Theophylline Attenuates the Release of Cardiovascular Disease-Related Triglyceride and Cholesterol by Inhibiting the Activity of Microsomal Triglyceride Transfer Protein in Rat Hepatocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Selection and Characteristics of Rat Diets for the Induction of TG and Cholesterol in Hepatocytes
2.3. Experimental Design and Animal Administration
2.4. Determination of Theophylline Dose for Measuring Lipid Profiles and MTP Activity
2.5. Rational for Using Hepatocytes to Study Blood Lipid Profiles
2.6. Preparation of Rat Hepatocytes and Their Culture
2.7. Determination of Lipid Levels in Hepatocyte Culture Medium
2.8. Preparation of Hepatocellular Lysates and Measurement of MTP Activity
2.9. Protein Assay
2.10. Statistical Analysis
3. Results
3.1. Intake of Feed and Lipids Producing Materials
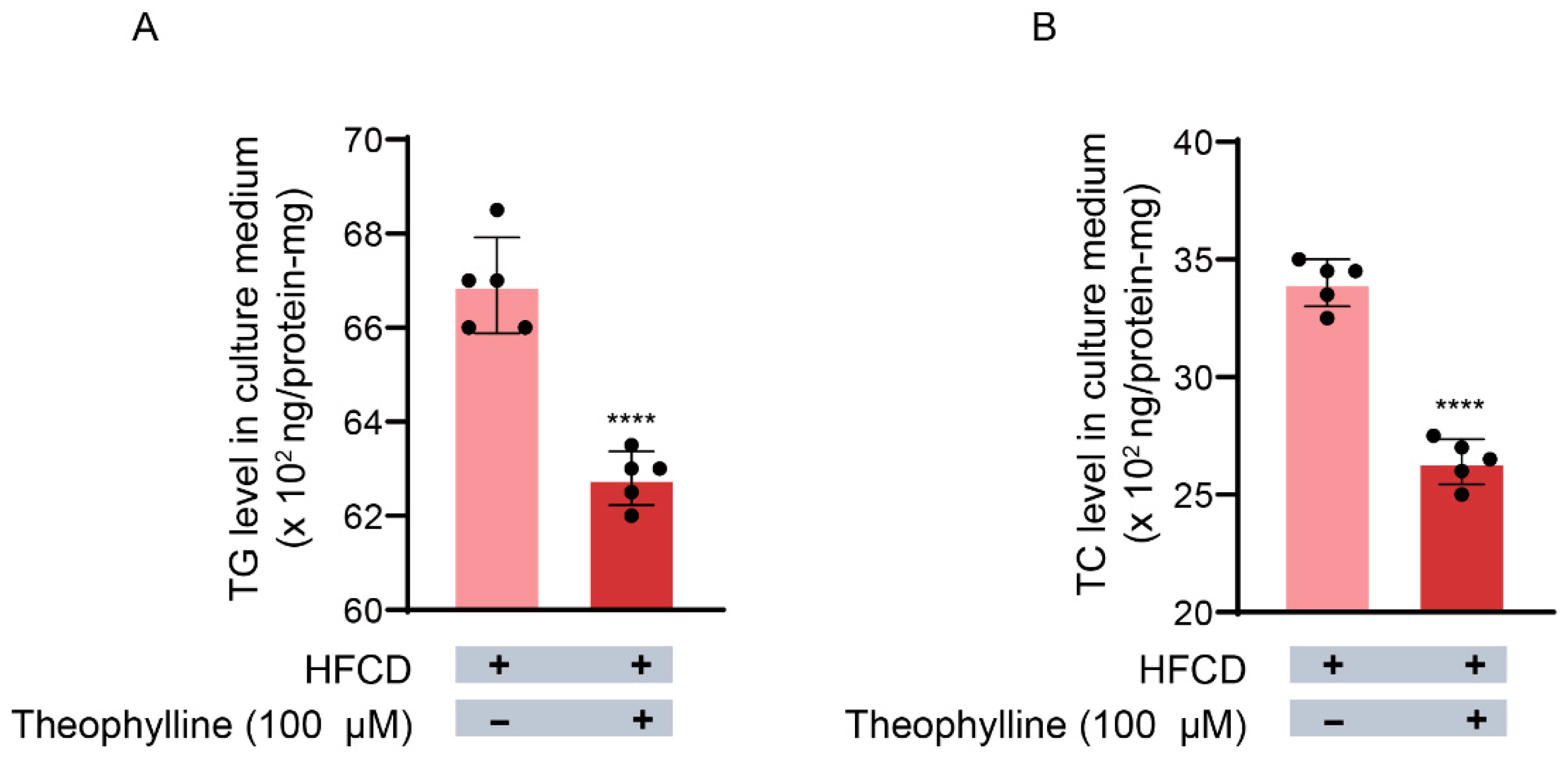
3.2. Effects of Theophylline on TG Release from Hepatocytes into the Culture Medium
3.3. Effects of Theophylline on TC Release from Hepatocytes into the Culture Medium
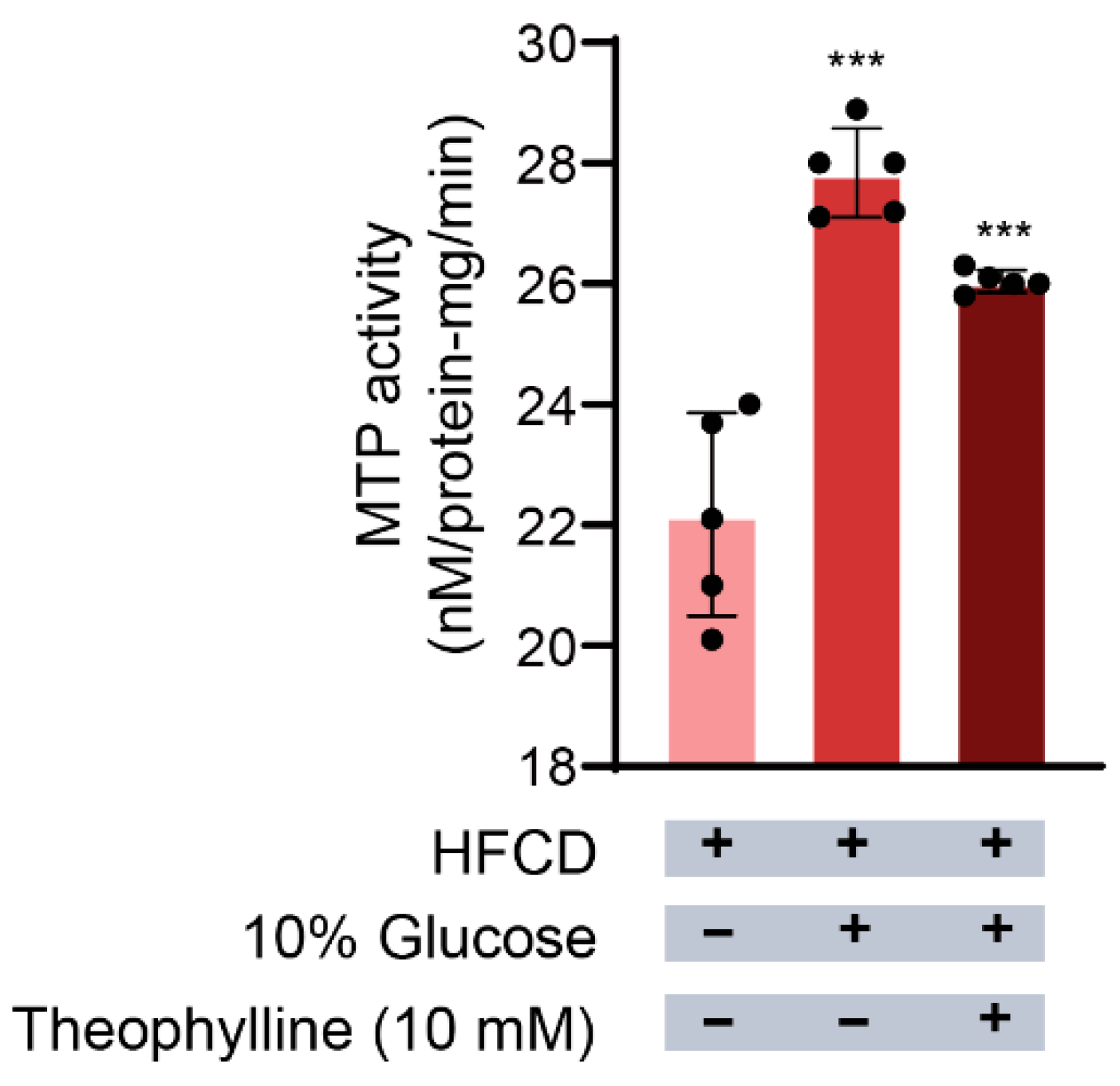
3.4. Effect of Theophylline on MTP Activity in Hepatocytes
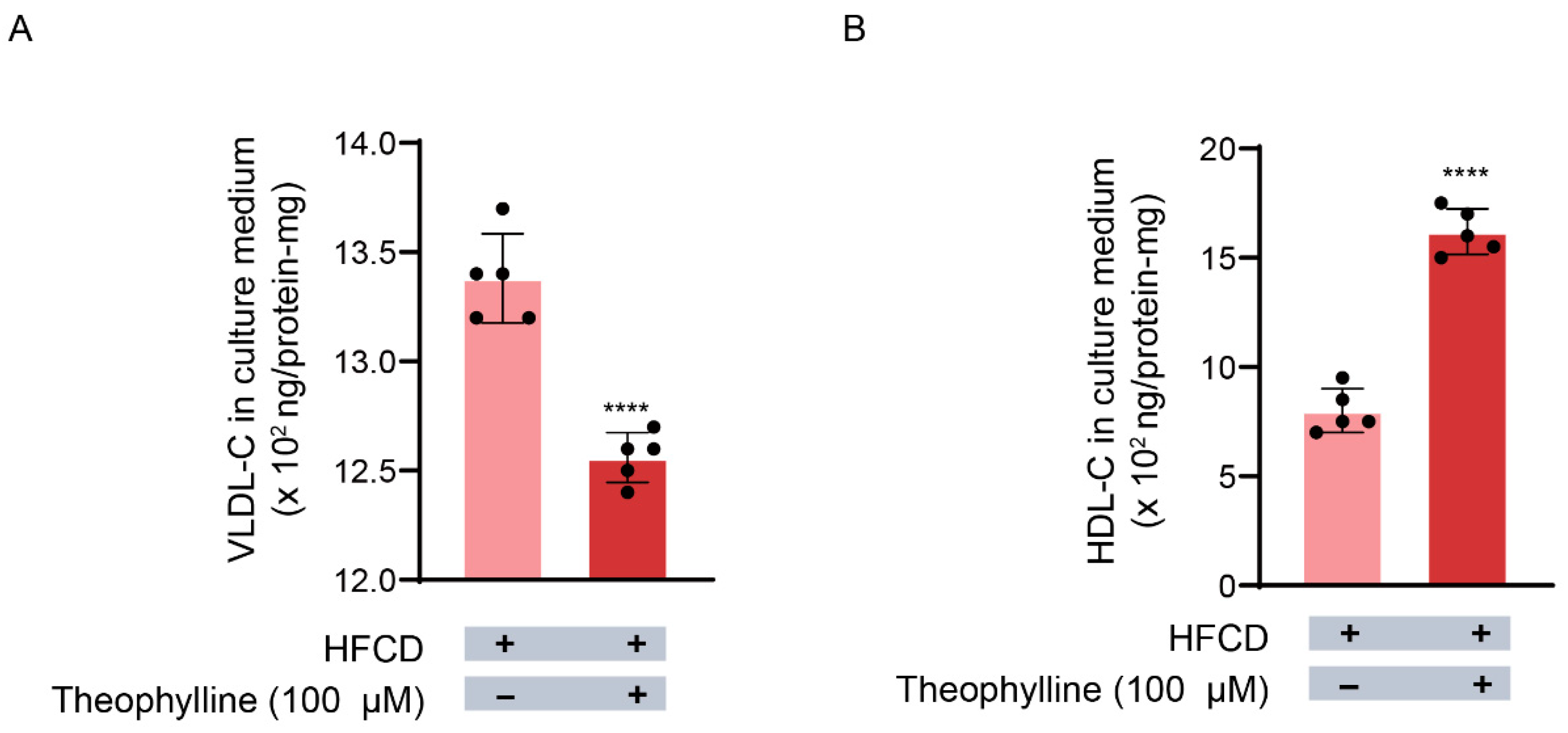
3.5. Effects of Theophylline on VLDL Release from Hepatocytes into the Culture Medium
3.6. Theophylline’s Effect on HDL Release from Hepatocytes
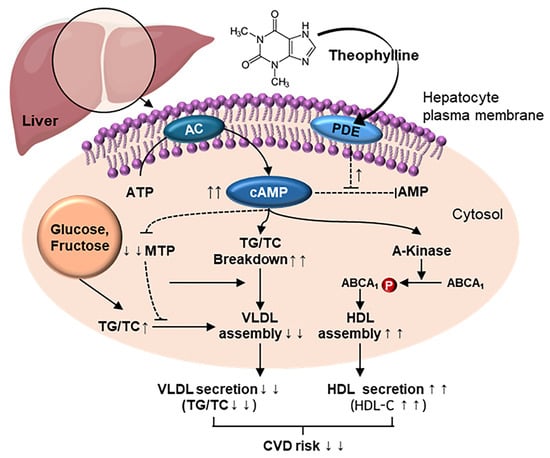
3.7. Theophylline’s Inhibitory Effect on CVD Risk Lipoproteins and Their Lipids
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CVD | Cardiovascular disease |
| VLDL | Very low density lipoprotein |
| MTP | Microsomal triglyceride transfer protein |
| cAMP | Cyclic adenosine monophosphate |
| HFCD | High-fat, high-carbohydrate diet |
| TG | Triglyceride |
| TC | Total cholesterol |
| VLDL-C | VLDL-cholesterol |
| HDL-C | HDL-cholesterol |
| AI | Atherogenic index |
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart disease and stroke statistics—2023 update: A report from the American Heart Association. Circulation 2023, 147, e93. [Google Scholar] [CrossRef]
- Higgins, J.A.; Hutson, J.L. The roles of Golgi and endoplasmic reticulum in the synthesis and assembly of lipoprotein lipids in rat hepatocytes. J. Lipid Res. 1984, 25, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, G.F. Assembly and secretion of hepatic very-low-density lipoprotein. Biochem. J. 1990, 268, 1–13. [Google Scholar] [CrossRef]
- Vance, J.E.; Vance, D.E. Lipoprotein assembly and secretion by hepatocytes. Annu. Rev. Nutr. 1990, 10, 337–356. [Google Scholar] [CrossRef]
- Griffin, B.A.; Zampelas, A. Influence of dietary fatty acids on the atherogenic lipoprotein phenotype. Nutr. Res. Rev. 1995, 8, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Benoist, F.; Nicodeme, E.; Grand-Perret, T. Microsomal triacylglycerol transfer protein prevents presecretory degradation of apolipoprotein B-100: A dithiothreitol-sensitive protease is involved. Eur. J. Biochem. 1996, 240, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.A.; Jamil, H. Progress towards understanding the role of microsomal triglyceride transfer protein in apolipoprotein-B lipoprotein assembly. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2000, 1486, 72–83. [Google Scholar] [CrossRef]
- Atzel, A.; Wetterau, J.R. Mechanism of microsomal triglyceride transfer protein catalyzed lipid transport. Biochemistry 1993, 32, 10444–10450. [Google Scholar] [CrossRef]
- Cho, H.-J.; Kang, H.-C.; Choi, S.-A.; Ju, Y.-C.; Lee, H.-S.; Park, H.-J. The possible role of Ca2+ on the activation of microsomal triglyceride transfer protein in rat hepatocytes. Biol. Pharm. Bull. 2005, 28, 1418–1423. [Google Scholar] [CrossRef]
- Cho, H.-J.; Kang, H.-C.; Ju, Y.-C.; Lee, H.-S.; Kim, H.-S.; Park, H.-J. Augmentation of Ca2+ induced microsomal triglyceride transfer protein activity by glucose supply enhances hypertriglyceridemia in vivo. Biol. Pharm. Bull. 2006, 29, 889–895. [Google Scholar] [CrossRef]
- Cho, H.-J.; Kim, H.-S.; Yu, Y.-B.; Kang, H.-C.; Lee, D.-H.; Rhee, M.-H.; Cho, J.-Y.; Park, H.-J. The opposite correlation between calcium ion and cyclic-AMP regarding the activation of microsomal triglyceride transfer protein in rat liver. BMB Rep. 2009, 42, 642–647. [Google Scholar] [CrossRef]
- Mulay, V.; Wood, P.; Rentero, C.; Enrich, C.; Grewal, T. Signal transduction pathways provide opportunities to enhance HDL and apoAI-dependent reverse cholesterol transport. Curr. Pharm. Biotechnol. 2012, 13, 352–364. [Google Scholar] [CrossRef]
- Uzuner, N.; Karaman, Ö.; Saydam, N.; Güner, G. Lipoprotein profile in long term theophylline administration in children with asthma. Allergol. Immunopathol. 2002, 30, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Apgar, J.L.; Tarka, S.M. Methylxanthine composition and consumption patterns of cocoa and chocolate products. In Caffeine; CRC Press: Boca Raton, FL, USA, 2019; pp. 163–192. [Google Scholar] [CrossRef]
- Inoue, H.; Kobayashi-Hattori, K.; Horiuchi, Y.; Oishi, Y.; Arai, S.; Takita, T. Regulation of the body fat percentage in developmental-stage rats by methylxanthine derivatives in a high-fat diet. Biosci. Biotechnol. Biochem. 2006, 70, 1134–1139. [Google Scholar] [CrossRef] [PubMed]
- Allayee, H.; Hartiala, J.; Lee, W.; Mehrabian, M.; Irvin, C.G.; Conti, D.V.; Lima, J.J. The effect of montelukast and low-dose theophylline on cardiovascular disease risk factors in asthmatics. Chest 2007, 132, 868–874. [Google Scholar] [CrossRef]
- Rustaeus, S.; Lindberg, K.; Stillemark, P.; Claesson, C.; Asp, L.; Larsson, T.; Borén, J.; Olofsson, S.-O. Assembly of very low density lipoprotein: A two-step process of apolipoprotein B core lipidation. J. Nutr. 1999, 129, 463S–466S. [Google Scholar] [CrossRef] [PubMed]
- Shelness, G.S.; Ingram, M.F.; Huang, X.F.; Delozier, J.A. Apolipoprotein B in the rough endoplasmic reticulum: Translation, translocation and the initiation of lipoprotein assembly. J. Nutr. 1999, 129, 456S–462S. [Google Scholar] [CrossRef]
- Song, Y.; Lee, S.-J.; Jang, S.-H.; Ha, J.H.; Song, Y.M.; Ko, Y.-G.; Kim, H.-D.; Min, W.; Kang, S.N.; Cho, J.-H. Sasa borealis stem extract attenuates hepatic steatosis in high-fat diet-induced obese rats. Nutrients 2014, 6, 2179–2195. [Google Scholar] [CrossRef]
- Jung, M.-S.; Lee, S.-J.; Song, Y.; Jang, S.-H.; Min, W.; Won, C.-K.; Kim, H.-D.; Kim, T.H.; Cho, J.-H. Rubus crataegifolius Bunge regulates adipogenesis through Akt and inhibits high-fat diet-induced obesity in rats. Nutr. Metab. 2016, 13, 29. [Google Scholar] [CrossRef]
- Ondee, T.; Pongpirul, K.; Udompornpitak, K.; Sukkummee, W.; Lertmongkolaksorn, T.; Senaprom, S.; Leelahavanichkul, A. High fructose causes more prominent liver steatohepatitis with leaky gut similar to high glucose administration in mice and attenuation by Lactiplantibacillus plantarum dfa1. Nutrients 2023, 15, 1462. [Google Scholar] [CrossRef]
- Mokhtari, I.; Moumou, M.; Mokhtari, C.; Harnafi, M.; Milenkovic, D.; Amrani, S.; Harnafi, H. Nutritional composition and effect of loquat fruit (Eriobotrya japonica L. var. Navela) on lipid metabolism and liver steatosis in high-fat high-sucrose diet-fed mice. Prev. Nutr. Food Sci. 2024, 29, 256–265. [Google Scholar] [CrossRef]
- Kim, H.-J.; Son, J.; Jin, E.; Lee, J.; Park, S. Effects of exercise and L-arginine inake on inflammation in fat diet induced obese rats. J. Exerc. Nutr. B 2016, 20, 36–40. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, J. Exercise training suppresses vascular fibrosis in aging obesity induced rats. J. Exerc. Nutr. Biochem. 2014, 18, 175–180. [Google Scholar] [CrossRef]
- Yang, L.Y.; Kuksis, A.; Myher, J.J.; Steiner, G. Contribution of de novo fatty acid synthesis to very low density lipoprotein triacylglycerols: Evidence from mass isotopomer distribution analysis of fatty acids synthesized from [2H6] ethanol. J. Lipid Res. 1996, 37, 262–274. [Google Scholar] [CrossRef]
- Nossen, J.O.; Rustan, A.C.; Drevon, C.A. Calcium-antagonists inhibit secretion of very-low-density lipoprotein from cultured rat hepatocytes. Biochem. J. 1987, 247, 433–439. [Google Scholar] [CrossRef]
- Burr, I.M.; Balant, L.; Stauffacher, W.; Renold, A.E. Perifusion of rat pancreatic tissue in vitro: Substrate modification of theophylline-induced biphasic insulin release. J. Clin. Investig. 1970, 49, 2097–2105. [Google Scholar] [CrossRef]
- Berry, M.N.; Friend, D.S. High-yield preparation of isolated rat liver parenchymal cells: A biochemical and fine structural study. J. Cell Biol. 1969, 43, 506–520. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Kessler, R.J.; Fanestil, D.D. Interference by lipids in the determination of protein using bicinchoninic acid. Anal. Biochem. 1986, 159, 138–142. [Google Scholar] [CrossRef]
- Morton, R.E.; Evans, T.A. Modification of the bicinchoninic acid protein assay to eliminate lipid interference in deyermining lipoprotein protein content. Anal. Biochem. 1992, 204, 332–334. [Google Scholar] [CrossRef]
- Thermo Scientific. Introduction Pierce® BCA Protein Assay Kit; Thermo Scientific: Waltham, MA, USA, 2013. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Thierer, J.H.; Foresti, O.; Yadav, P.K.; Wilson, M.H.; Moll, T.O.; Shen, M.-C.; Busch-Nentwich, E.M.; Morash, M.; Mohlke, K.L.; Rawls, J.F. Pla2g12b drives expansion of triglyceride-rich lipoproteins. Nat. Commun. 2024, 15, 2095. [Google Scholar] [CrossRef]
- Wu, B.-N.; Kuo, K.-K.; Chen, Y.-H.; Chang, C.-T.; Huang, H.-T.; Chai, C.-Y.; Dai, Z.-K.; Chen, I.-J. Theophylline-based KMUP-1 improves steatohepatitis via MMP-9/IL-10 and lipolysis via HSL/p-HSL in obese mice. Int. J. Mol. Sci. 2016, 17, 1345. [Google Scholar] [CrossRef]
- Chong, M.F.; Fielding, B.A.; Frayn, K.N. Metabolic interaction of dietary sugars and plasma lipids with a focus on mechanisms and de novo lipogenesis. Proc. Nutr. Soc. 2007, 66, 52–59. [Google Scholar] [CrossRef]
- Hickie, R.A.; Walker, C.M.; Datta, A. Increased activity of low-Km cyclic adenosine 3′:5′-monophosphate phosphodiesterase in plasma membranes of Morris hepatoma 5123tc (h). Cancer Res. 1975, 35, 601–605. [Google Scholar]
- Quiroga, A.D.; Lehner, R. Liver triacylglycerol lipases. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2012, 1821, 762–769. [Google Scholar] [CrossRef]
- Schott, M.B.; Rasineni, K.; Weller, S.G.; Schulze, R.J.; Sletten, A.C.; Casey, C.A.; McNiven, M.A. β-Adrenergic induction of lipolysis in hepatocytes is inhibited by ethanol exposure. J. Biol. Chem. 2017, 292, 11815–11828. [Google Scholar] [CrossRef]
- Wahlang, B.; McClain, C.J.; Barve, S.; Gobejishvili, L. Role of cAMP and phosphodiesterase signaling in liver health and disease. Cell. Signal. 2018, 49, 105–115. [Google Scholar] [CrossRef]
- Botham, K.M. Cyclic AMP and the regulation of cholesterol metabolism. Biochem. Soc. Trans. 1992, 20, 454–459. [Google Scholar] [CrossRef]
- Hoang, V.-Q.; Suckling, K.E.; Cho-Chui, Y.; Botham, K.M. The effect of cyclic AMP analogues and glucagon on cholesteryl ester synthesis and hydrolysis in cultured hamster hepatocytes. FEBS Lett. 1993, 329, 17–20. [Google Scholar] [CrossRef]
- Botham, K.M.; Hoang, V.-Q.; Jones, A.K.; Martinez, M.J.; Ochoa, B.; Suckling, K.E. Comparison of the effects of cyclic AMP analogues on cholesterol metabolism in cultured rat and hamster hepatocytes. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1996, 113, 185–191. [Google Scholar] [CrossRef]
- Hernández, M.L.; Martínez, M.J.; Ruiz, J.I.; Ochoa, B. Stimulation of microsomal cholesterol ester hydrolase by glucagon, cyclic AMP analogues, and vasopressin in isolated rat hepatocytes. Lipids 1996, 31, 269–276. [Google Scholar] [CrossRef]
- Björnsson, O.G.; Sparks, J.D.; Sparks, C.E.; Gibbons, G.F. Regulation of VLDL secretion in primary culture of rat hepatocytes: Involvement of cAMP and cAMP-dependent protein kinases. Eur. J. Clin. Investig. 1994, 24, 137–148. [Google Scholar] [CrossRef]
- Taskinen, M.-R.; Kuusi, T. Enzymes involved in triglyceride hydrolysis. Baillieres Clin. Endocrinol. Metab. 1987, 1, 639–666. [Google Scholar] [CrossRef] [PubMed]
- Tsujita, M.; Wu, C.-A.; Abe-Dohmae, S.; Usui, S.; Okazaki, M.; Yokoyama, S. On the hepatic mechanism of HDL assembly by the ABCA1/apoA-I pathway. J. Lipid Res. 2005, 46, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Du, X.; Yang, R.; Shan, L.; Lu, X.; Shen, Y.; Li, Y.; Duan, S.; Du, Z.; Fueng, J.; et al. Tramnscriptomic analysis revels the mechanisms underlying the differential effects of caffeine, theophylline, and theobromine in regulating hepatic fat accumulation. Food Funct. 2025, 16 (Suppl. 1), 2503–2514. [Google Scholar] [CrossRef]
- Liu, T.-T.; Liu, X.-T.; Huang, G.-L.; Liu, L.; Chen, Q.-X.; Wang, Q. Theophylline extracyed from Fu Brick Tea affects the metabolism of preadipocytes and body fat in mice as a pancreatic lipase inhibitor. Int. J. Mol. Sci. 2022, 23, 2525. [Google Scholar] [CrossRef]
- Hussein, R.M.; Elsirafy, M.; Wahba, Y.S.; Kawy, H.S.A.; Hasanin, A.H.; Galal, G. Theophylline, an old drug with multi-faceted effects: Its potential benefits in immunological liver injury in rats. Life Sci. 2015, 136, 100–107. [Google Scholar] [CrossRef]


| Ingredient | Contents | ||
|---|---|---|---|
| (g/kg) | (%) | ||
| Fat and oil | Soybean oil | 25 | 65.4 |
| Lard | 245 | ||
| Subtotal | 270 | ||
| Carbohydrate | Maltodextrin | 125 | |
| Sucrose | 68.8 | ||
| Cellulose | 50 | ||
| Subtotal | 243.8 | ||
| Protein and amino acid | Casein | 200 | 25.8 |
| L-Cystine | 3 | ||
| Mineral and electrolytes | Mineral mix | 10 | 7.3 |
| Dicalcium | 13 | ||
| Phosphate | 5.5 | ||
| Calcium | 16.5 | ||
| Carbonate | 10 | ||
| Potassium citrate | 2 | ||
| Vitamins | Vitamin mix | 10 | 1.5 |
| Choline bitartrate | 2 | ||
| Total | 785.8 | 100 | |
| Animal Group | Feed Intake (g/d) | Body Weight Gain (g/d) | FER | Intake of Lipid-Producing Materials of Feed (g/d) | |||
|---|---|---|---|---|---|---|---|
| Fat and Oil (270 g/kg-HFCD) | Carbohydrates (243.8 g/kg-HFCD) | Glucose (g/d) | Total | ||||
| HFCD | (1) 20.4 ± 1.5 | 3.3 ± 0.1 | 0.16 ± 0.1 | (3) 5.51 ± 0.41 | (5) 4.97 ± 0.41 | - | 10.48 ± 0.8 |
| HFCD + glucose | (2) 14.8 ± 1.7 *** | 3.6 ± 0.9 * | 0.24 ± 0.5 | (4) 4.00 ± 0.46 | (6) 3.60 ± 0.41 ** | 10.6 ± 0.9 | 18.2 ± 1.7 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, M.-K.; Jang, H.; Bae, J.-S.; Shin, J.-H.; Park, H.-J. Theophylline Attenuates the Release of Cardiovascular Disease-Related Triglyceride and Cholesterol by Inhibiting the Activity of Microsomal Triglyceride Transfer Protein in Rat Hepatocytes. Biomedicines 2025, 13, 2579. https://doi.org/10.3390/biomedicines13112579
Park M-K, Jang H, Bae J-S, Shin J-H, Park H-J. Theophylline Attenuates the Release of Cardiovascular Disease-Related Triglyceride and Cholesterol by Inhibiting the Activity of Microsomal Triglyceride Transfer Protein in Rat Hepatocytes. Biomedicines. 2025; 13(11):2579. https://doi.org/10.3390/biomedicines13112579
Chicago/Turabian StylePark, Min-Kyu, Hyeonha Jang, Jeong-Soo Bae, Jae-Ho Shin, and Hwa-Jin Park. 2025. "Theophylline Attenuates the Release of Cardiovascular Disease-Related Triglyceride and Cholesterol by Inhibiting the Activity of Microsomal Triglyceride Transfer Protein in Rat Hepatocytes" Biomedicines 13, no. 11: 2579. https://doi.org/10.3390/biomedicines13112579
APA StylePark, M.-K., Jang, H., Bae, J.-S., Shin, J.-H., & Park, H.-J. (2025). Theophylline Attenuates the Release of Cardiovascular Disease-Related Triglyceride and Cholesterol by Inhibiting the Activity of Microsomal Triglyceride Transfer Protein in Rat Hepatocytes. Biomedicines, 13(11), 2579. https://doi.org/10.3390/biomedicines13112579