Preoperative Prediction of Axillary Lymph Node Metastasis in Breast Cancer Using Radiomics Features of Voxel-Wise DCE-MRI Time-Intensity-Curve Profile Maps
Abstract
1. Introduction
2. Materials and Methods
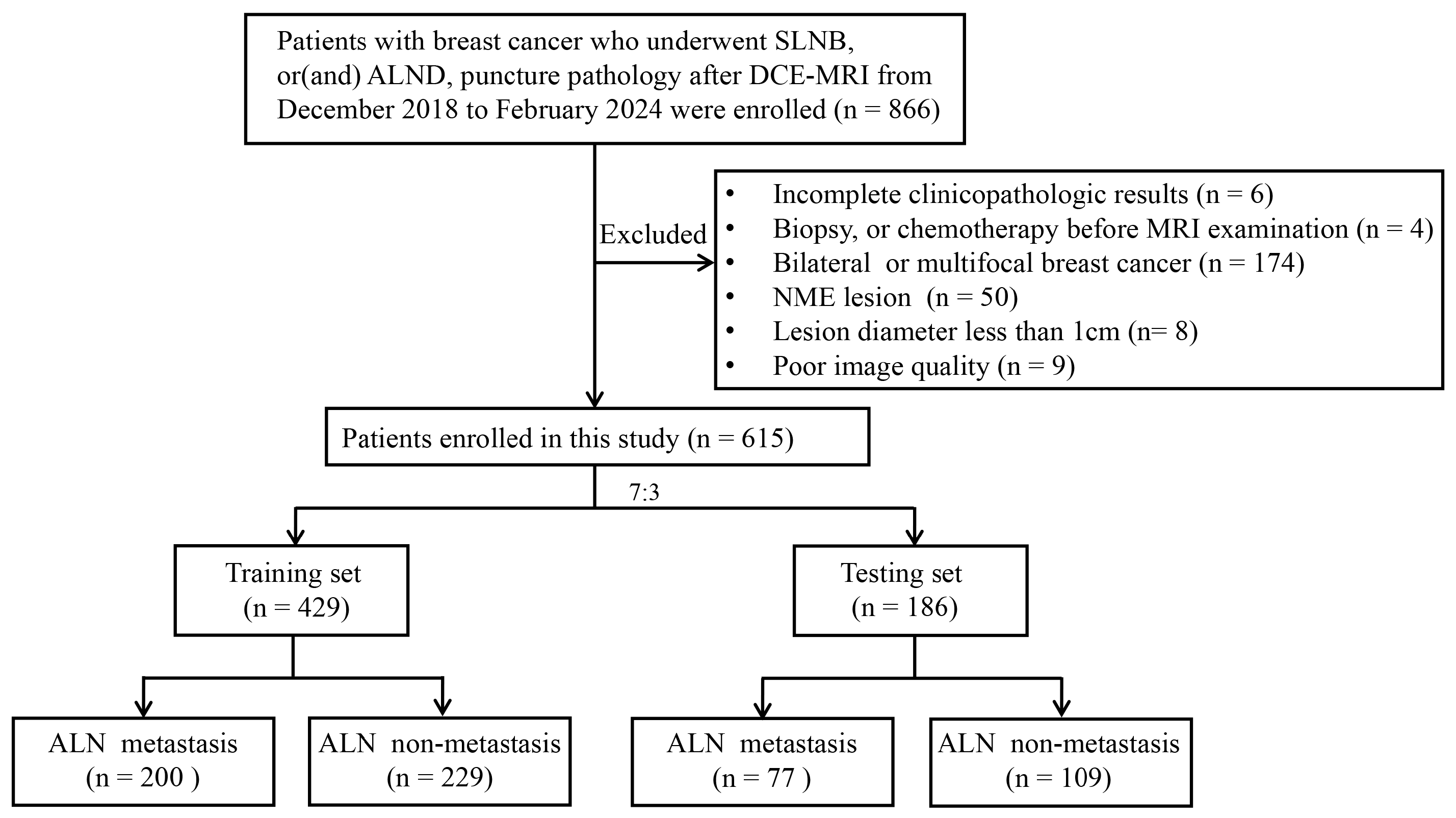
2.1. Patients
2.2. Clinical and Histopathology Data
2.3. MRI Data Collection
2.4. Tumor ROI Segmentation and Image Preprocessing
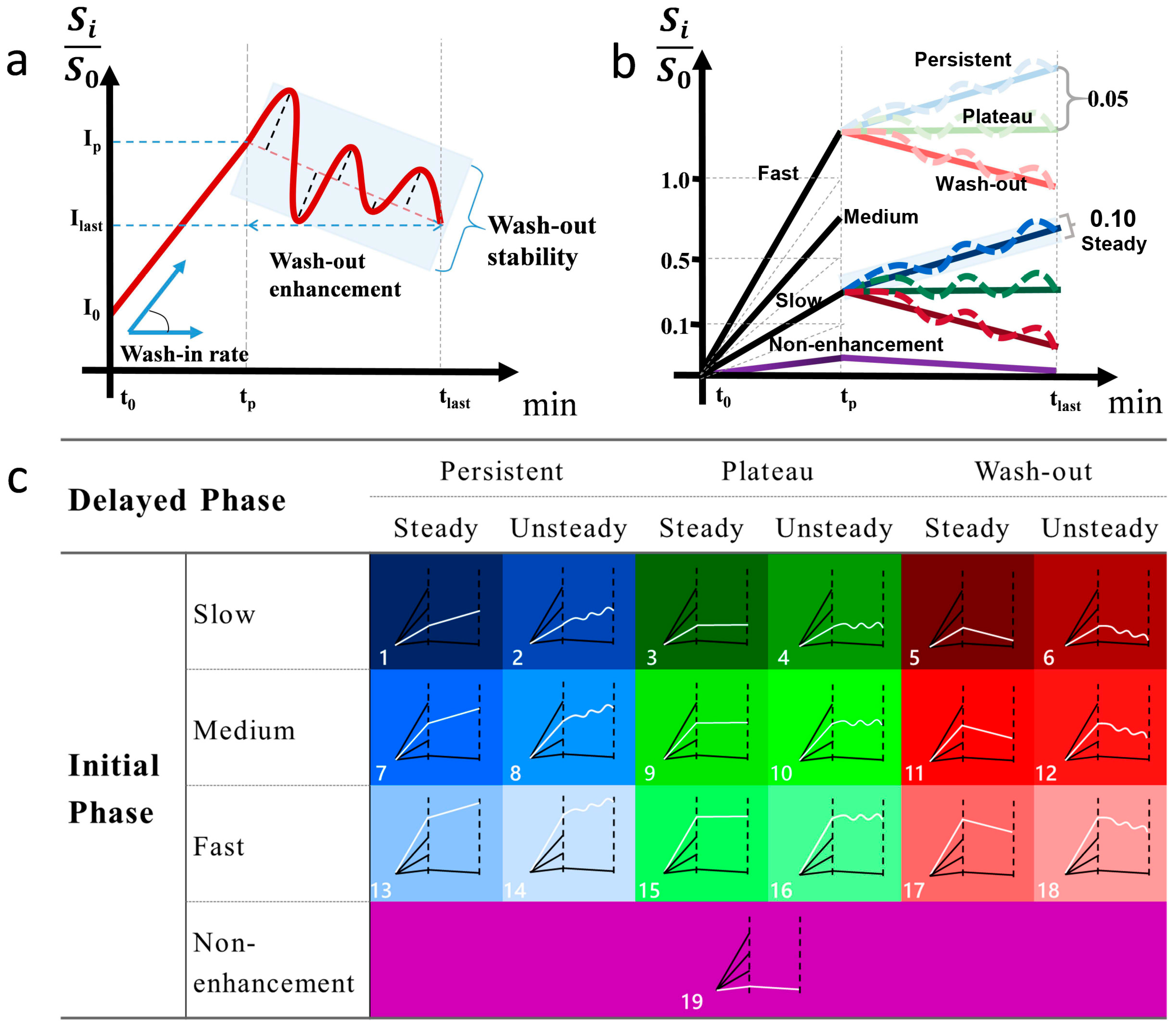
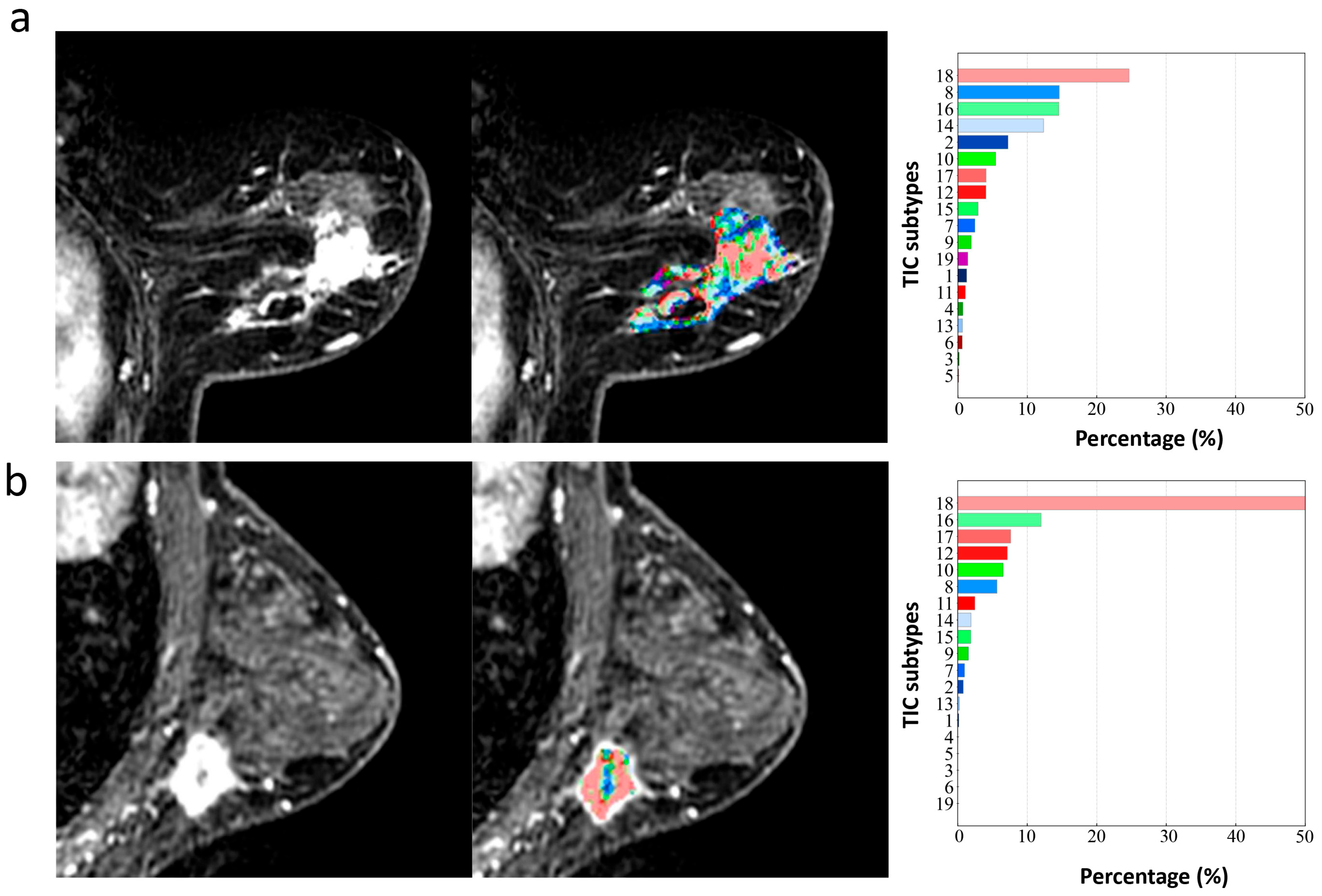
2.5. Definition of Type-19
- Wash-in rate = , where I0 and Ip indicate the baseline and peak signal intensity, respectively, and TP refers to the time used for the signal intensity to reach the peak within 3 phases. The wash-in rate of initial phase was divided into non-enhanced [<0.1], slow [0.1 to 0.5], medium [0.5 to 1.0], and fast [>1.0].
- Wash-out enhancement = , where Ilast and Ip refer to the peak signal intensity within 3 phases and signal intensity at the last phase, respectively. Wash-out enhancement is divided into persistent [>0.05], plateau [−0.05 to 0.05], and decline [<−0.05].
- Wash-out stability: The residual sum of squares (RSS) was used to calculate the degree in the oscillation of wash-out enhancement: wash−out stability = , where i ∈ {p,…, n}, p is the number of phases corresponding to TP and n is the number of the last phase. Ii is the signal intensity in phase i, and f(Ii) is the linear predicted signal intensity of Ii. Wash-out stability is divided into steady [<0.1] and unsteady [≥0.1]).
2.6. Feature Extraction
2.6.1. Type-19 Feature Set
2.6.2. Phase-3-Radiomics Feature Set and Type-19-Radiomics Feature Set
2.6.3. Type-19-Combined Feature Set
2.7. Feature Selection and Ranking
2.8. Model Construction
2.9. Statistical Analysis
3. Results
3.1. Clinicopathologic Characteristics
3.2. Feature Extraction and Selection
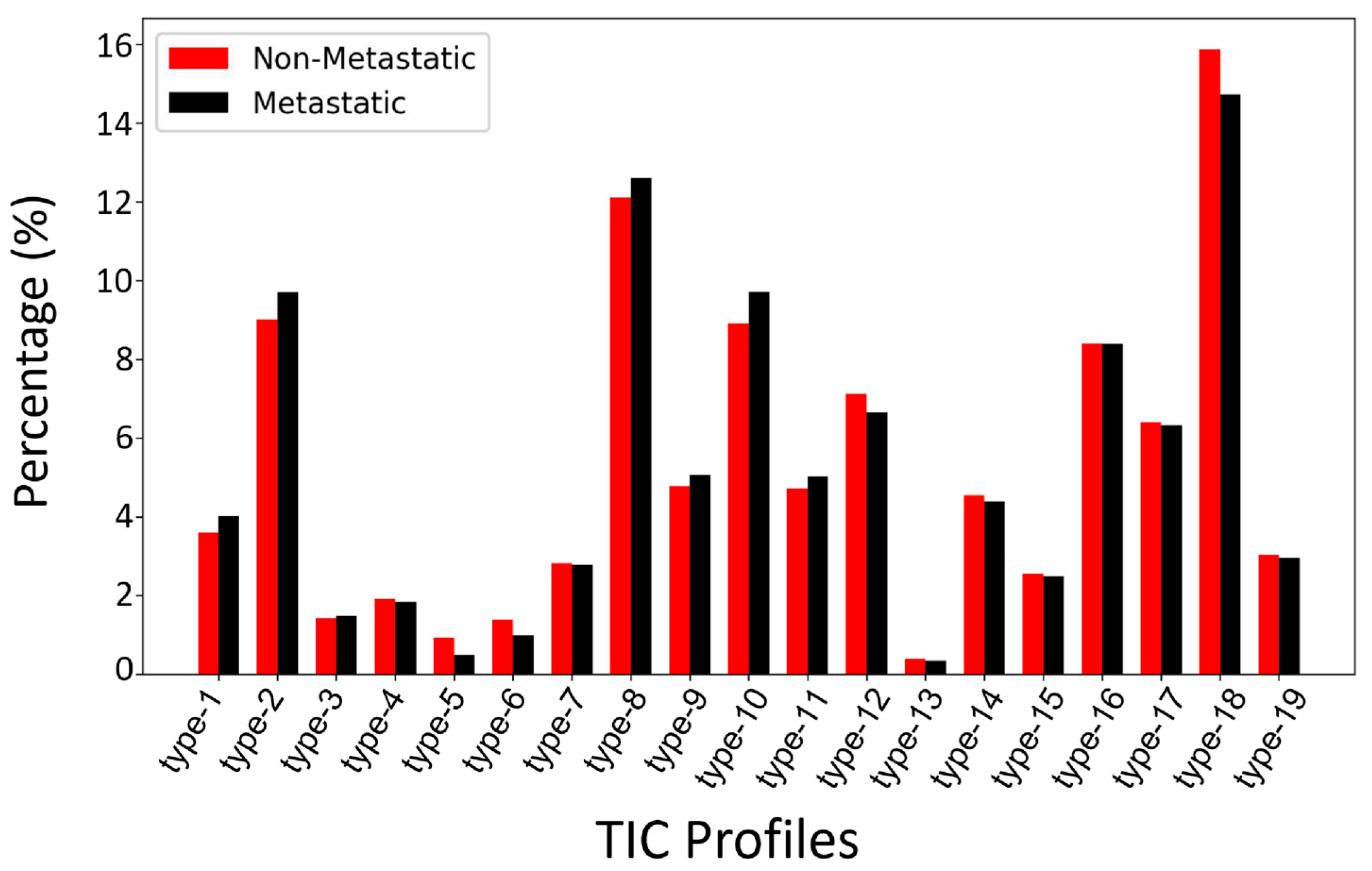
3.2.1. Type-19 Feature Set
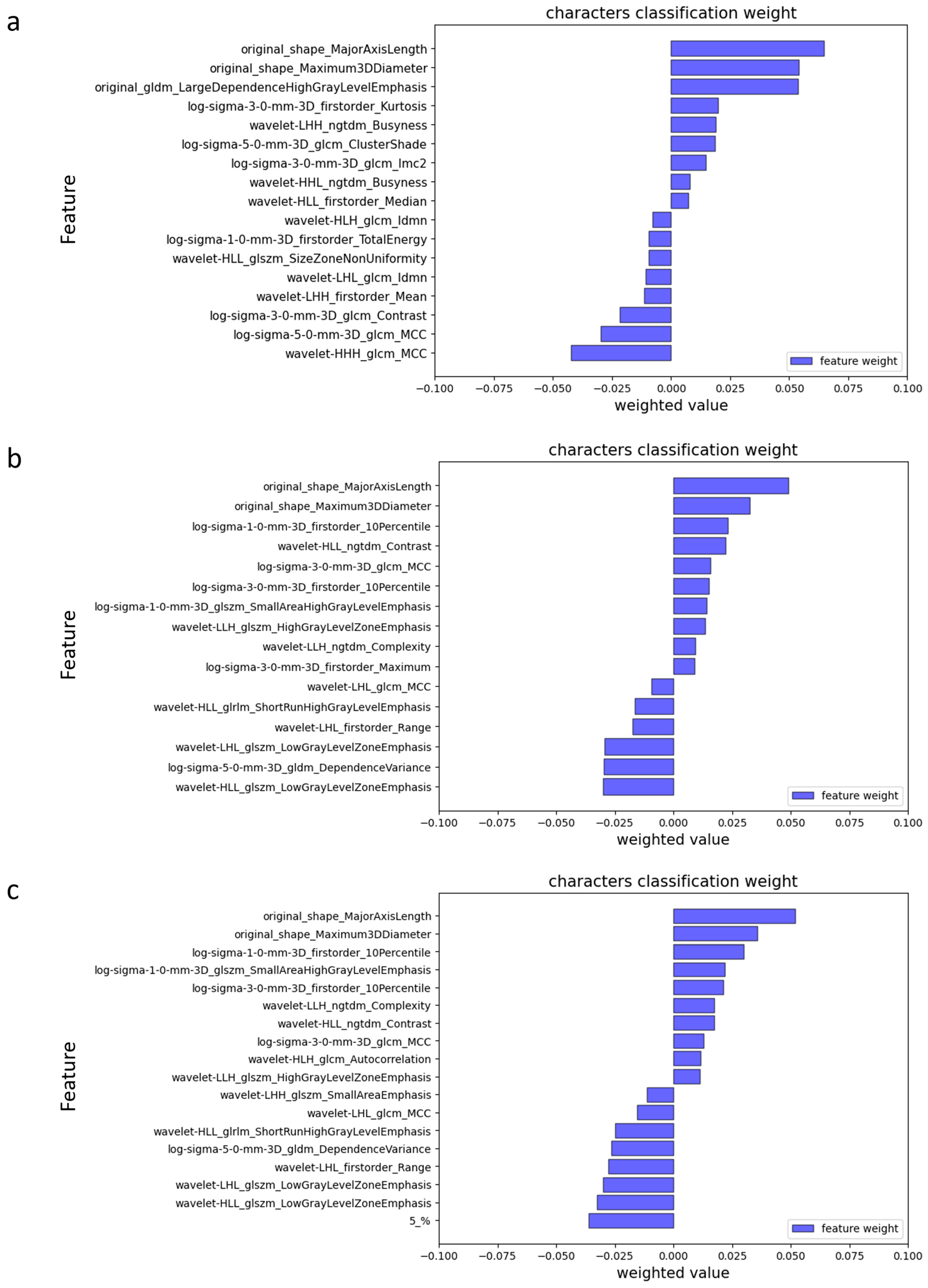
3.2.2. Radiomics Feature Selection and Ranking
3.3. Model Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DCE-MRI | Dynamic contrast-enhanced magnetic resonance imaging |
| TIC | Time-intensity curve |
| ALN | Axillary lymph node |
| ALND | Axillary lymph node dissection |
| SLNB | Sentinel lymph node biopsy |
| TR | Repetition time |
| TE | Echo time |
| AUC | Area under the curve |
| ROC | Receiver operating characteristic |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Danko, M.E.; Bennett, K.M.; Zhai, J.; Marks, J.R.; Olson, J.A.J. Improved staging in node-positive breast cancer patients using lymph node ratio: Results in 1788 patients with long-term follow-up. J. Am. Coll. Surg. 2010, 210, 797–805.e1, 805–807. [Google Scholar] [CrossRef]
- Ahmed, M.; Purushotham, A.D.; Douek, M. Novel techniques for sentinel lymph node biopsy in breast cancer: A systematic review. Lancet Oncol. 2014, 15, e351–e362. [Google Scholar] [CrossRef]
- Weigel, M.T.; Dowsett, M. Current and emerging biomarkers in breast cancer: Prognosis and prediction. Endocr. Relat. Cancer 2010, 17, R245–R262. [Google Scholar] [CrossRef] [PubMed]
- Langer, I.; Guller, U.; Berclaz, G.; Koechli, O.R.; Schaer, G.; Fehr, M.K.; Hess, T.; Oertli, D.; Bronz, L.; Schnarwyler, B.; et al. Morbidity of sentinel lymph node biopsy (SLN) alone versus SLN and completion axillary lymph node dissection after breast cancer surgery: A prospective Swiss multicenter study on 659 patients. Ann. Surg. 2007, 245, 452–461. [Google Scholar] [CrossRef]
- Schrenk, P.; Rieger, R.; Shamiyeh, A.; Wayand, W. Morbidity following sentinel lymph node biopsy versus axillary lymph node dissection for patients with breast carcinoma. Cancer 2000, 88, 608–614. [Google Scholar] [CrossRef]
- Pesek, S.; Ashikaga, T.; Krag, L.E.; Krag, D. The false-negative rate of sentinel node biopsy in patients with breast cancer: A meta-analysis. World J. Surg. 2012, 36, 2239–2251. [Google Scholar] [CrossRef]
- Choi, E.J.; Youk, J.H.; Choi, H.; Song, J.S. Dynamic contrast-enhanced and diffusion-weighted MRI of invasive breast cancer for the prediction of sentinel lymph node status. J. Magn. Reson. Imaging 2020, 51, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Ya, G.; Wen, F.; Xing-Ru, L.; Zhuan-Zhuan, G.; Jun-Qiang, L. Difference of DCE-MRI Parameters at Different Time Points and Their Predictive Value for Axillary Lymph Node Metastasis of Breast Cancer. Acad. Radiol. 2022, 29 (Suppl. 1), S79–S86. [Google Scholar] [CrossRef]
- Goto, M.; Ito, H.; Akazawa, K.; Kubota, T.; Kizu, O.; Yamada, K.; Nishimura, T. Diagnosis of breast tumors by contrast-enhanced MR imaging: Comparison between the diagnostic performance of dynamic enhancement patterns and morphologic features. J. Magn. Reson. Imaging 2007, 25, 104–112. [Google Scholar] [CrossRef]
- Liu, H.-L.; Zong, M.; Wei, H.; Lou, J.-J.; Wang, S.-Q.; Zou, Q.-G.; Shi, H.-B.; Jiang, Y.-N. Differentiation between malignant and benign breast masses: Combination of semi-quantitative analysis on DCE-MRI and histogram analysis of ADC maps. Clin. Radiol. 2018, 73, 460–466. [Google Scholar] [CrossRef]
- Winfield, J.M.; Payne, G.S.; Weller, A.; deSouza, N.M. DCE-MRI, DW-MRI, and MRS in Cancer: Challenges and Advantages of Implementing Qualitative and Quantitative Multi-parametric Imaging in the Clinic. Top. Magn. Reson. Imaging 2016, 25, 245–254. [Google Scholar] [CrossRef]
- Liu, Z.; Yao, B.; Wen, J.; Wang, M.; Ren, Y.; Chen, Y.; Hu, Z.; Li, Y.; Liang, D.; Liu, X.; et al. Voxel-wise mapping of DCE-MRI time-intensity-curve profiles enables visualizing and quantifying hemodynamic heterogeneity in breast lesions. Eur. Radiol. 2024, 34, 182–192. [Google Scholar] [CrossRef]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.T.H.M.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Yang, F.; Zhang, Y.; Guo, Y.; Qu, Y.; Zhang, X.; Zhu, Y.; Cui, S. Dynamic contrast-enhanced MRI radiomics nomogram for predicting axillary lymph node metastasis in breast cancer. Cancer Imaging 2022, 22, 17. [Google Scholar] [CrossRef]
- Mao, N.; Dai, Y.; Lin, F.; Ma, H.; Duan, S.; Xie, H.; Zhao, W.; Hong, N. Radiomics Nomogram of DCE-MRI for the Prediction of Axillary Lymph Node Metastasis in Breast Cancer. Front. Oncol. 2020, 10, 541849. [Google Scholar] [CrossRef]
- Chen, H.; Lan, X.; Yu, T.; Li, L.; Tang, S.; Liu, S.; Jiang, F.; Wang, L.; Huang, Y.; Cao, Y.; et al. Development and validation of a radiogenomics model to predict axillary lymph node metastasis in breast cancer integrating MRI with transcriptome data: A multicohort study. Front. Oncol. 2022, 12, 1076267. [Google Scholar] [CrossRef] [PubMed]
- Tagliafico, A.S.; Piana, M.; Schenone, D.; Lai, R.; Massone, A.M.; Houssami, N. Overview of radiomics in breast cancer diagnosis and prognostication. Breast 2020, 49, 74–80. [Google Scholar] [CrossRef]
- Han, X.; Gong, Z.; Guo, Y.; Tang, W.; Wei, X. Exploration of a noninvasive radiomics classifier for breast cancer tumor microenvironment categorization and prognostic outcome prediction. Eur. J. Radiol. 2024, 175, 111441. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.-B.; Liu, Y.-Y.; Wang, C.-H.; Qing, H.-M.; Wang, M.; Zhang, X.; Chen, X.-Y.; Xu, G.-H.; Zhou, P.; Ren, J. Radiomic features of axillary lymph nodes based on pharmacokinetic modeling DCE-MRI allow preoperative diagnosis of their metastatic status in breast cancer. PLoS ONE 2021, 16, e0247074. [Google Scholar] [CrossRef]
- Liu, J.; Sun, D.; Chen, L.; Fang, Z.; Song, W.; Guo, D.; Ni, T.; Liu, C.; Feng, L.; Xia, Y.; et al. Radiomics Analysis of Dynamic Contrast-Enhanced Magnetic Resonance Imaging for the Prediction of Sentinel Lymph Node Metastasis in Breast Cancer. Front. Oncol. 2019, 9, 980. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, J.; Zhang, J.; Yu, Z.; Jiang, H. Radiomic Nomogram for Predicting Axillary Lymph Node Metastasis in Patients with Breast Cancer. Acad. Radiol. 2024, 31, 788–799. [Google Scholar] [CrossRef]
- Wang, C.; Chen, X.; Luo, H.; Liu, Y.; Meng, R.; Wang, M.; Liu, S.; Xu, G.; Ren, J.; Zhou, P. Development and Internal Validation of a Preoperative Prediction Model for Sentinel Lymph Node Status in Breast Cancer: Combining Radiomics Signature and Clinical Factors. Front. Oncol. 2021, 11, 754843. [Google Scholar] [CrossRef]
- Shan, Y.-N.; Xu, W.; Wang, R.; Wang, W.; Pang, P.-P.; Shen, Q.-J. A Nomogram Combined Radiomics and Kinetic Curve Pattern as Imaging Biomarker for Detecting Metastatic Axillary Lymph Node in Invasive Breast Cancer. Front. Oncol. 2020, 10, 1463. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Mao, N.; Ma, H.; Dong, J.; Zhang, K.; Che, K.; Duan, S.; Zhang, X.; Shi, Y.; Xie, H. Pharmacokinetic parameters and radiomics model based on dynamic contrast enhanced MRI for the preoperative prediction of sentinel lymph node metastasis in breast cancer. Cancer Imaging 2020, 20, 65. [Google Scholar] [CrossRef]
- Liu, C.; Ding, J.; Spuhler, K.; Gao, Y.; Serrano Sosa, M.; Moriarty, M.; Hussain, S.; He, X.; Liang, C.; Huang, C. Preoperative prediction of sentinel lymph node metastasis in breast cancer by radiomic signatures from dynamic contrast-enhanced MRI. J. Magn. Reson. Imaging 2019, 49, 131–140. [Google Scholar] [CrossRef]
- Arneth, B. Tumor Microenvironment. Medicina 2019, 56, 15. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, X.; Zhu, L.; Zhao, Z.; Wang, T.; Zhang, X.; Cai, B.; Li, L.; Ma, M.; Ma, X.; et al. Preoperative Prediction of Axillary Lymph Node Metastasis in Breast Cancer Based on Intratumoral and Peritumoral DCE-MRI Radiomics Nomogram. Contrast Media Mol. Imaging 2022, 2022, 6729473. [Google Scholar] [CrossRef]
- Junttila, M.R.; de Sauvage, F.J. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 2013, 501, 346–354. [Google Scholar] [CrossRef]
- Dong, X.; Chunrong, Y.; Hongjun, H.; Xuexi, Z. Differentiating the lymph node metastasis of breast cancer through dynamic contrast-enhanced magnetic resonance imaging. BJR Open 2019, 1, 20180023. [Google Scholar] [CrossRef] [PubMed]
- Schacht, D.V.; Drukker, K.; Pak, I.; Abe, H.; Giger, M.L. Using quantitative image analysis to classify axillary lymph nodes on breast MRI: A new application for the Z 0011 Era. Eur. J. Radiol. 2015, 84, 392–397. [Google Scholar] [CrossRef]


| Characteristic | ALN Metastasis (n = 277) | ALN Non-Metastasis (n = 338) |
|---|---|---|
| Age, (mean ± SD), year | 51.13 ± 10.63 | 50.14 ± 11.13 |
| Menopausal status, n (%) | ||
| Premenopause | 117 (42.2%) | 172 (50.9%) |
| Postmenopause | 160 (57.8%) | 166 (49.1%) |
| Tumor size, (mean ± SD), cm | 3.20 ± 1.66 | 2.42 ± 1.22 |
| Histological type, n (%) | ||
| Invasive ductal carcinoma | 264 (95.3%) | 287 (84.9%) |
| Invasive lobular carcinoma | 6 (2.2%) | 8 (2.4%) |
| Others | 7 (2.5%) | 43 (12.7%) |
| Histological grade, n (%) | ||
| Grade I | 8 (2.9%) | 18 (5.3%) |
| Grade II | 112 (40.4%) | 132 (39.1%) |
| Grade III | 143 (51.6%) | 147 (43.5%) |
| Not available | 14 (5.1%) | 41 (12.1%) |
| ER status | ||
| Negative | 78 (28.2%) | 94 (27.8%) |
| Positive | 199 (71.8%) | 244 (72.2%) |
| PR status | ||
| Negative | 93 (33.6%) | 124 (36.7%) |
| Positive | 184 (66.4%) | 214 (63.3%) |
| HER-2 status | ||
| Negative | 174 (62.8%) | 250 (74.0%) |
| Positive | 103 (37.2%) | 88 (26.0%) |
| Ki-67 status | ||
| <14 | 33 (11.9%) | 50 (14.8%) |
| ≥14 | 244 (88.1%) | 288 (85.2%) |
| Molecular subtypes | ||
| Luminal A | 16 (5.8%) | 33 (9.8%) |
| Luminal B | 192 (69.3%) | 210 (62.1%) |
| HER-2 positive | 42 (15.2%) | 39 (11.5%) |
| Triple negative | 27 (9.7%) | 56 (16.6%) |
| Accuracy | Sensitivity | Specificity | Precision | F1 Score | AUC | ||
|---|---|---|---|---|---|---|---|
| 10-fold cross-validation set | Phase-3-radiomics | 0.6527 | 0.5512 | 0.7375 | 0.6462 | 0.5883 | 0.6982 |
| Type-19-radiomics | 0.6930 | 0.6926 | 0.6950 | 0.6573 | 0.6689 | 0.7638 | |
| Type-19 | 0.5178 | 0.2860 | 0.7071 | 0.4259 | 0.3375 | 0.5406 | |
| Type-19-combined | 0.7332 | 0.6621 | 0.7933 | 0.7379 | 0.6881 | 0.7793 | |
| Independent testing set | Phase-3-radiomics | 0.6186 | 0.4337 | 0.7549 | 0.5902 | 0.5000 | 0.5585 |
| Type-19-radiomics | 0.6595 | 0.6024 | 0.7059 | 0.6250 | 0.6135 | 0.6565 | |
| Type-19 | 0.4811 | 0.2530 | 0.6667 | 0.3138 | 0.3043 | 0.4352 | |
| Type-19-combined | 0.6703 | 0.5904 | 0.7353 | 0.6447 | 0.6164 | 0.6742 |
| 10-Fold Cross-Validation | Independent Testing Set | |
|---|---|---|
| Type-19-radiomics vs. phase-3-radiomics | p = 0.002 | p = 0.037 |
| Type-19-radiomics vs. type-19 | p < 0.001 | p < 0.001 |
| Type-19-combined vs. phase-3-radiomics | p < 0.001 | p = 0.006 |
| Type-19-combined vs. type-19 | p < 0.001 | p < 0.001 |
| Type-19-combined vs. type-19-radiomics | p = 0.156 | p = 0.522 |
| Type-19 vs. phase-3-radiomics | p < 0.001 | p = 0.028 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; Chen, K.; Wang, M.; Wen, J.; Feng, S.; Luo, H.; He, C.; Guo, Y.; Luo, D.; Liu, X.; et al. Preoperative Prediction of Axillary Lymph Node Metastasis in Breast Cancer Using Radiomics Features of Voxel-Wise DCE-MRI Time-Intensity-Curve Profile Maps. Biomedicines 2025, 13, 2562. https://doi.org/10.3390/biomedicines13102562
Ren Y, Chen K, Wang M, Wen J, Feng S, Luo H, He C, Guo Y, Luo D, Liu X, et al. Preoperative Prediction of Axillary Lymph Node Metastasis in Breast Cancer Using Radiomics Features of Voxel-Wise DCE-MRI Time-Intensity-Curve Profile Maps. Biomedicines. 2025; 13(10):2562. https://doi.org/10.3390/biomedicines13102562
Chicago/Turabian StyleRen, Ya, Kexin Chen, Meng Wang, Jie Wen, Sha Feng, Honghong Luo, Cuiju He, Yuan Guo, Dehong Luo, Xin Liu, and et al. 2025. "Preoperative Prediction of Axillary Lymph Node Metastasis in Breast Cancer Using Radiomics Features of Voxel-Wise DCE-MRI Time-Intensity-Curve Profile Maps" Biomedicines 13, no. 10: 2562. https://doi.org/10.3390/biomedicines13102562
APA StyleRen, Y., Chen, K., Wang, M., Wen, J., Feng, S., Luo, H., He, C., Guo, Y., Luo, D., Liu, X., Liang, D., Zheng, H., Zhang, N., & Liu, Z. (2025). Preoperative Prediction of Axillary Lymph Node Metastasis in Breast Cancer Using Radiomics Features of Voxel-Wise DCE-MRI Time-Intensity-Curve Profile Maps. Biomedicines, 13(10), 2562. https://doi.org/10.3390/biomedicines13102562






