A Systematic Review of Literature on the Association Among Sleep, Cortisol Level and Cardiovascular Health Within the Healthcare Shift Worker Population
Abstract
1. Introduction
2. Methods
2.1. Study Selection Criteria
2.2. Search Strategy
2.3. Study Selection Process
2.4. Data Extraction Process for Each Study
2.5. Study Quality
3. Results
3.1. Studies Characteristics
3.2. Cortisol Level in the Morning and in the Evening in Healthcare Shift Workers
3.3. Relationship Between Cortisol Level and Shift Schedule
3.4. Relationship Between Cortisol and Sleep Quality
3.5. Relationship Between Cardiovascular Health and Sleep Quality
3.6. Shift and Sleep
4. Discussion
4.1. Sleep Health and Cortisol Level
4.2. Sleep Health and Cardiovascular Health
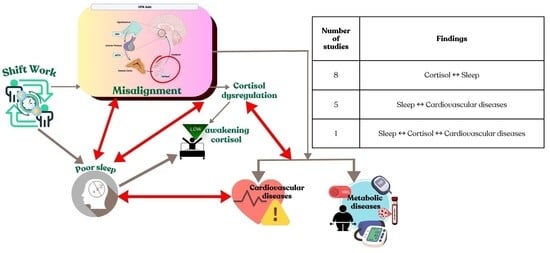
4.3. The Sleep, Cortisol and Cardiovascular Feedback Loop
5. Limitation
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAR | Cortisol awakening response |
| CARi | Cortisol levels |
| CPSQI | Chinese version of the Pittsburgh Sleep Quality Index cortisol awakening response |
| PSQI | Pittsburgh Sleep Quality Index |
| ESS | Epworth Sleepiness Scale |
| LSQ | Low sleep quality |
| HSQ | High sleep quality |
| TST | Total sleep time |
| SE | Sleep efficiency |
| WASO | Wake after sleep onset |
| SOL | Sleep onset latency |
| CVS | Cardiovascular |
| CVD | Cardiovascular disease |
| LF/HF | Low frequency/high frequency |
| LF | Low frequency |
| HF | High frequency |
| SDNN | Standard Deviation of NN intervals |
| RMSSD | Root Mean Square of Successive Differences |
| LLLT | Low-level LED light therapy |
| NN50 | Number of successive NN pairs differing by more than 50 ms |
| pNN50 | Percentage of NN intervals differing by greater than 50 ms |
| HR | Heart rate |
References
- Vallières, A.; Crawford, M.R. Changing the organizational work schedule of shift workers leads to improved sleep-an editorial. Sleep 2024, 11, 47. [Google Scholar] [CrossRef]
- Boivin, D.B.; Boudreau, P.; Kosmadopoulos, A. Disturbance of the Circadian System in Shift Work and Its Health Impact. J. Biol. Rhythm. 2022, 37, 3–28. [Google Scholar] [CrossRef] [PubMed]
- Costa, G. Shift work and health: Current problems and preventive actions. Saf. Health Work. 2010, 1, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Gurubhagavatula, I.; Barger, L.K.; Barnes, C.M.; Basner, M.; Boivin, D.B.; Dawson, D.; Drake, C.L.; Flynn-Evans, E.E.; Mysliwiec, V.; Patterson, P.D.; et al. Guiding principles for determining work shift duration and addressing the effects of work shift duration on performance, safety, and health: Guidance from the American Academy of Sleep Medicine and the Sleep Research Society. J. Clin. Sleep Med. 2021, 17, 2283–2306. [Google Scholar] [CrossRef]
- Reddy, S.; Reddy, V.; Sharma, S. Physiology, Circadian Rhythm; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Lee, Y.; Wisor, J.P. Multi-Modal Regulation of Circadian Physiology by Interactive Features of Biological Clocks. Biology 2021, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Al-Hrinat, J.; Al-Ansi, A.M.; Hendi, A.; Adwan, G.; Hazaimeh, M. The impact of night shift stress and sleep disturbance on nurses quality of life: Case in Palestine Red Crescent and Al-Ahli Hospital. BMC Nurs. 2024, 8, 23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mohd-Azmi, N.A.S.; Juliana, N.; Mohd Fahmi Teng, N.I.; Azmani, S.; Das, S.; Effendy, N. Consequences of Circadian Disruption in Shift Workers on Chrononutrition and their Psychosocial Well-Being. Int. J. Environ. Res. Public Health 2020, 17, 2043. [Google Scholar] [CrossRef]
- Chellappa, S.L.; Vujovic, N.; Williams, J.S.; Scheer, F.A.J.L. Impact of Circadian Disruption on Cardiovascular Function and Disease. Trends Endocrinol. Metab. 2019, 10, 767–779. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Byrne, N.A.; Yuen, F.; Butt, W.Z.; Liu, P.Y. Sleep and Circadian Regulation of Cortisol: A Short Review. Curr. Opin. Endocr. Metab. Res. 2021, 18, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Bidlingmaier, M.; Petru, R.; Pedrosa Gil, F.; Loerbroks, A.; Angerer, P. Impact of shift work on the diurnal cortisol rhythm: A one-year longitudinal study in junior physicians. J. Occup. Med. Toxicol. 2018, 13, 23. [Google Scholar] [CrossRef]
- McDowall, K.; Murphy, E.; Anderson, K. The impact of shift work on sleep quality among nurses. Occup. Med. 2017, 67, 621–625. [Google Scholar] [CrossRef]
- Huang, X.X.; Jiang, X.M.; Zheng, Q.X.; Chen, X.Q. The association between circadian rhythm of cortisol and shift work regularity among midwives-A multicenter study in Southeast China. Front. Public. Health 2022, 10, 965872. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Niu, S.F.; Chung, M.H.; Chu, H.; Tsai, J.C.; Lin, C.C.; Liao, Y.M.; Ou, K.L.; O’Brien, A.P.; Chou, K.R. Differences in cortisol profiles and circadian adjustment time between nurses working night shifts and regular day shifts: A prospective longitudinal study. Int. J. Nurs. Stud. 2015, 52, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.P.; Peng, Y.X. Differences between fixed day shift workers and rotating shift workers in gastrointestinal problems: A systematic review and meta-analysis. Ind. Health 2021, 59, 66–77. [Google Scholar] [CrossRef]
- Hsu, H.C.; Lee, H.F.; Lin, M.H. Exploring the Association between Sleep Quality and Heart Rate Variability among Female Nurses. Int. J. Environ. Res. Public Health 2021, 18, 5551. [Google Scholar] [CrossRef] [PubMed]
- Grandner, M.A. Sleep, Health, and Society. Sleep Med. Clin. 2017, 1, 1–22. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Andreadi, A.; Andreadi, S.; Todaro, F.; Ippoliti, L.; Bellia, A.; Magrini, A.; Chrousos, G.P.; Lauro, D. Modified Cortisol Circadian Rhythm: The Hidden Toll of Night-Shift Work. Int. J. Mol. Sci. 2025, 5, 2090. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Belloir, J.; Makarem, N.; Shechter, A. Sleep and Circadian Disturbance in Cardiovascular Risk. Curr. Cardiol. Rep. 2022, 12, 2097–2107. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Covassin, N.; Singh, P. Sleep Duration and Cardiovascular Disease Risk: Epidemiologic and Experimental Evidence. Sleep Med. Clin. 2016, 1, 81–89. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cunningham, T.R.; Guerin, R.J.; Ferguson, J.; Cavallari, J. Work-related fatigue: A hazard for workers experiencing disproportionate occupational risks. Am. J. Ind. Med. 2022, 11, 913–925. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hammer, B.; Virgili, E.; Bilotta, F. Evidence-based literature review: De-duplication a cornerstone for quality. World J. Methodol. 2023, 5, 390–398. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, X.; Dai, X.; Jiao, J.; Lee, S.Y. Impact of sleep-wake features on fatigue among female shift work nurses. Ann. Med. 2023, 55, 2210843. [Google Scholar] [CrossRef]
- Vivarelli, S.; Italia, S.; Teodoro, M.; Pollicino, M.; Vitello, C.; De-Vita, A.; Alibrandi, A.; Costa, C.; Fenga, C. Salivary Biomarkers Analysis and Neurobehavioral Assessment in Nurses Working Rotation Shifts: A Pilot Study. Int. J. Environ. Res. Public Health. 2023, 20, 5376. [Google Scholar] [CrossRef]
- Minelli, A.; Di-Palma, M.; Rocchi, M.B.L.; Ponzio, E.; Barbadoro, P.; Bracci, M.; Pelusi, G.; Prospero, E. Cortisol, chronotype, and coping styles as determinants of tolerance of nursing staff to rotating shift work. Chronobiol. Int. 2021, 38, 666–680. [Google Scholar] [CrossRef]
- Ljevak, I.; Vasilj, I.; Lesko, J.; Neuberg, M.; Perić, O.; Ćurlin, M. The impact of shift work on the metabolism and circadian rhythm in nurses and medical technicians. Acta Clin. Croat. 2020, 60, 476–482. [Google Scholar] [CrossRef]
- Bani-Issa, W.; Radwan, H.; Al-Marzooq, F.; Al-Awar, S.; Al-Shujairi, A.M.; Samsudin, A.R.; Khasawneh, W.; Albluwi, N. Salivary Cortisol, Subjective Stress and Quality of Sleep Among Female Healthcare Professionals. J. Multidiscip. Healthc. 2020, 13, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.Y.; Hsu, J.Y.; Lin, C.H.; Kuo, Y.C.; Chen, C.H.; Chen, H.Y.; Liu, S.J.; Chien, K.L. Association of stress hormones and the risk of cardiovascular diseases systematic review and meta-analysis. Int. J. Cardiol. Cardiovasc. Risk Prev. 2024, 10, 200305. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.P. Influence of shift type on sleep quality of female nurses working monthly rotating shifts with cortisol awakening response as mediating variable. Chronobiol. Int. 2018, 35, 1503–1512. [Google Scholar] [CrossRef]
- Lowson, E.; Middleton, B.; Arber, S.; Skene, D.J. Night work impact on nurses and families. Sleep Biol. Rhythm. 2013, 11, 7–13. [Google Scholar] [CrossRef]
- Lajoie, P.; Aronson, K.J.; Day, A.; Tranmer, J. A cross-sectional study of shift work, sleep quality and cardiometabolic risk in female hospital employees. BMJ Open 2015, 5, e007327. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Silva-Costa, A.; Griep, R.H.; Rotenberg, L. Disentangling the effects of insomnia and night work on cardiovascular diseases: A study in nursing professionals. Braz. J. Med. Biol. Res. 2015, 48, 120–127. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Panwar, A.; Bagla, R.K.; Mohan, M.; Rathore, B.B. Influence of shift work on sleep quality and circadian patterns of heart rate variability among nurses. J. Family Med. Prim. Care 2024, 8, 3345–3349. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liao, Y.H.; Tai, C.J.; Ming, J.L.; Lin, L.H.; Chien, L.Y. The Effectiveness of Low-Level LED Light Therapy for Sleep Problems, Psychological Symptoms, and Heart Rate Variability in Shift-Work Nurses: A Randomized Controlled Trial. J. Nurs. Manag. 2025, 86, 6478834. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roy, S.; Gupta, S.; Mukherjee, S.; Das, M. A Study to Assess Changes in Cortisol Level and Heart Rate Variability between Fixed Duty and Shift Duty Health Care Providers in a Tertiary Care Hospital A Cross-Sectional Study. J. Clin. Diagn. Res. 2023, 17, CC06–CC12. [Google Scholar] [CrossRef]
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological Quality of Case Series Studies: An Introduction to the Jbi Critical Appraisal Tool. JBI Evid. Synth. 2020, 18, 2127–2133. [Google Scholar] [CrossRef]
- Tsai, J.C.; Chou, K.R.; Tsai, H.T.; Yen, Y.C.; Niu, S.F. Effects of Nocturnal Sleep Quality on Diurnal Cortisol Profiles and Attention in Nurses: A Cross-Sectional Study. Biol. Res. Nurs. 2019, 21, 510–518. [Google Scholar] [CrossRef]
- Mason, I.C.; Qian, J.; Adler, G.K.; Scheer, F.A.J.L. Impact of circadian disruption on glucose metabolism: Implications for type 2 diabetes. Diabetologia 2020, 63, 462–472. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morris, C.J.; Purvis, T.E.; Hu, K.; Scheer, F.A. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc. Natl. Acad. Sci. USA 2016, 113, E1402–E1411. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stafford, M.; Ben-Shlomo, Y.; Cooper, C.; Gale, C.; Gardner, M.P.; Geoffroy, M.C.; Power, C.; Kuh, D.; Cooper, R. Diurnal cortisol and mental well-being in middle and older age: Evidence from four cohort studies. BMJ Open 2017, 7, e016085. [Google Scholar] [CrossRef]
- Hackett, R.A.; Kivimäki, M.; Kumari, M.; Steptoe, A. Diurnal Cortisol Patterns, Future Diabetes, and Impaired Glucose Metabolism in the Whitehall II Cohort Study. J. Clin. Endocrinol. Metab. 2016, 101, 619–625. [Google Scholar] [CrossRef]
- James, S.M.; Honn, K.A.; Gaddameedhi, S.; Van-Dongen, H.P.A. Shift Work: Disrupted Circadian Rhythms and Sleep-Implications for Health and Well-Being. Curr. Sleep Med. Rep. 2017, 3, 104–112. [Google Scholar] [CrossRef]
- Jarcho, M.R.; Slavich, G.M.; Tylova-Stein, H.; Wolkowitz, O.M.; Burke, H.M. Dysregulated diurnal cortisol pattern is associated with glucocorticoid resistance in women with major depressive disorder. Biol. Psychol. 2013, 93, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Walker, W.H., II; Walton, J.C.; DeVries, A.C.; Nelson, R.J. Circadian rhythm disruption and mental health. Transl. Psychiatry 2020, 10, 28. [Google Scholar] [CrossRef]
- Niu, S.F.; Chung, M.H.; Chen, C.H.; Hegney, D.; O’Brien, A.; Chou, K.R. The effect of shift rotation on employee cortisol profile, sleep quality, fatigue, and attention level: A systematic review. J. Nurs. Res. 2011, 19, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Garraio, C.; Matias, M.; Matos, P.M. Working time arrangements and exhaustion: The role of recovery experiences and satisfaction with the schedule. Scand. J. Psychol. 2023, 64, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Moreno, C.R.C.; Marqueze, E.C.; Sargent, C.; Wright, K.P., Jr.; Ferguson, S.A.; Tucker, P. Working Time Society consensus statements: Evidence-based effects of shift work on physical and mental health. Ind. Health 2019, 57, 139–157. [Google Scholar] [CrossRef]
- Lo-Martire, V.; Caruso, D.; Palagini, L.; Zoccoli, G.; Bastianini, S. Stress & sleep: A relationship lasting a lifetime. Neurosci. Biobehav. Rev. 2020, 117, 65–77. [Google Scholar] [CrossRef]
- Folkard, S. Do permanent night workers show circadian adjustment? A review based on the endogenous melatonin rhythm. Chronobiol. Int. 2008, 25, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Günther, A.; Witte, O.W.; Hoyer, D. Autonomic dysfunction and risk stratification assessed from heart rate pattern. Open Neurol. J. 2010, 4, 39–49. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xhyheri, B.; Manfrini, O.; Mazzolini, M.; Pizzi, C.; Bugiardini, R. Heart rate variability today. Prog. Cardiovasc. Dis. 2012, 55, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Itani, O.; Jike, M.; Watanabe, N.; Kaneita, Y. Short sleep duration and health outcomes: A systematic review, meta-analysis, and meta-regression. Sleep Med. 2017, 32, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Cappuccio, F.P.; Cooper, D.; D’Elia, L.; Strazzullo, P.; Miller, M.A. Sleep duration predicts cardiovascular outcomes: A systematic review and meta-analysis of prospective studies. Eur. Heart J. 2011, 12, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; An, X.; Yang, C.; Sun, W.; Ji, H.; Lian, F. The crucial role and mechanism of insulin resistance in metabolic disease. Front. Endocrinol 2023, 14, 1149239. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morris, C.J.; Yang, J.N.; Garcia, J.I. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc. Natl. Acad. Sci. USA 2016, 113, E528–E535. [Google Scholar] [CrossRef] [PubMed]
- Dressle, R.J.; Feige, B.; Spiegelhalder, K.; Schmucker, C.; Benz, F.; Mey, N.C.; Riemann, D. HPA axis activity in patients with chronic insomnia: A systematic review and meta-analysis of case-control studies. Sleep Med. Rev. 2022, 62, 101588. [Google Scholar] [CrossRef] [PubMed]
- Hirotsu, C.; Tufik, S.; Andersen, M.L. Interactions between sleep, stress, and metabolism: From physiological to pathological conditions. Sleep Sci. 2015, 8, 143–152. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caruso, C.C. Negative impacts of shiftwork and long work hours. Rehabil. Nurs. 2014, 39, 16–25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


| Author | Sample Size (n) | Exposure of Working Shift | Cortisol Measures | Sleep Quality Measures | Outcome | Conclusions |
|---|---|---|---|---|---|---|
| Zhang, 2023 (China) [23] | Nurses (n = 152) | Type of shift: 8 h (day–evening–night) and 12 h (day–night) | Saliva samples were collected between: 7:00–8:00 and 19:00–20:00 | Sleep-wake indexes are analyzed by actigraphy data | Compared with the 8 h shift nurses, the 12 h shift nurses had: Cortisol result: Higher saliva cortisol levels before the day shift (median 0.54 vs. 0.31, p < 0.005) and after the night shift (median 0.51 vs. 0.31, p < 0.005). Sleep result: Significantly more TST (456 vs. 364 min) Longer reaction time before the night shift (286 vs. 277 min). | Fast rapid rotation in shift workers disturbed circadian rhythm and reduced total sleep time. |
| Vivarelli, 2023 (Italy) [24] | Nurses (n = 102) | Type of shift: Night shift with forward rotating shift. Morning shift followed by an afternoon shift and a night shift, then followed by a rest day | Saliva: morning and evening | PSQI ESS | Cortisol result: 17% had above the threshold limit of morning salivary cortisol. 91% had below the threshold limit of evening cortisol. Sleep result: PSQI: 75% good sleep, 21.6% poor sleep. 2.9% bad sleep. ESS: 85.3% no daytime sleepiness | There was a positive relationship between cortisol and sleepiness, even if not significant. |
| Minelli, 2021 (Italy) [25] | Nurses (n = 30) | Type of shift: Morning shift 06:00 Afternoon shift 14:00 Night shift 22:00 | Saliva: Four salivary samples were collected at 21:00, 24:00, 03:00 and 06:00 | PSQI | Cortisol result: Cortisol concentration is low around 21:00 and 24:00, then increasing during early morning between 03:00 and 06:00. Cortisol output during the early night shift failed to correlate with PSQI score. Cortisol output during night shifts positively correlates with basal cortisol diurnal secretion measured during non-working days. Sleep result: PSQI: 76% had good sleep. | Rotating shift workers showed that low total cortisol during night work is associated with circadian misalignment. |
| Ljevak, 2020 (Croatia) [26] | Subjects (n = 157): 135 female nurses. 22 male medical technicians | Control group: Morning shifts (7:30 to 14:30) Experimental group: 12 working hours to 24 h off-work and 12 working night shift hours to 48 h off-work | Blood: collected at 7.30 a.m. | Standard Shiftwork Index | Cortisol result: 5% of shift workers had morning cortisol levels lower than the reference values, and 5% had morning cortisol levels higher than the reference values. Sleep result: Shift workers showed significantly higher levels of sleep difficulties than the first-shift workers. A strong correlation was found in the rate of sleep disturbances between morning shift workers and those participants working a rotating shift system. | Shift workers had impaired cortisol levels and higher levels of sleep difficulties. |
| Bani-Issa, 2020 (UAE) [27] | Healthy adult women healthcare professionals (n = 335) | Day or night shifts | Saliva samples were collected at: (7:00–8:00) and (19:00–20:00) | PSQI | Cortisol result: Morning cortisol level: 36.1% had impaired below average levels. Bedtime cortisol level:14.3% had high bedtime cortisol levels. There was a significant correlation between morning and bedtime cortisol levels (r = 0.196, p = 0.001). Sleep result: 60.3% had poor sleep quality. Morning and bedtime cortisol levels were not significantly correlated with quality of sleep (r = 0.26, p = 0.57 for morning cortisol; r = 0.013, p = 0.92 for bedtime cortisol). | Shift workers had impaired morning and bedtime cortisol level with poor sleep quality. Cortisol levels in the morning and at night were independently correlated with sleep quality. |
| Tsai, 2019 (Taiwan) [28] | Female nurse (n = 61) | Worked 8 h per shift. 4 weeks of regular day-shift work (8:00–16:00) | 4 saliva samples collected at: awakening and after awakening 0 min, 30 min, 6 h, 12 h. | PSQI Actigraphy data | Cortisol result: LSQ had a higher cortisol concentration at awakening (0 min) than did the HSQ. LSQ had a flatter CAR and smaller diurnal cortisol slope 30 min to 12 h than HSQ. HSQ had significantly higher cortisol concentration at 30 min after awakening (11.79 + 5.80 ng/mL) than LSQ. HSQ had significantly lower mean cortisol concentration at 12 h after awakening (2.40 + 2.48 ng/mL) than LSQ. Sleep result: 54.3% LSQ (CPSQI > 5), 45.7% HSQ (CPSQI 5), HSQ had greater TST, higher SE, and shorter WASO and SOL than the LSQ. | Low sleep quality showed flatter cortisol awakening response (CAR). |
| Chang, 2018 (Taiwan) [29] | Female nurses (n = 128) | Types of shifts: 08:00–16:00 16:00–24:00 00:00–08:00 | Saliva: Collected after waking and 30 min after waking. | PSQI | Cortisol result: CARi night shift and the evening shift workers is significantly lower than the day shift. Shift type significantly influenced CARi (F = 19.66, p < 0.001). Sleep result: Evening shift or night shift workers have significantly poorer sleep quality than those working the day shift. Shift type significantly influenced sleep quality (F = 15.13, p < 0.001). | There was a significant correlation between CARi and sleep quality; nurses with higher CARi had better sleep quality. |
| Lowson, 2013 (UK) [30] | Nurses (n = 20) | Types of shifts: Nurses (n = 15) 7 to 8 h and night shifts of 10 to 12 h, 07.00 to 21.30 h and finishing between 07.00 to 08.00. Nurses (n = 5) 12 h shifts with shift changes 20:00 and 08:00 | Saliva: Collected just after waking in the morning and just before sleeping in the evening. | PSQI | Cortisol result: Cortisol level soon after waking significantly lower (time 07:58 ± 38 min) (12.5 ± 5.6 nmol/L) (p < 0.001) than the late evening 21:53 ± 1 h 17 min) 4.0 ± 5.2 nmol/L During periods of night work: The early morning cortisol levels for nurses was 10.7 ± 5.7 nmol/L which was significantly lower (p < 0.05) than the early morning cortisol level (14.2 ± 5.0 nmol/L) for other times the early morning saliva samples were collected significantly later (p < 0.01) compared with other mornings (08:16 ± 34 min) following night shifts compared with 07:41 ± 34 min for other mornings). For late evening cortisol levels, higher cortisol levels (4.9 ± 7.2 nmol/L) were found during periods of night work (non-significant) (p = 0.102) compared with other times (3.0 ±1.5 nmol/L). Sleep result: During periods of night work, sleep quality worse (nonsignificant) and more sleepiness (Karolinska Sleepiness Scale) before (p < 0.01) and after (p < 0.001) their main period of day sleep compared with night-time sleep-in periods of no night work. | Night shift workers reported to have worse sleep quality (non-significant) and lower early morning cortisol levels than other mornings. |
| Author (Country) | Sample Size (n) | Exposure of Working Shift | Cardiovascular Measures | Sleep Quality Measures | Outcome | Conclusions |
|---|---|---|---|---|---|---|
| Lajoie P., 2015 (Canada) [31] | Shift female hospital employees (n = 121) | Rotating 12 h day/night shifts | Metabolic syndrome | PSQI -sleep latency -sleep efficiency | CVS result: Rotating 12 h shift work was associated with two fold increased risk of metabolic syndrome (OR = 2.29) among female hospital workers. Sleep result: Night shift workers had significant poor sleep quality than day workers with 47.9% scoring > 5 on the PSQI compared to 32.7% of day workers (p < 0.01). They also reported poor sleep latency (42% vs. 27%) and lower sleep efficiency, indicating reduced sleep quality. | Shift work strongly associated with metabolic syndrome among female workers however sleep quality was not a significant mediator and no significant association with metabolic syndrome. |
| Silva-Costa A, 2015 (Brazil) [32] | Night shift nurses (n = 340) | Work night shifts at least once a week or four times per month | Self-reported physician diagnosed cardiovascular disease: hypertension, coronary disease, myocardial infarction, or heart failure | Insomnia | CVS result: The proportion of self-reported physician diagnosed CVD was 18% among day workers and 21% among night workers. Sleep result: The prevalence among night workers reported that 24% had nocturnal insomnia, 35% had diurnal insomnia, 13% experienced both while 23% of day workers reported nocturnal insomnia. | Night workers who had insomnia during both sleep episodes had three times higher odds of developing cardiovascular disease compared to those without insomnia. |
| Hsu HC, 2021 (Taiwan) [16] | Female shift work nurses (n = 393) | Heart rate variability (HRV): TP, LF, HF, LF/HF, SDNN, RMSSD | PSQI | CVS result: Reduced autonomic function (lower TP) and higher mean heart rate are moderately predictive of poor sleep quality. Lower low frequency and total power and higher high frequency showed an altered autonomic balance. Sleep result: 95.9% had poor sleep quality, scoring 10.2. The majority had sleep efficiency less than 85%, and 2.8% reported no difficulty falling back asleep after waking. | Poor sleep quality correlated with lower TP and LF. Poor sleep in nurses is linked to autonomic imbalance, potentially elevating cardiovascular risk. | |
| Panwar A, 2024 (India) [33] | Female nurses (n = 38) | Morning (9 a.m. to 2 p.m.) shift and night shift (9 p.m.–9 a.m.) | HRV: frequency and time domains | PSQI | CVS result: Decrease in standard deviation of normal-to-normal intervals, total power, and high-frequency power while increase in LF/HF ratio. Sleep result: Night shift nurses had significantly poorer sleep quality than morning shift nurses. | Night shift work is related to poor sleep quality and significant disruptions of heart rate variability. |
| Liao YH, 2025 (Taiwan) [34] | Shift-work nurses (Intervention group: 32; Control group: 32) (n = 64) | 4-week shift work schedule in the last 6 months (day, evening, night shift) LLLT using red and near-infrared light, administered three times a week for one month | Heart rate variability | Insomnia Severity Index | CVS result: No significant difference between in HRV outcomes. Sleep result: Intervention group resulted 4.3, controls 12.6 (p < 0.001), reflecting significantly less severe insomnia in the LED-LLLT group. | LLLT was effective in improving in shift-work nurses with insomnia. However, it did not cause significant changes in heart rate variability. |
| Author | Sample Size (n) | Exposure of Working Shift | Cortisol Measure | Cardiovascular Measure | Sleep Quality Measure | Outcome | Conclusions |
|---|---|---|---|---|---|---|---|
| Roy, 2023 (India) [35] | Healthcare providers: 60 fixed duty and 60 shift duty (n = 120) | Fixed time schedule: 10.00–17.00 (Monday to Friday) Shift duty schedule: Two morning (6:00–14:00), two evening (14:00–22:00), two night duties (22:00–6:00) | Blood | HRV (SDNN, RMSSD, NN50, pNN50, mean HR) | Athens Insomnia Scale | Cortisol result: Shift duty workers had significantly higher evening cortisol levels (9.4 ± 2.36 mcg/dL) than fixed duty workers (3.74 ± 1.7 mcg/dL) (p = 0.036). Cardiovascular result: Low HF power. LF/HF ratio significantly higher (p < 0.001) in shift workers Sleep result: Shift duty workers had a higher chance of having insomnia (Athen’s score > 6) (p < 0.001) than fixed duty workers. | Long duration of shift work increases evening cortisol level and increases the chance of having insomnia and impaired cardiovascular regulation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukor, A.N.A.; Juliana, N.; Abdul Hamid, N.; Teng, N.I.M.F.; Ithnin, M.; Azmani, S.; Kasim, S.S. A Systematic Review of Literature on the Association Among Sleep, Cortisol Level and Cardiovascular Health Within the Healthcare Shift Worker Population. Biomedicines 2025, 13, 2539. https://doi.org/10.3390/biomedicines13102539
Sukor ANA, Juliana N, Abdul Hamid N, Teng NIMF, Ithnin M, Azmani S, Kasim SS. A Systematic Review of Literature on the Association Among Sleep, Cortisol Level and Cardiovascular Health Within the Healthcare Shift Worker Population. Biomedicines. 2025; 13(10):2539. https://doi.org/10.3390/biomedicines13102539
Chicago/Turabian StyleSukor, Aslah Nabilah Abdull, Norsham Juliana, Nazefah Abdul Hamid, Nur Islami Mohd Fahmi Teng, Muslimah Ithnin, Sahar Azmani, and Sazzli Shahlan Kasim. 2025. "A Systematic Review of Literature on the Association Among Sleep, Cortisol Level and Cardiovascular Health Within the Healthcare Shift Worker Population" Biomedicines 13, no. 10: 2539. https://doi.org/10.3390/biomedicines13102539
APA StyleSukor, A. N. A., Juliana, N., Abdul Hamid, N., Teng, N. I. M. F., Ithnin, M., Azmani, S., & Kasim, S. S. (2025). A Systematic Review of Literature on the Association Among Sleep, Cortisol Level and Cardiovascular Health Within the Healthcare Shift Worker Population. Biomedicines, 13(10), 2539. https://doi.org/10.3390/biomedicines13102539