], Vafadarnejad et al. (2020) [34] |
| Periodontitis-related MI | GM-CSF/TGF-β on predisposed bone marrow neutrophils; PPARγ | Long-lived, apoptosis-resistant; Directly deposit Collagen I and Fibronectin; Activate fibroblasts via TNFα. | Pathogenic | Wang et al. (2025) [36] | |
| Kidney | Fibrosis (UUO, ADR, I/R) | T cells and Tubular epithelial cells (GM-CSF and TGF-β1) | Directly produce Collagen I (COL1A1); Activate fibroblasts (TGF-β1, TNF-α, IL-1β). | Pathogenic (profibrotic) | Ryu et al. (2022) [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheen, K.; Choi, T.; Kim, M.S. Context-Dependent Roles of Siglec-F+ Neutrophils. Biomedicines 2025, 13, 2601. https://doi.org/10.3390/biomedicines13112601
Sheen K, Choi T, Kim MS. Context-Dependent Roles of Siglec-F+ Neutrophils. Biomedicines. 2025; 13(11):2601. https://doi.org/10.3390/biomedicines13112601
Chicago/Turabian StyleSheen, Kisung, Taesoo Choi, and Man S. Kim. 2025. "Context-Dependent Roles of Siglec-F+ Neutrophils" Biomedicines 13, no. 11: 2601. https://doi.org/10.3390/biomedicines13112601
APA StyleSheen, K., Choi, T., & Kim, M. S. (2025). Context-Dependent Roles of Siglec-F+ Neutrophils. Biomedicines, 13(11), 2601. https://doi.org/10.3390/biomedicines13112601






